Abstract
Peroxidases are the ubiquitous enzyme and reported to be present in all living genera. They catalyses reduction of peroxide and generate reactive oxygen species. In the present study we demonstrated that insect infestation induces peroxidase activity in sap and total soluble protein (TSP) of plant leaves. Three important crop plants viz. tomato, cowpea and cotton were used for this study. After infestation of chewing insect, Peroxidase activity in the sap and TSP of all the studied plants were enhanced in the range of 1.6 to 3.14 fold. Similar observations were also obtained with feeding of sap sucking insects, in which increment in peroxidase activity of sap and TSP was in the range of 1.8 to 2.53 fold. Enhanced peroxidase activity was reconfirmed by in-gel peroxidase assay. Enzyme kinetic study showed turn over efficiency of peroxidase from cotton (~101.3 min-1) was almost similar to tomato (~100.8 min-1) but higher than cowpea (~98.21min-1). MS/MS analysis of observed band showed significant similarity with the reported peroxidases in database.
Introduction
Plants are major feeding source for insects and other living organisms. Insects feed nearly all parts of plants by different feeding methods and cause severe damages especially to the crop plants.1 Coleopteran and lepidopteran insects damage crop by direct chewing or biting of different plant parts however homopteran insects cause direct (by sucking phloem sap) as well as indirect damage (by spreading various viral diseases). Plant insect interaction is reported for the past hundreds of years. Both insect and plant exchange chemical and other signals which establish interaction between them. The integrated responses in plants direct the production of phytochemicals against the feeding arthropods.2
Plants are sessile organism therefore mainly depend on chemical or other phenolic compound for defense against insect attack. They use toxic secondary metabolites, proteins, hormones, and several types of structural and phytochemical changes to repel the insects. Overexpression of some oxidative peroxidases has also been reported as defense responsive system in plants. Several reports are available in which activity of oxidative enzymes like peroxidase found to be increased after insect attack.3,4 Reactive oxygen species (ROS) signaling and hormone-signaling pathways are strongly linked with plant–insect interactions.5 Differential protein expression analysis of rice after infestation with brown plant hopper showed upregulation of numerous peroxidases along with many other enzymes.6 Further, transcriptomic studies of susceptible and resistant cultivars of Sorghum and Brassica oleracea in response to insect attack have shown remarkable changes in oxidative stress related transcripts.7,8 Sitka spruce also showed overexpression of many proteins including a number of peroxidases after infestation with budworm and pine weevils.9 Maffei et al. (2006)10 observed that when Lima bean leaves challenged with Spodoptera littoralis the peroxidase activity increased significantly than the unchallenged and mechanically wounded plants.
Peroxidases oxidize several compounds by using H2O2. They are generally heme group containing glycoproteins and divided into acidic, basic and neutral types in plants. Plants peroxidases have many forms, which are encoded by multi gene families. Several utilities of peroxidases have been reported in plants, like degradation of H2O2, removal of toxic compounds, defense against insect herbivore and many other stress related responses.11
In present study we have analyzed differential enhancement in peroxidase activity in response to 2 major types of insect pests (chewing and sucking) on 3 agriculturally important crop plants (cotton, tomato and cowpea). The major objective of the present study was to decipher, if any, role of insect attack (chewing or sucking) on stress enzyme (peroxidases) as well as to establish that defensive peroxidase activity enhancement was varied with each crop. Further MS-MS analysis revealed the presence of 4 different forms of peroxidaes in in-gel peroxidase activity and their presence is also varied according to the plant species.
Results
Quantification of total sap protein
Sap was extracted from 3 different plants (cowpea, cotton and tomato) before and after 48 h of insect infestation (chewing type; Spodoptera litura, sucking type; Aphis craccivora and Bemisia tabaci) and total protein content was estimated by Bradford method. Before infestation, quantity of total proteins in the sap of cotton, tomato and cowpea was 133, 69 and 34 µg/ml, respectively. Protein content in all plants was decreased after infestation of chewing as well as sap sucking insects. After infestation with sap sucking insect, protein content in sap of cowpea, cotton and tomato was decreased by 30, 41.66, and 17.70%, respectively (Table 1), however in the case of chewing insect it was declined by 26.47, 24.24, and 10.14%, respectively.
Table1. Quantity of protein in plant sap.
| Plant | Infected by | Protein quantity in sap (µg/ml) |
|---|---|---|
| Cowpea | - | 34.00 ± 2.10 |
| Sucking insect | 23.80 ± 1.02 | |
| Chewing insect | 25.05 ± 1.80 | |
| Cotton | - | 133.00 ± 3.21 |
| Sucking insect | 77.65 ± 2.09 | |
| Chewing insect | 100.75 ± 1.21 | |
| Tomato | - | 68.90 ± 0.97 |
| Sucking insect | 54.80 ± 1.41 | |
| Chewing insect | 61.90 ± 2.11 |
Quantification of total soluble protein (TSP) from plants leaves
Bradford assay revealed that, cowpea having maximum amount (3.78 mg/ml) of TSP which was decreased after insect attack (48 h). TSP of Cowpea leaves was decreased by 27.29 and 48.14% after the infestation of sap sucking (2.75 mg/ml) and chewing (1.96 mg/ml) insects, respectively. Further, TSP content of cotton leaves was decreased by 32.8 and 45.8% after the infestation of sap sucking (1.73 mg/ml) and chewing insect (1.38 mg/ml) respectively, over control (2.55 mg/ml). Minimum TSP content was found in tomato plant (2.48 mg/ml) which was also decreased after insect feeding. Feeding of sap sucking insect (1.85mg/ml) decreased TSP content by 25.40% while chewing insect (1.68 mg/ml) decreased by 32.25% (Table 2).
Table 2. Total soluble protein contained in the leaves of plant.
| Plant | Infected by | TSP in leaves (mg/ml) |
|---|---|---|
| Cowpea | - | 3.78 ± 0.42 |
| Sucking insect | 2.75 ± 0.38 | |
| Chewing insect | 1.96 ± 0.21 | |
| Cotton | - | 2.55 ± 0.35 |
| Sucking insect | 1.73 ± 0.27 | |
| Chewing insect | 1.38 ± 0.57 | |
| Tomato | - | 2.48 ± 0.37 |
| Sucking insect | 1.85 ± 0.29 | |
| Chewing insect | 1.68 ± 0.22 |
Effect of insect infestation on Peroxidase activity
Sap and TSP of all the 3 plants were analyzed for peroxidase enzyme assay in microtiter plate using TMB/H2O2 as substrate.
Effect of chewing insect
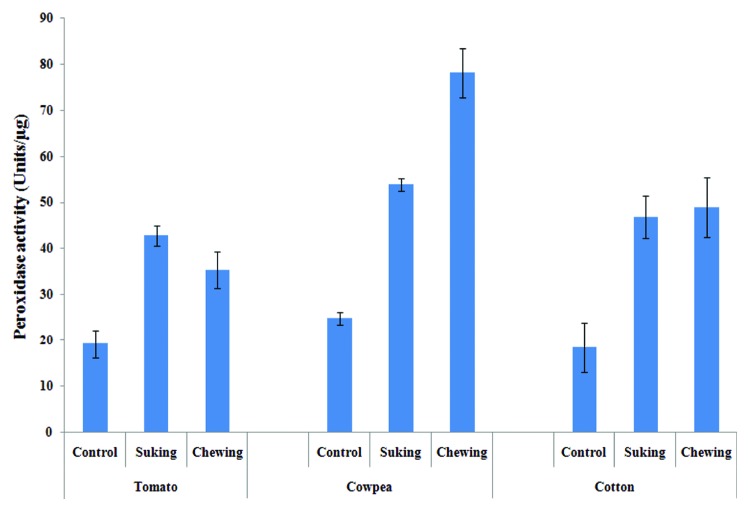
Plants were infested with chewing insect (Spodoptera) for 48 h and then peroxidase activity was measured. Peroxidase activity in the sap of all 3 tested crop plants was significantly enhanced after insect feeding. Sap of tomato, cotton and cowpea having 19.16, 18.44 and 24.77 units/µg peroxidase activity which was 1.8 (35.38 U/µg), 2.65 (48.87 U/µg), and 3.14 (78.01 U/µg) fold increased after Spodoptera infestation (Fig. 1). Similarly, peroxidase activity was also raised in TSP of plants; tomato, cotton and cowpea having 2.12, 2.49, and 2.01 units/µg peroxidase activity which was later enhanced by 2.4 (5.11 U/µg), 1.69 (4.08 U/µg), and 2.19 (4.42 U/µg) fold upon Spodoptera infestation (Fig. 2)
Figure 1. Peroxidase activity in the sap of plants after insect infestation. Graph showed the enhancement of peroxidase activity in the sap of all 3 plants (tomato, cowpea, and cotton) over their control (uninfested) plant.
Figure 2. Peroxidase activity in total soluble protein (TSP) of plant’s leaves after insect infestation. Enhancement of peroxidase activity was observed in the TSP of all 3 plants (tomato, c owpea, and cotton) compared with their control (uninfested) plant.
Effect of sap sucking insect
Insect were allowed to feed on plant for a constant time interval (48 h) and then peroxidase activity was calculated in sap as well as TSP of plants. In tomato plant enzyme activity was 2.23 (42.75 U/µg) and 1.9 (4.15 U/µg) fold elevated over control (described earlier) in sap and TSP, respectively. However, enhancement of peroxidase activity in the sap and TSP of cowpea plant was 2.17 (53.82 U/µg) and 2.35 (4.66 U/µg) fold respectively after insect infestation. Pattern of change in peroxidase activity after insect feeding was also similar in cotton plant, activity was raised by 2.53 (46.81 U/µg) and 1.81 (4.38 U/µg) fold in sap and TSP of plant (Figs.1 and 2).
Increment of peroxidase activity in sap and TSP of all plants after the feeding of chewing as well as sucking insect was again reconfirmed by in-gel peroxidase assay. In-gel assay clearly indicated the enrichment in enzyme activity. Intensity of band was considerably high in infested lane in comparison to control 1. Faint band in control lane correspond to the basal level of peroxidase in plants, dense bands in insect infested lane clearly confirmed the enhancement of enzyme activity (Fig. 3). Observed band was excised out from the gel and preceded for MALDI-TOF-TOF analysis.
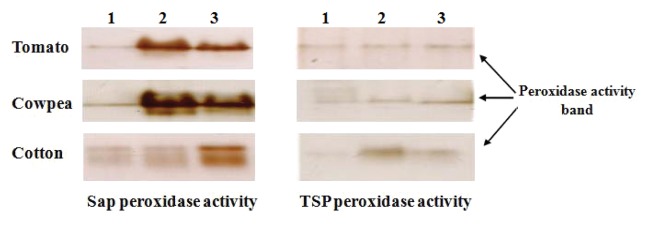
Figure 3. In-gel peroxidase assay with sap protein and TSP of plant’s leaves. 1, 2 and 3 lane represent un-infested (control), infested with sucking insect (whiteflies and aphid) and infested with chewing insect (Spodoptera), respectively.
Comparative enzyme kinetics of Peroxidase from plants
Peroxidase activities of all three plants followed standard Michaelis–Menten enzyme kinetics. Velocity of the enzyme catalyzed reaction increased continuously by increasing substrate concentration and became constant after a definite point (Fig. 4). GraphPad prism-6 analysis revealed that, Km value of cowpea’s enzyme (0.385 ± 0.02 mM) was minimum in all 3 tested enzyme followed by tomato (0.475 ± 0.04 mM) and cotton (0.622 ± 0.03 mM). However, Vmax of peroxidases of cowpea, tomato and cotton plants were 70.81 ± 1.32, 82.18 ± 2.23, and 75.09 ± 1.52 µM min−1 µg−1 , respectively. Turn over efficiency (Kcat) of peroxidase from cotton (~101.3 min−1) was almost similar to tomato (~100.8 min−1) but higher then cowpea (~98.21min−1).
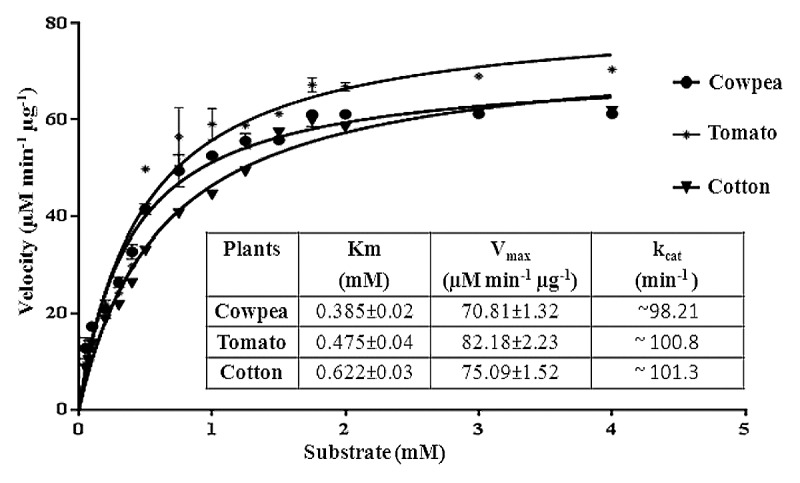
Figure 4. Michaelis–Menten curve of peroxidase catalyzed reaction of all 3 plants. The kinetic parameter of peroxidase from cowpea, cotton and tomato are also showed in table (inset).
Mass spectrometric analysis
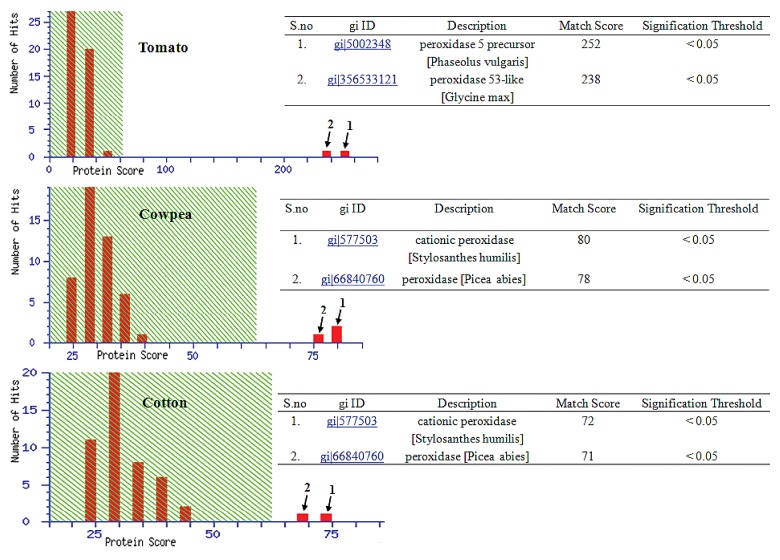
Excised protein band was reduced by DTT and then alkylated with iodoacetamide. Afterward, protein was digested with trypsin and peptides were examined on MALDI-TOF-TOF platform. Generated MS/MS data was analyzed on MASCOT search engine via a MASCOT MS/MS Ion Search. Obtained result confirmed that observed band on gel was peroxidase (Fig. 5). MS/MS data of tomato showed significant similarity with two protein i.e., peroxidase 5 precursor of Phaseolus vulgaris (gi|5002348; match score, 252) and peroxidase 53-like protein of Glycine max (gi|356533121; match score, 238) while MS/MS profiles of both cowpea and cotton were significantly matches with cationic peroxidase of Stylosanthes humilis (gi|577503) and peroxidase of Picea abies (gi|66840760).
Figure 5. MS/MS analysis of band observed in in-gel peroxidase assay. Detail of top 2 hits of matched protein of database are shown in image (inset table).
Discussion
Peroxidases are the key enzyme of defense related pathways in plants12 and play core role in response to wide range of pathogens.13 They also participate in many primary metabolic processes such as auxin metabolism, lignin and suberin formation, cross-linking of cell wall components, phytoalexin synthesis, and in metabolism of ROS and RNS.14 Peroxidases are coded by a large multigenic family. There are 138 and 73 members of peroxidases reported in rice and Arabidopsis, respectively.15,16
Present study reports that peroxidases were elicited by the infestation of chewing and sap sucking insects indicating their role in defense against phytophagous insects. Three crop plants (tomato, cowpea and cotton) were taken for the study and peroxidase activity measured after insect feeding in sap protein as well as TSP of plant leaves. Sap of Tomato, Cowpea and Cotton has 69, 34, and 133 µg/ml of protein, respectively. However it is already reported that, xylem sap of apple has 300 µg/ml of protein, whereas peach and pear xylem sap contained approximately 100 µg/ml of protein.17 TSP in the leaves of tested crop plant (tomato, 2.48%; cowpea, 3.78 mg/ml; cotton, 2.55 mg/ml) is 20–100 fold higher than its sap protein, as the sap is known for less protein content and mainly involved in transport of minerals, amino acids, organic acids, sugars, and sugar alcohol through sieve elements (SEs).18 SEs are the dead cells and not able to synthesize RNA and proteins. They only bring macromolecules through specialized plasmodesmata connecting SEs to the adjacent companion cells.19,20
Protein content in sap as well as leaves are significantly decreased after insect infestation because in biotic stresses plant de-accelerates the rate of protein synthesis21,22 and whole translation machinery shifted to produce defense related proteins.22 It can be the reason that protein content was decreased in both sap and leaves but the peroxidase activity was enhanced. Peroxidases are pathogenesis-related proteins (PRs) and having potential roles in the interaction between plants and insects.23 Our result suggested that peroxidase activity in sap of plants (tomato, 19.16; cotton, 18.44 and cowpea 24.77 units/µg) is much higher than TSP of leaves (tomato, 2.12; cotton, 2.49 and cowpea 2.01 units/µg), but fold change in activity of peroxidase after insect attack is almost similar in both the cases (Sap and TSP). Approximate ~1.6–~3 fold enrichment in peroxidase activity was observed in both sap and TSP. It suggested that each part of plant respond similarly upon insect attack. Level of peroxidase activity was enhanced after feeding of both chewing and sap sucking insect which showed that the similar pathway was activated in which peroxidase plays a critical role. In-gel assay data revealed that peroxidases are the major protein after insect attack in sap because level of total protein in sap was decreased but level of peroxidase enzyme was increased.
Enzyme kinetic study confirmed that peroxidase of all three tested plant follow the Michaelis–Menten enzyme kinetics. The turn over efficiency of cowpea peroxidase (98.21 min−1) was lowest among three examined plants because it catalyzes reaction with minimum velocity (70.81 ± 1.32µM min−1 µg−1). This might be a reason for low enhancement of sap peroxidase activity (2.17 fold) in sap sucking insect infested plants. However, maximum turnover efficiency of peroxidase from cotton (101.3 min−1) was resulted into the highest enhancement in peroxidase activity (2.53 fold) in sap sucking insect infested plants. The results confirmed that the level of peroxidase activity enhancement after insect infestation was directly correlated with the efficiency of enzyme.
MS/MS profile of cowpea and cotton showed significant similarity by maximum score with cationic peroxidase of Stylosanthes humilis while in tomato it matches with peroxidase-5-precursor of Phaseolus vulgaris by maximum score. Young et al. (1995)24 also demonstrated that cationic peroxidases were accumulates in xylem vessels during incompatible interactions with Xanthomonas oryzae in rice. However, it was also reported earlier that overexpression of anionic peroxidase confers resistance against Lepidopteran and Coleopteran insects.11
Present study suggested that defense mechanism of plants against sap sucking and chewing insect was similarly induced by both the insects and involves peroxidase enzyme as a key player. Peroxidases may be used to raise insect resistant plants against broad range of insects by transgenic approach.
Materials and Methods
Plant material
Cowpea (Vigna ungucuilata), cotton (Gosypium hirsutum) and tomato (Lycopersicum esculentum) plants were grown in pots containing garden soil under controlled growth chamber conditions (16 h light, 26 ± 2 °C, 75% relative humidity). Three-week-old plants were used for the experiment.
Insect rearing
Insect were reared in their native host plant. Larvae of Spodoptera litura were reared on castor while aphids (Aphis craccivora) and whiteflies (Bemisia tabaci) were reared on cowpea and cotton plants, respectively. Temperature was maintained at 26 ± 2 °C with 16 h photoperiod and 75% relative humidity.25,26
Insect infestation
All 3 different crop plants were infested with chewing as well as sap sucking insects, separately. Second instar larvae of S. litura (previously reared on castor leaves) were taken as model for chewing insect due to their versatile host range. Whiteflies, one of the most emerging sap sucking insect, were used for cotton and tomato plants while aphids were used for cowpea because of their native host.
Fifteen healthy plants of each species grown in growth chamber (to nullify all the environmental effects) were selected for experiment. Three lots of 5 plants were infested with chewing insects (50 insects/plant), 5 with sap sucking insects (500 insects/plant) and remaining 5 were taken as control (non-infested). Plants were covered with perforated polybag to retain the insects, maintain proper aeration and humidity. Experiment was performed at condition similar to insect rearing and peroxidase activity of plant calculated after 48 h of infestation.
Extraction of sap from plants
Sap was extracted from infested and uninfested plants using pressure chamber. Plant’s stems were cut across from lower portion, washed thoroughly with milliQ water, blot dried and fixed in pressure extractor (Model 3005, Soil moisture equipment corp.). Initially low pressure (3 bars) was applied which gradually increased (till 10 bars) using commercial nitrogen gas. The exuding fluid (1.5–2 ml) was collected and stored on ice.
Extraction of total soluble protein (TSP) from plants leaves
One gram leaf tissue per sample was powdered using liquid nitrogen, homogenized in 3 ml extraction buffer (20 mM HEPES, 10% glycerol, 1mM EDTA 100µM PMSF, 5 mM DTT, and 1 mM benzamidine) and centrifuged (16,000 × g, 10 min, 4 °C). Supernatant was collected in fresh tube, dialyzed against 20 mM Tris-Cl and stored at 4 °C for further experiments.
Quantification of proteins in plant sap and leaves TSP
Protein concentrations were determined by Bradford method using bovine serum albumin (BSA) as standard. Standard curve was plotted by series of BSA concentrations and used for the calculation of concentration of unknown protein sample.
Peroxidase assay
Peroxidase activity was analyzed by spectrophotometer as well as in-gel assay.
Spectrophotometric assay
Protein samples (100 ng) were coated on microtiter plate by overnight incubation at 4 °C. Standard peroxidase enzyme (obtained from Sigma) was also coated at different dilution on Elisa plate in similar way. Plate was washed with water and assay performed by using 100 µl TMB (3, 3′, 5, 5′ Tetramethylbenzidine)/ H2O2 (Hydrogen peroxide) as substrate. Reaction with peroxidase results in the conversion of the substrate into a soluble blue product. The rate of blue color development is a direct measure of the rate of the peroxidase reaction. Reaction was stopped by adding 50 µl of 100 mM H2SO4 after 5 min which transforms blue product into a super-oxidized soluble yellow product. Peroxidase activity was measured by determining the absorbance of yellow product at 450 nm. Increase in absorbance was directly proportional to the peroxidase activity. A standard curve was plotted with the absorbance data of standard peroxidase enzyme and used for the calculation of peroxidase activity in experimental plant samples.
In-gel peroxidase assay
In-gel assay was performed on SDS-polyacrylamide gel following the protocol established in our laboratory with slight modification27. The protein samples were prepared without DTT and heat denaturation, and resolved on 12% SDS-PAGE. Gel was washed twice with 2.5% Triton × 100 and then with distilled water. Peroxidase band was developed by using diaminebenzidine/H2O2 (DAB) substrate according to manufacturer’s protocol (GeneI, Merck).
Calculation of kinetic parameter of Peroxidase
Kinetic parameters (Km, Vmax, and Kcat) were calculated for peroxidases isolated from the sap of all three tested plants. To compare enzyme activity of peroxidase enzymatic reactions were performed with different concentrations (0.05–4mM) of substrate (Tmb/H2O2) at regular intervals. The obtained data was analyzed by Michaelis-Menten equation on nonlinear regression using GraphPad Prism-6 software.
Mass spectrometric analysis
Peroxidase band developed in in-gel assay was used as marker to excise band from a duplicate gel and used for mass spectrometric analysis. The excised protein band was reduced with 10 mM DTT (56 °C, 30min), alkylated with 50 mM iodoacetamide (room temperature, 30 min) and digested with trypsin (Sequencing grade, Porcine, Promega) in 50 mM ammonium bicarbonate buffer (pH 8.0) 37 °C for overnight.25 The digested peptides were extracted by sonication in extraction solution (60% ACN and 1% TFA) for several times,dried by centrifugal evaporation and suspended in 5 μl resuspension solution (50% ACN and 0.1% TFA). Suspended peptides (0.5 µl) were spotted on MALDI plate followed by 0.5 μl of CHCA matrix (10 mg/mL in 50% ACN, 0.1% TFA). Spots were dried completely and used for PMF on MALDI-TOF-TOF platform (model 4800, ABsciex). The peptides with higher signal intensity were selected for MS/MS analysis. The spectra obtained from MS/MS were analyzed by searching against the MSDB, Swiss-Prot and NCBI database with the following parameters: fixed precursor ion mass tolerance of 20 ppm, fragment ion mass tolerance of 0.05 Da, calibration error of 0.005 Da, one missed cleavage, carbamidomethylation of cysteines and possible oxidation of methionine. The spectra of common contaminants were removed by searching against contaminant database, and the remaining data were used for the identification of proteins.
Statistical analysis
All the experiments were performed in triplicates and the average was calculated. All the statistical analysis was done on SPSS software.
Acknowledgments
Authors are grateful to the Council of Scientific and Industrial Research, Government of India. CSIR-EMPOWER program for the financial support. HS is thankful to CSIR-India for senior research fellowship.
Glossary
- Peroxidases are the ubiquitous enzyme and reported to be present in all living genera. They catalyses reduction of peroxide and generate reactive oxygen species. In the present study we demonstrated that insect infestation induces peroxidase activity in sap and total soluble protein (TSP) of plant leaves. Three important crop plants viz. tomato
cowpea and cotton were used for this study. After infestation of chewing insect, peroxidase activity in the sap and TSP of all the studied plants were enhanced in the range of 1.6–3.14 fold. Similar observations were also obtained with feeding of sap sucking insects, in which increment in peroxidase activity of sap and TSP was in the range of 1.8–2.53 fold. Enhanced peroxidase activity was reconfirmed by in-gel peroxidase assay. Enzyme kinetic study showed turn over efficiency of peroxidase from cotton (~101.3 min−1) was almost similar to tomato (~100.8 min−1) but higher than cowpea (~98.21 min−1). MS/MS analysis of observed band showed significant similarity with the reported peroxidases in database
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25615
Reference
- 1.Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 2.Maffei ME, Mithöfer A, Boland W. Insects feeding on plants: rapid signals and responses preceding the induction of phytochemical release. Phytochemistry. 2007;68:2946–59. doi: 10.1016/j.phytochem.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Allison SD, Schultz JC. Differential activity of peroxidase isozymes in response to wounding, gypsy moth, and plant hormones in northern red oak (Quercus rubra L.) J Chem Ecol. 2004;30:1363–79. doi: 10.1023/B:JOEC.0000037745.66972.3e. [DOI] [PubMed] [Google Scholar]
- 4.Heng-Moss TM, Sarath G, Baxendale FP, Novak D, Bose S, Ni X, et al. Characterization of oxidative enzyme changes in buffalograsses challenged by Blissus occiduus. J Econ Entomol. 2004;97:1086–95. doi: 10.1603/0022-0493(2004)097[1086:COOECI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Kerchev PI, Fenton B, Foyer CH, Hancock RD. Plant responses to insect herbivory: interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ. 2012;35:441–53. doi: 10.1111/j.1365-3040.2011.02399.x. [DOI] [PubMed] [Google Scholar]
- 6.Wei Z, Hu W, Lin Q, Cheng X, Tong M, Zhu L, et al. Understanding rice plant resistance to the Brown Planthopper (Nilaparvata lugens): a proteomic approach. Proteomics. 2009;9:2798–808. doi: 10.1002/pmic.200800840. [DOI] [PubMed] [Google Scholar]
- 7.Park SJ, Huang Y, Ayoubi P. Identification of expression profiles of sorghum genes in response to greenbug phloem-feeding using cDNA subtraction and microarray analysis. Planta. 2006;223:932–47. doi: 10.1007/s00425-005-0148-1. [DOI] [PubMed] [Google Scholar]
- 8.Broekgaarden C, Poelman EH, Steenhuis G, Voorrips RE, Dicke M, Vosman B. Responses of Brassica oleracea cultivars to infestation by the aphid Brevicoryne brassicae: an ecological and molecular approach. Plant Cell Environ. 2008;31:1592–605. doi: 10.1111/j.1365-3040.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 9.Ralph SG, Yueh H, Friedmann M, Aeschliman D, Zeznik JA, Nelson CC, et al. Conifer defence against insects: microarray gene expression profiling of Sitka spruce (Picea sitchensis) induced by mechanical wounding or feeding by spruce budworms (Choristoneura occidentalis) or white pine weevils (Pissodes strobi) reveals large-scale changes of the host transcriptome. Plant Cell Environ. 2006;29:1545–70. doi: 10.1111/j.1365-3040.2006.01532.x. [DOI] [PubMed] [Google Scholar]
- 10.Maffei ME, Mithöfer A, Arimura G, Uchtenhagen H, Bossi S, Bertea CM, et al. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006;140:1022–35. doi: 10.1104/pp.105.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowd PF, Lagrimini LM. The role of peroxidase in host insect defenses. In: Carozzi N and Koziel M (eds), Advances in Insect Control: The Role of Transgenic Plants. 1997; 195–223 Taylor and Francis, London. [Google Scholar]
- 12.El-Sayed M, Verpoorte R. Growth, metabolic pro□ling and enzymes activities of Catharanthus roseus seedlings treated with plant growth regulators. Plant Growth Regul. 2004;44:53–8. doi: 10.1007/s10725-004-2604-5. [DOI] [Google Scholar]
- 13.van Loon LC, Rep M, Pieterse CMJ. Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol. 2006;44:135–62. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 14.Almagro L, Gómez Ros LV, Belchi-Navarro S, Bru R, Ros Barceló A, Pedreño MA. Class III peroxidases in plant defence reactions. J Exp Bot. 2009;60:377–90. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- 15.Passardi F, Longet D, Penel C, Dunand C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry. 2004;65:1879–93. doi: 10.1016/j.phytochem.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Welinder KG. Superfamily of plant, fungal and bacterial peroxidases. Curr Opin Struct Biol. 1992;2:388–93. doi: 10.1016/0959-440X(92)90230-5. [DOI] [Google Scholar]
- 17.Biles CL, Abeles FB. Xylem sap proteins. Plant Physiol. 1991;96:597–601. doi: 10.1104/pp.96.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmermann MH, Ziegler H. Transport in plants: phloem transport. In: Encyclopedia of plant physiology, Vol. 1. New York: Springer 1975: 480–503. [Google Scholar]
- 19.Clark AM, Jacobsen KR, Bostwick DE, Dannenhoffer JM, Skaggs MI, Thompson GA. Molecular characterization of a phloem-specific gene encoding the filament protein, phloem protein 1 (PP1), from Cucurbita maxima. Plant J. 1997;12:49–61. doi: 10.1046/j.1365-313X.1997.12010049.x. [DOI] [PubMed] [Google Scholar]
- 20.Dannenhoffer JM, Schulz A, Skaggs MI, Bostwick DE, Thompson GA. Expression of the phloem lectin is developmentally linked to vascular differentiation in cucurbits. Planta. 1997;201:405–14. doi: 10.1007/s004250050083. [DOI] [Google Scholar]
- 21.Bilgin DD, Zavala JA, Zhu J, Clough SJ, Ort DR, DeLucia EH. Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ. 2010;33:1597–613. doi: 10.1111/j.1365-3040.2010.02167.x. [DOI] [PubMed] [Google Scholar]
- 22.Dubey RC. Handbook of Plant and Crop Stress, Second Edition, edited by Mohammad Pessarakli 2002; 365-397. [Google Scholar]
- 23.Kehr J. Phloem sap proteins: their identities and potential roles in the interaction between plants and phloem-feeding insects. Journal of Experimental Botany. 2006;57:767–774. doi: 10.1093/jxb/erj087. [DOI] [PubMed] [Google Scholar]
- 24.Young SA, Cuo A, Cuikema JA, White FF, Leach JE . Rice Cationic Peroxidase Accumulates in Xylem Vessels during lncompatible lnteractions with Xanthomonas oryzae pv oryzae. Plant Physiol. 1995;107:1333–1 341. doi: 10.1104/pp.107.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upadhyay SK, Mishra M, Singh H, Ranjan A, Chandrashekar K, Verma PC, et al. Interaction of Allium sativum leaf agglutinin with midgut brush border membrane vesicles proteins and its stability in Helicoverpa armigera. Proteomics. 2010;10:4431–40. doi: 10.1002/pmic.201000152. [DOI] [PubMed] [Google Scholar]
- 26.Upadhyay SK, Chandrashekar K, Thakur N, Verma PC, Borgio JF, Singh PK, et al. RNA interference for the control of whiteflies (Bemisia tabaci) by oral route. J Biosci. 2011;36:153–61. doi: 10.1007/s12038-011-9009-1. [DOI] [PubMed] [Google Scholar]
- 27.Upadhyay SK, Chandrashekar K. Interaction of salivary and midgut proteins of Helicoverpa armigera with soybean trypsin inhibitor. Protein J. 2012;31:259–64. doi: 10.1007/s10930-012-9402-0. [DOI] [PubMed] [Google Scholar]