Abstract
Aim:
To investigate whether curcumin (Cur) suppressed lipopolysaccharide (LPS)-induced inflammation in vascular smooth muscle cells (VSMCs) of rats, and to determine its molecular mechanisms.
Methods:
Primary rat VSMCs were treated with LPS (1 μg/L) and Cur (5, 10, or 30 μmol/L) for 24 h. The levels of MCP-1, TNF-α, and iNOS were measured using ELISA and real-time RT-PCR. NO level was analyzed with the Griess reaction. Western-blotting was used to detect the activation of TLR4, MAPKs, IκBα, NF-κB p65, and the p47phox subunit of NADPH oxidase in the cells.
Results:
Treatment of VSMCs with LPS dramatically increased expression of inflammatory cytokines MCP-1 and TNF-α, expression of TLR4 and iNOS, and NO production. LPS also significantly increased phosphorylation of IκBα, nuclear translocation of NF-κB (p65) and phosphorylation of MAPKs in VSMCs. Furthermore, LPS significantly increased production of intracellular ROS, and decreased expression of p47phox subunit of NADPH oxidase. Pretreatment with Cur concentration-dependently attenuated all the aberrant changes in LPS-treated VSMCs. The LPS-induced overexpression of MCP-1 and TNF-α, and NO production were attenuated by pretreatment with the ERK inhibitor PD98059, the p38 MAPK inhibitor SB203580, the NF-κB inhibitor PDTC or anti-TLR4 antibody, but not with the JNK inhibitor SP600125.
Conclusion:
Cur suppresses LPS-induced overexpression of inflammatory mediators in VSMCs in vitro via inhibiting the TLR4-MAPK/NF-κB pathways, partly due to block of NADPH-mediated intracellular ROS production.
Keywords: curcumin, vascular smooth muscle cell, atherosclerosis, inflammation, lipopolysaccharide, monocyte chemotactic protein-1 (MCP-1), TNF-α, Toll-like receptor 4 (TLR4), mitogen-activated protein kinases (MAPK), NF-κB, reactive oxygen species (ROS), NADPH
Introduction
Long-lasting, low-grade inflammation plays an important role in the progression of atherosclerosis, which is implicated in many cardiovascular diseases1. The pathological proliferation and inflammatory responses of vascular smooth muscle cells (VSMCs) can potentially contribute to atherosclerosis and arterial restenosis2. After stimulation by lipopolysaccharides (LPS) or other inflammatory inducers, activated VSMCs overexpress various inflammatory cytokines, including TNF-α and MCP-1. The overexpression of such factors has been clinically linked to the acceleration of atherosclerosis and the formation of vulnerable plaques3,4. Therefore, using pharmacological treatments to inhibit VSMCs' excessive inflammatory responses presents a powerful strategy to control the progression of atherosclerotic plaques within arterial walls.
Curcumin (Cur) is a natural polyphenol that is responsible for the yellow color of turmeric. Turmeric commonly appears today in ordinary diets, but it has been used since ancient times in herbal remedies to treat various diseases5. Cur has diverse pharmacological effects, such as anti-inflammatory, anti-carcinogenic, and anti-oxidative effects, and it also provides cardiovascular protection. Cur harbors these benefits because it regulates certain molecular targets, including pro-inflammatory cytokines, growth factors, factors involved in proliferation and apoptosis, adhesion molecules, and transcription factors, associated with many different cell types6,7,8,9. In vascular cells specifically, Cur has been found to inhibit oxidized low-density lipoprotein (ox-LDL) -induced overexpression of pro-inflammatory cytokines in VSMCs4. However, the molecular mechanisms underlying Cur's inhibition of LPS-induced inflammatory responses in VSMCs are not well known.
Toll-like receptor 4 (TLR4), belonging to a family of pathogen-associated molecular-pattern-recognition molecules, plays a key role in the initiation and acceleration of inflammation10. Evidence is mounting for the argument that TLR4 plays an important role in the progression of atherosclerosis: one study demonstrated that TLR4 is markedly overexpression in human atherosclerotic plaques, while in other experiments involving TLR4 knockouts, scientists were able to reduce area and vulnerability of atherosclerotic plaques11,12. Furthermore, Cur has been found to attenuate inflammation via inhibition of the TLR4 receptor in experimental colitis13. The NF-κB signaling pathway, being downstream of TLR4-mediated signaling, plays a critical role in amplifying inflammatory responses by upregulating the expression of various pro-inflammatory cytokines genes14. Mitogen-activated protein kinases (MAPKs) are a group of signaling molecules to transport diverse extracellular stimuli into intracellular regions to regulate proliferation, inflammatory responses, differentiation, and apoptosis, among other things15,16,17,18. LPS has been shown to induce overproduction of IL-6 by suppressing the activation of extracellular signal-regulated kinase 1/2 (ERK1/2), p38 MAPK, and NF-κB in VSMCs19. There is also a growing evidences interest in the pro-inflammatory effects of intracellular reactive oxygen species (ROS), which are regarded as secondary messengers that amplify inflammation by upregulating kinase cascades and activating transcription factors in VSMCs20,21. However, few studies have demonstrated whether Cur can effectively inhibit LPS-induced inflammation via TLR4-mediated ROS-relative MAPK/NF-κB signaling pathways in VSMCs.
Therefore, our present study was aimed at uncovering the molecular mechanisms by which Cur inhibits LPS-induced overexpression of pro-inflammatory mediators and intracellular ROS in VSMCs.
Materials and methods
Regents
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were provided by Gibco BRL (Carlsbad, CA, USA). Penicillin, streptomycin, Tris, curcumin, EDTA, LPS from Escherichia coli 0111:B4, PD98059, SB203580, pyrrolidinedithiocarbamate (PDTC), 2′,7′-dichlorodihydrofluororescein diacetate (DCFH-DA), diphenyleneiodonium (DPI), SP600125, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma Chemical Co (St Louis, MO, USA). Antibodies against TLR4, anti-TLR4, ERK1/2, p38 MAPK, c-Jun N-terminal kinase1/2 (JNK1/2), IκBα, phospho-IκBα (p-IκBα), phospho-ERK1/2 (p-ERK1/2), phospho-p38MAPK (p-p38MAPK), phospho-JNK1/2 (p-JNK1/2), and NF-κB (p65) were purchased from Cell Signaling Technology (Beverly, MA, USA); TRIzol, EasyScript Reverse Transcriptase, TransStrat Green Qpcr SuperMix, and a β-actin antibody were purchased from TransGen Biotechnology (Beijing, China). MCP-1 and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were bought from Thermo Fisher Scientific (Rockford, IL, USA). The histone antibody, polyclonal anti-rat iNOs antibody, and the p47phox antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
VSMCs culture
The study was carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No 85–23, revised 1996). Male Sprague-Dawley rats (weight 140–180 g) were obtained from the Laboratory Animal Institute in the School of Medicine at Xi-an Jiaotong University. According to a previously described method22, VSMCs were isolated from the thoracic aorta of rats. Cells were cultured in DMEM containing 15% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Morphological examination was carried out to identify VSMCs. Cells between passage 3 and passage 10 were used for all experiments. When the cells reached 80%-90% confluence, the medium was replaced with serum-free medium and cells were cultured for 12-16 h before conducting the experiments.
Cell viability assay
Cells were seeded at a density of 4000 cell/well in 96-well plates. Cell viability was determined by the MTT reduction assay. After various indicated treatments for 24 h, the medium was removed and cells were incubated with MTT (5 mg/mL) for 4 h at 37 °C. The dark blue formazan crystals that formed in intact cells were solubilized with DMSO, and then the absorbance was measured at 490 nm on a microplate reader (Bio-Rad, Hercules, CA, USA).
Enzyme-linked immunosorbent assay (ELISA) for MCP-1 and TNF-α
VSMCs of 5×106 cells/well were plated onto 6-well plates. VSMCs were pretreated with different concentrations of Cur (5, 10, or 30 μmol/L) for 1 h, and then LPS (1 μg/mL) was added to the VSMCs culture medium for 24 h. In another experiment, VSMCs were pretreated with anti-TLR4, DPI (20 μmol/L), PD98059 (50 μmol/L), SB203580 (25 μmol/L), SP600125 (15 μmol/L) and PDTC (80 μmol/L) for 1 h, and then incubated with LPS (1 μg/ml) for another 24 h. The concentrations of MCP-1 and TNF-α in the culture medium were measured by ELISA kits according to the manufacturer's instructions.
Measurement of nitrite
Nitrite, a stable precursor of NO, was analyzed using the Griess reaction23. Fifty microliters of the culture supernatant was mixed with an equal volume of Griess reagent (0.1% naphthyl-ethylenediamine, 1% sulfanylamide, and 2.5% phosphoric acid). Absorbance was measured at 540 nm, using a calibration curve with sodium nitrite standards.
Real-time reverse-transcriptase polymerase chain reaction (real-time RT-PCR)
Total RNA was extracted using TRIzol reagent, and DNA was removed using the DNA-free kit (Ambion, Austin, TX, USA). The quality of mRNA was checked by performing denaturing agarose gel electrophoresis containing 1.5% formaldehyde. The total RNA concentration and purity were determined by UV–Vis spectroscopy with the Bio-Rad SmartSpec 3000 system (Bio-Rad, Hercules, CA, USA). To synthesize cDNA, 1 μg of total RNA was used in a 20 μL reaction using oligo(dT)18 Primer and TransScript Reverse Transcriptase (Transgen Biotech, Beijing, China). Primers for rat TNF-α, MCP-1, TLR4, iNOS, and β-actin were chosen using the Beacon designer v4.0 (Premier Biosoft, CA, USA). β-Actin was used as an endogenous control. The mRNA levels of TNF-α, MCP-1, TLR4, iNOS, and β-actin were examined by real-time RT-PCR with the ABI PRISM 7000 sequence detection PCR system (Applied Biosystems, Foster City, CA, USA). One melting peak confirmed the presence of a single product. Results were expressed as fold differences relative to the level of β-actin using the 2-ΔΔCT method (Table 1).
Table 1. Primers used for real-time PCR analysis.
| Gene | Oligonucleotide primer sequences (5′–3′) |
|---|---|
| MCP-1 | Forward: GGCCTGTTGTTCACAGTTGCT |
| Reverse: TCTCACTTGGTTCTGGTCCAGT | |
| TLR4 | Forward: GGCATCATCTTCATTGTCCTTG |
| Reverse: AGCATTGTCCTCCCACTCG | |
| TNF-α | Forward: TCCCAACAAGGAGGAGAAGT |
| Reverse: TGGTATGAAGTGGCAAATCG | |
| iNOS | Forward: CCACGCTCTTCTGTCTACTGAAC |
| Reverse: ACGGGCTTGTCACTCGAG | |
| β-Actin | Forward: ATCGGCAATGAGCGGTTCC |
| Reverse: AGCACTGTGTTGGCATAGAGG |
Western-blot
VSMC lysates were prepared with 200 μL of ice-cold lysis buffer (pH 7.4) (50 mmol/L HEPES, 5 mmol/L EDTA, 100 mmol/L NaCl, 1% Triton X-100, protease inhibitor cocktail; Roche, Mannheim, Germany) in the presence of phosphatase inhibitors (50 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate, 10 mmol/L sodium pyrophosphate, 1 nmol/L microcystin). The activated NF-κB (p65) protein, located in the nucleus, was extracted using the Pierce NE-PER kit (Pierce, Rockford, IL, USA). The BCA protein assay kit was used to determine protein concentrations. Samples underwent 10% SDS-PAGE and were then transferred onto a polyvinylidene difluoride membrane in a semi-dry system (Bio-Rad), which was blocked with 5% fat-free milk in TBST buffer (20 mmol/L Tris-HCl, 137 mmol/L NaCl and 0.1% Tween 20), then incubated with primary antibodies against TLR4 (1:200), iNOs (1:200), IκBα (1:400), p-IκBα (1:200), NF-κB (p65) (1:200), p38MAPK (1:500), p-p38MAPK (1:500), ERK1/2 (1:500), p-ERK1/2 (1:500), JNK (1:200), p-JNK (1:200), p47phox (1:400), β-actin (1:1000) and histone (1:500) in TBST buffer overnight, then washed and incubated with secondary antibodies for 120 min. The optical densities of bands were quantified by using Gel-Pro Analyzer v4.0 (Media Cybernetics, Rockville, MD, USA). β-Actin was the endogenous control, and results were expressed relative to that control.
Stable free radical scavenging activity
Stable free radical scavenging activity was identified using the method reported by Jeong et al24. Briefly, 100 μmol/L of DPPH radical solution was dissolved in 100% ethanol. The mixture was shaken vigorously and allowed to stand for 10 min in the dark. The test materials (100 μL each) were added to 900 μL of the DPPH radical solution. After incubation at room temperature for 30 min, the absorbance at 517 nm was measured using the SPECTRA (shell) Reader.
Intracellular ROS assay
The level of intracellular ROS was measured by the DCFH-DA method, based on the ROS-dependent oxidation of DCFH to the highly fluorescent DCF. DCFH was dissolved in methanol at 10 mmol/L and then diluted by a factor of 500 in Hanks' balanced salt solution (HBSS) to give DCFH at 20 μmol/L. The cells were incubated with DCFH-DA for 1 h and then treated with HBSS containing Cur (5, 10, or 30 μmol/L) or LPS (1μg/mL) for another 2 h. The fluorescence was measured immediately at wave lengths of 485 nm for excitation and 528 nm for emission on the iMark™ Microplate Absorbance Reader (Bio-Rad, Laboratories, Inc, China)
Statistical analysis
Results are expressed as the mean±SEM of three experiments. Differences between groups were assessed by one-way ANOVA followed by post hoc tests. Statistical tests were performed using SPSS 17.0 software (SPSS Inc, Chicago, IL, USA). A value of P<0.05 was considered to be statistically significant.
Results
Effect of Cur on VSMCs viability
To detect whether Cur was toxic to VSMCs, cells were treated with different concentrations of curcumin (0-80 μmol/L) or LPS (1 μg/mL) for 24 h, and were then subjected to the MTT assay to determine cell viability. The results showed that both Cur at concentrations between 0-80 μmol/L and LPS (1 μg/mL) did not affect VSMC viability.
Effect of Cur on LPS-induced expression of MCP-1 and TNF-α in VSMCs
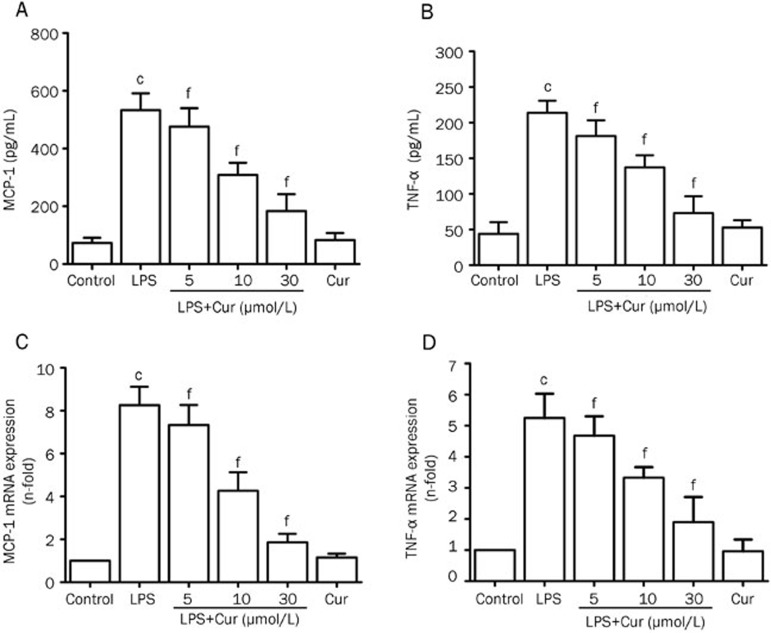
Stimulation of VSMCs with LPS caused a significant increase in MCP-1 and TNF-α secretion in the conditioned media. Cur inhibited LPS-induced overexpression of MCP-1 and TNF-α in a concentration-dependent manner in VSMCs (Figure 1A and 1B). Cur also suppressed an LPS-induced increase in the mRNA expression of MCP-1 and TNF-α, also in a concentration-dependent manner. However, treating VSMCs with Cur alone had no effect on the expression of MCP-1 and TNF-α (Figure 1C and 1D).
Figure 1.
Inhibitory effect of Cur on LPS-induced MCP-1 and TNF-α production in VSMCs. VSMCs were pretreated with the indicated concentration of Cur (5, 10, and 30 μmol/L) for 1 h, and then treated with LPS (1 μg/mL) for another 24 h. Some cells were treated with Cur (30 μmol/L) alone for 24 h. Culture medium was collected and the levels of MCP-1 and TNF-α were measured with commercial ELISA kits (A and B). Total RNA was extracted after the same treatment for 6 h, and the levels of MCP-1 mRNA and TNF-α mRNA were detected by quantitative real-time PCR (C and D). Data are presented as mean±SEM of three independent experiments. cP<0.01 vs control; fP<0.01 vs LPS.
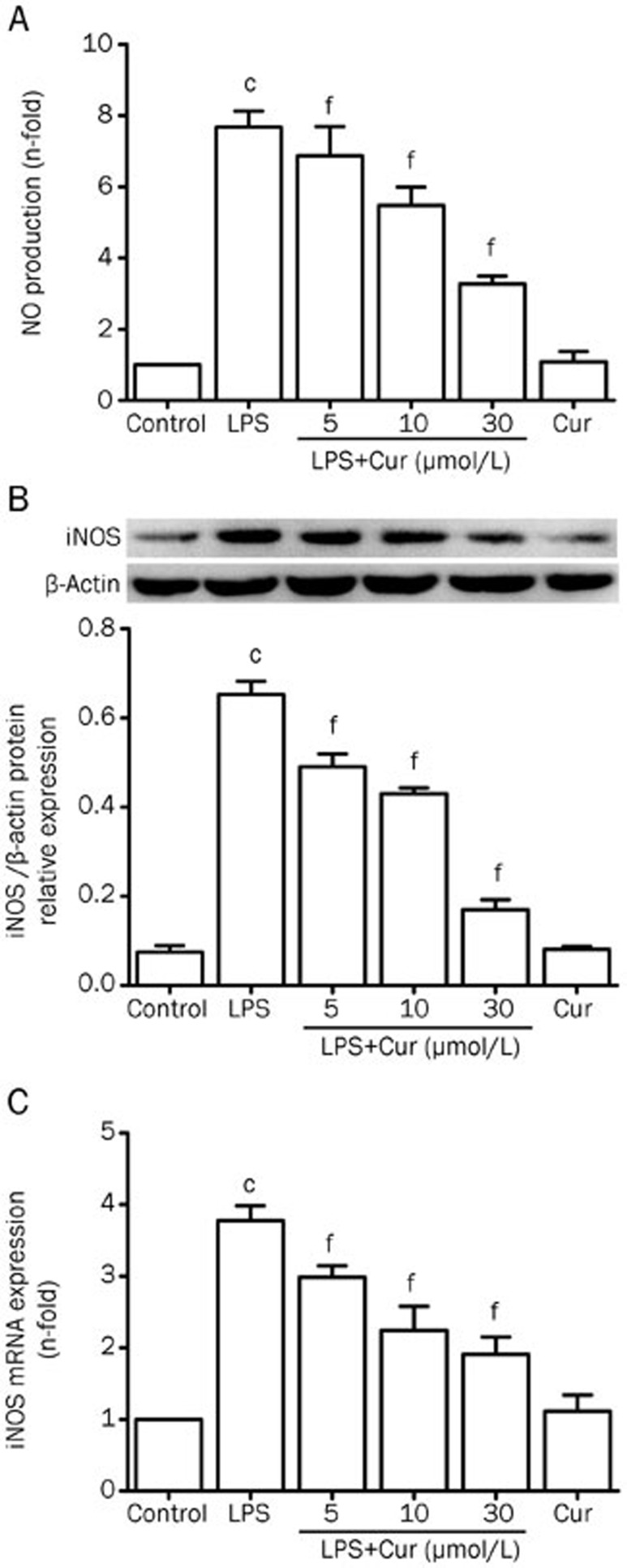
Effect of Cur on LPS-induced NO production and iNOS expression
NO levels in the supernatant were detected by the Griess assay. Cur remarkably decreased LPS-induced secretion of NO in VSMCs, in a concentration-dependent manner. In the absence of LPS stimulation, Cur showed no effect on NO secretion (Figure 2A). Cur inhibited LPS-induced overexpression of iNOS mRNA and protein levels in VSMCs, while treatment with Cur in the absence of LPS stimulation again had no effect on mRNA and protein expression of iNOS (Figure 2B and 2C). These results indicate that Cur reduced the production of NO in part by suppressing the activation of iNOS in LPS-stimulated VSMCs.
Figure 2.
Effect of Cur on LPS-induced NO production and iNOS expression in VSMCs. VSMCs were pretreated with the indicated concentration of Cur (5, 10, and 30 μmol/L) for 1 h, and then treated with LPS (1 μg/mL) for another 24 h. Some cells were treated with Cur (30 μmol/L) alone for 24 h. Cell supernatants were collected to measure NO production (A). The levels of iNOS mRNA and iNOS protein were respectively analyzed by quantitative real-time PCR after normalization to β-actin and Western blotting, in which β-actin was used as an internal control (B and C). Data are presented as mean±SEM of three independent experiments. cP<0.01 vs control; fP<0.01 vs LPS.
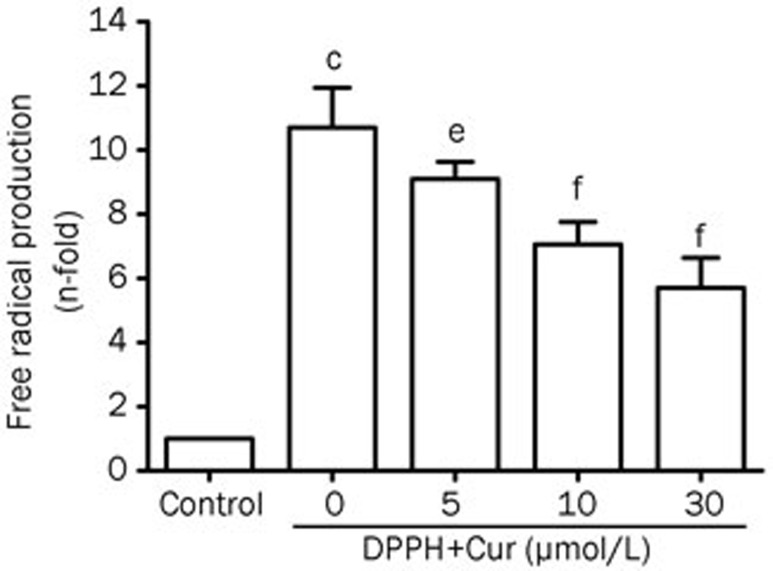
Effect of Cur on the free radical scavenging activity
The DPPH reduction assay was used to detect the scavenging activity of Cur on LPS-stimulated VSMCs. Cur was able to effectively scavenge the DPPH free radical caused by ROS, in a concentration-dependent manner, suggesting that Cur might be a powerful free radical scavenger (Figure 3).
Figure 3.
Inhibitory effect of Cur on LPS-induced free radical scavenging activity in VSMCs. DPPH at a concentration of 100 μmol/L was dissolved in 100% ethanol. VSMCs were incubated with the DPPH solution alone or DPPH in conjunction with Cur (5, 10, and 30 μmol/L) for 30 min at room temperature. The absorbance at 517 nm was measured using a SPECTRA (shell) Reader. Data are presented as mean±SEM of three independent experiments. cP<0.01 vs control; eP<0.05, fP<0.01 vs DPPH.
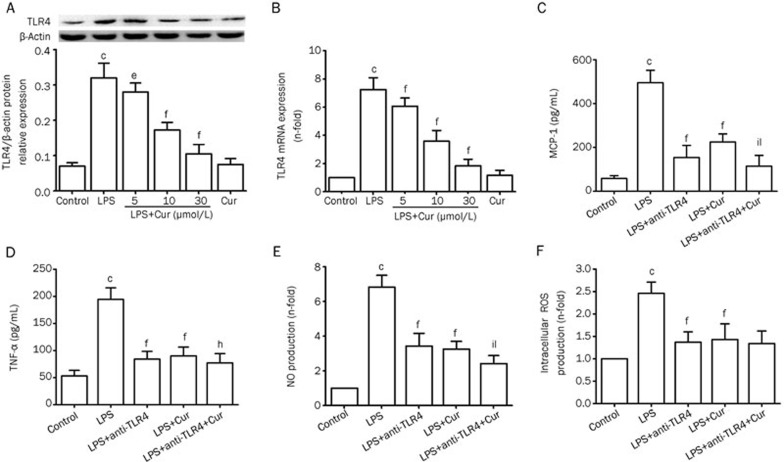
The relationship between Cur's inhibitory effect on LPS-induced inflammation and TLR4 in VSMCs
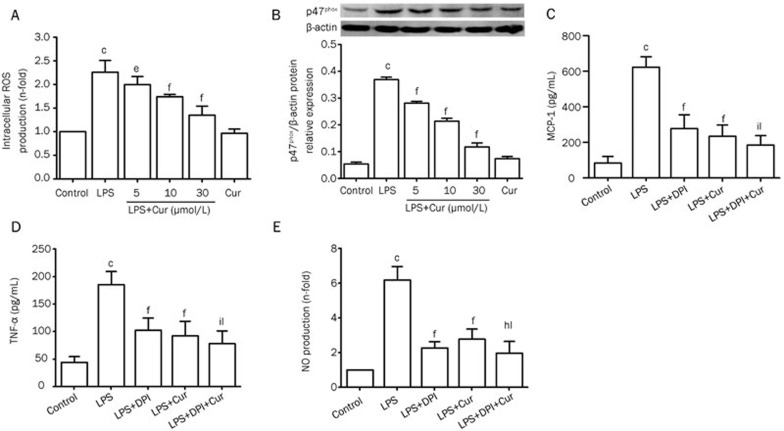
After VSMCs were stimulated with LPS (1 μg/mL) for 24 h, the expression of TLR4 significantly increased. Pretreating VSMCs with Cur suppressed LPS-induced overexpression of TLR4 mRNA and protein, in a dose-dependent manner. Again, Cur alone did not affect the expression of TLR4 (Figure 4A and 4B). To evaluate whether Cur inhibited the inflammatory responses through TLR4, VSMCs were pretreated with or without anti-TLR4 neutralizing antibody (5 μg/mL) for 1 h before adding Cur (30 μmol/L) for 1 h, and then cells were stimulated with LPS (1 μg/mL) for 24 h. The TLR4 inhibitor (anti-TLR4 antibody) and Cur both partially suppressed LPS-induced overexpression of MCP-1, TNF-α, and NO. Furthermore, pretreating VSMCs with a combination of anti-TLR4 antibody and Cur synergistically reversed the LPS-induced expression of MCP-1, TNF-α, and NO (Figure 4C, 4D, and 4E). We also proved that the TLR4 inhibitor (anti-TLR4 antibody) and Cur both partially suppressed LPS-induced overexpression of intracellular ROS (Figure 4F). The results suggest that TLR4 may be involved in Cur's reduction of LPS-induced inflammation in VSMCs.
Figure 4.
Relationship between Cur's inhibitory effects on LPS-induced inflammation and TLR4 in VSMCs. VSMCs were pretreated with the indicated concentration of Cur (5, 10, or 30 μmol/L) for 1 h, and then treated with LPS (1 μg/mL) for another 24 h. The levels of TLR4 mRNA and TLR4 protein were respectively analyzed by quantitative real-time PCR and Western blotting (A and B). VSMCs were pretreated with or without the anti-TLR4 antibody (5 μg/mL) for 1 h before adding Cur (30 μg/mL) for 1 h, and subsequently treated with LPS (1 μg/mL) for 24 h. The conditioned media were collected to measure the concentration of MCP-1 (C), TNF-α (D), and NO (E); intracellular ROS production was measured by DCHF-DA method (F). Data are presented as mean±SEM of three independent experiments. cP<0.01 vs control; fP<0.01 vs LPS; hP<0.05, iP<0.01 vs LPS+anti-TLR4; lP<0.01 vs LPS+Cur.
Effects of Cur on intracellular ROS production and p47phox expression in LPS-stimulated VSMCs
Treating VSMCs with LPS (1 μg/mL) for 24 h significantly increased their intracellular ROS production, while pretreatment with Cur (5, 10, or 30 μmol/L) remarkably attenuated these effects (Figure 5A). The p47phox subunit is a key component of NADPH oxidase which is involved in intracellular ROS production in VSMCs. Our data showed that Cur inhibited LPS-induced overexpression of p47phox in VSMCs, in a dose-dependent manner (Figure 5B). To evaluate whether LPS-induced overexpression of cytokines in VSCMs occurred because of an increase in ROS, cells were pretreated with or without DPI (20 μmol/L), which inhibits NADPH oxidase, for 1 h before adding Cur (30 μmol/L) for 1 h, and then cells were stimulated with LPS (1 μg/mL) for 24 h. DPI and Cur both partially decreased LPS-induced overexpression of MCP-1, TNF-α, and NO. Pretreating VSMCs with a combination of DPI and Cur synergistically suppressed the LPS-induced increase of inflammatory cytokines. These results therefore suggest that Cur's inhibitory effect on LPS-induced inflammation is, in part, dependent on suppressing the production of NADPH-mediated intracellular ROS in VSMCs.
Figure 5.
Effect of Cur on LPS-induced intracellular ROS production and p47phox expression in VSMCs. Cell were pretreated with Cur (5, 10, or 30 μmol/L) for 100 min, and then incubated with 1 μg/mL LPS for another 2 h. Some cells were treated with Cur (30 μmol/L) alone for 2 h. The intracellular ROS levels were determined by DCFH oxidation (A). Cells were treated with Cur in the presence or absence of LPS (1 μg/mL) for 9 h. The expression of p47phox was detected by Western blotting (B). VSMCs were pretreated with or without DPI (20 μmol/L) for 1 h before the addition of Cur (30 μg/mL) for 1 h, and subsequently treated with LPS (1 μg/mL) for 2 h. The conditioned media were collected to measure the concentration of MCP-1 (C), TNF-α (D), and NO (E). Each bar represents the means±SEM of three independent experiments. cP<0.01 vs control; eP<0.05, fP<0.01 vs LPS; hP<0.05, iP<0.01 vs LPS+anti-TLR4; lP<0.01 vs LPS+Cur.
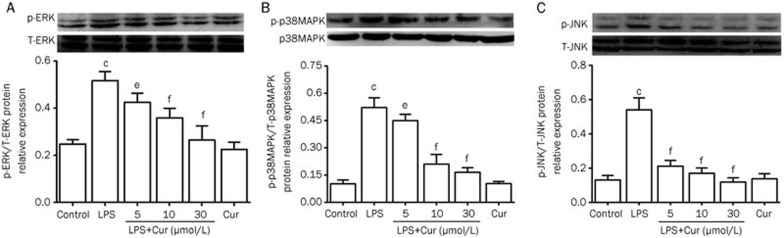
Effect of Cur on the MAPK signaling pathway in LPS-stimulated VSMCs
The MAPK signaling pathway is an important cascade that translates extracellular signals into intracellular activities. In order to determine whether the MAPK signaling pathway was involved in Cur's inhibition of inflammatory responses in LPS-stimulated VSMCs, we identified activated MAPK signaling molecules. Because the MAPK signaling molecules investigated here are activated by phosphorylation, we detected the phosphorylated forms of ERK1/2, p38 MAPK, and JNK1/2 by Western-blotting. After VSMCs were treated with LPS (1 μg/mL) for 30 min, the phosphorylation of ERK1/2, p38MAPK and JNK1/2 was significantly increased compared with their respective control groups. However, pretreating VSMCs with Cur for 1 h remarkably attenuated these effects, in a concentration-dependent manner. These results suggest that the MAPK signaling pathway is involved in Cur's inhibition of LPS-induced inflammation in VSMCs (Figure 6).
Figure 6.
Effect of Cur on phosphorylation of the MAPK signaling pathway in LPS-stimulated VSMCs. Cells were pretreated with the indicated concentration of Cur (5, 10, and 30 μmol/L) for 1 h, and then stimulated with LPS (1 μg/mL) for 30 min. Some cells were treated with Cur (30 μmol/L) alone for 30 min. Western blot analysis was subsequently used to detect the expression of ERK, p38 MAPK, and JNK. Phosphorylated proteins were also detected. Cur suppressed LPS-induced phosphorylation of ERK, p38 MAPK, and JNK in VSMCs. Data are presented as mean±SEM of three independent experiments. cP<0.01 vs control; eP<0.05, fP<0.01 vs LPS.
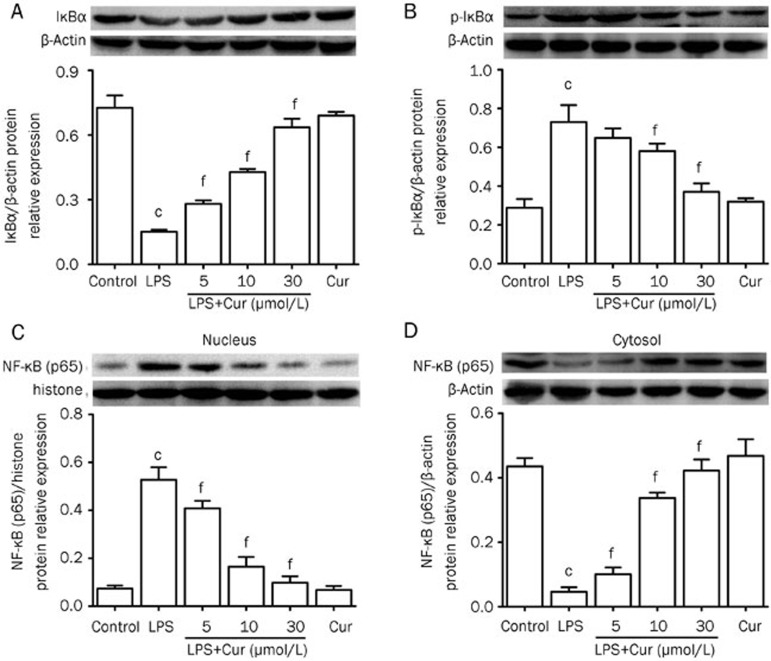
Effect of Cur on LPS-induced phosphorylation of IκBα and nuclear translocation of NF-κB (p65) in VSMCs
The activation of NF-κB plays a critical role in upregulating cellular inflammation by controlling the expression of inflammation-related genes. Activation of NF-κB occurs when it is isolated from IκBα. Once IκBα is phosphorylated, it degrades and allows NF-κB to become activated, after which NF-κB is translocated from the cytosol into the nucleus where it can control DNA transcription. Therefore, we wanted to observe if Cur could inhibit the LPS-induced phosphorylation of IκBα and subsequent nuclear translocation of NF-κB (p65). Our results showed that treating VSMCs with LPS (1 μg/mL) for 24 h significantly increased IκBα phosphorylation and degradation, and also heightened nuclear translocation of NF-κB (p65). Cur worked to inhibit IκBα phosphorylation and degradation, and also reduced the nuclear translocation of NF-κB (p65) in a concentration-dependent manner (Figure 7).
Figure 7.
Effect of Cur on LPS-induced phospho-rylation of IκBα and nuclear translocation of NF-κB (p65) in VSMCs. VSMCs were treated with Cur in the presence or absence of LPS (1 μg/mL) for 24 h. Some cells were treated with Cur (30 μmol/L) alone for 24 h. Protein expression of IκBα (A), p-IκBα (B), and NF-κB (p65) in the nucleus (C) and the cytosol (D) were respectively measured by Western blot. The quantification of relative band intensities was determined by densitometry. Data are presented as mean±SEM of three independent experiments. cP<0.01 vs control; fP<0.01 vs LPS.
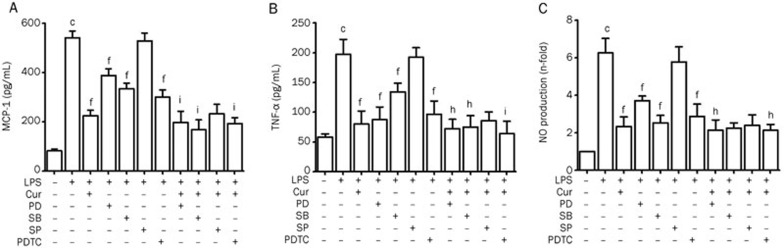
Effect of MAPK and NF-κB inhibitors on LPS-induced over-expression of inflammatory cytokines and NO
As shown previously, Cur inhibited the activation of MAPK signaling molecules and the nuclear translocation of NF-κB. To observe the role that MAPKs and NF-κB (p65) played in LPS-induced overexpression of inflammatory cytokines and NO, inhibitors of MAPKs and NF-κB (p65) were used in VSMC culture conditions. ELISA and NO assays quantified the effects of these inhibitors. The ERK inhibitor PD98059, the p38 MAPK inhibitor SB203580, and the NF-κB inhibitor PDTC, all inhibited the expression of inflammatory cytokines and NO in LPS-induced VSMC. In combination with Cur, their inhibitory effects were synergistically strengthened. The only inhibitor that did not suppress inflammatory cytokines or NO was SP600125, a JNK inhibitor. These results indicate that ERK, p38 MAPK, and NF-κB (p65) may play a critical role in the LPS-induced inflammation process, and using Cur can significantly suppress this inflammation (Figure 8).
Figure 8.
Effect of inhibitors to MAPKs and NF-κB on LPS-induced overexpression of inflammatory cytokines and NO. Cells were pretreated with PD98059 (50 μmol/L), SB203580 (25 μmol/L), SP600125 (15 μmol/L), and PDTC (80 μmol/L) for 1 h before adding Cur (30 μmol/L), and then incubated with 1 μg/mL LPS for another 24 h. The production of MCP-1 and TNF-α was measured by ELISA kits (A and B), and cell supernatants were collected to measure NO concentration (C). Each bar represents the means±SEM of three independent experiments. cP<0.01 vs control; fP<0.01 vs LPS; hP<0.05, iP<0.01 vs LPS+Cur.
Discussion
In the present study, we have shown that Cur is capable of attenuating LPS-induced overexpression of NO, MCP-1, and TNF-α in VSMCs in vitro. We have demonstrated that Cur's ability to inhibit LPS-induced inflammation works by reducing overexpression of TLR4, suppressing nuclear translocation of NF-κB-interfering with the MAPK signaling pathway, and curtailing the overexpression of intracellular ROS, which is an important secondary messenger that triggers inflammatory responses. These findings exhibit a novel linkage between Cur's anti-inflammatory effect and TLR4-mediated inflammatory signaling pathway in LPS-induced VSMCs, which expand the understanding of Cur's anti-inflammatory activities and outline potential molecular mechanisms that explain Cur's protective cardiovascular effects.
Numerous papers have demonstrated that atherosclerosis can be caused by multiple factors and that long-lasting, low-grade inflammatory responses play an important role in the progression of atherosclerosis and plaque instability25,26. MCP-1 is a powerful monocyte chemoattractant cytokine, that can recruit monocytes or macrophages into the tunica intima and media of arteries walls27. TNF-α, regarded as a potent pro-inflammatory mediator, can increase the production of other inflammatory cytokines, encourage the expression of adhesion molecules, and accelerate VSMCs apoptosis, which strongly promote progression of atherosclerosis and plaque instability28,29,30. Although interlerkin (IL-6), C-reactive protein, or other pro-inflammatory cytokines have been proved to promote the progression of atherosclerosis recently, MCP-1 and TNF-α are still regarded as two classic and powerful cytokines to trigger the inflammation within atherosclerotic plaques. LPS promotes inflammatory and immune responses via activation of almost all immune system cells, such as macrophages, mast cells, monocytes and VSMCs, which can cause over inflammation within atherosclerotic plaques. In VSMCs, LPS has been shown to induce the overexpression of many pro-inflammatory cytokines, including TNF-α, nitric oxide synthase, and MCP-13,31,32. Cur has been found to inhibit LPS-induced overexpression of vascular cell adhesion molecule-1 (VCAM-1), which plays a critical role in inflammatory responses within atherosclerotic plaques by regulating the adhesion of macrophages to endothelial cells in order to suppress inflammation33. In our study, we found that LPS markedly increased the expression of TNF-α and MCP-1 in VSMCs, which is consistent with findings in previous reports. More important, Cur effectively inhibited LPS-induced overexpression of pro-inflammatory cytokines in a concentration-dependent manner. Additionally, some studies have demonstrated that Cur also inhibits ox-LDL-, TNF-α-, or PMA-induced inflammation in different cell types, so we believe that Cur's anti-inflammatory effect is multiple, and not specially LPS-dependent4,34.
Although endothelial cell production of NO expression by the enzyme ecNOS has been found to mitigate endothelial cell dysfunction, which is beneficial in preventing atherosclerosis35, high NO production is known to promote atherosclerosis via increasing oxidative stress. Oxidative stress increases when overabundant NO reacts with superoxide to form peroxynitrite, a powerful oxidizing agent36. Peroxynitrite has the ability to modify numerous intracellular proteins and lipids37. An especially destructive modification is the nitrosylation of tyrosines, which can lead to the blockage of intracellular transcription signals by interfering with the normal phosphorylation and dephosphorylation activities required for signal propagation through various pathways, including the NF-κB and MAPK signaling pathways38. In vivo experiments using ApoE−/− mice having reduced levels of iNOS reported fewer instances of atherosclerosis and lower levels of plasma lipid peroxides than mice with normal iNOS levels. Furthermore, ONO1714, a selective inhibitor of iNOS, was found to retard atherosclerosis induced by a high-cholesterol diet39,40. These results imply that inhibiting the overexpression of iNOS and the subsequent overproduction of NO may help slow the progression of atherosclerosis. In our experiment, we found that Cur remarkably inhibited NO generation in LPS-stimulated VSMCs, while also reducing the amount of iNOS (and iNOS mRNA) present within LPS-induced VSMCs. These results indicate that Cur can effectively downregulate iNOS-mediated NO production in LPS-stimulated VSMCs, highlighting a novel molecular mechanism by which Cur may produce anti-atherosclerotic effects.
LPS upregulates the expression of TLR4, which accelerates the progression of atherosclerosis and increases plaque instability through the TLR4-mediated inflammatory signaling pathway, in turn producing many types of pro-inflammatory cytokines41. The expression of TLR4 has been observed to increase in human atherosclerotic plaques. An in vivo study in mice further supported this finding: a genetic deficiency of TLR4 in high-fat fed ApoE−/− mice led to a decrease in atherosclerotic plaque area and greater plaque stability11,12. In addition, the activation of TLR4 signaling may play a part in the overexpression of NO in LPS-induced VSMCs42. In our study, we first observed that Cur inhibited LPS-induced overexpression of TLR4 (and TLR4 mRNA) in VSMCs. Our data also indicate that a TLR4-specific monoclonal antibody was able to partially suppress LPS-induced inflammatory responses; adding Cur resulted in even further suppression. These results suggest that TLR4, a specific receptor for LPS, was involved in the mechanism by which Cur inhibits LPS-induced inflammation, specifically inhibiting overproduction of MCP-1, TNF-α, and NO in VSMCs.
During an inflammation response, the phosphorylation of intracellular signaling pathways followed by activation of transcription factors often triggers the overexpression of proinflammatory mediators. The phosphorylation of MAPKs (ERK1/2, p38 MAPK, and JNK1/2), which are signaling molecules involved in upstream regulation of several transcription factors, has been observed to increase in LPS-stimulated VSMCs43. Meng et al reported that the activation of NF-κB was also partially dependent on the phosphorylation of MAPKs44. NF-κB upregulates the expression of various pro-inflammatory cytokines and enzymes by binding with DNA and controlling the transcription of target genes, but only when NF-κB is activated and translocated into the nucleus. NF-κB is normally located in the cytosol and complexed with IκBα, which renders NF-κB inactive. When IκBα is phosphorylated, it degrades, allowing NF-κB to become active and translocate to the nucleus. Recently, it has been found that in VSMCs Cur inhibits oxLDL-induced phosphorylation of the MAPK signaling pathway, which in turn inhibits the activation of NF-κB4. Our data support these previous studies. We found that Cur powerfully inhibited the phosphorylation of ERK1/2, p38 MAPK, and JNK1/2, and suppressed the degradation of IκBα as well as the nuclear translocation of NF-κB (p65) in LPS-stimulated VSMCs. Experiments involving selective inhibitors of ERK1/2, p38 MAPK, and NF-κB showed the same inhibitory effect on LPS-induced inflammation as Cur. When used together, Cur and the selective inhibitors reduced inflammation even more. These results indicate that the ERK1/2, p38 MAPK, and NF-κB pathways are involved in the overproduction of inflammatory proteins in LPS-stimulated VSMCs.
The common risk factors for atherosclerosis, such as smoking, hypercholesterolemia, and hypertension, are known to cause overexpression of ROS in VSMCs, which triggers multiple pathological responses related to atherosclerosis, such as the proliferation and migration of VSMCs, oxidation of lipids, and overproduction of pro-inflammatory cytokines. Our data show that LPS significantly increased intracellular ROS production, in accordance with previous studies. When pretreated with Cur, we observed that Cur effectively attenuated the LPS-induced increase of intracellular ROS and exhibited potent free radical scavenging activity. ROS have been observed to participate in the TLR4-mediated inflammatory signaling pathway45. In our study, we inhibited the action of NADPH oxidase, which produces ROS, using DPI. We found that DPI-inhibition of NADPH oxidase attenuated LPS-induced inflammation in VSMCs, an effect that was strengthened when DPI was used in combination with Cur. These results suggest that ROS, acting as a secondary messenger, trigger the activation of TLR4-mediated signaling. Therefore, targeting ROS is a key mechanism by which Cur produces its anti-inflammatory effects on LPS-induced VSMCs.
Recently, many groups have focused on the pathological effects of the membrane-associated enzyme NADPH oxidase, which is a major producer of intracellular ROS in the cardiovascular system. Many cell types in the cardiovascular system exhibit NADPH oxidase-dependent activity, including VSMCs, cardiomyocytes, cardiac fibroblasts, and endothelial cells46. It is well known that p47phox is an important subunit of NADPH oxidase's cytosolic component. Furthermore, excessive NADPH oxidase activity, caused by proinflammatory stimuli, can further increase p47phox expression47. Results from in vivo studies using ApoE−/− mice (fed a high-fat diet) with dysfunctional p47phox have reported smaller areas of atherosclerotic plaques compared to mice with normally functioning p47phox48. Several other studies reported that the inhibitory effects of Cur on LPS-induced intracellular ROS production depend on the suppression of NADPH oxidase expression49,50. These results indicate that NADPH oxidase plays an important role in inflammatory responses that are induced by diverse stimuli. Our data show that Cur significantly inhibited LPS-induced overproduction of p47phox in VSMCs, and that pretreatment with DPI, a selective NADPH oxidase inhibitor, also had an inhibitory effect. These results indicate that Cur's suppressive effect on the generation of intracellular ROS is associated with the activity of NADPH oxidase. In addition to NADPH oxidase, the mitochondrial electron chain also participates in the formation of intracellular ROS. Therefore, it might be interesting to determine if Cur affects other related signaling pathways during inhibition of LPS-induced intracellular ROS production in VSMCs.
In conclusion, our results indicate that Cur attenuates LPS-induced overexpression of MCP-1, TNF-α, and NO. Cur's ability to inhibit the inflammatory response may depend, in part, on suppressing the activation of TLR4, inhibiting phosphorylation of ERK1/2 and p38 MAPK, preventing nuclear translocation of NF-κB, and reducing NADPH-mediated intracellular ROS production. In general, the present study provides a beneficial experimental basis for using Cur as a therapeutic agent to treat chronic inflammatory diseases such as atherosclerosis.
Author contribution
Most of the experiments were conducted by Zhe MENG, who also wrote this paper. Chao YAN and Qian DENG also conducted some of the experiments. Deng-feng GAO and Xiao-lin NIU designed the experiments together.
Acknowledgments
This study was supported by the National Natural Science Foundation of China [NSFC 81070219 to Xiao-lin NIU and NSFC 30900617 to Deng-feng GAO].
References
- Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JH, Kim WS, Rihal CS, Lerman A. Individual measurement and significance of carotid intima, media, and intima-media thickness by B-mode ultrasonographic image processing. Arterioscler Thromb Vasc Biol. 2006;26:2380–5. doi: 10.1161/01.ATV.0000240420.36229.f9. [DOI] [PubMed] [Google Scholar]
- Ji Y, Liu J, Wang Z, Li Z. PPARgamma agonist rosiglitazone ameliorates LPS-induced inflammation in vascular smooth muscle cells via the TLR4/TRIF/IRF3/IP-10 signaling pathway. Cytokine. 2011;55:409–19. doi: 10.1016/j.cyto.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Liu T, Guo Z. Curcumin inhibits ox-LDL-induced MCP-1 expression by suppressing the p38MAPK and NF-kappaB pathways in rat vascular smooth muscle cells. Inflamm Res. 2012;61:61–7. doi: 10.1007/s00011-011-0389-3. [DOI] [PubMed] [Google Scholar]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–98. [PubMed] [Google Scholar]
- Masuda T, Hidaka K, Shinohara A, Maekawa T, Takeda Y, Yamaguchi H. Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin. J Agric Food Chem. 1999;47:71–7. doi: 10.1021/jf9805348. [DOI] [PubMed] [Google Scholar]
- Sikora E, Scapagnini G, Barbagallo M. Curcumin, inflammation, ageing and age-related diseases. Immun Ageing. 2010;7:1. doi: 10.1186/1742-4933-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszanecki R, Jawień J, Gajda M, Mateuszuk L, Gebska A, Korabiowska M, et al. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J Physiol Pharmacol. 2005;56:627–35. [PubMed] [Google Scholar]
- den Dekker WK, Cheng C, Pasterkamp G, Duckers HJ. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis. 2010;209:314–20. doi: 10.1016/j.atherosclerosis.2009.09.075. [DOI] [PubMed] [Google Scholar]
- Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–61. [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbad A, Oriowo MA, Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem. 2009;322:127–35. doi: 10.1007/s11010-008-9949-4. [DOI] [PubMed] [Google Scholar]
- Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–62. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Luo XH, Guo LJ, Yuan LQ, Xie H, Zhou HD, Wu XP, et al. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005;309:99–109. doi: 10.1016/j.yexcr.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- Xing H, Zhang S, Weinheimer C, Kovacs A, Muslin AJ. 14-3-3 proteins block apoptosis and differentially regulate MAPK cascades. EMBO J. 2000;19:349–58. doi: 10.1093/emboj/19.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YH, Jeong YT, Lee KA, Choi KH, Kim SM, Rhim BY, et al. Roles of MAPK and NF-kappaB in interleukin-6 induction by lipopolysaccharide in vascular smooth muscle cells. J Cardiovasc Pharmacol. 2008;51:71–7. doi: 10.1097/FJC.0b013e31815bd23d. [DOI] [PubMed] [Google Scholar]
- San Martin A, Foncea R, Laurindo FR, Ebensperger R, Griendling KK, Leighton F. Nox1-based NADPH oxidase-derived superoxide is required for VSMC activation by advanced glycation end-products. Free Radic Biol Med. 2007;42:1671–9. doi: 10.1016/j.freeradbiomed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol. 2007;150:963–76. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Taubman MB, Akers M, Mendlowitz M, Alexander RW. Characterization of phosphatidylinositol-specific phospholipase C from cultured vascular smooth muscle cells. J Biol Chem. 1991;266:15498–504. [PubMed] [Google Scholar]
- Cho JY, Baik KU, Jung JH, Park MH. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000;398:399–407. doi: 10.1016/s0014-2999(00)00337-x. [DOI] [PubMed] [Google Scholar]
- Jeong JM, Choi CH, Kang SK, Lee IH, Lee JY, Jung H. Antioxidant and chemosensitizing effects of flavonoids with hydroxy and/or methoxy groups and structure-activity relationship. J Pharm Pharm Sci. 2007;10:537–46. doi: 10.18433/j3kw2z. [DOI] [PubMed] [Google Scholar]
- Levi M, van der Poll T, Schultz M. Infection and inflammation as risk factors for thrombosis and atherosclerosis. Semin Thromb Hemost. 2012;38:506–14. doi: 10.1055/s-0032-1305782. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–51. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken N A, Coughlin S R, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–7. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JJ, Weissberg PL, Bennett MR. Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler Thromb Vasc Biol. 2003;23:1553–8. doi: 10.1161/01.ATV.0000086961.44581.B7. [DOI] [PubMed] [Google Scholar]
- Barks JL, Mcquillan JJ, Iademarco MF. TNF-alpha and IL-4 synergistically increase vascular cell adhesion molecule-1 expression in cultured vascular smooth muscle cells. J Immunol. 1997;159:4532–8. [PubMed] [Google Scholar]
- Ortego M, Bustos C, Hernández-Presa MA, Tuñón J, Díaz C, Hernández G, et al. Atorvastatin reduces NF-kappaB activation and chemokine expression in vascular smooth muscle cells and mononuclear cells. Atherosclerosis. 1999;147:253–61. doi: 10.1016/s0021-9150(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Heo SK, Yun HJ, Noh EK, Park WH, Park SD. LPS induces inflammatory responses in human aortic vascular smooth muscle cells via Toll-like receptor 4 expression and nitric oxide production. Immunol Lett. 2008;120:57–64. doi: 10.1016/j.imlet.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Jiang P, Xu J, Zheng S, Huang J, Xiang Q, Fu X, et al. 17Beta-estradiol down-regulates lipopolysaccharide-induced MCP-1 production and cell migration in vascular smooth muscle cells. J Mol Endocrinol. 2010;45:87–97. doi: 10.1677/JME-09-0166. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dhawan S, Hardegen NJ, Aggarwal BB. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem Pharmacol. 1998;55:775–83. doi: 10.1016/s0006-2952(97)00557-1. [DOI] [PubMed] [Google Scholar]
- Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–7. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- Wever RM, Luscher TF, Cosentino F, Rabelink TJ. Atherosclerosis and the two faces of endothelial nitric oxide synthase. Circulation. 1998;97:108–12. doi: 10.1161/01.cir.97.1.108. [DOI] [PubMed] [Google Scholar]
- Bunderson M, Coffin JD, Beall HD. Arsenic induces peroxynitrite generation and cyclooxygenase-2 protein expression in aortic endothelial cells: possible role in atherosclerosis. Toxicol Appl Pharmacol. 2002;184:11–8. [PubMed] [Google Scholar]
- Rubbo H, Trostchansky A, O'Donnell VB. Peroxynitrite-mediated lipid oxidation and nitration: mechanisms and consequences. Arch Biochem Biophys. 2009;484:167–72. doi: 10.1016/j.abb.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Schmidt K, Mayer B. Dityrosine formation outcompetes tyrosine nitration at low steady-state concentrations of peroxynitrite. Implications for tyrosine modification by nitric oxide/superoxide in vivo. J Biol Chem. 2000;275:6346–52. doi: 10.1074/jbc.275.9.6346. [DOI] [PubMed] [Google Scholar]
- Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation. 2001;103:3099–104. doi: 10.1161/01.cir.103.25.3099. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Matsui-Hirai H, Fukatsu A, Sumi D, Kano-Hayashi H, Rani PJ, et al. Selective iNOS inhibitor, ONO1714 successfully retards the development of high-cholesterol diet induced atherosclerosis by novel mechanism. Atherosclerosis. 2006;187:316–24. doi: 10.1016/j.atherosclerosis.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Li H, Sun B. Toll-like receptor 4 in atherosclerosis. J Cell Mol Med. 2007;11:88–95. doi: 10.1111/j.1582-4934.2007.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo SK, Yun HJ, Noh EK, Park WH, Park SD. LPS induces inflammatory responses in human aortic vascular smooth muscle cells via Toll-like receptor 4 expression and nitric oxide production. Immunol Lett. 2008;120:57–64. doi: 10.1016/j.imlet.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Son YH, Jeong YT, Lee KA, Choi KH, Kim SM, Rhim BY, et al. Roles of MAPK and NF-kappaB in interleukin-6 induction by lipopolysaccharide in vascular smooth muscle cells. J Cardiovasc Pharmacol. 2008;51:71–7. doi: 10.1097/FJC.0b013e31815bd23d. [DOI] [PubMed] [Google Scholar]
- Meng XL, Yang JY, Chen GL, Wang LH, Zhang LJ, Wang S, et al. Effects of resveratrol and its derivatives on lipopolysaccharide-induced microglial activation and their structure-activity relationships. Chem Biol Interact. 2008;174:51–9. doi: 10.1016/j.cbi.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Yang FL, Yang YL, Liao PC, Chou JC, Tsai KC, Yang AS, et al. Structure and immunological characterization of the capsular polysaccharide of a pyrogenic liver abscess caused by Klebsiella pneumoniae: activation of macrophages through Toll-like receptor 4. J Biol Chem. 2011;286:21041–51. doi: 10.1074/jbc.M111.222091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madamanchi NR, Runge MS. NADPH oxidases and atherosclerosis: unraveling the details. Am J Physiol Heart Circ Physiol. 2010;298:H1–H2. doi: 10.1152/ajpheart.01020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin EL, Touyz RM. Inflammation and vascular hypertrophy induced by angiotensin II: role of NADPH oxidase-derived reactive oxygen species independently of blood pressure elevation. Arterioscler Thromb Vasc Biol. 2003;23:707–9. doi: 10.1161/01.ATV.0000069907.12357.7E. [DOI] [PubMed] [Google Scholar]
- Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, et al. p47phox is required for atherosclerotic lesion progression in ApoE−/− mice. J Clin Invest. 2001;108:1513–22. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wu C, Zhao S, Yuan D, Lian G, Wang X, et al. Demethoxycurcumin, a natural derivative of curcumin attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-kappaB signaling pathways in N9 microglia induced by lipopolysaccharide. Int Immunopharmacol. 2010;10:331–8. doi: 10.1016/j.intimp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- He LF, Chen HJ, Qian LH, Chen GY, Buzby JS. Curcumin protects pre-oligodendrocytes from activated microglia in vitro and in vivo. Brain Res. 2010;1339:60–9. doi: 10.1016/j.brainres.2010.04.014. [DOI] [PubMed] [Google Scholar]