Abstract
The potato cyst nematode Globodera rostochiensis is a biotrophic pathogen that secretes effector proteins into host root cells to promote successful plant parasitism. In addition to the role in generating within root tissue the feeding cells essential for nematode development,1 nematode secreted effectors are becoming recognized as suppressors of plant immunity.2-4 Recently we reported that the effector ubiquitin carboxyl extension protein (GrUBCEP12) from G. rostochiensis is processed into free ubiquitin and a 12-amino acid GrCEP12 peptide in planta. Transgenic potato lines overexpressing the derived GrCEP12 peptide showed increased susceptibility to G. rostochiensis and to an unrelated bacterial pathogen Streptomyces scabies, suggesting that GrCEP12 has a role in suppressing host basal defense or possibly pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) during the parasitic interaction.3 To determine if GrCEP12 functions as a PTI suppressor we evaluated whether GrCEP12 suppresses flg22-induced PTI responses in Nicotiana benthamiana. Interestingly, we found that transient expression of GrCEP12 in N. benthamiana leaves suppressed reactive oxygen species (ROS) production and the induction of two PTI marker genes triggered by the bacterial PAMP flg22, providing direct evidence that GrCEP12 indeed has an activity in PTI suppression.
Keywords: plant-parasitic nematode, Globodera rostochiensis, PAMP-triggered immunity, flg22, reactive oxygen species, PTI maker gene
Plants are equipped with a robust immune system to defeat pathogen attack. The first layer of immunity involves the recognition of pathogen-associated molecular patterns (PAMPs) by surface-localized pattern recognition receptors and is referred to as PAMP-triggered immunity (PTI).5 Perception of PAMPs such as bacterial flagellin or its derivative flg22 triggers numerous downstream responses, including production of reactive oxygen species (ROS), activation of mitogen-activated protein kinases, cell wall callose deposition and increased expression of defense-related genes.6 Although the role of PTI in plant-nematode interactions remains to be elucidated, accumulating evidence suggests that plant-parasitic nematodes actively utilize their secreted effectors to manipulate PTI to enable successful infection. For example, overexpression of nematode effectors in host plants often resulted in increased susceptibility to nematode infection with some cases also to infection by other adapted plant pathogens.3,4,7 The enhanced susceptibility to adapted pathogens is often a result of PTI suppression.8-10 Transcriptome analysis also revealed that defense-related genes are repressed in nematode-induced feeding cells.11-13 Most importantly, the calreticulin Mi-CRT effector from the root-knot nematode Meloidogyne incognita was recently shown to suppress PTI responses including callose deposition and defense gene expression mediated by the PAMP elf18,4providing the first direct evidence for a role of nematode effectors in PTI suppression. The GrCEP12 peptide released from G. rostochiensis during the parasitic interaction is a unique 12-amino acid peptide having no obvious similarity to any known peptides.3 Interestingly, however, transgenic potato lines overexpressing GrCEP12 showed enhanced susceptibility to G. rostochiensis, which is likely due to a function of GrCEP12 in PTI suppression during nematode parasitism. To evaluate this hypothesis, we used the Agrobacterium-mediated transient expression in N. benthamiana leaves to investigate whether GrCEP12 can suppress flg22-mediated PTI responses.
GrCEP12 Suppresses flg22-Mediated ROS Production in N. benthamiana
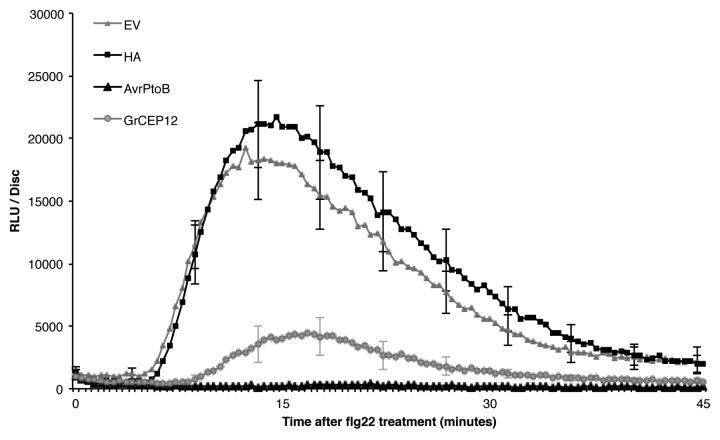
A ROS burst is one of the earliest PTI responses.6 We therefore assessed whether GrCEP12 suppresses ROS production induced by flg22. Agrobacterium strain GV3101 carrying the GrCEP12 construct was infiltrated into N. benthamiana leaves. Forty-eight hours after infiltration, leaf discs were collected and then challenged with flg22 at a concentration of 100 nM. ROS production was measured using a luminol-based assay.14 The bacterial effector AvrPtoB, a demonstrated suppressor of PTI,15 as well as the empty vector (EV) and the construct expressing the 9-amino acid HA tag peptide3 were included as positive and negative controls, respectively. We observed that, similarly to AvrPtoB, GrCEP12 dramatically suppressed flg22-induced ROS production in leaf discs when compared with the negative controls (Fig. 1).
Figure 1. GrCEP12 suppresses flg22-mediated ROS production in N. benthamiana. Agrobacterium tumefaciens strain GV3101 carrying GrCEP12, AvrPtoB, HA or the empty vector (EV) construct was infiltrated into leaves of 3-wk-old N. benthamiana plants. Infiltrated leaf discs were collected 48 h post-agroinfiltration and assayed for ROS production as described.14 Values indicated are average of relative luminescence units (RLUs) ± SD of 18–24 leaf discs. The experiment was repeated four times with similar results.
GrCEP12 Suppresses flg22-Triggered Marker Gene Expression in N. benthamiana
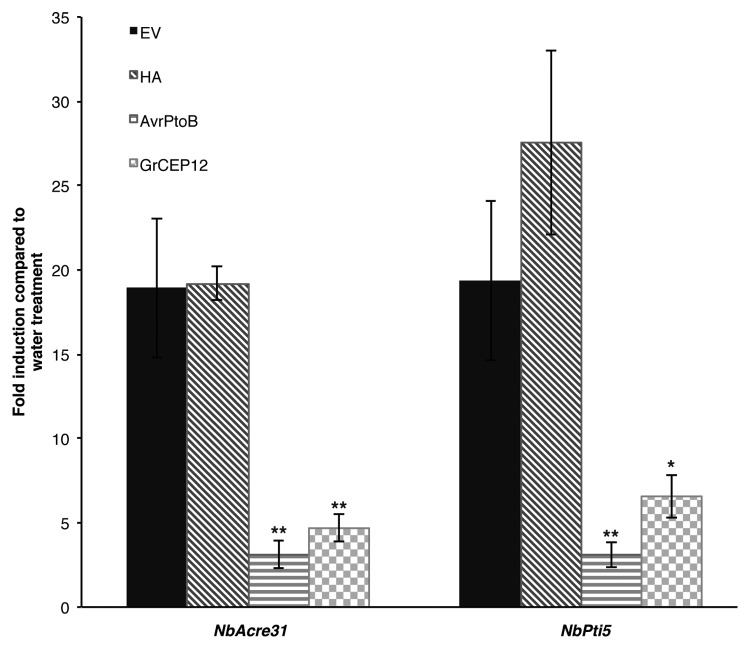
One of the responses to PAMP perception is transcriptional reprogramming of plant cells.6 NbPti5 and NbACRE31genes have been demonstrated to be markers of PTI in N. benthamiana,16 we therefore evaluated whether GrCEP12 affects the expression of these two marker genes after flg22 treatment. Agrobacterium strain GV3101 carrying GrCEP12 was infiltrated into N. benthamiana leaves and leaf discs were collected 24 h post-agroinfiltration. After soaking overnight in water, leaf discs were incubated with 100 nM flg22 or distilled water for 30 min. mRNA was isolated from treated leaf tissue and gene expression was determined by quantitative RT-PCR.3 In this assay, we also included AvrPtoB as well as the EV and the HA constructs as positive and negative controls, respectively. Both NbPti5 and NbCRE31 were upregulated in leaf tissue infiltrated with the EV or the HA construct after flg22 treatment (Fig. 2). In contrast, flg22-induced upregulation of NbPti5 and NbCRE31 was significantly reduced in GrCEP12 and AvrPtoB infiltrated leaf tissues as compared with those infiltrated with the negative controls (Fig. 2).
Figure 2. GrCEP12 suppresses flg22-induced expression of PTI marker genes in N. benthamiana. N. benthamiana leaf discs expressing GrCEP12, AvrPtoB, HA or the empty vector (EV) construct were collected 24 h post-agroinfiltration and soaked overnight in water. Leaf discs were then treated with 100 nM flg22 or distilled water for 30 min. mRNA was extracted from treated leaf discs and quantitative RT-PCR was conducted to determine the expression of NbPti5 and NbACRE31 genes in flg22-treated leaf discs relative to water-treated leaf discs. All samples were normalized against the reference gene NbEF1α.17 Values are means ± SD of three independent experiments with three technical replicates of each. Asterisks indicate statistically significant difference compared with the EV control (*p < 0.05; **p < 0.01; Student’s t-test).
Taken together, our results clearly demonstrated an activity of the GrCEP12 peptide in PTI suppression. PTI appears to be conserved across the plant kingdom. Although no nematode PAMPs have been identified to date, it is possible that signaling pathways induced by the bacterial PAMP flg22 in N. benthamiana overlap with signaling pathways induced by an unknown nematode PAMP(s) in potato, a host of G. rostochiensis. It is therefore conceivable that GrCEP12 is a genuine suppressor of PTI during G. rostochiensis infection of potato, consistent with the increased nematode susceptibility observed in transgenic potato overexpressing GrCEP12. It is worth noting that, to our knowledge, GrCEP12 represents the smallest peptide effector from plant microbial pathogens showing an activity in PTI suppression. Identifying host plant target(s) of GrCEP12 may help uncover the mechanism of PTI suppression by GrCEP12 and provide insights into the role of PTI in plant-nematode interactions.
Acknowledgments
This work was supported by funding from US Department of Agriculture—Agricultural Research Service. We thank Dr Gregory Martin at Boyce Thompson Institute for Plant Research, Ithaca, NY for providing the AvrPtoB construct and Dr Suma Chakravarthy at the Department of Plant Pathology and Plant-Microbe Biology, Cornell University for critical comments of the original manuscript.
Glossary
Abbreviations:
- PTI
PAMP-triggered immunity
- ROS
reactive oxygen species
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25359
References
- 1.Hussey RS. Disease-inducing secretions of plant-parasitic nematodes. Annu Rev Phytopathol. 1989;27:123–41. doi: 10.1146/annurev.py.27.090189.001011. [DOI] [Google Scholar]
- 2.Postma WJ, Slootweg EJ, Rehman S, Finkers-Tomczak A, Tytgat TOG, van Gelderen K, et al. The effector SPRYSEC-19 of Globodera rostochiensis suppresses CC-NB-LRR-mediated disease resistance in plants. Plant Physiol. 2012;160:944–54. doi: 10.1104/pp.112.200188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chronis D, Chen S, Lu S, Hewezi T, Carpenter SCD, Loria R, et al. A ubiquitin carboxyl extension protein secreted from a plant-parasitic nematode Globodera rostochiensis is cleaved in planta to promote plant parasitism. Plant J. 2013;74:185–96. doi: 10.1111/tpj.12125. [DOI] [PubMed] [Google Scholar]
- 4.Jaouannet M, Magliano M, Arguel MJ, Gourgues M, Evangelisti E, Abad P, et al. The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol Plant Microbe Interact. 2013;26:97–105. doi: 10.1094/MPMI-05-12-0130-R. [DOI] [PubMed] [Google Scholar]
- 5.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–9. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 6.Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 7.Hewezi T, Baum TJ. Manipulation of plant cells by cyst and root-knot nematode effectors. Mol Plant Microbe Interact. 2013;26:9–16. doi: 10.1094/MPMI-05-12-0106-FI. [DOI] [PubMed] [Google Scholar]
- 8.Fabro G, Steinbrenner J, Coates M, Ishaque N, Baxter L, Studholme DJ, et al. Multiple candidate effectors from the oomycete pathogen Hyaloperonospora arabidopsidis suppress host plant immunity. PLoS Pathog. 2011;7:e1002348. doi: 10.1371/journal.ppat.1002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, Felix G, et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–7. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 10.Nekrasov V, Li J, Batoux M, Roux M, Chu Z-H, Lacombe S, et al. Control of the pattern-recognition receptor EFR by an ER protein complex in plant immunity. EMBO J. 2009;28:3428–38. doi: 10.1038/emboj.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ithal N, Recknor J, Nettleton D, Maier T, Baum TJ, Mitchum MG. Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol Plant Microbe Interact. 2007;20:510–25. doi: 10.1094/MPMI-20-5-0510. [DOI] [PubMed] [Google Scholar]
- 12.Szakasits D, Heinen P, Wieczorek K, Hofmann J, Wagner F, Kreil DP, et al. The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. Plant J. 2009;57:771–84. doi: 10.1111/j.1365-313X.2008.03727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barcala M, García A, Cabrera J, Casson S, Lindsey K, Favery B, et al. Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. Plant J. 2010;61:698–712. doi: 10.1111/j.1365-313X.2009.04098.x. [DOI] [PubMed] [Google Scholar]
- 14.Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, Rathjen JP. Hierarchy and roles of pathogen-associated molecular pattern-induced responses in Nicotiana benthamiana. Plant Physiol. 2011;156:687–99. doi: 10.1104/pp.110.171249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindeberg M, Cunnac S, Collmer A. Pseudomonas syringae type III effector repertoires: last words in endless arguments. Trends Microbiol. 2012;20:199–208. doi: 10.1016/j.tim.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen HP, Chakravarthy S, Velásquez AC, McLane HL, Zeng L, Nakayashiki H, et al. Methods to study PAMP-triggered immunity using tomato and Nicotiana benthamiana. Mol Plant Microbe Interact. 2010;23:991–9. doi: 10.1094/MPMI-23-8-0991. [DOI] [PubMed] [Google Scholar]
- 17.Heese A, Hann DR, Gimenez-Ibanez S, Jones AME, He K, Li J, et al. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA. 2007;104:12217–22. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]