Abstract
CD8+ T cells in progressing tumors frequently fail to mount an effective antitumor response often in association with the expression of inhibitory receptors, including programmed cell death-1 (PD-1) and lymphocyte-activation gene 3 (Lag3). Using a lymphoma tumor model, we demonstrate that tumor-infiltrating CD8+ T cells from growing tumors co-express inhibitory receptors and co-stimulatory receptors, including 4-1BB (TNFRSF9) as well as high levels of 2 transcription factors, Eomesodermin (Eomes) and T-bet (Tbx21), critical determinants of CD8+ T cell fate. Immunotherapy with an agonistic anti-4–1-BB antibody altered the ratio of Eomes to T-bet expression in tumor-infiltrating CD8+ T cells by increasing Eomes and decreasing T-bet expression. 4-1BB-agonist immunotherapy was also associated with downregulated expression of the inhibitory receptors PD-1 and Lag3 on tumor-infiltrating CD8+ T cells, a molecular phenotype associated with subsequent attenuation of tumor growth. Furthermore, 4-1BB-agonist immunotherapy failed to effect tumor progression in mice with Eomes deficient T cells. However, upon resumption of tumor growth, tumor-infiltrating CD8+ T cells from treated animals continued to express high levels of Eomes as well as elevated levels of the inhibitory receptors PD-1 and Lag3. Our data suggest that tumor-infiltrating CD8+ T cells are poised between activation and inhibition as dictated by expression of both co-stimulatory receptors and inhibitory receptors and demonstrate that T cell expression of Eomes is necessary, but not sufficient, for efficacious 4-1BB-agonist-mediated immunotherapy.
Keywords: T cells, tumor immunotherapy, 4-1BB, eomesodermin, PD-1, Lag3
Introduction
Current anticancer immunotherapeutic strategies encompass those aiming to manipulate T cell receptor signaling and include the infusion of agonistic antibodies to the T-cell co-stimulatory molecule tumor necrosis factor (TNF) receptor superfamily member 9 (TNFRSF9), best known as 4-1BB.1-4 Activation of 4-1BB signaling has demonstrated immunotherapeutic benefit in the treatment of a variety of cancer types and a humanized agonistic anti-4-1BB antibody is currently undergoing evaluation in clinical trials.5 While evidence supports a principal role for CD8+ T cells in the cancer inhibitory effects mediated by 4-1BB-directed immunotherapy, much remains obscure regarding the precise mechanisms underlying the CD8+ T cell tumor immunity occurring downstream of 4-1BB ligation.3,6-8 Gaining further insight into these immunologic manifestations will foster the optimization of 4-1BB-directed cancer treatment regimens, as well as promote the development of novel immunotherapeutic modalities in the future.
4-1BB signaling in T cells is initiated by the recruitment of TNF receptor-associated factors 1 and 2, (TRAF1 and TRAF2, respectively) with the subsequent activation of the downstream signaling cascade, including the oncogenic NF-κB (Rel) transcription factors, and mitogen-activated protein kinase (MAPK) family members ERK, JNK, and p38 MAPK.9 While NF-κB activity leads to increased expression of the anti-apoptotic BCL2-related proteins Bfl-1 (Bcl2A1) and Bcl-XL (Bcl2L1), ERK promotes degradation of the pro-apoptotic regulatory protein Bim, thereby culminating in increased T-cell survival.10,11 More recently, a connection between 4-1BB signaling and the T-cell master transcription factor eomesodermin (Eomes) has been inferred from observations of high expression levels of Eomes in KLRG1hi CD4+ and CD8+ tumor-infiltrating T cells after 4-1BB agonist antibody therapy in a mouse melanoma model.12
Eomes and T-bet (Tbx21), both members of the T-box family of transcription factors, have been implicated as critical determinants of effector cell activity and memory cell specification among CD8+ T cells responding to various acute infectious challenges.13-15 Eomes and T-bet also appear to play a role in modulating T cell differentiation occurring during immunological responses to chronic viral infection. In support, 2 disparate subsets of CD8+ T cells marked by distinct expression patterns of T-bet and Eomes were found to be necessary for efficacious containment of chronic viral infection.16 These findings led us to wonder whether tumor-infiltrating CD8+ T cells occurring during tumor progression and 4-1BB-mediated tumor regression could be similarly divided into functionally unique subsets on the basis of differential Eomes and T-bet expression.
To address how the expression of Eomes and T-bet expression contributes to the immunologic activities of tumor-infiltrating CD8+ T cells, either untreated developing tumors and those responding to anti-4-1BB agonist antibody (α4-1BB) therapy, we interrogated a mouse model of thymic lymphoblastic malignancy in the setting of deficiency in Eomes, T-bet, or both. We found that α4-1BB-antibody therapy increased expression of Eomes and decreased expression of T-bet in tumor-infiltrating CD8+ T cells. We further observed that α4-1BB immunotherapy was associated with a decrease in T cell expression of the inhibitory receptors programmed cell death-1 (PD-1) and lymphocyte-activation gene 3 (Lag3) and, α4-1BB antibody-mediated antitumor immunity was ineffective in mice harboring T cells lacking Eomes. Our findings substantiate those of the recently published mouse melanoma study12 providing solid evidence for the α4-1BB immunotherapy-mediated elicitation of Eomes expressing T cells with distinct antitumor immunologic properties. We further extend these findings by elucidating a correlation between the downregulation of the inhibitory receptors PD-1 and Lag3 on tumor-infiltrating CD8+ T cells and beneficial therapeutic response to α4-1BB antibody treatment.
Results
Tumor infiltrating CD8+ CD44hi T cells co-express Eomes and T-bet concurrently with select inhibitory and co-stimulatory receptors
CD8+ tumor-infiltrating lymphocytes (TILs) and CD8+ T cells responding to chronic viral infection are both subject to persistent antigen exposure. In the case of chronic viral infection, functionally distinct CD8+ T cell subsets distinguishable by variable expression levels of T-bet and Eomes have been previously identified.16 To determine whether CD8+ tumor-infiltrating lymphocytes (TILs) could be divided into distinct sub-populations based on Eomes and T-bet expression, we measured the expression of both transcription factors by intracellular staining and cytofluorimetric analysis of tumor-infiltrating CD8+ T cells from EG7 lymphoma-bearing mice.17 We observed a single population of CD8+ CD44hi TILs with elevated expression levels of both Eomes and T-bet relative to that observed in splenic memory (CD44hi) CD8+ T cells (Fig. 1A).

Figure 1. Co-expression of Eomes, T-bet, PD-1, Lag3, 4-1BB, and OX40 by CD8+ CD44hi infiltrating lymphocytes in EG7 tumors. (A–C) Cytofluorometric analysis of tumor-infiltrating lymphocytes (TILs) present in harvested EG7 tumors 21 d after tumor cell s.c. inoculation into the flank of C57BL/6 host mice. Tumors were enzymatically dissociated to a single cell suspension and cells were stained with fluorophore-conjugated antibodies against the indicated markers. Flow cytometry was performed by gating on CD8+ cells. (A) Dot plot showing the expression of T-bet and Eomes in CD8+ CD44hi cells among TILs vs. splenic controls. (B) Histograms of the expression of the indicated proteins in either CD8+ CD44lo or CD8+ CD44hi tumor or spleen control cells, as indicated. Data in (A) and (B) are representative of at least 3 independent experiments. (C) 5 × 104 OVA-specific CD8+ T cells magnetically enriched from spleen preparations from CD45.1 OT-1 donor mice were adoptively transferred to each mouse by i.v. injection on the same day as EG7 flank tumor inoculation. TILs were harvested 21 d later and analyzed by flow cytometry, as above. Dot plots show expression of the indicated marker in CD45.1+ (donor derived) and CD45.1− (host derived) subsets of CD8+ CD44hi cells. Data are representative of three independent experiments. 4-1BB/TNFRSF9, tumor necrosis factor receptor superfamily member 9; Eomes, eomesodermin; CTLA4, cytotoxic T-lymphocyte antigen 4; Lag3, lymphocyte-activation gene 3; PD-1, programmed cell death-1; T-bet/Tbx21, T-box 21.
Eomes and T-bet are both associated with CD8+ effector T-cell activity and their expression in CD8+ T cells within growing tumors led us to examine whether these lymphocytes co-expressed inhibitory receptors associated with T-cell exhaustion. We found increased expression levels of the T cell inhibitory receptor PD-1 on CD8+ CD44hi TILs relative to that of CD8+ CD44hi splenocytes (Fig. 1B). Furthermore, we consistently observed expression of an additional inhibitory receptor Lag3 on a proportion of CD8+CD44hi TILs, although cytotoxic T-lymphocyte antigen 4 (CTLA-4), another negative regulator of T-cell responses, was not expressed. In contrast to the phenotypically distinct CD44high subset of CD8+ TILs, naïve (CD44lo) CD8+ TILs resembled naïve CD8+ T cells from the spleen (Fig. 1B).
Although we found that CD8+ CD44hi TILs in our system do express T cell inhibitory receptors, co-stimulation through members of the TNF receptor superfamily, specifically 4-1BB, OX40 (Tnfrsf4), and CD40, could nevertheless, cause immunologic tumor regression as previously shown in mouse tumor models similar to EG7.18-20 In order to determine this immunotherapeutic potential in EG7 lymphomas, we examined the expression of these 3 TNF receptor superfamily members and found that 4-1BB and OX40 were expressed on CD8+ CD44hi TILs from EG7 tumors, whereas CD40 was not (Fig. 1B).
The distinct phenotype of CD8+ CD44hi TILs from EG7 tumors compared with those from splenocytes suggests that the TIL population is not composed solely of non-specific memory CD8+ T cells residing in the tumor. We next sought to determine whether the observed robust T-bet and Eomes expressivity and surface marker expression profile (PD-1+Lag3+4-1BB+OX40+) of the CD8+ CD44hi TILs matched that of tumor antigen-specific TILs. To measure the relative expression levels of these molecules in tumor-antigen specific TILs vs. CD8+ CD44hi TILs, we adoptively transferred T cells from OT-1 donor mice transgenic for a T cell receptor recognizing chicken ovalbumin-derived peptide (OVA) expressed by EG7 cells.21 Five × 104 CD8+ T cells from OT-1 mice, distinguishable by expression of the congenic marker CD45.1 as opposed to expression of CD45.2 by endogenous T cells, were intravenously injected into tumor recipient mice coincidently with flank tumor inoculation. Analysis of TILs derived from resulting EG7 tumors revealed similar patterns of expression of the T-bet and Eomes transcription factors as well as all inhibitory and co-stimulatory receptors evaluated between OT-1 tumor-antigen specific TILs and endogenous CD8+ CD44hi TILs (Fig. 1C).
The data show that CD8+CD44hi TILs in EG7 tumors are phenotypically distinct from memory CD8+ T cells while naïve CD8+ T cells in EG7 tumors resemble naïve CD8+ T cells in the spleen. CD8+ CD44hi TILs are marked by high expression of Eomes and T-bet and selective co-expression of inhibitory receptors and co-stimulatory receptors, a profile corresponding to that of tumor antigen-specific TILs. Taken together, our results suggest that although CD8+ CD44hi TILs express inhibitory receptors associated with T-cell exhaustion, they retain potential for activation through 4-1BB or OX40 ligation.
CD8+ CD44hi TIL expression of Lag3 is decreased in the absence of Eomes
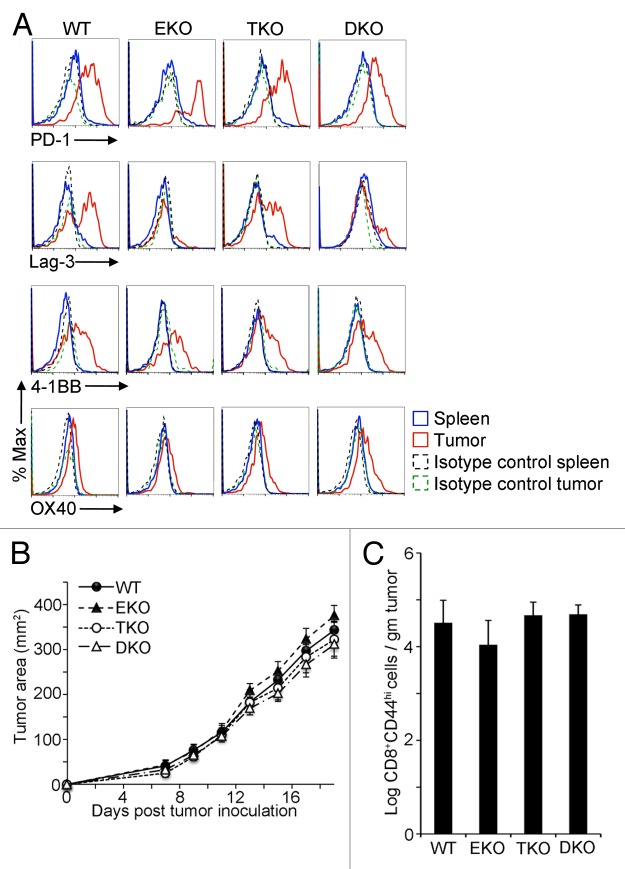
Considering the pattern of expression of inhibitory and co-stimulatory receptors occurring in conjunction with elevated Eomes and T-bet in CD8+ CD44hi TILs, we next sought to determine whether the expression of these cell surface molecules was Eomes or T-bet dependent. We analyzed CD8+ CD44hi TILs from EG7 tumors in CD4-Cre Eomesflox/flox (Eomes KO), Tbx21−/− (T-bet KO), or CD4-Cre Eomesflox/flox Tbx21−/− (double KO) mice. As shown in Figure 2A, whereas the expression of PD-1, 4-1BB, and OX40 by CD8+CD44hi TILs was not significantly altered by the lack of Eomes, T-bet, or both, Lag3 levels were decreased specifically in CD8+ CD44hi TILs lacking Eomes.
Figure 2. Diminished Lag3 expression on Eomes-deficient CD8+ CD44hi TILs. (A–C) 1 × 106 EG7 cells were subcutaneously injected into the flank of 6–12 wk old C57BL/6 wild-type (WT), Eomes KO (EKO), T-bet KO (TKO) or double KO (DKO) recipient mice. Data are representative of at least 3 independent experiments. (A) Cytofluorometric analysis of immunofluorescence stained, dissociated tumor cells 21 d after tumor cell inoculation. Histograms of the expression level of the indicated marker in CD8+ CD44hi splenocytes or EG7 tumor-infiltrating lymphocytes (TILs). (B) Growth curves for EG7 tumors in C57BL/6 controls vs. EKO, TKO, and DKO mice. Each point represents the mean ± SEM of tumor measurements (n = 8 per group). Statistical analyses were performed by Student t test with no significant differences between WT and EKO, TKO, or DKO at any time point (P > 0.05). (C) Quantitation of CD8+ CD44hi TILs in EG7 tumors from mice in (B), 21 d post tumor inoculation as determined by flow cytometry. Bars represent the mean ± SEM of the log CD8+ CD44hi TILs per gram tumor (n = 8 per group). Statistical analyses were performed by Student t test with no significant differences found between WT and EKO, TKO, or DKO (P > 0.05). 4-1BB/ TNFRSF9, tumor necrosis factor receptor superfamily member 9; Eomes, eomesodermin; KO, knockout; Lag3, lymphocyte-activation gene 3; PD-1, programmed cell death-1; Tbet, T-bet/Tbx21, T-box 21;
In light of the scarce effect of Eomes and T-bet expression on the levels of CD8+ CD44hi TIL surface molecules, we next sought to determine the impact of Eomes and T-bet on EG7 tumor development and the frequency of TILs. As shown in Figure 2B–C, the observed tumor growth rates as well as the number of CD8+ CD44hi TILs were similar irrespective of the presence of Eomes, T-bet, or both. These findings suggest that although Eomes and T-bet are both expressed at high levels in CD8+ CD44hi TILs, the initial anti-EG7 tumor response is not substantially altered in the absence of either or both transcription factors, despite diminished expression of Lag3 in the absence of Eomes.
4-1BB ligation leads to increased Eomes and diminished T-bet expression in CD8+ CD44hi TILs
Variable tumor regression has been observed following administration of an agonistic anti-4-1BB directed antibody (α4-1BB), such that treatment is efficacious only in certain tumor models. Beneficial responses have been observed to occur in tumor models resembling EG7, whereas in other systems α4-1BB treatment has been shown to be ineffective or efficacious only when given in combination with another form of immunotherapy.18,22,23 To determine the efficacy of α4-1BB in our model of thymic lymphoblastic malignancy, we injected EG7 tumor-bearing mice with α4-1BB or control rat IgG2A and measured tumor growth over time. We observed no significant difference between the 2 groups in the first week after injection, however, subsequent stabilization of tumor size was observed in the α4-1BB group the following week (Fig. 3A). Tumors in the α4-1BB group typically remained stable in size for approximately one additional week prior to growth resumption. To determine whether the observed temporary arrest in tumor progression was associated with a change in T-box transcription factor levels, we analyzed CD8+ CD44hi TILs for expression of Eomes and T-bet 6 d after injection of α4-1BB. As shown in Figure 3B, Eomes expression in CD8+ CD44hi TILs, already elevated relative to the levels observed in memory CD8+ T cells, was further increased after α4-1BB administration. In contrast, T-bet expression was slightly diminished by the α4-1BB therapeutic regimen.
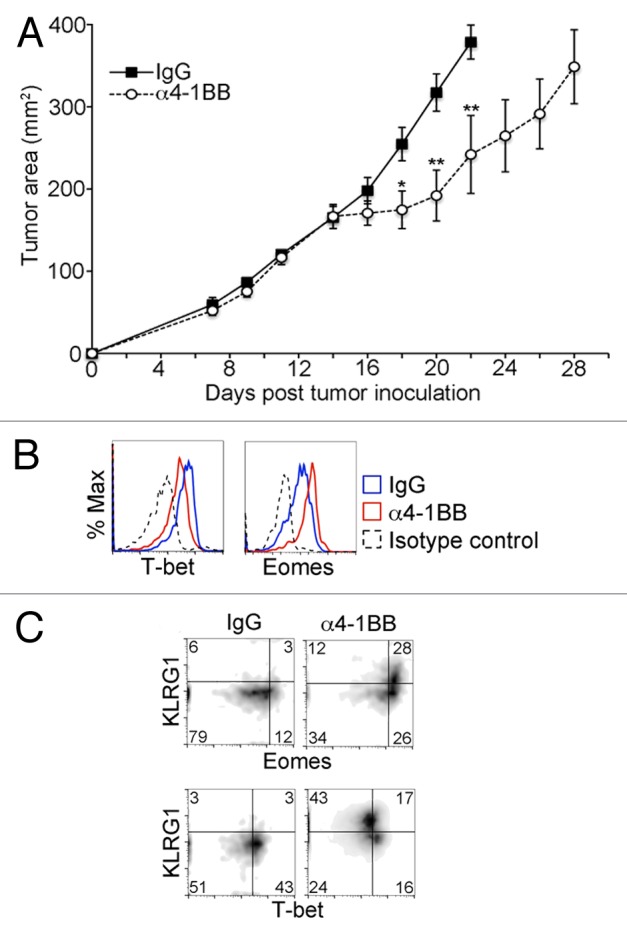
Figure 3. Enhanced Eomes expression and diminished T-bet expression in CD8+ CD44hi TILs after in vivo administration of α4-1BB. (A–C) C57BL/6 mice bearing EG7 tumors were administered 100 μg α4-1BB antibody or control rat IgG on day 7 and day 10 after tumor cell inoculation. (A) Tumor growth curves. Each data point represents the mean ± SEM of tumor measurements (n = 8 per group). Data are from one of four independent experiments). Statistical analyses were performed by Student t test; *P < 0.05 and **P < 0.01. (B–C) Cytofluorometric analysis of immunofluorescence stained, dissociated tumor cells 13 d after tumor cell inoculation and 3 d after the second dose of α4-1BB antibody or IgG control. Data are representative of three independent experiments. (B) Histograms of the expression level of T-bet (Tbx21, T-box 21) and Eomes (eomesodermin) in CD8+ CD44hi gated tumor-infiltrating lymphocytes (TILs) in EG7 tumors from α4-1BB (red line in B) or rat IgG (blue line in B) treated animals. Isotype control staining of CD8+ CD44hi gated TILs from IgG-treated animal is shown for comparison (black dashed line in B). (C) Dot plot showing change in distribution of Eomes, T-bet and KLRG1 expression in CD8+ CD44hi TILs in response to the indicated treatment.
In CD4+ T cells, combined OX40 and 4-1BB co-stimulation has been previously shown to induce both Eomes and T-bet expression.24 More recently, another study demonstrated upregulation of Eomes in a KLRG1-expressing subset of B16 melanoma tumor-infiltrating T cells in response to immunotherapy with a tumor vaccine and a 4-1BB agonist antibody.12 KLRG1 is a cadherin receptor that has previously been shown to be associated with terminal differentiation and senescence in T cells and may also act as a T cell inhibitory receptor.25,26 To determine if there was a correlation between KLRG1 and Eomes in TILs responding to α4-1BB in our tumor model, we measured KLRG1 expression relative to Eomes and T-bet in CD8+ CD44hi TILs after α4-1BB administration in EG7 tumor-bearing mice (Fig. 3C). We observed high levels of KLRG1 expression in approximately half of the CD8+ CD44hi TILs and enriched among CD8+ CD44hi TILs in which Eomes was more abundant. The CD8+ CD44hi TILs that had diminished expression of T-bet, however, were almost uniformly KLRG1hi. Taken together, these results demonstrate that Eomes and T-bet expression in CD8+ CD44hi TILs is altered by α4-1BB immunotherapy in EG7 tumor bearing mice. Considering the marked upregulation of Eomes expression observed in response to α4-1BB immunotherapy, we hypothesized that Eomes expression plays an important role in the delay in tumor progression elicited by this treatment regimen.
Eomes is required for α4-1BB-mediated EG7 tumor growth retardation
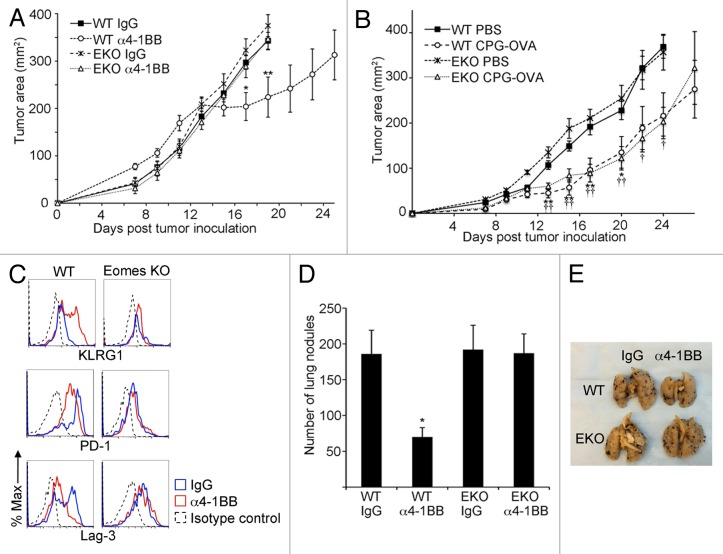
In order to determine whether Eomes expression in CD8+ CD44hi TILs is essential for the attenuation of EG7 tumor growth in response to 4-1BB stimulation, we treated tumor-bearing mice from Eomes proficient or Eomes KO backgrounds with α4-1BB therapeutic antibody and monitored changes in tumor size over time. Whereas in Eomes proficient mice in which we observed tumor growth arrest with the same kinetics as in our prior experiments, Eomes KO mouse tumors continued to grow at the same rate as those in rat IgG2a control treated tumor-bearing hosts from either background (Fig. 4A).
Figure 4. Eomes is required for α4-1BB mediated delay in tumor progression. (A) C57BL/6 (WT) or Eomes KO (EKO) mice bearing EG7 tumors were administered 100 μg α4-1BB or control rat IgG on d7 and d10 after tumor cell inoculation. Tumor growth curves are shown, with each point representing the mean ± SEM of the tumor area (n = 8 per group). Data are from one of three independent experiments. Statistical analyses were performed by Student t test; IgG vs. α4-1BB in WT mice *P < 0.05 and **P < 0.01 whereas in EKO mice no significant differences were detected between treatment groups. (B) Tumor growth curves for s.c. flank EG7 tumors in WT or EKO mice immunized by s.c. injection of phosphate-buffered saline (PBS) or CpG oligodeoxynucleotide and ovalbumin (CPG-OVA) 14d prior to tumor inoculation and on the day of tumor cell inoculation. Each point represents the mean ± SEM of the tumor area (n = 4 per group). Data are from one of two independent experiments. Statistical analyses were performed by Student t test for differences between PBS and CPG-OVA groups in WT mice (*P < 0.05 and **P < 0.01) or EKO mice (†P < 0.05 and ††P < 0.01). (C) Cytofluorometric analysis of immunofluorescence stained, dissociated tumor cells for KLRG1, PD-1 and Lag3 in CD8+ CD44hi gated TILs from EG7 tumors 13d after tumor inoculation and 3d after the second dose of α4-1BB antibody (red line) or rat IgG (blue line) treatment in vivo. Isotype control staining of CD8+ CD44hi gated TILs from IgG-treated animal is shown for comparison (black dashed line). Data are representative of 3 independent experiments. (D) Quantitation of lung nodules from WT or EKO mice 14 d after i.v. injection of B16 cells followed by administration of 100 μg α4-1BB or control rat IgG on d6 and d9 after B16 injection. Bars represent mean ± SEM of lung nodule counts (n = 5 per group). Data are from 1 of 2 independent experiments. Statistical analyses were performed by Student t test; IgG vs. α4-1BB groups *P < 0.05 in WT mice whereas no significant differences were detected between treatment groups in EKO mice. (E) Photo of representative lungs from each of the four groups from (D).
We next investigated whether Eomes is required for a different form of antitumor immunotherapy. We tested whether immunization against the ovalbumin epitope expressed by EG7 tumor cells could alter tumor growth in Eomes KO mice. In sharp contrast to our findings with α4-1BB agonist antibody, immunization against ovalbumin co-administered with CpG oligodeoxynucleotide adjuvant slowed EG7 tumor growth in both Eomes proficient and Eomes deficient mice (Fig. 4B).
Eomes deficient CD8+ CD44hi TILs harvested 3 d after the second dose of α4-1BB antibody, a time frame coincident with tumor growth retardation, showed no increase in KLRG1 expression in response to α4-1BB (Fig. 4C). Our earlier experiments showed that CD8+ CD44hi TILs from untreated EG7 tumors co-express at least 2 inhibitory receptors, PD-1 and Lag3, along with 4-1BB. Following α4-1BB immunotherapy, we observed diminished expression of both PD-1 and Lag3 in CD8+ CD44hi TILs from WT animals (Fig. 4C). However, PD-1 expression remained stable in CD8+ CD44hi TILs from tumor-bearing Eomes KO mice given α4-1BB treatment relative to those derived from the IgG control-treated hosts. The expression of Lag3, which was not elevated in Eomes KO CD8+ CD44hi TILs even in large, growing tumors, was similarly not altered after α4-1BB antibody injection.
To establish whether Eomes is necessary for α4-1BB-mediated immunotherapy in a less immunogenic tumor model of non-lymphoid origin, we used B16 melanoma cells in an experimental lung metastasis model.27 Fourteen days after i.v. inoculation with B16 cells, α4-1BB antibody therapy resulted in a diminished number of B16 nodules occurring in the lungs of C57BL/6 mice whereas there was no effect on metastatic burden in Eomes KO mice (Fig. 4D–E). Taken together, our findings demonstrate that 4-1BB-agonist therapy can impair tumor growth through an Eomes-dependent mechanism and suggest that 4-1BB-agonist therapy has the potential to reverse inhibitory receptor expression in tumor-infiltrating CD8+ T cells.
CD8+ CD44hi TILs express high levels of Eomes, PD-1, and Lag3 during tumor progression following α4-1BB immunotherapy
We next analyzed TILs from EG7 tumors that had resumed growth after α4-1BB immunotherapy to determine whether the observed increase in Eomes expression and corresponding decrease in inhibitory receptor expression were lost. In fact, the elevated Eomes expression in CD8+ CD44hi TILs occurring after α4-1BB antibody administration persisted in TILs derived from tumors that had resumed growth post immunotherapy (Fig. 5). Furthermore, the Eomes expression profile in CD8+ CD44hi TILs derived from α4-1BB antibody vs. control rat IgG2a treated animals appeared similar upon tumor progression (12 d after therapy and 22 d post tumor-inoculation) to that of CD8+ CD44hi TILs derived from growth inhibited tumors (3 d after therapy and 13 d post tumor-inoculation).
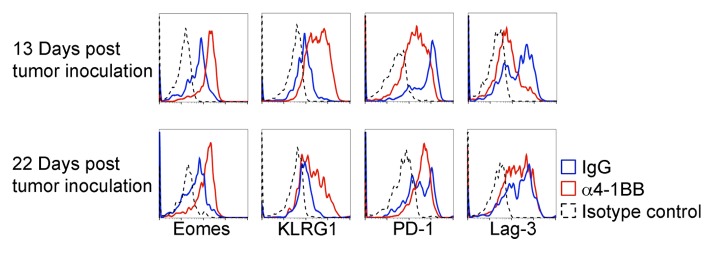
Figure 5. Analysis of Eomes, PD-1 and Lag3 expression in CD8+ CD44hi TILs from tumors progressing after α4-1BB immunotherapy. Immunofluorescent staining and cytofluorometric analysis for expression of Eomes, KLRG1, PD-1, or Lag3 expression in CD8+ CD44hi gated tumor-infiltrating lymphocytes (TILs) derived from EG7 tumors during α4-1BB antibody mediated tumor stasis/regression (d13 post tumor inoculation and d3 after the second dose of α4-1BB or control rat IgG) and upon resumption of tumor growth (d22 post tumor inoculation and d12 after the second dose of α4-1BB). All data are representative of at least three experiments.
In contrast to the increase in Eomes expression, the increased expression of KLRG1 and reduced expression of the inhibitory receptors, PD-1 and Lag3 was not maintained in CD8+ CD44hi TILs analyzed from progressing EG7 tumors in α4-1BB injected mice (Fig. 5). Twenty-two days after tumor inoculation, KLRG1 expression remained higher in CD8+ CD44hi TILs from α4-1BB treated mice than in those from control rat IgG2a injected mice, but seemed diminished relative to 9 d prior. Although initially distinct, by 22 d after tumor inoculation (12 d after the second dose of α4-1BB antibody), the expression of PD-1 and Lag3 in CD8+ CD44hi TILs after α4-1BB administration were similar to those observed in TILs derived from control rat IgG2a treated animals (Fig. 5). These data demonstrate that the effects of α4-1BB immunotherapy on CD8+ CD44hi TIL expression of Eomes, KLRG1, and inhibitory receptors are not absolutely linked, and suggest that upregulation of Eomes in CD8+ CD44hi TILs is not sufficient to maintain effective antitumor immunity.
Discussion
We report here a co-expression of 4-1BB stimulatory and inhibitory receptors on the surface of CD8+ CD44hi TILs. We further demonstrate that α4-1BB agonist immunotherapy leads to an increase in Eomes expression and, that an Eomes-dependent delay in tumor progression correlates with a transient decrease in the expression of the inhibitory receptors PD-1 and Lag3. This study corroborates and extends recently published findings in a B16 melanoma tumor model system showing Eomes-dependent differentiation of highly active, tumor infiltrating KLRG1hi T cells after 4-1BB directed therapy.12 In this study, authors showed that α4-1BB-agonist antibody effects multiple cell types in vivo, including CD4+ T cells, macrophages and dendritic cells, such that it remains possible that some of the effects we observed in CD8+ T cells after α4-1BB administration are indirect manifestations of α4-1BB antibody via other immune cells. Since the use of α4-1BB agonist antibody as an anticancer immunotherapy was first proposed, it has been assayed in a number of different tumor model systems with variable results.3,28-30 Some of these models require co-administration of additional therapy along with α4-1BB antibody.12,31-34 In this study, in response to α4-1BB agonist treatment alone was observed in mice bearing EG7 tumors similar to its use in early clinical trials.1,3,29,30
Attempts to identify the cellular targets of α4-1BB immunotherapy by selective cell depletion in vivo have consistently identified CD8+ T cells as essential to the therapeutic effect of α4-1BB.8,35-38 Depletion of NK cells has yielded inconsistent results, with loss of α4-1BB antibody mediated antitumor effects observed in some studies in contrast to no effect in others.8,28,36,38,39 CD4+ T cells appear to be unnecessary for α4-1BB mediated tumor immunity, with some evidence actually supporting superior activity when α4-1BB antibody is combined with a depleting anti-CD4 antibody.8,36-38 In a different approach, Lin et. al. examined the ability of α4-1BB to exert antitumor effects on the growth of EG7 tumors in contexts in which 4-1BB expression was restricted to specific immune cell populations.6 Specifically, 4-1BB expression on αβ T cells was found to be necessary and sufficient for tumor inhibitory responses to α4-1BB. Expression of 4-1BB restricted to tumor-specific CD8+ T cells transferred into a 4-1BB deficient host was also found to be sufficient for α4-1BB mediated antitumor immunity.6 Therefore, these data suggest that our observations are due, at least in part, to the direct effects of α4-1BB-agonist antibody on CD8+ T cells.
It remains unclear whether there is a threshold of T-bet and/or Eomes expression in CD8+ TILs that is either necessary or sufficient for long-lasting antitumor immunity. Prior work has shown relatively stable antitumor immune responses by tumor-vaccine stimulated CD8+ T cells lacking either T-bet or Eomes, although a deficiency in tumor infiltration has been demonstrated when T cells lack both transcription factors.40 In addition to the evinced induction of Eomes and KLRG1 co-expressing TILs in response to α4-1BB antibody, there is further evidence linking Eomes expression to T-cell anticancer activity. Induction of Eomes in CD4 T cells by co-stimulation with agonist antibodies directed against the co-stimulatory receptors, 4-1BB and OX-40 has been previously shown to be associated with antitumor activity in a melanoma model.24 The T cell inhibitory receptor, CTLA-4, has been shown to repress Eomes expression and antibody mediated blockade CTLA-4 has therapeutic activity in animal models and in cancer patients.41-43 While these data suggest that high Eomes expression in TILs is associated with tumor regression, Eomes alone is insufficient to ablate tumor development as our data show tumor progression despite continued expression of Eomes.
Our data do show a correlation between α4-1BB immunotherapy and transient decreased expression of the inhibitory receptors PD-1 and Lag3 on TILs. Insights into the complex interactions between T cell co-stimulatory pathways and inhibitory receptor signaling are highly relevant to the design of approaches to combinatorial immunotherapy.44 Pre-clinical studies in which α4-1BB agonist therapy has been combined with PD-1 or PD-L1 blockade in mouse models of hepatocellular carcinoma and chronic viral infection have demonstrated potential synergy between the two approaches.45,46 Our observation of diminished expression of PD-1 and Lag3 soon after α4-1BB therapy and increased expression upon tumor progression suggests that the timing and dosing of 4-1BB agonist and PD-1 antagonist approaches may have a significant impact on antitumor efficacy. This view is supported by the observation of sensitivity to relative dosing of α4-1BB vs. αPD-L1 antibodies eliciting optimal CD8+ T-cell response to chronic viral infection.45
In addition to the temporary alteration of PD-1 and Lag3 expression after α4-1BB antibody administration, our data suggest a potential relationship between Eomes and Lag3 expression in CD8+ CD44hi TILs. Prior work has established a link between T-bet, which shares partial homology with Eomes, and the inhibitory receptor PD-1.47 In this case, T-bet has been shown to inhibit the expression of PD-1, whereas our data suggests that Eomes may positively regulate the expression of Lag3. However, the nature of the relationship between Eomes and Lag3 is confounded by the observation of increased Eomes expression and decreased Lag3 expression immediately after α4-1BB therapy. Nevertheless, the observation of high Eomes expression and high Lag3 expression on TILs upon tumor progression after α4-1BB therapy suggests that the earlier increase in Eomes expression and decrease in Lag3 expression may not be linked events.
In the setting of pathogen infection such as lymphocytic choriomeningitis virus (LCMV), control of chronic infection is dependent on the existence of 2 sub-populations of responding CD8+ T cells, a T-bethi Eomeslo and a Tbetlo Eomeshi population.16 Loss of either population leads to a loss of viral control and increased viral burden. In our EG7 tumors, we initially observed a single population of CD8+ T cells with regard to high levels of T-bet and Eomes expression. Immediately after α4-1BB therapy and coincident with controlled tumor growth, we observed an Eomeshi population that had relatively low expression of T-bet, and an Eomeslo population that had relatively higher expression of T-bet. Upon progression of EG7 tumor growth after α4-1BB therapy, we observed a single population of CD8+ T cells with elevated expression of Eomes and co-expression of the inhibitory receptors PD-1 and Lag3. In the LCMV model, T-bet ablation in T cells during chronic infection leads to high levels of Eomes and PD-1 expression in virus-reactive T cells, a molecular phenotype correlating with failure to contain the virus. The Eomeshi CD8+ T cell population in the setting of uncontrolled chronic LCMV infection was found to be phenotypically and functionally exhausted, and it is possible that T cell exhaustion accounts for the eventual failure of α4-1BB therapy in our system as well. Multiple strategies for combating T cell exhaustion in cancer are under development and α4-1BB directed therapy may be most effective in combination with an approach designed to prevent T cell exhaustion.48-51
Re-expression of PD-1 and Lag3 in CD8+ CD44hi TILs with continued high levels of Eomes after α4-1BB immunotherapy demonstrates that Eomes expression is not sufficient to suppress the expression of inhibitory receptors. Although we did not observe downregulation of PD-1 in Eomes deficient TILs after α4-1BB therapy, the relationship between PD-1 expression and Eomes may be indirect in this setting. 4-1BB signaling has been shown to increase the survival of CD8+ T cells through the upregulation of the pro-survival Bcl-2 family members Bcl-2, Bfl-1 and Bcl-XL, and downregulation of the pro-apoptotic family member Bim.10,11,52,53 Our observations would be in keeping with a scenario in which Eomes is necessary for a subset of α4-1BB mediated effects, such as α4-1BB stimulated TIL survival, but does not directly influence PD-1 downregulation. Overall our results are consistent with a model in which TILs can exist in a state poised between inhibition and reactivation with simultaneous expression of multiple inhibitory and co-stimulatory receptors. 4-1BB-agonist immunotherapy leads to increased expression of the master transcription factor Eomes and decreased expression of inhibitory receptors, tipping the balance toward TIL reactivation.
Materials and Methods
Mice strains
Mice were bred, housed and utilized in accordance with the University of Maryland School of Medicine Institutional Animal Care and Use Guidelines. C57BL/6 mice, OT-1 mice, and CD45.1 mice on a C57BL/6 background were initially obtained from The Jackson Laboratory. OT-1 mice and CD45.1 mice were crossed to obtain CD45.1 OT-1 mice. Tbx21−/− (T-bet KO) and CD4-Cre Eomesflox/flox (Eomes KO) mice on a C57BL/6 background were a generous gift of Dr. Steven Reiner (Columbia University).14 Tbx21−/− mice and CD4-Cre Eomesflox/flox mice were crossed to obtain Tbx21−/− CD4-Cre Eomesflox/flox (double KO) mice.
Antibodies
Fluorochrome-labeled antibodies to CD8, CD44, PD-1, Lag3, CTLA4, 4-1BB, CD40, OX40, KLRG1, CD45.1, T-bet, and Eomes were purchased from eBioscience. Anti-4-1BB antibody (rat IgG2A) utilized for in vivo applications has been previously described18 and was produced from hybridoma line 2A with permission from Dr. Lieping Chen (Yale University).
Cell staining and flow cytometry
Cells were stained with fluorochrome-labeled antibodies to cell surface molecules for 30 minutes at 4oC prior to fixation and permeabilization (FoxP3 / Transcription Factor Staining Buffer Set, eBiocience) and staining with fuorochrome-labeled antibodies to intracellular antigens. 1 × 106 events per sample were acquired on an Accuri C6 (BD Biosciences) flow cytometer. Gating based on CD8 and CD44 surface staining (simultaneous) and analysis was performed using FlowJo software (Treestar).
Cell lines
EG7 cells were purchased from ATCC and grown in RPMI 1640 (Life Technologies) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin G, 100 μg/mL streptomycin sulfate, 0.05 mM 2-mercaptoethanol, and 0.4 mg/mL G418. B16 melanoma cells were also purchased from ATCC and grown in Dulbecco’s modified Eagle’s medium (Life Technologies) supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin G, and 100 μg/mL streptomycin sulfate.
Tumor transplantation models, immunotherapy and monitoring of tumor growth
In the lymphoma model, 1 × 106 EG7 cells in 200 μL phosphate-buffered saline (PBS) were subcutaneously injected into flank of 6–12 wk old C57BL/6, Eomes KO (EKO), T-bet KO (Tbx21−/−) mice or double KO recipient mice. Tumors were measured in both the vertical and horizontal dimensions using a caliper and the measurements were used to calculate tumor area at the indicated time points. In some experiments, EG7 tumor-bearing wild-type C57BL/6, Eomes KO (EKO), T-bet KO (Tbx21−/−) mice or double KO were treated with i.p. administration of 100 μg α4-1BB or control rat IgG on day 7 and day 10 after tumor inoculation. In the lung nodule model, 3 × 105 B16 cells in 200 μL PBS were intravenously injected into 6–12 wk old C57BL/6 mice. After 14 d mice were euthanized and lungs were harvested and fixed in Fekete’s solution prior to counting of surface tumor nodules.
Immunization
Ovalbumin (OVA) and unmethylated CpG oligodinucleotide (CPG) were both purchased from Invivogen. Injected solution contained 1 mg/mL OVA and 0.5 mg/mL CPG in PBS. 100 μL of this solution (vs. PBS control) was administered by s.c. injection 14 d prior to tumor inoculation and again on the day of tumor cell inoculation.
Tumor harvesting and preparation of tumor-infiltrating T cells
For analyses of TILs in the absence of immunotherapy (as in Figs. 1 and 2) tumors were harvested 21 d after inoculation. For analyses of TILs responding to antibody administration (as in Figs. 3 and 4) tumors were harvested 3 d after the final dose of agonistic anti-4-1BB antibody corresponding to 13 d after the initial tumor cell inoculation. For analysis of TILs responding to antibody administration vs. TILs from treatment-resistant tumors that resumed growth after antibody administration (as in Fig. 5) tumors were harvested 6 or 15 d after the first of 2 doses of antibody (13 or 22 d after initial tumor inoculation), as indicated. Tumors were measured prior to sacrifice and excised tumors were weighed. Tumors were minced, digested with Collagenase Type 2 (Life Technologies) and DNAase (Roche), and homogenized to a single cell suspension.
Adoptive T cell transfer
Spleens were collected from CD45.1 OT-1 donor mice and CD8+ T cells were isolated magnetically using a CD8+ T cell isolation kit (Miltenyi). CD8+ T cells were resuspended in PBS at a concentration of 2.5 × 105 cells/ml. 200 μL of the cell suspension was transferred to each recipient by i.v. injection.
Statistical Analysis
Statistical analyses were performed as indicated using the 2-tailed Student t test. A P value of less than 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by National Institute of Health grants (P30CA134274, K08HL93207), the Gabrielle’s Angel Foundation for Cancer Research, and the Marlene and Stewart Greenebaum Cancer Center. We thank Curt Civin for assistance and discussion.
Glossary
Abbreviations:
- 4-1BB/TNFRSF9
tumor necrosis factor receptor superfamily member 9
- α4-1BB
agonistic anti-4-1BB antibody
- Eomes
eomesodermin
- Lag3
lymphocyte-activation gene 3
- LCMV
lymphocytic choriomeningitis virus
- PD-1
programmed cell death-1
- Tbet
T-bet/Tbx21, T-box 21
- TNFRS
tumor necrosis family receptor superfamily
Citation: Song C, Sadashivaiah K, Furusawa A, Davila E, Tamada K, Banerjee A. Eomesodermin is required for antitumor immunity mediated by 4-1BB-agonist immunotherapy. OncoImmunology 2014; 3:e27680; 10.4161/onci.27680
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27680
References
- 1.Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19:1044–53. doi: 10.1158/1078-0432.CCR-12-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol. 2013;25:230–7. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol Cancer Ther. 2012;11:1062–70. doi: 10.1158/1535-7163.MCT-11-0677. [DOI] [PubMed] [Google Scholar]
- 4.Peggs KS, Quezada SA, Allison JP. Cancer immunotherapy: co-stimulatory agonists and co-inhibitory antagonists. Clin Exp Immunol. 2009;157:9–19. doi: 10.1111/j.1365-2249.2009.03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37:508–16. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Lin GH, Liu Y, Ambagala T, Kwon BS, Ohashi PS, Watts TH. Evaluating the cellular targets of anti-4-1BB agonist antibody during immunotherapy of a pre-established tumor in mice. PLoS One. 2010;5:e11003. doi: 10.1371/journal.pone.0011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May KF, Jr., Chen L, Zheng P, Liu Y. Anti-4-1BB monoclonal antibody enhances rejection of large tumor burden by promoting survival but not clonal expansion of tumor-specific CD8+ T cells. Cancer Res. 2002;62:3459–65. [PubMed] [Google Scholar]
- 8.Miller RE, Jones J, Le T, Whitmore J, Boiani N, Gliniak B, Lynch DH. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 10.Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J Immunol. 2008;180:8093–101. doi: 10.4049/jimmunol.180.12.8093. [DOI] [PubMed] [Google Scholar]
- 11.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169:4882–8. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 12.Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, Sun JC, Allison JP. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med. 2013;210:743–55. doi: 10.1084/jem.20121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185:4988–92. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–11. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–21. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–5. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–85. doi: 10.1016/S0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, Strome SE, Pease LR, Chen L. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–9. doi: 10.1172/JCI0214184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172:4821–5. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 20.Tutt AL, O’Brien L, Hussain A, Crowther GR, French RR, Glennie MJ. T cell immunity to lymphoma following treatment with anti-CD40 monoclonal antibody. J Immunol. 2002;168:2720–8. doi: 10.4049/jimmunol.168.6.2720. [DOI] [PubMed] [Google Scholar]
- 21.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim JA, Averbook BJ, Chambers K, Rothchild K, Kjaergaard J, Papay R, Shu S. Divergent effects of 4-1BB antibodies on antitumor immunity and on tumor-reactive T-cell generation. Cancer Res. 2001;61:2031–7. [PubMed] [Google Scholar]
- 23.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 2011;6:e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Ménoret A, Mittler RS, Gordon SM, Reiner SL, Vella AT, et al. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J Immunol. 2011;187:3555–64. doi: 10.4049/jimmunol.1101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henson SM, Akbar AN. KLRG1--more than a marker for T cell senescence. Age (Dordr) 2009;31:285–91. doi: 10.1007/s11357-009-9100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tessmer MS, Fugere C, Stevenaert F, Naidenko OV, Chong HJ, Leclercq G, Brossay L. KLRG1 binds cadherins and preferentially associates with SHIP-1. Int Immunol. 2007;19:391–400. doi: 10.1093/intimm/dxm004. [DOI] [PubMed] [Google Scholar]
- 27.Overwijk WW, Restifo NP. B16 as a mouse model for human melanoma. Curr Protoc Immunol 2001; Chapter 20:Unit 20 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 29.Snell LM, Lin GH, McPherson AJ, Moraes TJ, Watts TH. T-cell intrinsic effects of GITR and 4-1BB during viral infection and cancer immunotherapy. Immunol Rev. 2011;244:197–217. doi: 10.1111/j.1600-065X.2011.01063.x. [DOI] [PubMed] [Google Scholar]
- 30.Lynch DH. The promise of 4-1BB (CD137)-mediated immunomodulation and the immunotherapy of cancer. Immunol Rev. 2008;222:277–86. doi: 10.1111/j.1600-065X.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- 31.Ito F, Li Q, Shreiner AB, Okuyama R, Jure-Kunkel MN, Teitz-Tennenbaum S, Chang AE. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64:8411–9. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
- 32.Choi BK, Kim YH, Kang WJ, Lee SK, Kim KH, Shin SM, Yokoyama WM, Kim TY, Kwon BS. Mechanisms involved in synergistic anticancer immunity of anti-4-1BB and anti-CD4 therapy. Cancer Res. 2007;67:8891–9. doi: 10.1158/0008-5472.CAN-07-1056. [DOI] [PubMed] [Google Scholar]
- 33.Ko E, Luo W, Peng L, Wang X, Ferrone S. Mouse dendritic-endothelial cell hybrids and 4-1BB costimulation elicit antitumor effects mediated by broad antiangiogenic immunity. Cancer Res. 2007;67:7875–84. doi: 10.1158/0008-5472.CAN-06-1744. [DOI] [PubMed] [Google Scholar]
- 34.Kim YH, Choi BK, Oh HS, Kang WJ, Mittler RS, Kwon BS. Mechanisms involved in synergistic anticancer effects of anti-4-1BB and cyclophosphamide therapy. Mol Cancer Ther. 2009;8:469–78. doi: 10.1158/1535-7163.MCT-08-0993. [DOI] [PubMed] [Google Scholar]
- 35.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–72. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 36.Martinet O, Ermekova V, Qiao JQ, Sauter B, Mandeli J, Chen L, Chen SH. Immunomodulatory gene therapy with interleukin 12 and 4-1BB ligand: long- term remission of liver metastases in a mouse model. J Natl Cancer Inst. 2000;92:931–6. doi: 10.1093/jnci/92.11.931. [DOI] [PubMed] [Google Scholar]
- 37.Houot R, Goldstein MJ, Kohrt HE, Myklebust JH, Alizadeh AA, Lin JT, Irish JM, Torchia JA, Kolstad A, Chen L, et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114:3431–8. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murillo O, Dubrot J, Palazón A, Arina A, Azpilikueta A, Alfaro C, Solano S, Ochoa MC, Berasain C, Gabari I, et al. In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb. Eur J Immunol. 2009;39:2424–36. doi: 10.1002/eji.200838958. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Iuchi T, Jure-Kunkel MN, Chang AE. Adjuvant effect of anti-4-1BB mAb administration in adoptive T cell therapy of cancer. Int J Biol Sci. 2007;3:455–62. doi: 10.7150/ijbs.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Y, Ju S, Chen E, Dai S, Li C, Morel P, Liu L, Zhang X, Lu B. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J Immunol. 2010;185:3174–83. doi: 10.4049/jimmunol.1000749. [DOI] [PubMed] [Google Scholar]
- 41.Hegel JK, Knieke K, Kolar P, Reiner SL, Brunner-Weinzierl MC. CD152 (CTLA-4) regulates effector functions of CD8+ T lymphocytes by repressing Eomesodermin. Eur J Immunol. 2009;39:883–93. doi: 10.1002/eji.200838770. [DOI] [PubMed] [Google Scholar]
- 42.Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C, O’Day SJ, Hoos A, Humphrey R, Berman DM, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci. 2013;1291:1–13. doi: 10.1111/nyas.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 44.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 45.Vezys V, Penaloza-MacMaster P, Barber DL, Ha SJ, Konieczny B, Freeman GJ, Mittler RS, Ahmed R. 4-1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J Immunol. 2011;187:1634–42. doi: 10.4049/jimmunol.1100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao H, Huang B, Yuan Y, Li D, Han LF, Liu Y, Gong W, Wu FH, Zhang GM, Feng ZH. Soluble PD-1 facilitates 4-1BBL-triggered antitumor immunity against murine H22 hepatocarcinoma in vivo. Clin Cancer Res. 2007;13:1823–30. doi: 10.1158/1078-0432.CCR-06-2154. [DOI] [PubMed] [Google Scholar]
- 47.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–71. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goding SR, Wilson KA, Xie Y, Harris KM, Baxi A, Akpinarli A, Fulton A, Tamada K, Strome SE, Antony PA. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol. 2013;190:4899–909. doi: 10.4049/jimmunol.1300271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakuishi K, Ngiow SF, Sullivan JM, Teng MW, Kuchroo VK, Smyth MJ, Anderson AC. TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology. 2013;2:e23849. doi: 10.4161/onci.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldberg MV, Drake CG. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–78. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Speiser DE. A molecular profile of T-cell exhaustion in cancer. Oncoimmunology. 2012;1:369–71. doi: 10.4161/onci.18342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernandez-Chacon JA, Li Y, Wu RC, Bernatchez C, Wang Y, Weber JS, Hwu P, Radvanyi LG. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother. 2011;34:236–50. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Wen T, Routy JP, Bernard NF, Sekaly RP, Watts TH. 4-1BBL induces TNF receptor-associated factor 1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. J Immunol. 2007;179:8252–63. doi: 10.4049/jimmunol.179.12.8252. [DOI] [PubMed] [Google Scholar]