Abstract
Aim:
Sesamin is one of the major lignans in sesame seeds with antihyperlipidemic, antioxidative and antihypertensive activities. The aim of this study was to examine the effects of sesamin on arterial function in spontaneously hypertensive rats (SHRs).
Methods:
SHRs were orally administered sesamin (40, 80 and 160 mg·kg−1·d−1) for 16 weeks. After the rats were killed, thoracic aortas were dissected out. The vasorelaxation responses of aortic rings to ACh and nitroprusside were measured. The expression of eNOS and NADPH oxidase subunits p47phox and p22phox in aortas were detected using Western blotting and immunohistochemistry. Aortic nitrotyrosine was measured with ELISA. The total antioxidant capacity (T-AOC) and MDA levels in aortas were also determined.
Results:
The aortic rings of SHRs showed significantly smaller ACh-induced and nitroprusside-induced relaxation than those of control rats. Treatment of SHRs with sesamin increased both the endothelium-dependent and endothelium-independent relaxation of aortic rings in a dose-dependent manner. In aortas of SHRs, the level of T-AOC and the expression of nitrotyrosine, p22phox and p47phox proteins were markedly increased, while the level of MDA and the expression of eNOS protein were significantly decreased. Treatment of SHRs with sesamin dose-dependently reversed these biochemical and molecular abnormalities in aortas.
Conclusion:
Long-term treatment with sesamin improves arterial function in SHR through the upregulation of eNOS expression and downregulation of p22phox and p47phox expression.
Keywords: sesamin, spontaneously hypertensive rat, aorta, vasorelaxation, endothelium, eNOS, NADPH oxidase, malondialdehyde, ACh, nitroprusside
Introduction
The endothelial nitric oxide synthase (eNOS) within normal endothelium contributes to the regulation of systolic blood pressure by the production of nitric oxide (NO) from L-arginine. Spontaneously hypertensive rats (SHR) are characterized by arterial dysfunction resulting from the increased generation of reactive oxygen species (ROS), which subsequently causes NO breakdown and the suppression of eNOS expression1,2,3,4,5. Several studies have shown that eNOS expression is significantly decreased in dysfunctional arteries from SHR6,7. An exaggerated production of the superoxide anion (·O2−) by the vasculature has been observed in SHR8, which is predominantly derived from NADPH oxidase9. NO can be scavenged by ·O2− to form peroxynitrite, effectively reducing the bioavailability of endothelium-derived NO10. Thus, diminished NO has been linked to decreased eNOS expression and increased ·O2− in hypertension and may lead to arterial dysfunction11,12,13,14.
Sesamin (Ses), a major lignan in sesame seeds, has antihyperlipidemic, antioxidative and antihypertensive pharmacological properties in different murine models15,16,17,18,19,20,21,22,23. Recent studies have demonstrated that sesamin metabolites induce an endothelial NO-dependent vasorelaxation through their antioxidative property-independent mechanisms22,24. Furthermore, sesamin feeding leads to enhanced endothelium-dependent relaxation by suppressing aortic NADPH oxidase in deoxycorticosterone acetate (DOCA)-salt hypertensive rats25. Kong18 et al and Wu26 et al reported that sesamin can improve endothelial function and exert reno-protective effects by enhancing NO bioactivity in renovascular hypertensive rats fed with a high-fat, high-sucrose diet. However, no study to date has reported the effects of sesamin on the aortas from SHR.
Therefore, the purpose of this study was to evaluate the arterio-protective effects of sesamin in SHR. Additionally, we examined the roles of p22phox, p47phox, eNOS and nitrotyrosine (the footprint of NO interaction with ·O2-) expression to explore the mechanisms behind sesamin-mediated arterial protection.
Materials and methods
Drugs and reagents
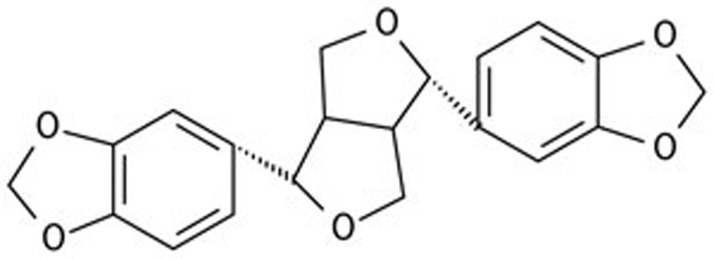
Sesamin (Figure 1, >94%) was provided by Tianyi Lvbao Technology Co, Ltd (Chinese invention patent number ZL 03113181.6 Wuhu, China). Norepinephrine, phenylephrine, acetylcholine (ACh, an endothelium-dependent vasodilator), and nitroprusside (an endothelium-independent vasodilator) were purchased from Sigma (St Louis, MO, USA). Krebs solution was made from the following reagents (in mmol/L): 118.3 NaCl, 25 NaHCO3, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgSO4, and 11.1 glucose.
Figure 1.
The structure of sesamin.
Experimental animals, grouping and treatment
The experiments were performed on aortas from age- (16 weeks) and weight-matched male SHR and Wistar-Kyoto rats (WKY) [SCXK (Shanghai) 2007–0005, Shanghai SLAC Laboratory Animal Co, Ltd]. Twenty-eight SHR were randomly assigned to a model group (SHR-untreated, n=7), three treatment groups (SHR-ses 160 mg/kg, SHR-ses 80 mg/kg, SHR-ses 40 mg/kg, n=7 each) or a control group (WKY-untreated, n=7). Animals had free access to a standardized diet and tap water. Sesamin was suspended in 0.5% carboxymethylcellulose sodium and orally administered between 5:00 and 6:00 PM every day for 16 weeks in the three treatment groups. Untreated groups received an equal volume of carboxymethylcellulose sodium as a control. All animals were housed in a room with standardized temperature (21±1 °C) and exposed to a 12 h dark-light cycle.
Sample collection
At the end of the experiment, the rats were fasted overnight and anesthetized by an intraperitoneal injection of sodium pentobarbital (50 mg/kg, ip). Thoracic aortas from the rats were carefully removed, cleaned of adhering tissue and divided into two parts. One part contained the descending thoracic aorta, which was cut into two transverse rings (3–4 mm in length), was used for the vascular reactivity experiments. Portions of the arcus aortae were either used for immunohistochemistry or were quickly frozen in liquid nitrogen and stored at −80 °C for future processing.
Vascular reactivity studies
The thoracic aorta was quickly removed and cut into rings 3 mm in length. Two rings were taken from every rat aorta and were suspended between two triangular-shaped stainless steel stirrups in two jacketed organ chambers (A and B, Model ALC-M, Alcott Biotech Co, Ltd, Shanghai, China) containing 10 mL of Krebs solution at 37 °C that was continuously oxygenated with 95% O2 and 5% CO2. Pre-load (2 g) was applied to the rings, and the vessels were allowed to equilibrate for 60 min (with 4 washes). Changes in isometric tension were detected by force transducers and recorded via a MAP2000 polygraph (Alcott Biotech Co, Ltd, Shanghai, China). The rings were stimulated with norepinephrine (3×10−7mol/L) to evaluate their viability and were serially washed back to baseline levels and equilibrated once again. In chambers A or B, the concentration-relaxation response curves to acetylcholine (10−8 to 10−5mol/L) or nitroprusside (10−9to 10−6mol/L) were performed in aortic rings, which were precontracted by phenylephrine (10−6mol/L), respectively.
Malondialdehyde determination
Malondialdehyde (MDA), a degradation product of lipid peroxidation, is a class of thiobarbituric acid reactive substances. Aortic MDA levels were measured by a thiobarbituric acid assay proposed by Rodriguez-Martinez and Ruiz-Torres27 and modified by Manso28 et al.
Total antioxidant capacity assays
Total antioxidant capacity (T-AOC) was determined in rat aortic homogenate using commercial kits from the Beyotime Institute of Biotechnology (Haimen, China). The frozen aortas were homogenized in 10 mmol/L Tris buffer (pH 7.4) containing 1% Triton X-100, 1 mmol/L EDTA, 1 mmol/L phenylmethyl sulfonyl fluoride (PMSF), 10 mg/mL aprotinin and 10 mg/mL leupeptin. T-AOC was determined by the ferric-reducing ability of plasma (FRAP) method29. Protein concentration was determined by the bicinchoninic acid assay (Beyotime Institute of Biotechnology, Haimen, China).
ELISA
Nitrotyrosine is a stable end product of peroxynitrite-mediated oxidation/nitration, which can be used as a footprint of in vivo peroxynitrite (ONOO−) formation and a surrogate index of in vivo-uncoupled eNOS-dependent damage30. Peroxynitrite is a strong oxidant formed in the reaction between NO and superoxide (·O2−) and the subsequent reaction of peroxynitrite with protein, which results in the formation of nitrotyrosine. Aortic nitrotyrosine was measured using the NWLSS nitrotyrosine ELISA kit (Northwest Life Science Specialties, LLC) according to the manufacturer's protocol. The protein concentration was determined by the bicinchoninic acid assay (Beyotime Institute of Biotechnology, Haimen, China).
Immunohistochemistry
Immunostaining for p22phox, p47phox, and eNOS were performed on 4% paraformaldehyde-fixed rat aorta sections according to previously described protocols31,32,33. Thoracic aortas embedded in paraffin were cut into 5-μm sections. Slides were incubated in methanol containing 0.3 % H2O2for 30 min and blocked with normal goat serum in PBS (containing 0.1% BSA and 0.01% Tween 20) for 30 min. Primary antibodies against p22phox, p47phox (Santa Cruz Biotechnology, Santa Cruz, USA), or eNOS (Boster Bio-engineering, Wuhan, China) diluted in PBS containing 0.1% BSA and 0.01% Tween 20 were applied to slides for 12 h at 4 °C. After two washes with PBS containing 0.01% Tween 20, slides were processed using the ABC and DAB staining kit (Vector Laboratories, Burlingame, CA, USA). After counterstaining with hematoxylin, slides were dehydrated and permanently mounted. Sections (five microscopic fields per slice with one slice/animal) were digitized using an Olympus BX51 microscope (Olympus Optical Co, Ltd, Tokyo, Japan). Digital images were processed using Image-Pro Plus 6.0 software (Media Cybernetics Inc, Maryland, USA), which was used to determine the integral optical density (IOD) and average optical density (AOD, AOD=positive area×OD/total area) of p22phox, p47phox, and eNOS as described previously34,35.
Western blotting analysis
P22phox, p47phox, and eNOS protein levels in the aortas were detected by western blotting as previously described36,37,38. Aorta samples were homogenized in ice-cold RIPA buffer (Beyotime Institute of Biotechnology, Haimen, China). The protein concentrations were determined using a BCA protein assay kit (Beyotime Institute of Biotechnology, Jiangsu and China). Aortic extracts (30 μg of protein per lane) were mixed with sample loading buffer and separated on a 12% SDS-polyacrylamide gel. Proteins were electro-transferred on PVDF membranes (0.45 μm, Beyotime Institute of Biotechnology, Haimen, China). The membranes were blocked in TBST (1×TBS, 0.1% Tween-20) containing 5% nonfat dry milk for one hour at room temperature and incubated with the following antibodies: p22phox, p47phox (Santa Cruz Biotechnology, Santa Cruz, USA), eNOS (Boster Bio-engineering, Wuhan, China) and β-actin (Bioss Biotechnology, Beijing, China) at 4 °C overnight. The bands were detected using a chemiluminescence assay (ECL Plus, Beyotime Institute of Biotechnology, Haimen, China) and evaluated by densitometry using Image-Pro Plus 6.0 software (Media Cybernetics Inc, USA). The relative density values of each band normalized to β-actin were presented.
Statistical analysis
Data were expressed as the mean±SD. For statistical analysis, one-way ANOVA was used followed by Newman-Keuls tests. A P-value of P<0.05 was considered to be statistically significant.
Results
Vascular activity ex vivo
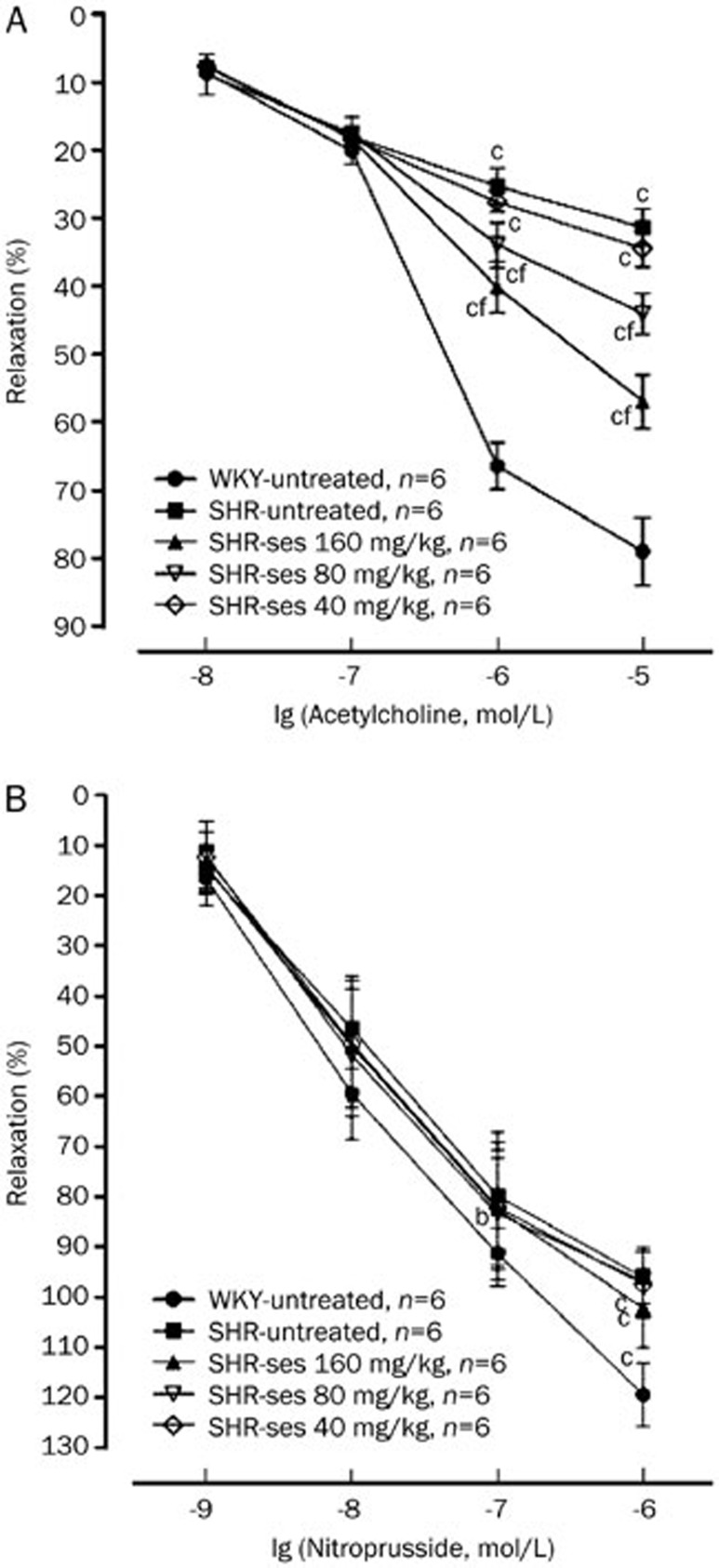
Acetylcholine elicited a concentration-dependent relaxation in phenylephrine precontracted aortic rings in all experimental groups. Acetylcholine-induced vasorelaxation was significantly decreased (by 25.27%, 31.34% for 10−6, 10−5mol/L acetylcholine, respectively; P<0.01) in the SHR-untreated group compared with the WKY-untreated group. The aortic rings from the SHR-ses 160 mg/kg and SHR-ses 80 mg/kg groups showed significant increases (by 25.57% and 12.73% for 10−5mol/L acetylcholine, respectively; P<0.01) in vasodilatation induced by acetylcholine as compared to those from the SHR-untreated group (Figure 2A). The endothelium-independent vasorelaxation induced by sodium nitroprusside was significantly decreased (by 11.50%, 23.42% for 10−7, 10−6 mol/L sodium nitroprusside, respectively; P<0.01) in the SHR-untreated group compared with the WKY-untreated group. The vasorelaxation induced by sodium nitroprusside was increased in SHR-ses 160 mg/kg and SHR-ses 80 mg/kg groups (by 6.13%, 5.83% for 10−6 mol/L sodium nitroprusside, respectively; P>0.05) compared with the SHR-untreated group (Figure 2B). These results indicated that sesamin may significantly improve endothelium-dependent dysfunction and weakly increase endothelium-independent relaxation.
Figure 2.
Effects of sesamin on the aortic relaxant responses. (A) ACh induced relaxation responses. (B) SNP induced relaxation responses. bP<0.05, cP<0.01 vs WKY-untreated. fP<0.01 vs SHR-untreated.
Measurement of aortic T-AOC and MDA
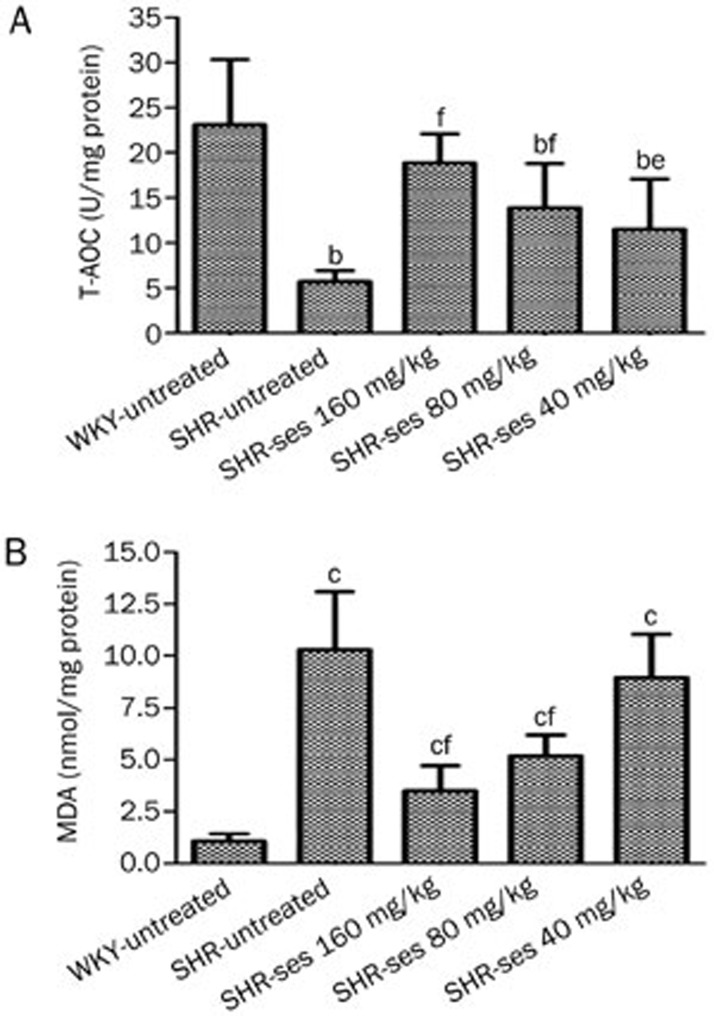
As shown in Figure 3, the decrease of aortic T-AOC content and increase in aortic MDA content confirmed that oxidative damage had been induced in untreated SHR (P<0.01 vs WHY-untreated). The level of aortic T-AOC in the SHR-ses 160 mg/kg, SHR-ses 80 mg/kg, and SHR-ses 40 mg/kg groups were significantly higher than those in the SHR-untreated group (P<0.01 or P<0.05). The level of aortic MDA in SHR-ses 160 mg/kg and SHR-ses 80 mg/kg groups was significantly lower than that in the SHR-untreated group (P<0.01). These results confirmed previous evidence, which demonstrated that sesamin has antioxidative properties.
Figure 3.
Effect of sesamin on T-AOC and MDA level in aortas. n=7. bP<0.05, cP<0.01 vs WKY-untreated. eP<0.05, fP<0.01 vs SHR-untreated.
Measurement of aortic nitrotyrosine
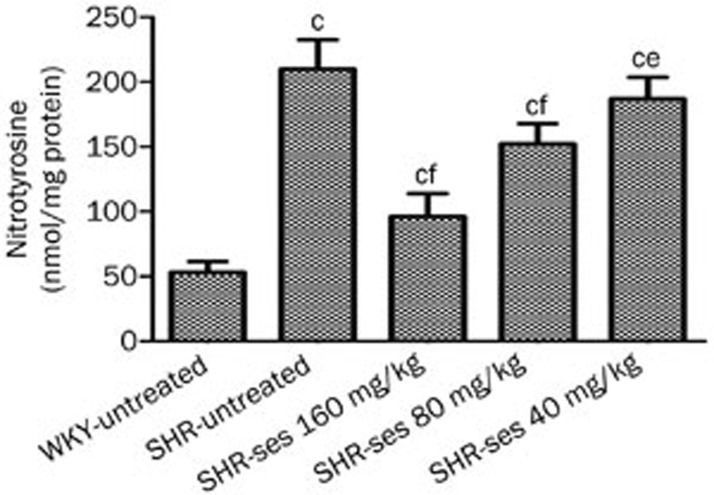
Aortic nitrotyrosine was significantly higher in the SHR-untreated group compared to the WKY-untreated group (Figure 4). Aortic nitrotyrosine levels were significantly lower in SHR-ses 160 mg/kg, SHR-ses 80 mg/kg, and SHR-ses 40 mg/kg groups. These results demonstrate that sesamin treatment decreases peroxynitrite production by reducing the reaction between superoxide and NO, thereby increasing NO bioavailability.
Figure 4.
Effect of sesamin on vascular nitrotyrosine content measured by ELISA. n=7. cP<0.01 vs WKY-untreated. fP<0.01 vs SHR-untreated.
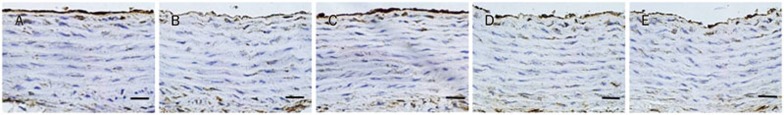
Expression of eNOS by immunohistochemistry
SHR exhibited a significant reduction of eNOS protein expression in aortic endothelium when compared with WKY. Treatment with sesamin was able to enhance protein expression of aortic eNOS (Figures 5, 6).
Figure 5.
Effect of sesamin on the protein expression of eNOS in aortic endothelium of SHRs. (A) WKY-untread. (B) SHR-untreated. (C) SHR-ses 160 mg/kg. (D) SHR-ses 80 mg/kg. (E) SHR-ses 40 mg/kg. Brown staining in aortic endothelium represents eNOS in the aortas. ×400. Bar=50 μm.
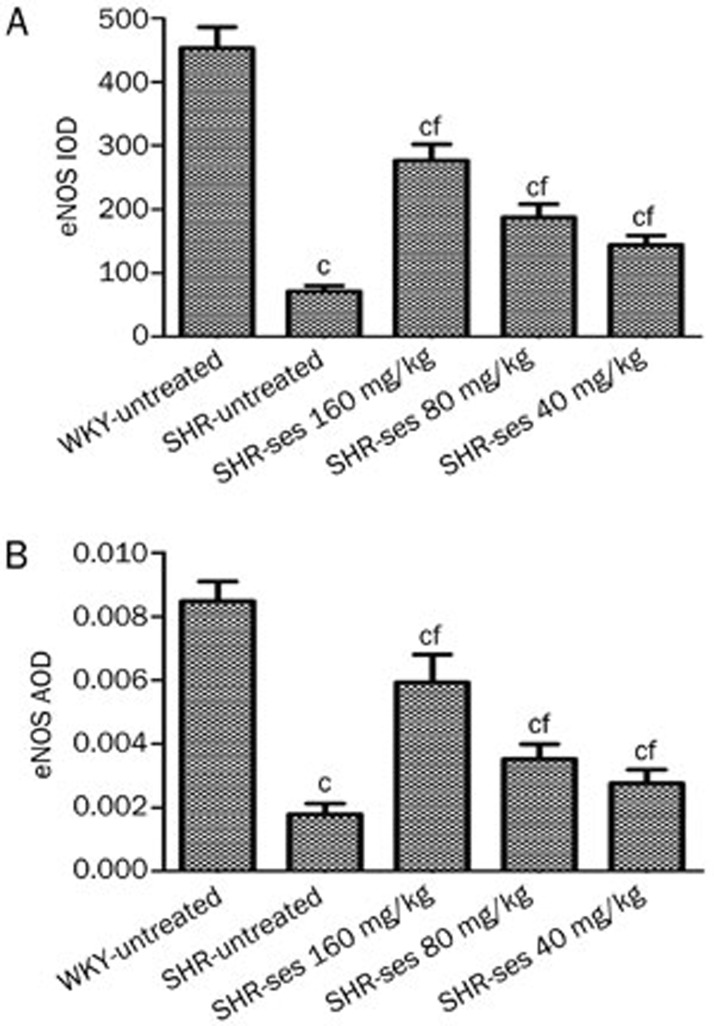
Figure 6.
Effect of sesamin on the integral optical density (IOD, A) and average optical density (AOD, B) of eNOS in aortic endothelium. Mean±SEM. n=7. cP<0.01 vs WKY-untreated. fP<0.01 vs SHR-untreated.
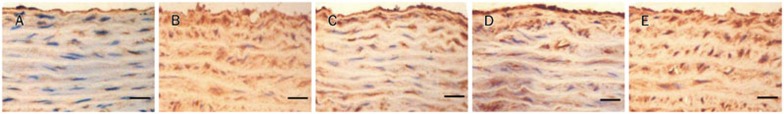
Expression of p22phox by immunohistochemistry
As shown in Figures 7 and 8, p22phox protein expressions in aortic tissues were significantly higher in the SHR-untreated group when compared with the WKY-untreated group. These abnormalities were essentially reversed by treatment with 160 mg/kg and 80 mg/kg sesamin for 16 weeks.
Figure 7.
Effect of sesamin on the protein expression of p22phoxin aortas. (A) WKY-untreated. (B) SHR-untreated. (C) SHR-ses 160 mg/kg. (D) SHR-ses 80 mg/kg. (E) SHR-ses 40 mg/kg. Brown staining represents p22phox in the aortas. ×400. Bar=50 μm.
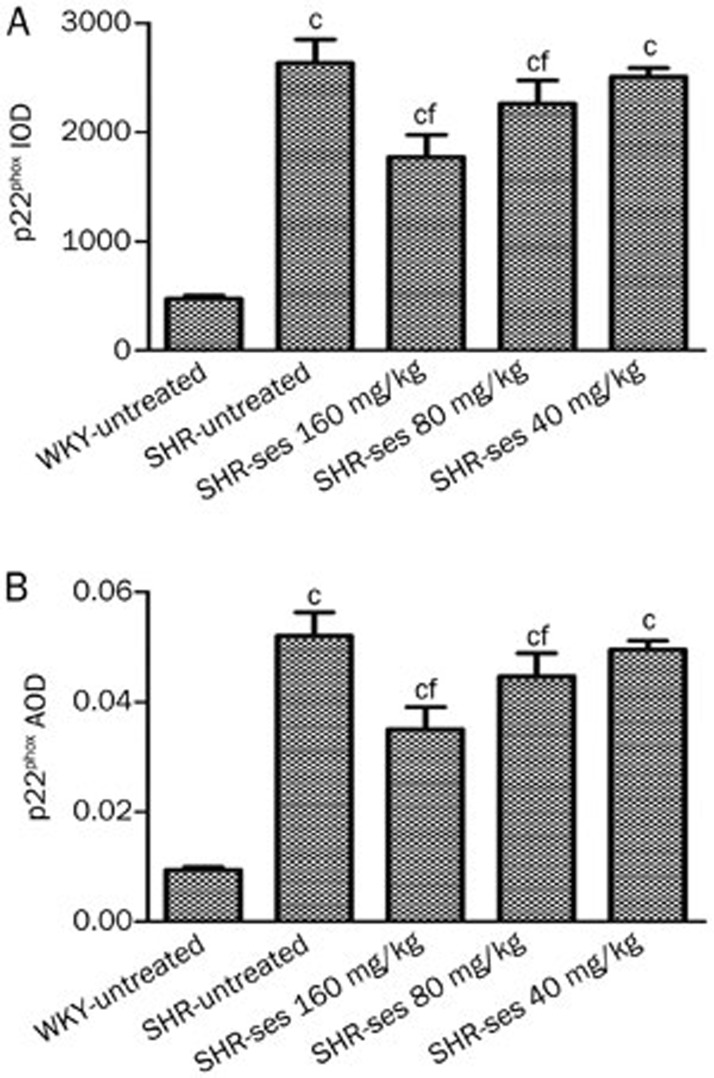
Figure 8.
Effect of sesamin on the IOD (A) and AOD (B) of p22phox in aortas. Data are presented as mean±SEM. n=7. cP<0.01 vs WKY-untreated. fP<0.01 vs SHR-untreated.
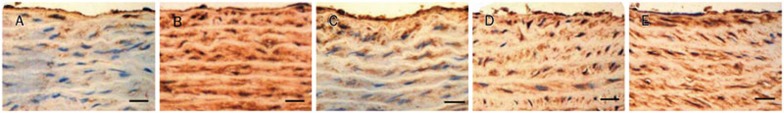
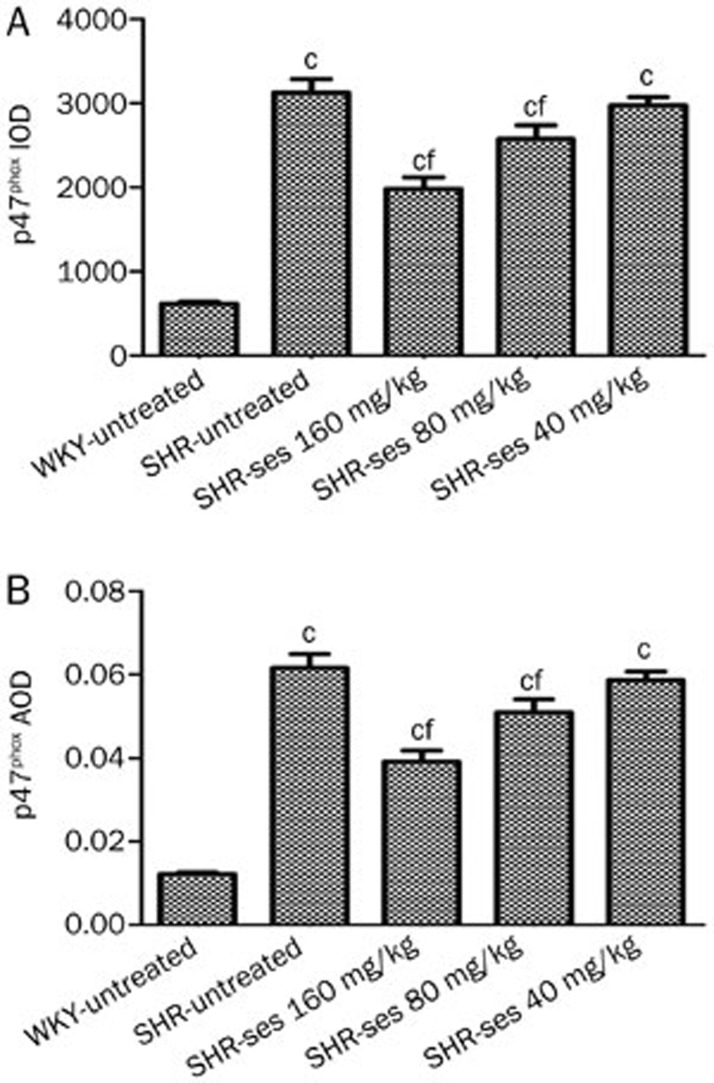
Expression of p47phox by immunohistochemistry
The SHR-untreated group exhibited a notable rise of p47phoxprotein expression (P<0.01 vs WKY-untreated) in Figures 9 and 10. Abnormal p47phox protein expression levels were reversed by treatment with 160 mg/kg and 80 mg/kg of sesamin for 16 weeks.
Figure 9.
Effect of sesamin on the protein expression of p47phox in aortas. (A) WKY-untreated. (B) SHR-untreated. (C) SHR-ses 160 mg/kg. (D) SHR-ses 80 mg/kg. (E) SHR-ses 40 mg/kg. Brown staining represents p47phox in the aortas. ×400. Bar=50 μm.
Figure 10.
Effect of sesamin on the IOD (A) and AOD (B) of p47phox in aortas. Data are presented as mean±SEM. n=7. cP<0.01 vs WKY-untreated. fP<0.01 vs SHR-untreated.
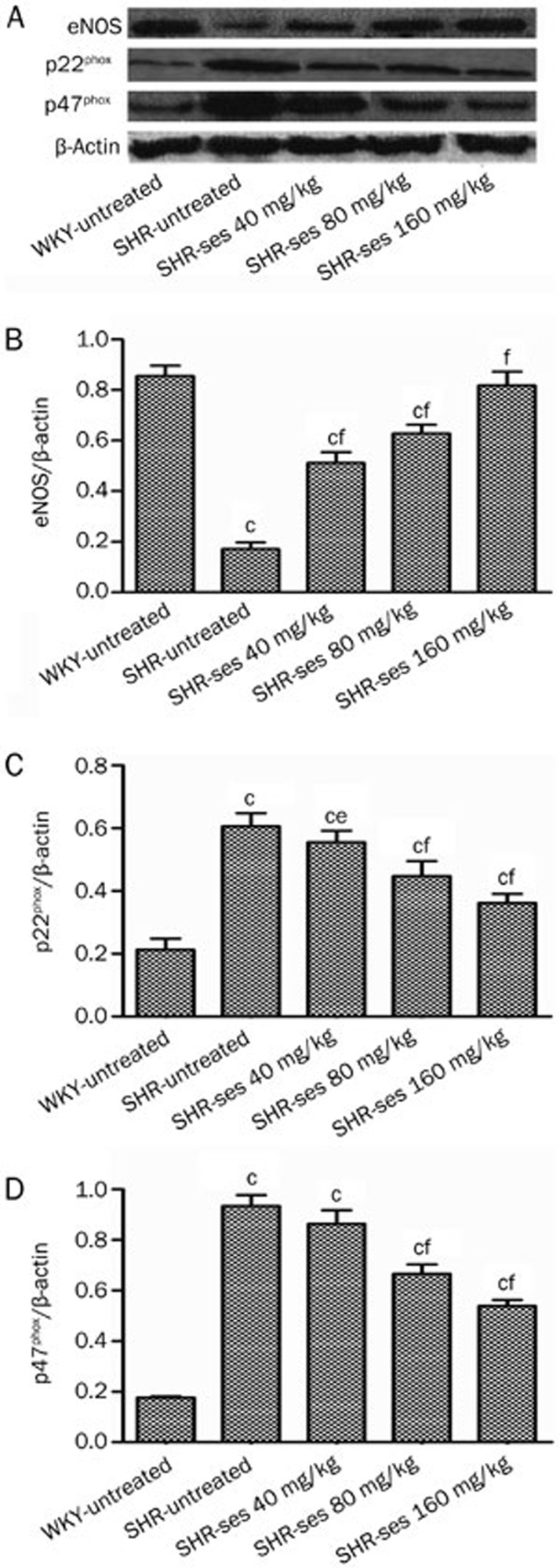
Expression of p22phox, p47phox, and eNOS by Western blotting
The aortas from the SHR-untreated group exhibited a significant reduction in eNOS protein expression with a notable rise in p22phox and p47phox protein expression (P<0.01 vs WKY-untreated). The abnormal expression of p22phox, p47phox, and eNOS were reversed by treatment with sesamin for 16 weeks (Figure 11).
Figure 11.
Effect of sesamin on the protein expression of eNOS (B), p22phox (C), and p47phox (D) in aortas by Western blotting. Histograms represent densitometric values normalized to the corresponding β-actin. n=7. bP<0.05, cP<0.01 vs WKY-untreated. eP<0.05, fP<0.01 vs SHR-untreated.
Discussion
In the present study, we observed that acetylcholine-induced endothelium-dependent vasorelaxation and nitroprusside-induced endothelium-independent vasorelaxation were significantly decreased in the aortas from untreated SHR. These data indicated that there was arterial dysfunction in 32-week-old male SHR. These results were consistent with previous reports39,40. After 16 weeks of treatment with sesamin, the endothelium-dependent dysfunction had improved, and the endothelium-independent relaxation was weakly increased. These findings suggest that chronic treatment with sesamin significantly improves arterial dysfunction in SHR. Thus, it is important to ascertain how sesamin could produce these effects in SHR.
The eNOS within the normal endothelium contributes to the regulation of systolic blood pressure by the production of NO from L-arginine. Endothelium-derived NO is an important endogenous vasodilator, which plays a key role in the regulation of vascular tone in addition to other various endothelium-derived molecules11,41. NO has opposing, dose-dependent effects on endothelial cell apoptosis; low concentrations of NO protect endothelial cells from apoptosis-inducing stimuli and high concentrations of NO can induce apoptosis42,43. Importantly, the diminished capacity to produce NO by the endothelium in hypertension has been linked to decreased eNOS expression11,44. We found that sesamin treatment improved the aortic arterial function by increasing eNOS expression in the endothelium of SHR. Several recent findings have supported this conclusion: sesamin enhanced eNOS expression in the aorta of 2K1C rats fed with a HFS; sesamin metabolites induced NO-dependent vasorelaxation; sesamin feeding had no antihypertensive action in chronically L-NOARG-treated rats or DOCA-salt-treated mice; sesamin induced eNOS mRNA and protein expression and enhanced NOS activity in human umbilical vein endothelial cells18,24,45. The present experiment demonstrated that sesamin enhanced eNOS expression in the aortic endothelium of SHR and subsequently increased NO levels. Consequently, arterial function was improved. These results confirm and expand upon previous studies demonstrating sesamin mediated improvement in endothelial function18,22.
In addition, vascular oxidative stress plays an important pathophysiological role in the development of arterial dysfunction46,47,48,49. Oxidative stress occurs when the generation of ROS overwhelms antioxidant defense systems50,51. There are several parameters of oxidative stress including MDA and T-AOC. MDA, the product of lipid peroxidation, is the most frequently used biomarker for assessing oxidative stress52. T-AOC provides an overview of the biological interactions between individual antioxidant species, which is composed of some enzymes, such as SOD, GSH-Px, CAT, and non-enzymatic antioxidants53. In this study, we showed that sesamin effectively increased T-AOC and decreased MDA levels in the aortas from SHR. Therefore, the antioxidative effect of sesamin may account for the improvement in arterial function.
The NADPH oxidases appear to be critical sources of hypertensive ROS54. Vascular ·O2− is produced predominantly from multisubunit NADPH oxidase in the endothelium, VSMCs and the adventitia55,56,57,58,59,60,61,62,63,64. Genetic models of hypertension, such as SHR, exhibit enhanced NADPH oxidase-mediated ·O2− generation in the aorta8. These processes are associated with increased expression of NADPH oxidase subunits, particularly p22phox and p47phox, and increased activity of the enzyme64. NO can interact with ·O2− to form the highly reactive peroxynitrite65,66,67. Peroxynitrite is a weak vasodilator compared with NO and a powerful oxidant. Furthermore, it can suppress eNOS expression via activation of RhoA5,68 and lead to eNOS uncoupling (inactivation of eNOS), which results in reduced NO production, increased ·O2− by the enzyme69,70,71 and, ultimately, vascular dysfunction.
In our experimental conditions, we found the elevation of aortic nitrotyrosine and upregulation of p22phox and p47phox protein expression in the vascular tissues of untreated SHR. These results were consistent with previous reports64,72. Indeed, the increased nitrotyrosine protein expression from the untreated SHR group was tangible evidence for the presence of oxidative stress leading to enhance NO inactivation. The increased p22phox and p47phox expression could increase ·O2− production and contribute to the elevation of nitrotyrosine expression and NO inactivation in SHR. Several reports have recently suggested that long-term sesamin treatment decreased aortic nitrotyrosine and p47phox protein expression in renovascular hypertensive rats that were fed with a high-fat, high-sucrose diet18, and sesamin feeding decreased aortic superoxide production and mRNA expression of NADPH oxidase subunits (p22phox, gp91phox, Nox1, and Nox4) in DOCA-salt hypertensive rats22,25. In the present study, we showed that sesamin effectively reduced the expression of nitrotyrosine, p22phox and p47phox in the aortas from SHR. These results demonstrated that the antioxidative effects of sesamin were due to reduced p22phox and p47phox expression. Subsequently, the reaction between NADPH oxidase-mediated ·O2− and NO was suppressed, and eNOS expression was increased because of reduced peroxynitrite. Consequently, these physiological changes led to enhanced levels and bioactivity of NO. Ultimately, these findings demonstrated that sesamin improved arterial function, which may be attributed to decreased p22phox and p47phox expression and increased eNOS expression.
Author contribution
Jie-ren YANG and Jun-xiu ZHANG designed research; Jun-xiu ZHANG, Wen-xing LI, Li-juan TANG and Hui YANG performed research; Guo-xiang CHEN and Xiang KONG contributed new analytical tools and reagents; Jun-xiu ZHANG and Jie-ren YANG analyzed data; Jun-xiu ZHANG wrote the paper.
Acknowledgments
This work was supported by grants from the Key Program of Natural Science Foundation of Education Department of Anhui Province (No 2006kj099A), the Program of Universities Excellent Young Talent Foundation of Anhui Province (No 2009SQRZ184) and the Young and Middle-aged Research Foundation of Wannan Medical College (No WK201206, WK201212).
References
- Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–52. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Rizzoni D, Castellano M, Porteri E, Bettoni G, Muiesan ML, Agabiti-Rosei E. Vascular structural and functional alterations before and after the development of hypertension in SHR. Am J Hypertens. 1994;7:193–200. doi: 10.1093/ajh/7.2.193. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Swei A, Zweifach BW, Schmid-Schönbein GW. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension. 1995;25:1083–9. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Tawfik HE, Matragoon S, Pillai B, Caldwell RB, Caldwell RW. Peroxynitrite mediates diabetes-induced endothelial dysfunction: Possible role of Rho kinase activation. Exp Diabetes Res. 2010;2010:247861. doi: 10.1155/2010/247861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M, Galisteo M, Vera R, Villar IC, Zarzuelo A, Tamargo J, et al. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens. 2006;24:75–84. doi: 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- Ülker S, McKeown PP, Bayraktutan U. Vitamins reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension. 2003;41:534–9. doi: 10.1161/01.HYP.0000057421.28533.37. [DOI] [PubMed] [Google Scholar]
- Zalba G, Beaumont FJ, San José G, Fortuño A, Fortuño MA, Etayo JC, et al. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000;35:1055–61. doi: 10.1161/01.hyp.35.5.1055. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance. Hypertension. 2004;44:248–52. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923–39. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre M, Bohr DF, Dominiczak AF. Endothelial function in hypertension: the role of superoxide anion. Hypertension. 1999;34:539–45. doi: 10.1161/01.hyp.34.4.539. [DOI] [PubMed] [Google Scholar]
- Hirose N, Inoue T, Nishihara K, Sugano M, Akimoto K, Shimizu S, et al. Inhibition of cholesterol absorption and synthesis in rats by sesamin. J Lipid Res. 1991;32:629–38. [PubMed] [Google Scholar]
- Ide T, Hong DD, Ranasinghe P, Takahashi Y, Kushiro M, Sugano M. Interaction of dietary fat types and sesamin on hepatic fatty acid oxidation in rats. Biochim Biophys Acta. 2004;1682:80–91. doi: 10.1016/j.bbalip.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Kita S, Matsumura Y, Morimoto S, Akimoto K, Furuya M, Oka N, et al. Antihypertensive effect of sesamin. II. Protection against two-kidney, one-clip renal hypertension and cardiovascular hypertrophy. Biol Pharm Bull. 1995;18:1283–5. doi: 10.1248/bpb.18.1283. [DOI] [PubMed] [Google Scholar]
- Kong X, Yang JR, Guo LQ, Xiong Y, Wu XQ, Huang K, et al. Sesamin improves endothelial dysfunction in renovascular hypertensive rats fed with a high-fat, high-sucrose diet. Eur J Pharmacol. 2009;620:84–9. doi: 10.1016/j.ejphar.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Kita S, Morimoto S, Akimoto K, Furuya M, Oka N, et al. Antihypertensive effect of sesamin. I. Protection against deoxycorticosterone acetate-salt-induced hypertension and cardiovascular hypertrophy. Biol Pharm Bull. 1995;18:1016–9. doi: 10.1248/bpb.18.1016. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Kita S, Tanida Y, Taguchi Y, Morimoto S, Akimoto K, et al. Antihypertensive effect of sesamin. III. Protection against development and maintenance of hypertension in stroke-prone spontaneously hypertensive rats. Biol Pharm Bull. 1998;21:469–73. doi: 10.1248/bpb.21.469. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Aono H, Toyoda-Ono Y, Maeda H, Kiso Y, Moriyama K. Antihypertensive effects of sesamin in humans. J Nutr Sci Vitaminol (Tokyo) 2009;55:87–91. doi: 10.3177/jnsv.55.87. [DOI] [PubMed] [Google Scholar]
- Nakano D, Itoh C, Ishii F, Kawanishi H, Takaoka M, Kiso Y, et al. Effects of sesamin on aortic oxidative stress and endothelial dysfunction in deoxycorticosterone acetate-salt hypertensive rats. Biol Pharm Bull. 2003;26:1701–5. doi: 10.1248/bpb.26.1701. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Sasagawa S, Murakami T, Yoshizumi H, Shimizu S, Yamada H. Effects of sesamin on serum lipoprotein metabolism in normocholesterolemic and hypercholesterolemic stroke-prone SHR. Ann N Y Acad Sci. 1993;676:338–9. doi: 10.1111/j.1749-6632.1993.tb38748.x. [DOI] [PubMed] [Google Scholar]
- Nakano D, Kwak CJ, Fujii K, Ikemura K, Satake A, Ohkita M, et al. Sesamin metabolites induce an endothelial nitric oxide-dependent vasorelaxation through their antioxidative property-independent mechanisms: possible involvement of the metabolites in the antihypertensive effect of sesamin. J Pharmacol Exp Ther. 2006;318:328–35. doi: 10.1124/jpet.105.100149. [DOI] [PubMed] [Google Scholar]
- Nakano D, Kurumazuka D, Nagai Y, Nishiyama A, Kiso Y, Matsumura Y. Dietary sesamin suppresses aortic NADPH oxidase in DOCA salt hypertensive rats. Clin Exp Pharmacol Physiol. 2008;35:324–6. doi: 10.1111/j.1440-1681.2007.04817.x. [DOI] [PubMed] [Google Scholar]
- Wu XQ, Kong X, Zhou Y, Huang K, Yang JR, Li XL. Sesamin exerts renoprotective effects by enhancing NO bioactivity in renovascular hypertensive rats fed with high-fat-sucrose diet. Eur J Pharmacol. 2012;683:231–7. doi: 10.1016/j.ejphar.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martinez MA, Ruiz-Torres A. Homeostasis between lipid peroxidation and antioxidant enzyme activities in healthy human aging. Mech Ageing Dev. 1992;66:213–322. doi: 10.1016/0047-6374(92)90137-3. [DOI] [PubMed] [Google Scholar]
- Manso MA, Miguel M, Even J, Hernandez R, Aleixandre MA, López-Fandiño R. Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats. Food Chemistry. 2008;109:361–7. doi: 10.1016/j.foodchem.2007.12.049. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- ter Steege JC, Koster-Kamphuis L, van Straaten EA, Forget PP, Buurman WA. Nitrotyrosine in plasma of celiac disease patients as detected by a new sandwich ELISA. Free Radic Biol Med. 1998;25:953–63. doi: 10.1016/s0891-5849(98)00184-1. [DOI] [PubMed] [Google Scholar]
- Patterson C, Ruef J, Madamanchi NR, Barry-Lane P, Hu Z, Horaist C, et al. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47 (phox) may participate in forming this oxidase in vitro and in vivo. J Biol Chem. 1999;274:19814–22. doi: 10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- Potenza MA, Gagliardi S, De Benedictis L, Zigrino A, Tiravanti E, Colantuono G, et al. Treatment of spontaneously hypertensive rats with rosiglitazone ameliorates cardiovascular pathophysiology via antioxidant mechanisms in the vasculature. Am J Physiol Endocrinol Metab. 2009;297:E685–94. doi: 10.1152/ajpendo.00291.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägg U, Andersson I, Naylor AS, Grönros J, Jonsdottir IH, Bergström G, et al. Voluntary physical exercise-induced vascular effects in spontaneously hypertensive rats. Clin Sci (Lond) 2004;107:571–81. doi: 10.1042/CS20040171. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang F, Wang B, Gong B, Zhang J, Zhang X, et al. Cilostazol protects diabetic rats from vascular inflammation via nuclear factor-kappa B-dependent down-regulation of vascular cell adhesion molecule-1 expression. J Pharmacol Exp Ther. 2006;318:53–8. doi: 10.1124/jpet.106.101444. [DOI] [PubMed] [Google Scholar]
- Gu K, Zhao JD, Ren ZG, Ma NY, Lai ST, Wang J, et al. A natural process of cirrhosis resolution and deceleration of liver regeneration after thioacetamide withdrawal in a rat model. Mol Biol Rep. 2011;38:1687–96. doi: 10.1007/s11033-010-0281-1. [DOI] [PubMed] [Google Scholar]
- López-Sepúlveda R, Jiménez R, Romero M, Zarzuelo MJ, Sánchez M, Gómez-Guzmán M, et al. Wine polyphenols improve endothelial function in large vessels of female spontaneously hypertensive rats. Hypertension. 2008;51:1088–95. doi: 10.1161/HYPERTENSIONAHA.107.107672. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Vaziri ND, Wang XQ, Barnard RJ. Enhanced NO inactivation and hypertension induced by a high-fat, refined-carbohydrate diet. Hypertension. 2000;36:423–9. doi: 10.1161/01.hyp.36.3.423. [DOI] [PubMed] [Google Scholar]
- Xu C, Lee S, Singh TM, Sho E, Li X, Sho M, et al. Molecular mechanisms of aortic wall remodeling in response to hypertension. J Vasc Surg. 2001;33:570–8. doi: 10.1067/mva.2001.112231. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–8. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- Yang D, Félétou M, Boulanger CM, Wu HF, Levens N, Zhang JN, et al. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–10. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Förstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–54. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Shen YH, Wang XL, Wilcken DE. Nitric oxide induces and inhibits apoptosis through different pathways. FEBS Lett. 1998;433:125–31. doi: 10.1016/s0014-5793(98)00844-8. [DOI] [PubMed] [Google Scholar]
- Walford G, HLoscalzo J. Nitric oxide in vascular biology. J Thromb Haemost. 2003;1:2112–8. doi: 10.1046/j.1538-7836.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rodríguez R, Herrera MD, de Sotomayor MA, Ruiz-Gutierrez V. Pomace olive oil improves endothelial function in spontaneously hypertensive rats by increasing endothelial nitric oxide synthase expression. Am J Hypertens. 2007;20:728–34. doi: 10.1016/j.amjhyper.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Lee CC, Chen PR, Lin S, Tsaia SC, Wanga BW, Chena WW, et al. Sesamin induces nitric oxide and decreases endothelin-1 production in HUVECs: possible implications for its antihypertensive effect. J Hypertens. 2004;22:2329–38. doi: 10.1097/00004872-200412000-00015. [DOI] [PubMed] [Google Scholar]
- Ferroni P, Basili S, Paoletti V, Davì G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr Metab Cardiovasc Dis. 2006;16:222–33. doi: 10.1016/j.numecd.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Harrison DG, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. J Am Soc Hypertens. 2007;1:30–44. doi: 10.1016/j.jash.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care. 2008;31:S181–4. doi: 10.2337/dc08-s245. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Passalacqua W, Araya J, Orellana M, Rivera G. Implications of oxidative stress and homocysteine in the pathophysiology of essential hypertension. J Cardiovasc Pharmacol. 2003;42:453–61. doi: 10.1097/00005344-200310000-00001. [DOI] [PubMed] [Google Scholar]
- Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–70. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Juránek I, Bezek S. Controversy of free radical hypothesis: reactive oxygen species — cause or consequence of tissue injury. Gen Physiol Biophys. 2005;24:263–78. [PubMed] [Google Scholar]
- Janero DR. Malondialdehyde and thiobarbituric-acid reactivity as diagnostic indices of lipid peroxidation, and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Maxwell SR, Dietrich T, Chapple IL. Prediction of serum total antioxidant activity from the concentration of individual serum antioxidants. Clin Chim Acta. 2006;372:188–94. doi: 10.1016/j.cca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:274–8. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- Strålin P, Karlsson K, Johansson BO, Marklund SL. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol. 1995;15:2032–6. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. J Clin Invest. 1996;98:2572–9. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning) Free Radic Res. 1999;3:261–72. doi: 10.1080/10715769900300841. [DOI] [PubMed] [Google Scholar]
- Channon KM, Guzik TJ. Mechanisms of superoxide production in human blood vessels: relationship to endothelial dysfunction, clinical and genetic risk factors. J Physiol Pharmacol. 2002;53:515–24. [PubMed] [Google Scholar]
- Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, et al. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–35. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Haendeler J, Berk BC. Thioredoxin: a key regulator of cardiovascular homeostasis. Circ Res. 2003;93:1029–33. doi: 10.1161/01.RES.0000102869.39150.23. [DOI] [PubMed] [Google Scholar]
- Jones SA, O'Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–34. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- Azumi H, Inoue N, Takeshita S, Rikitake Y, Kawashima S, Hayashi Y, et al. Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation. 1999;100:1494–8. doi: 10.1161/01.cir.100.14.1494. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Lassègue B, HClempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–97. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- Tschudi M, Mesaros S, Luscher TF, Malinski T. Direct in situ measurement of nitric oxide in mesenteric resistance arteries: increased decomposition by superoxide in hypertension. Hypertension. 1996;27:32–5. doi: 10.1161/01.hyp.27.1.32. [DOI] [PubMed] [Google Scholar]
- List BM, Klösch B, Völker C, Gorren AC, Sessa WC, Werner ER, et al. Characterization of bovine endothelial nitric oxide synthase as a homodimer with down-regulated uncoupled NADPH oxidase activity: tetrahydrobiopterin binding kinetics and role of haem in dimerization. Biochem J. 1997;323:159–65. doi: 10.1042/bj3230159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers MJ, Harrison DG. Reactive oxygen species and the control of vasomotor tone. Curr Hypertens Rep. 1999;1:102–8. doi: 10.1007/s11906-999-0080-z. [DOI] [PubMed] [Google Scholar]
- Yao L, Romero MJ, Toque HA, Yang G, Caldwell RB, Caldwell RW. The role of RhoA/Rho kinase pathway in endothelial dysfunction. J Cardiovasc Dis Res. 2010;1:165–70. doi: 10.4103/0975-3583.74258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino F, Lüscher TF. Tetrahydrobiopterin and endothelial function. Eur Heart J. 1998;19:G3–8. [PubMed] [Google Scholar]
- Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–8. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- Szabó CH. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett. 2003;140:105–12. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- Hong HJ, Loh SH, Yen MH. Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. Br J Pharmacol. 2000;131:631–7. doi: 10.1038/sj.bjp.0703603. [DOI] [PMC free article] [PubMed] [Google Scholar]