Abstract
Somatic embryogenesis (SE) is the process by which cells become dedifferentiated and reprogram to follow an embryogenic pathway. It is important for regeneration of transgenic plants as well as for propagation of certain genotypes. However, competence for SE varies, even among genotypes of a species, and the basis for this variation is not understood. We have found that the MADS-box transcription factor (Glycine max) AGAMOUS-Like 15 [(Gm)AGL15] promotes SE in Arabidopsis and in soybean when overexpressed. In soybean, part of the promotion of SE is via GmAGL15-mediated control of ethylene biosynthesis and response. Addition of ACC, the precursor to ethylene, to culture media enhanced SE in Arabidopsis and soybean. Transcription factors important for embryogenesis responded directly to GmAGL15 and to ethylene accumulation. Here we correlate ethylene production and patterns of gene expression with SE potential of soybean genotypes. However, other results indicate that there is not a complete positive correlation between ethylene production and SE, indicating that the interactions between hormones, gene expression and developmental outcomes are complex.
Keywords: somatic embryo, MADS-domain, Glycine max, ethylene, AGL15, AGL18, Arabidopsis thaliana, overexpression
Somatic embryogenesis (SE) is a process by which somatic cells become dedifferentiated and are able to reprogram to follow an embryogenic pathway. It is important agronomically to regenerate plants from transformed cells and to propagate desirable genotypes. What determines competence for SE remains a mystery that was featured in “What don’t we know” in Science magazine several years ago.1 Explants from different tissues can exhibit very different competence for SE and this can vary with age of the tissue and particular genotype within a species. Some genes have been identified that can promote SE when ectopically expressed and these include the MADS-box transcription factor AGAMOUS-Like 15 (AGL15) that can promote SE in two systems in the model plant Arabidopsis thaliana.2,3 Furthermore, a Glycine max (soybean) ortholog of AGL15, designated GmAGL15, can promote recovery of transgenic plants when constitutively expressed via the 35S promoter, presumably by enhancing regeneration of the transformed cell by SE.3 Explants from these transgenic plants, as well as from plants overexpressing a related gene GmAGL18, show increased SE development when cultured as described in Meurer et al. (2001).4,5 To better understand the molecular basis of the increased SE in response to the 35Spro:GmAGL15 transgene, we performed qRT-PCR for select genes involved in embryogenesis as well as a microarray experiment to assess the transcriptomes more globally.5,6 Genes involved in ethylene biosynthesis and response were overrepresented among the genes showing differential expression.6 Furthermore we found that in two cultivars of soybean, Jack and Williams 82, presence of the 35Spro:GmAGL15 transgene led to increased ethylene production from developing embryos of the size used for SE (4–5 mm). Orthologs of a transcription factor related to MtSERF1, that is expressed in response to ethylene and was found to be necessary for SE in Medicago truncatula,7 were directly expressed in response to AGL15 in soybean and Arabidopsis.6 The soybean and Arabidopsis orthologs were also responsive to ethylene biosynthesis and perception.6
In soybean, some cultivars such as Jack are relatively embryogenic and others (e.g., Defiance) are recalcitrant for this process.4 To score embryo induction, immature cotyledon explants from embryos 4–5 mm in size were removed and placed onto D40 medium, so-called due to the concentration of 2,4-D (40 mg/L) that induces SE. Explants were scored at 30, 45 and 60 d after initiation of culture (dac) and were assigned a 0 if no embryos were present; 1 if 1–5 embryos developed on the explant; 2 if 6–15 embryos were observed; and 3 if there were more than 15 embryos on the explant. The average was calculated per plate by summing the scores and dividing by the number of explants on the plate (21–25 typically). The average over all plates for a given genotype is shown in Figure 1. In agreement with prior results,4 Jack had a significantly higher embryo induction score than did Defiance at all timepoints. Because addition of ACC, a precursor to ethylene, to D40 medium could enhance SE from Defiance,6 we tested ethylene production by Defiance compared with Jack. As shown in Figure 2a, Defiance had significantly reduced ethylene production. For this experiment, seeds containing 4–5 mm embryos were used to test ethylene production. This allowed us to test tissue of the stage used for culture, but not wound the tissue and therefore we could examine genotype separate from wounding effects. Consistent with reduced ethylene production and SE in Defiance is the observation that Defiance accumulated reduced transcript from GmAGL15 compared with Jack in developing seeds (Fig. 3) as prior work demonstrated that an ACC SYNTHASE and ACC OXIDASE (ethylene biosynthetic genes) are expressed in response to GmAGL15. GmAGL15 and ethylene were also found to correlate with expression of key embryo transcription factors GmAGL18, GmABI3 and GmFUS3.5,6 Interestingly, Defiance seeds showed reduced transcript accumulation from GmABI3 and GmFUS3, consistent with GmAGL15 directly upregulating these genes and/or generation of reduced ethylene compared with Jack (Fig. 3). Further, GmSERF1 also was decreased in Defiance relative to Jack (Fig. 3). GmSERF1 is a potential ortholog of MtSERF1 that has been shown to be necessary for SE in Medicago truncatula.7 An Arabidopsis ortholog of SERF1 (At5g61590) is at least partially responsible for increased SE in response to overexpression of AGL15 in Arabidopsis.6 SERF/At5g61590 are AP2/ERF transcription factors.6,7 Recently the AP2/ERF gene family expression patterns were characterized during SE in Hevia brasiliensis (rubber tree).8 HbERF-IXb2 from rubber tree was reported as most similar to Arabidopsis At5g07580, that along with At5g61590 are the most similar Arabidopsis proteins to MtSERF1. HbERF-IXb2 shows significant transcript increases in regenerant (e.g., highly embryogenic) lines of Hevia compared with embryogenic or non-embryogenic lines upon transfer of calli to SE induction medium.8
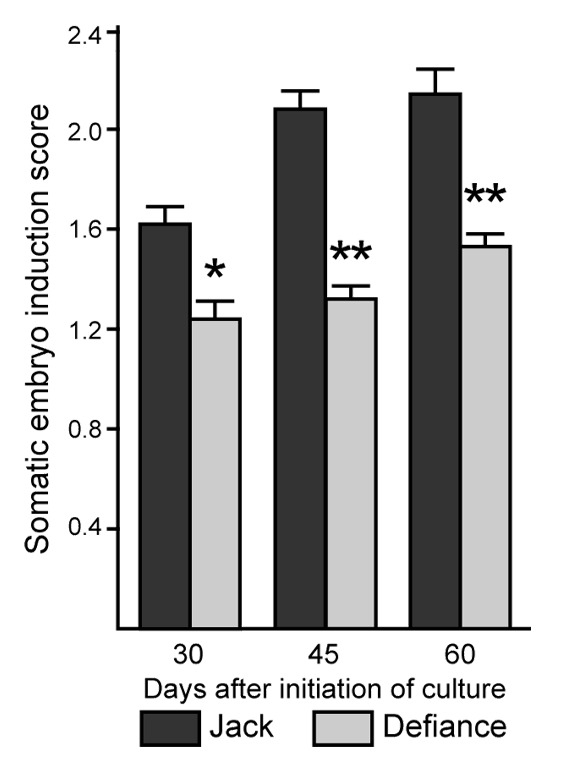
Figure 1. The soybean cultivar Defiance is less embryogenic than Jack. Immature cotyledon explants from 4–5 mm embryos were placed onto D40 SE initiation medium and the number of embryos scored at 30, 45 and 60 d. See the text for the scoring system. Means and standard error of the mean are shown for at least 11 plates of explants per genotype/timepoint. Significant differences between genotypes at a given timepoint at * p < 0.01; **p < 0.001.
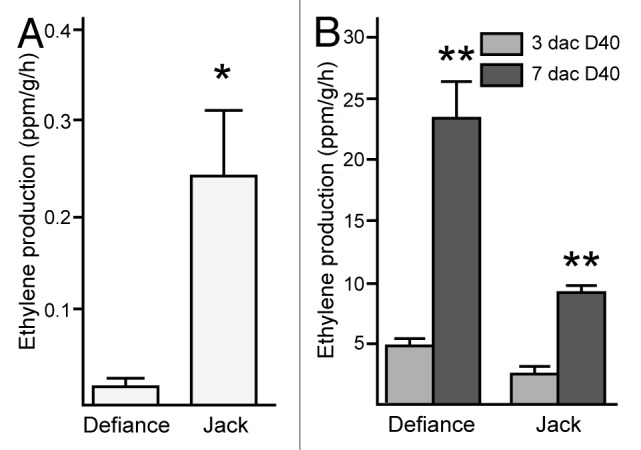
Figure 2. Ethylene production by cultivars of soybean from developing seeds containing 4–5 mm embryos without culture (A) or cotyledon explants from 4–5 mm embryos placed onto D40 medium for 3 or 7 d (B). Data shown are means and standard error of the mean for eight (A) or at least four (B) independent experiments. Asterisks indicate significant difference between the cultivars (A) or the timepoints within a cultivar (B) at *p < 0.05; **p < 0.01.
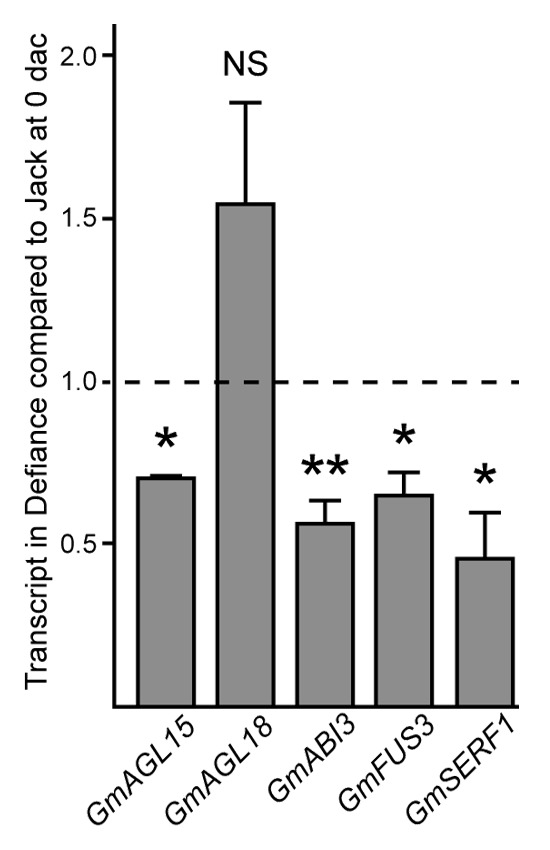
Figure 3. Transcript accumulation in isolated developing seeds containing 4–5 mm embryos. Transcripts from genes involved in the control of embryogenesis accumulate to lower amounts in the poorly embryogenic soybean cultivar Defiance compared with Jack that is more competent for somatic embryogenesis. Developing seeds of the stage used for SE but without any culture were used in this experiment. Means and standard error of the mean for at least three biological replicates are shown. Asterisks indicate significant difference between the cultivars as follows: * p < 0.05; **p < 0.01; NS, not significant.
Although ethylene and SE show a positive correlation at some levels in soybean and Arabidopsis, the situation was not this straight-forward. Developing seeds of Jack showed higher ethylene production than Defiance and explants from these seeds had higher SE (Fig. 1 and 2A). However, upon culturing explants on D40 medium for 3 and 7 d, both Jack and Defiance showed increased ethylene production as would be expected by exposure to the synthetic auxin 2,4-D9 and Defiance showed a greater increase in ethylene production (Fig. 2b), yet ultimately it had reduced embryo development compared with Jack (Fig. 1). Wounding alone does not cause the magnitude of ethylene production as does wounding with placement on 2,4-D, with the amount of ethylene remaining below 1 ppm/g/h when explants are cultured on medium lacking 2,4-D (data not shown). The lack of complete positive correlation between ethylene production and SE may be an issue of timing where the importance is ethylene concentration at the time of placement on D40. Alternatively, too much ethylene may inhibit somatic embryogenesis. Although 10 μM ACC, the highest concentration tested in the soybean system, significantly increased SE for Defiance,6 different effects were observed at different concentrations in an Arabidopsis SE system in which mature seeds are allowed to complete germination in medium containing 2,4-D and will, at some frequency, show somatic embryo (SE) development from the shoot apical meristem (SAM) region.10 As shown in Figure 4, some concentrations of ACC increased the fraction of seedlings with SAM SE development, but higher concentrations inhibited development. This is not unlike the situation with gibberellic acid where inhibition of biosynthesis of this hormone by paclobutrazol promotes SAM SE but higher amounts of paclobutrazol inhibit SAM SE for the low number of seeds able to complete germination.11 Similarly, when a GA catabolic enzyme is overexpressed in Arabidopsis that is a direct target of AGL15, lines with more moderate expression show increased SAM SE whereas more severe overexpression lacks SAM SE development.11 This type of opposing effects at different concentrations of a plant hormone is not unusual (for a review see ref. 12).
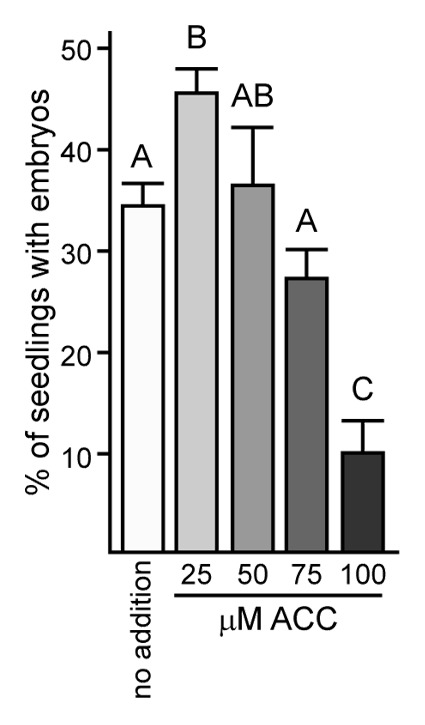
Figure 4. ACC, a precursor to ethylene, affects Arabidopsis SAM SE. Means and standard error of the mean are shown for two biological replicates of the experiment (n = 3–4 flasks scored per replicate). Different letters indicate significant difference at p < 0.001.
Acknowledgments
We thank Dr R. Geneve and Huihua Ji (University of Kentucky) for assistance with ethylene measurements. We also thank Jeanne Hartman, Olivia Jones and Rachel Mueller (University of Kentucky) for assistance with cultures and the USDA Soybean Germplasm Collection for the cv Defiance seed. This work was supported by the United Soybean Board (0282/1282/2282) and the National Science Foundation (IOS-0922845). This article (13-06-057) is published with the approval of the Director of the Kentucky Agricultural Experiment Station.
Glossary
Abbreviations:
- (Gm)AGL15
(Glycine max)AGAMOUS-Like15
- AGL18
AGAMOUS-Like18
- SE
somatic embryogenesis
- SAM
shoot apical meristem
- ABI3
ABA-INSENSITIVE3
- FUS3
FUSCA3
- ACC
aminocyclopropane-1-carboxylic-acid
- GA
gibberellic acid
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25422
References
- 1.Vogel G. How does a single somatic cell become a whole plant? Science. 2005;309:86. doi: 10.1126/science.309.5731.86. [DOI] [PubMed] [Google Scholar]
- 2.Harding EW, Tang W, Nichols KW, Fernandez DE, Perry SE. Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-Like 15. Plant Physiol. 2003;133:653–63. doi: 10.1104/pp.103.023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakare D, Tang W, Hill K, Perry SE. The MADS-domain transcriptional regulator AGAMOUS-LIKE15 promotes somatic embryo development in Arabidopsis and soybean. Plant Physiol. 2008;146:1663–72. doi: 10.1104/pp.108.115832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meurer CA, Dinkins RD, Redmond CT, McAllister KP, Tucker DT, Walker DR, et al. Embryogenic response of multiple soybean Glycine max (L.) Merr. cultivars across three locations. In Vitro Cell Dev Biol Plant. 2001;37:62–7. doi: 10.1007/s11627-001-0012-3. [DOI] [Google Scholar]
- 5.Zheng Q, Perry SE. Alterations in the transcriptome of Glycine max in response to enhanced somatic embryogenesis promoted by orthologs of AGAMOUS-Like 15 and AGAMOUS-Like 18. 2013 doi: 10.1104/pp.113.234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Q, Zheng Y, Perry SE. AGAMOUS-Like15 promotes somatic embryogenesis in Arabidopsis and soybean in part by the control of ethylene biosynthesis and response. Plant Physiol. 2013;161:2113–27. doi: 10.1104/pp.113.216275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantiri FR, Kurdyukov S, Lohar DP, Sharopova N, Saeed NA, Wang XD, et al. The transcription factor MtSERF1 of the ERF subfamily identified by transcriptional profiling is required for somatic embryogenesis induced by auxin plus cytokinin in Medicago truncatula. Plant Physiol. 2008;146:1622–36. doi: 10.1104/pp.107.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piyatrakul P, Putranto RA, Martin F, Rio M, Dessailly F, Leclercq J, et al. Some ethylene biosynthesis and AP2/ERF genes reveal a specific pattern of expression during somatic embryogenesis in Hevea brasiliensis. BMC Plant Biol. 2012;12:244. doi: 10.1186/1471-2229-12-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raghavan C, Ong EK, Dalling MJ, Stevenson TW. Regulation of genes associated with auxin, ethylene and ABA pathways by 2,4-dichlorophenoxyacetic acid in Arabidopsis. Funct Integr Genomics. 2006;6:60–70. doi: 10.1007/s10142-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 10.Mordhorst AP, Voerman KJ, Hartog MV, Meijer EA, van Went J, Koornneef M, et al. Somatic embryogenesis in Arabidopsis thaliana is facilitated by mutations in genes repressing meristematic cell divisions. Genetics. 1998;149:549–63. doi: 10.1093/genetics/149.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Caruso LV, Downie AB, Perry SE. The embryo MADS domain protein AGAMOUS-Like 15 directly regulates expression of a gene encoding an enzyme involved in gibberellin metabolism. Plant Cell. 2004;16:1206–19. doi: 10.1105/tpc.021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N. The dual-and-opposing-effect of ethylene on the negative gravitropism of Arabidopsis inflorescence stem and light-grown hypocotyls. Plant Sci. 2008;175:71–86. doi: 10.1016/j.plantsci.2008.02.001. [DOI] [Google Scholar]


