Abstract
Plant immunity is essential for plant survival and resistance (R) proteins serve essential roles in pathogen detection and defense signal initiation. A gain-of-function mutation in SNC1, a TIR-type R gene, results in a characteristic autoimmune phenotype in Arabidopsis. From a forward genetic suppressor screen using snc1, MOS2 (MODIFIER of snc1), which encodes an RNA-binding protein, was identified. When MOS2 function is lost, the autoimmunity caused by snc1 is abolished and basal resistance against virulent pathogens is attenuated. Recently, it was shown that mos2 mutants also have defects in miRNA processing. However, it is not known how the role of MOS2 in miRNA production is related to the suppression of snc1-mediated autoimmunity. Here, we show that MOS2 contributes to proper splicing of SNC1 transcript, agreeing with its potential association with the MOS4-associated complex (MAC). In addition, although mutant plants carrying a mutation in the MOS2 homolog MOS2H are wild-type like, the double mutant mos2 mos2h is lethal. These data suggest that MOS2 and MOS2H have unequally redundant functions. Overall, MOS2 and MOS2H probably have diverse functions in both alternative splicing and miRNA processing.
Keywords: Arabidopsis, MOS2, MOS2H, RNA-binding protein, SNC1, plant immunity, resistance protein, splicing
Like all organisms, plants face constant threats from pathogens in their environment. The plasma membrane layer of plant defense involves the recognition of molecules often conserved among large groups of microbial organisms, such as bacterial flagellin.1 However, successful pathogens are able to secrete or inject molecules termed effectors into plant cells to subvert this layer of defense. Through evolution, plants have evolved many resistance (R) genes, which encode proteins that are able to recognize the effectors directly or indirectly.2 Following recognition, a very strong immune response is usually triggered, which often includes accumulation of salicylic acid (SA), production of reactive oxygen species and hypersensitive response (HR), which is a localized cell death around the site of infection. R gene mediated defense is usually very effective in preventing establishment of infection and thus has been widely used by breeders for crop protection.
Despite the importance of R proteins in plant immunity, little is known about their activation mechanisms. A Modifier of snc1 (MOS) forward genetic screen was conducted to identify components required for R protein activation, using an autoimmune snc1 mutant.3 This allele encodes an altered form of a TIR-NB-LRR-type of R protein which is less vulnerable to protein degradation and therefore accumulates to higher levels in the plant.4 The over-accumulation of snc1 confers a dwarf, autoimmune phenotype. Mutants in several genes were identified that suppressed the autoimmunity of snc1, including MOS2.5,6 As mos2 suppresses the small stature, high constitutive SA accumulation and enhanced disease resistance characteristics of snc1, MOS2 is necessary for the function of snc1. Basal resistance and resistance conferred by other R proteins are also reduced in mos2 single mutants, suggesting a more general role for MOS2 in defense. Recently it was further shown that MOS2 is involved in micro RNA (miRNA) maturation by binding to long primary miRNA (pri-miRNA) transcripts and facilitating their recruitment to HYL1, a protein that assists in the production of miRNAs through the cleavage of pri-miRNA.7 However, how miRNA maturation affects snc1-mediated autoimmunty is unclear.
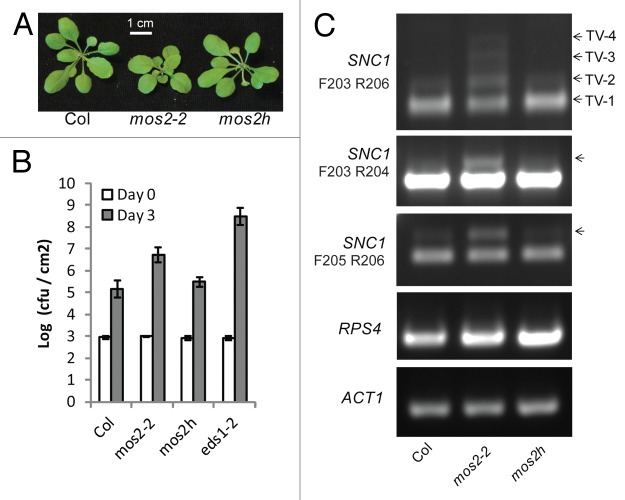
The Arabidopsis genome also contains a close homolog of MOS2, which was named MOS2H.5 The deduced amino acid sequences of these two proteins share 52% identity. While the null mos2-2 plants exhibit a slightly stunted morphology and round leaves, the mos2h-1 knockout mutant (SAIL_223_H01), which carries a T-DNA insertion in the exon of MOS2H, does not show any obvious morphological phenotypes (Fig. 1A). When we crossed mos2-2 with mos2h-1 plants, no homozygous mos2-2 mos2h plants were recovered in the F2 generation. Furthermore, several mos2-2/mos2-2 MOS2h/mos2h-1 plants were allowed to self-fertilize and their offspring were examined for the presence of a double mutant. However, no double mutant plants were recovered. This suggests that MOS2 and MOS2H are partially redundant and required for plant survival, with MOS2 playing a larger or broader role.
Figure 1.MOS2 has a role in basal immunity and SNC1 splicing. (A) Morphology of 3-wk-old soil-grown wild-type (Col), mos2-2 and mos2h plants. (B) Susceptibility of wild-type, mos2-2 and mos2h-1 to P.s.m. ES4326. Leaves of 4-wk-old soil-grown plants were infiltrated with a suspension of bacteria at OD600 = 0.001 and leaf discs from the infiltrated area were taken at 0 or 3 d to quantify the colony forming units (cfu). Bars represent the means of five replicates and the error bars represent the standard deviations. (C) Splicing pattern of SNC1 and RPS4, as determined through RT-PCR. cDNA was amplified using a combination of primers F203, R205, R204 and R206 for SNC1 and RPS4-F and RPS4-R for RPS4.10 Bands representing intron-retained splice variants are indicated by arrowheads. These bands were directly sequenced and confirmed to be intron-retained forms of SNC1.
In addition to the morphological phenotypes, mos2-2 plants also exhibit enhanced disease susceptibility to the virulent pathogen Pseudomonas syringae pv maculicola (P.s.m.) ES4326 (Fig. 1B), demonstrating a role for MOS2 in basal resistance. The growth of P.s.m ES4326 in mos2-2 was not as high as observed in eds1-2, which was used as a positive control for defects in basal immunity. However, the resistance of mos2h-1 plants is not different from wild-type, in agreement with their wild-type-like morphology.
MOS2 binds pri-miRNA and facilitates production of miRNA.7 Several miRNAs have been shown to be involved in antibacterial resistance.8,9 The function of MOS2 in defense may be partly explained by its role in miRNA maturation. However, it remains elusive whether MOS2 also can regulate resistance though additional mechanisms. MOS12, another gene recovered from the MOS screen, has previously been characterized as a positive regulator of immunity, with a role in the proper splicing of the R genes SNC1and RPS4.10 MOS12 seems to function together with the spliceosome-interacting MOS4-associated complex (MAC).10-12 Interestingly, the yeast MOS2 homolog Spp2 and human MOS2 homolog GPKOW/T54 were identified as putative Nineteen complex (NTC) components, with NTC as the equivalent MAC in those organisms.13,14 Therefore, we examined the contribution of MOS2 and MOS2H to R gene splicing. An increase in intron-retained splice forms of SNC1 was seen in mos2-2 mutants but not mos2h (Fig. 1C). Sequencing of the upper bands further confirmed that they are indeed intron-retained splice forms of SNC1. However, no difference was observed in the splicing pattern of RPS4. This suggests that like MOS12 and the MAC components, MOS2 is also required for the proper splicing of SNC1. The autoimmune phenotype of snc1 mutants is due to increased accumulation of snc1 protein, which activates the immune signaling pathway.4 A positive feedback loop then increases the transcriptional level of snc1, ultimately resulting in further elevated snc1 protein levels. Because of the reduced efficiency of snc1 mRNA splicing in mos2 mutant plants, snc1 protein probably cannot accumulate to levels high enough to cause autoimmunity. This partly explains the snc1-suppressing phenotype of mos2.
It is not known what role MOS2 performs in mRNA splicing, nor how this is related to its other known roles in miRNA biogenesis. It has been suggested that the RNA-binding activity of MOS2 may be involved in the recruitment of pri-miRNA molecules to HYL1 and other proteins responsible for processing into miRNAs.7 It is possible that MOS2 also binds mRNA and recruits it to the MAC, which further facilitates proper splicing of SNC1 transcript.10 Members of the MAC and other pre-mRNA splicing factors have also been implicated in control of RNA-directed DNA methylation, suggesting that splicing-related proteins may play roles in diverse cellular processes.15 Future in-depth transcriptome analysis of these mutants and refined biochemical dissection of the protein-protein interactions may help us reveal how MOS2 mechanistically regulates plant immunity and plant development.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25372
References
- 1.Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 2.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–9. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Goritschnig S, Dong X, Li X. A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell. 2003;15:2636–46. doi: 10.1105/tpc.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng YT, Li Y, Huang S, Huang Y, Dong X, Zhang Y, et al. Stability of plant immune-receptor resistance proteins is controlled by SKP1-CULLIN1-F-box (SCF)-mediated protein degradation. Proc Natl Acad Sci USA. 2011;108:14694–9. doi: 10.1073/pnas.1105685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Cheng YT, Bi D, Palma K, Li X. MOS2, a protein containing G-patch and KOW motifs, is essential for innate immunity in Arabidopsis thaliana. Curr Biol. 2005;15:1936–42. doi: 10.1016/j.cub.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Johnson KCM, Dong OX, Huang Y, Li X. A Rolling Stone Gathers No Moss, but Resistant Plants Must Gather Their MOSes. Cold Spring Harb Symp Quant Biol. 2013;77:1–10. doi: 10.1101/sqb.2013.77.014738. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Shi Y, Li J, Xu L, Fang Y, Li X, et al. A role for the RNA-binding protein MOS2 in microRNA maturation in Arabidopsis. Cell Res. 2013;23:645–57. doi: 10.1038/cr.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, et al. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;312:436–9. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Zhang Q, Zhang J, Wu L, Qi Y, Zhou JM. Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiol. 2010;152:2222–31. doi: 10.1104/pp.109.151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu F, Xu S, Wiermer M, Zhang Y, Li X. The cyclin L homolog MOS12 and the MOS4-associated complex are required for the proper splicing of plant resistance genes. Plant J. 2012;70:916–28. doi: 10.1111/j.1365-313X.2012.04906.x. [DOI] [PubMed] [Google Scholar]
- 11.Monaghan J, Xu F, Gao M, Zhao Q, Palma K, Long C, et al. Two Prp19-like U-box proteins in the MOS4-associated complex play redundant roles in plant innate immunity. PLoS Pathog. 2009;5:e1000526. doi: 10.1371/journal.ppat.1000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palma K, Zhao Q, Cheng YT, Bi D, Monaghan J, Cheng W, et al. Regulation of plant innate immunity by three proteins in a complex conserved across the plant and animal kingdoms. Genes Dev. 2007;21:1484–93. doi: 10.1101/gad.1559607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aksaas AK, Larsen AC, Rogne M, Rosendal K, Kvissel AK, Skalhegg BS. G-patch domain and KOW motifs-containing protein, GPKOW; a nuclear RNA-binding protein regulated by protein kinase A. J Mol Signal. 2011;6:10–14. doi: 10.1186/1750-2187-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy J, Kim K, Maddock JR, Anthony JG, Woolford JL., Jr. The final stages of spliceosome maturation require Spp2p that can interact with the DEAH box protein Prp2p and promote step 1 of splicing. RNA. 1995;1:375–90. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang CJ, Zhou JX, Liu J, Ma ZY, Zhang SW, Dou K, et al. The splicing machinery promotes RNA-directed DNA methylation and transcriptional silencing in Arabidopsis. EMBO J. 2013;32:1128–40. doi: 10.1038/emboj.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]