Abstract
Perlite is a generic name for an amorphous volcanic alumina–silicate rock that expands by a factor of 4–20 when rapidly heated to 1400–1800 °F (760–980 °C). Both the ore and the expanded product have extensive and widespread commercial applications. Limited data on the toxicology of perlite in animal studies indicate that the LD50 (oral ingestion) is more than 10 g/kg and, from a chronic inhalation study in guinea pigs and rats, that the NOAEL for the inhalation pathway is 226 mg/m3. Health surveillance studies of workers in US perlite mines and expansion plants (including some workers exposed to levels greater than prevailing occupational exposure limits (OELs) conducted over 20 years indicate that the respiratory health of workers is not adversely affected. Studies in Turkish mines and expanding plants had generally similar results, but are more difficult to interpret because of high smoking rates in these populations. A recent mortality study of permanent residents of the island of Milos (Greece) exposed to various mining dusts (including perlite) resulted in non-significant increases in standard mortality ratios for pneumonia and chronic obstructive pulmonary disease (COPD), whereas a companion morbidity study revealed elevated odds ratios for allergic rhinitis, pneumonia, and COPD when compared to another industrial area of Greece. Residents were exposed to other mining dusts and other possible causes or contributing factors and no ambient monitoring data were presented so it is not possible to use this study for risk calculations of perlite-exposed populations. Perlite is regulated as a “nuisance dust” in most countries.
Keywords: Applications, epidemiology, mining, occupational exposure, perlite, processing, toxicity
Introduction
This article provides a comprehensive review of perlite toxicology and epidemiology. The article is relevant because perlite is widely used for many applications and several new contributions have been written since the last authoritative review.
Perlite (ore CAS# 130885-09-5) is an amorphous volcanic alumina–silicate rock (in colors ranging from transparent light gray to glossy black) that has a relatively high water content (2–5% w/w), typically formed by the hydration of obsidian. According to Kadey (1983), the name is derived from the classical description (Johannsen, 1939) that this mineral was a “glassy rhyolite with a pearly luster and concentric onionskin parting”. 1
Perlite has the unusual and critical property of expanding (“popping”) by a factor of 4–20 (Doğan & Alkan, 2004; Ennis, 2011; USEPA, 1980, 1995a, b) when rapidly heated to 1400–1800 °F (760–980 °C) to create a product termed expanded perlite (CAS #093763-70-3). Expanded perlite has several attractive physical properties for commercial applications including low bulk density, low thermal conductivity, high heat resistance, low sound transmission, high surface area, and chemical inertness (Ennis, 2011; Health Council of the Netherlands, 2003). Both the ore and the expanded product are referred to as perlite.
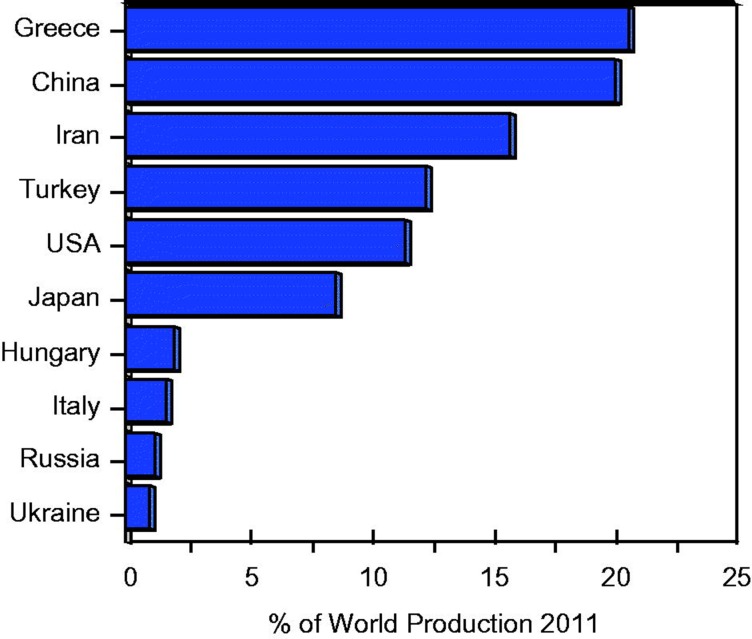
The use of perlite reportedly dates back to the 1800s and modern exploitation of this resource in the United States began in the 1940s (Austin & Barker, 1998; Ennis, 2011; Jaster, 1956; Shackley & Allen, 1992; Weber, 1963), but might have been used elsewhere as much as 2300 years ago (Kadey, 1983). Perlite mines are located in several countries of the world. Figure 1 shows that 10 countries accounted for approximately 95% of the world production of 3 470 000 metric tons in 2011 (British Geological Survey, 2013). Major world perlite producers include Greece, China, Iran, Turkey, the United States and Japan. Crushing, drying, and screening are typically done at the mining sites prior to shipment to expanding plants. The low density of expanded perlite favors the location of expanding plants near market areas to minimize transportation costs.
Figure 1.
Ten largest perlite producers in 2011 ranked in descending order of output (% of world production). These accounted for 95% of world production of perlite (3 470 000 metric) Source: British Geological Survey, 2013.
In the United States, crude perlite ore production (424 000 metric tons in 2012) came from eight mines operated by six companies in five Western States (see Bolen, 2012, 2013). New Mexico is the major producing state in the United States. Processed crude perlite was expanded at 50 plants in 27 states (Bolen, 2013). In 2012, the US imported (Bolen, 2013) an estimated 168 000 metric tons (chiefly from Greece) and exported 35 000 tons (chiefly to Canada). World reserves and resources of perlite are abundant.
For economic reasons, most perlite mines are surface mines; details of the mining process are reviewed in several publications (e.g. Kadey, 1983; USEPA, 1980). After removal of overburden, 2 Perlite mines use ripping, blasting, or both after which the ore is loaded on trucks or scrapers for transport to the processing plant. Crude perlite processing is limited to crushing, drying, grinding, screening, sizing, and possibly blending; commercial deposits of perlite have little overburden and no chemicals are used in the processing of perlite (Perlite Institute, 2009), which generates relatively modest environmental impacts compared to mining and beneficiation operations for many other ores.
Expanded Perlite production is done in expansion furnaces; after preheating and heating (where the volcanic glass is softened and entrapped water molecules turn to steam and expand the particles) a suction fan is used to draw the expanded particles (now with a white color) from the furnace and transport them pneumatically to a cyclone classifier system. The cyclone classifier system collects the expanded particles, and removes excessive fine particles (details on processing can be found in USEPA, 1995a, b). Expanded perlite can be manufactured to various densities (Austin & Barker, 1998) ranging from 2 lbs/ft3 (32 kg/m3) to 15 lbs/ft3 (240 kg/m3) and can be produced in various dimensions as required for specific applications. Producers use different nomenclature to describe perlite grades; some use terms such as fine, medium, coarse, while others define grades by application (e.g. horticultural, cryogenic, industrial, and construction). Grain sizes vary by grade and producer and, depending upon grade could range from ∼20 μm to as much as 10 mm. 3
Applications
Although the major perlite markets are for expanded perlite, crude perlite ore is used in certain industrial applications including sandblasting, as a slag coagulant, special casting sand, and metal finishing (Perlite Institute, 2009). Major markets for expanded perlite in the United States (Austin & Barker, 1998; Bolen, 2012; Ennis, 2011) vary by grade and include formed products (e.g. acoustic ceiling tiles, pipe insulations, and insulation boards), fillers (caulking compositions, paints, plastics, loose fill insulation, packing materials), horticultural aggregate (soil-less culture of plants, mixed with peat as a propagating medium), and as a filter aid (wastewater treatment, and to filter vegetable and fruit juices, soft drinks, and pharmaceutical applications). There are numerous other applications (Perlite Institute, 2009). Perlite has been studied as an ingredient in toothpaste (Collins et al., 2005; Stamm, 2007), a dietary supplement for broilers (Tatar et al., 2012), a component of swine-fodder (Duchstein, 1982), a component of landfill liners for the in situ leachate treatment of landfills (Ozel et al., 2012), for the removal of cadmium, nickel, and lead from aqueous solutions (Aminifard et al., 2011; Malakootian et al., 2011; Torab-Mostaedi et al., 2010), as a passive material in the form of ceiling tile for removal of ozone from indoor air (Cros et al., 2011), in mycotoxin detoxification of animal feed (Huwig et al., 2001), and as a component of a stormwater filter (Gironás et al., 2008).
Competitive commodities that can substitute for perlite include (depending upon application) polystyrene, diatomite, expanded clay and shale, pumice, slag, synthetic vitreous fibers, and vermiculite (Baker & Santini, 2006; Bolen, 2013; Papadopoulos, 2005).
Composition
Chemically, perlite ore consists of SiO2, Al2O3, and lesser amounts of several metal oxides (sodium, potassium, iron, calcium, and magnesium). Table 1 shows the composition of perlite ores from several countries. The endpoints of the composition ranges shown in Table 1 come from different mines in the countries referenced. The chemical composition of perlite from any particular mine is less variable than the ranges shown in Table 1.
Table 1.
Chemical composition of Perlite from various sources.
| Origin | US | Greece | Turkey | Hungary | Yemen | Korea | Bulgaria | Slovakia |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 65–77.5 | 71–75 | 71–75 | 68–75 | 65–75 | 68–69 | 70–80 | 68–73 |
| Al2O3 | 11–18 | 12–16 | 12.5–18 | 10–15 | 9.4–12.8 | 11.95–15.8 | 10–15 | 7.5–15 |
| Na2O | 2.4–4.6 | 3.0–4.0 | 2.9–4.0 | 2.8–4.5 | 3.37–4.25 | 3.2–4.4 | <10 | 2.5–5.0 |
| K2O | 1.4–5.7 | 4.0–5.0 | 4.0–5.0 | 3.2–4.5 | 3.6–4.1 | 2–3.94 | <10 | 2–5.5 |
| Fe2O3 | 0.5–2.2 | 0.5–2.0 | 0.1–1.5 | 1.0–2.5 | 2.68–3.66 | 0.7–1.63 | <1.5 | 1.0–2.0 |
| MgO | 0.1–0.7 | 0.2–0.7 | 0.03–0.5 | 0.2–1.5 | 0.5–0.93 | 0.35–0.48 | <1.0 | <1.0 |
| CaO | 0.5–3.6 | 0.5–1.5 | 0.5–2.0 | 1.5–2.0 | 0.87–1.84 | 1.57–1.89 | <1.5 | 0.5–2.0 |
| Loss on ignition | 2–5 | 2–5 | 4.1 | 2.0–5.0 | 0.35–3.94 | NR | <5 | 3.0–4.1 |
| Source | Diverse | Sampatakakis et al., 2013 | Doğan & Alkan, 2004; Kabra et al., 2013 | Mineralholding Ltd. | Sa’ad et al., 2010 | Noh & Boles, 1989 | Yanevaet al., 2012 | LB Minerals |
Mineralholding Ltd. Data available at http://www.mineralholding.hu/ipariasvanyok/en/raw_perlite.html.
Data for US taken from various mines, mineral deposit reports, and technical data sheets. Illustrative sources include Coombs, 1952; Ennis, 2011; USEPA, 1995b; Huntting, 1949; Jaster, 1956; Rotella & Simandl, 1995; and Simandi et al., 1995. Endpoints of ranges may come from different deposits. Ore from all countries may contain additional minor components (e.g. TiO2).
Various mineral impurities in relatively low concentrations can be found in most perlite deposits, including biotite, chert, feldspar, and oligoclase (see e.g. Austin & Barker, 1998; Sa’ad et al., 2010; Weber, 1963), but the most significant from a toxicological perspective is crystalline silica (quartz, cristobalite, and tridymite). Safety data sheets for commercial perlite report various percentages of crystalline silica (ranging from <0.05 to 5%). IARC (2012) concluded that respirable crystalline silica in the form of quartz or cristobalite dust is carcinogenic to humans (Group 1). Occupational exposure to crystalline silica can cause silicosis and increase the risk of pulmonary tuberculosis. These exposures have also been linked to the development of autoimmune disorders, chronic renal disease, and other adverse health effects (NIOSH, 2002).
Regulatory status and occupational exposure limits (OELs)
Table 2 provides a brief summary of information on the regulatory status of perlite and various regulatory or advisory exposure limits. Perlite is not listed as a carcinogen by IARC, NTP, ACGIH, OSHA, or California Prop. 65. Used as a filter aid, perlite is included in the US Food and Drug Administration (FDA, 1979) database as Generally Recognized as Safe (GRAS) according to the Select Committee of GRAS Substances (SCOGS, 1979) Report 61 (1979), which noted: “Estimates of the maximum quantities of minerals that might be extracted from perlite and diatomaceous earth used as filteraids in food processing indicates no hazard to public health”. Perlite is included in the Register of Feed Additives by the European Food Safety Authority (EFSA).
Table 2.
Summary information on perlite.
| Item | |
|---|---|
| CAS/EINECS NO. | CAS: 130885-09-5 (perlite ore); EINECS: Not listed CAS: 093763-70-3 (expanded perlite);EINECS: Not listed; RTECS No. SD5254000; REACH Exempted according to art.2 par 7b and Annex V.7. |
| NAICS (2012) Codes | Mining: 212399 (All other non-metallic mineral mines) Processing: 327992 (Ground or treated mineral and earth manufacturing) |
| Composition | Varies with source, see Table 1. |
| Carcinogenicity | Not listed as a carcinogen by IARC, NTP, OSHA, or CA Prop 65. ACGIH: A4 – Not Classifiable as a Human Carcinogen Perlite does not meet the criteria for classification as hazardous according to EC Regulation 1272/2008 and Directive 67/548/EC as amended. |
| WHMIS | Does not meet criteria |
| FDA | GRAS SCOGS Report #61, 1979 (http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm260952.htm) |
| EFSA | EFSA approved in subcategory of binders, anticaking agents, and coagulants E 599 Panel on Additives and Products (http://www.efsa.europa.eu/en/search/doc/68e.pdf). |
| OELs for selected countries | OSHA PNOR: 15 mg/m3 (total dust), 5 mg/m3 (respirable fraction) 8-h TWA NIOSH REL: 10 mg/m3 (total dust), 5 mg/m3 (respirable fraction) 8-h TWA ACGIH TLV: PNOS 10 mg/m3 (inhalable particles), 3 mg/m3 (respirable fraction) 8-h TWA Australia: 10 mg/m3, 8-h TWA Belgium: 10 mg/m3 (inhalable particles), 2 mg/m3 (respirable fraction) 8-h TWA Canada (Ontario, British Columbia): 10 mg/m3, 8-h TWA, http://www.e-laws.gov.on.ca/html/regs/english/elaws_regs_900833_e.htm and http://www2.worksafebc.com/publications/ohsregulation/GuidelinePart5.asp?ReportID = 32895. China: 8 mg/m3 (total dust), 4 mg/m3 (respirable fraction) 8-h TWA (Liang et al., 2003) Denmark: 10 mg/m3 (inhalable particles), 5 mg/m3 (respirable fraction) 8-h TWA Greece: 10 mg/m3 (inhalable particles), 5 mg/m3 (respirable fraction) 8-h TWA Ireland: 10 mg/m3 (inhalable particles), 5 mg/m3 (respirable fraction) 8-h TWAa Netherlands: 10 mg/m3, 8-h TWA, as inhalable dust Spain: 10 mg/m3 (inhalable particles), 3 mg/m3 (respirable fraction) 8-h TWAb UK: 10 mg/m3 (inhalable particles), 4 mg/m3 (respirable fraction) 8-h TWA |
| Potentially exposed cohort in the US | Total from NOES survey 215,854 persons in 1981–1983, see http://www.cdc.gov/noes/noes1/b0105sic.html. |
| Production (US) | Domestic production 424 000 metric tons; apparent consumption 557 000 metric tons (Bolen, 2013) |
| Major producing locations in US | The processed crude perlite was mined in eight mines located in Arizona, Idaho, Nevada, New Mexico, and Oregon (Bolen, 2012). Perlite was expanded at 50 plants located in 27 states (Bolen, 2013). |
From a regulatory standpoint, perlite is regarded as what was formerly termed a “nuisance dust” (now particulates not elsewhere regulated [PNOR]) by OSHA (a conclusion also reached more recently by the Health Council of the Netherlands (2003). In the United States, the applicable OSHA permissible exposure limit (PEL) is 15 mg/m3 (total dust), 5 mg/m3 (respirable fraction) 8-h time weighted average (TWA). (There is no applicable short-term exposure limit.) The current administrative occupational exposure limit for perlite in the Netherlands is 10 mg/m3 8-h TWA, as inhalable dust (Health Council of the Netherlands, 2003), a health-based recommended occupational exposure limit (HBROEL) that was supported by a no observed adverse effect level (NOAEL) derived from animal testing. Applicable OELs in several other countries are shown in Table 2.
It should be noted that exposure to any dust at levels significantly above the designated occupational exposure limit might result in adverse health effects. And, as written by Elmes (1987) in discussing “nuisance dusts”, “There is, of course, no scientific basis for the concept that ‘nuisance’ dusts are completely safe or are only dangerous if they contain quartz”. What is correct is that compared to others, PNOR, as a class, are likely to exhibit fewer and less severe health effects than exposures to other dusts, particularly if exposures are well controlled.
Exposed population in the United States
USGS estimates that 92 persons were engaged in perlite mining in the United States in 2013 (W. Bolen, USGS personal communication, 26 November 2013, 703-648-7727). There are no published data on the employment in perlite expansion plants per se, but the average employment per plant in NAICS 327992 (which includes facilities engaged in ground or treated mineral and earth manufacturing) was approximately 25 in the 2007 census, which equates to approximately 1250 workers employed at domestic expansion plants using Bolen’s (2013) estimate for the number of expansion plants in the US (see Table 2).
Between 1981 and 1983 NIOSH conducted the National Occupational Exposure Survey (NOES) that collected data on potential occupational exposures to chemical, physical and biological agents. The estimated population exposed to perlite in the United States was 215 854 persons, which ranked 385th among the agents included in the NOES database (http://www.cdc.gov/noes/noes3/empl0001.html). This survey has not been updated. Thus, it is clear that the majority of the perlite-exposed workers are employed by end-users.
Occupational exposures
There are only very limited published data available on workplace exposures in mining and expanding plants (see below) and only one study (Breum et al., 2003) that addresses end-user exposures. The Breum et al. (2003) study examines simulated perlite exposures during installation of loose fill building insulation. Exposures in this study were measured for only brief (8–10 min) periods (not 8-h TWAs) and (in the case of perlite) for only four samples; sample results for installation of attic insulation; installer (inhalable dust geometric mean (GM) 160 mg/m3) and helper (inhalable dust GM 4.5 mg/m3) and for insulation in the wall, installer (inhalable dust GM 98 mg/m3) and helper (inhalable dust GM 4.9 mg/m3). The authors noted that use of respirators would be required.
Health effects
Our knowledge of the industry permitted us to identify many relevant unpublished studies. Additionally, we performed an extensive online literature search to identify relevant studies of humans and laboratory animals. These are discussed below.
Studies of human populations occupationally exposed to perlite
There have been several studies of the health effects of occupational exposure to perlite. For the most part these studies have concluded that exposure to perlite results in similar effects to those resulting from exposure to most inert insoluble dusts.
One long-standing series of medical surveillance studies was sponsored by the Perlite Institute on behalf of several perlite producers (mines and expanding plants) in the United States (Cooper, 1975, 1976, 1980; Cooper & Sargent, 1986; Weill, 1990, 1994):
Cooper (1975) performed a radiographic survey of workers from 10 facilities (including mines and expansion plants) in Western US states. The available data did not permit a reconstruction of an exposure history for the workers studied, but limited data suggested that at least some workers were exposed to dust levels above the applicable OEL. Records were available for 285 men (100 in mines and 185 in expanding plants), including both current and past employees (“leavers”). Of these, X-ray films were available for 240 of the 285 workers with job tenures ranging from 1 to 23 years. The study found no individuals with definite X-ray evidence of pneumoconiosis in the cohort other than two workers with prior histories of working with diatomaceous earth. The author concluded “These results support the position that perlite does not produce pneumoconiosis” but cautioned “since no individuals had exposure for more than 25 years and, since some crude perlite ores contain as much as 6% crystalline silica (i.e. quartz), it is prudent to keep exposures at or below nuisance dust levels, and to maintain medical surveillance”. No data were provided on the respirable crystalline silica content of the ores, however.
Subsequently, Cooper (1976) studied the pulmonary function of workers with one or more years working at three plants in the San Louis valley of northern New Mexico and southern Colorado, one of which had a collocated expansion facility and the other two operated perlite mines. Among 135 eligible workers, pulmonary function was studied in 117 (87%); those not sampled included six workers on vacation, four with shift schedules that did not permit testing, three men on medical leave, two with illnesses in their families and three who refused the examination. The occupational tenure of those studied ranged from 1 to 23 years with an average of 7.9 years. Review of the chest X-rays confirmed the findings from previous study which showed no changes indicative of pneumoconiosis. Measurement of forced vital capacity (FVC) did not show reductions correlated with length of exposure, after effects of cigarette smoking had been taken into account. The study results indicated that there were slight but not statistically significant reductions in forced expiratory volume in one second (FEV1) and in FEV1/FVC, which were associated with years of employment. The author concluded that even though the data showed no evidence of pneumoconiosis by chest radiography or FVC, it was prudent to control exposures and to continue medical surveillance.
In 1983, Cooper & Sargent (1986) examined chest films from 152 workers who had been employed five or more years in perlite mining and processing – 42 of whom had worked in the industry more than 15 years and 19 more than 20 years. Nearly all of these workers were exposed to dust levels beneath the OEL, but some (those engaged in bagging of expanded perlite) were exposed to dust levels above the OEL. However, the data did not permit estimation of cumulative dust exposure. The authors concluded: “This review of 152 chest films of perlite workers with over 5 years of employment gave no indication that any workers are developing pneumoconiosis. There were no films with small opacities of profusion greater than 0/1”. Measurements of pulmonary function in 122 perlite workers showed a small reduction in FVC that correlated with employment duration, but stepwise regression analysis did not show this to be statistically significant. Rather the authors concluded “the most important factor influencing pulmonary function in perlite workers is cigarette smoking. This was supported by regression analysis and by comparing tests 8 years apart in 66 men with differing smoking patterns”.
In 1980, Cooper summarized earlier findings in an EPA workshop on substitutes for asbestos and indicated that preliminary indications of ongoing studies supported earlier findings that there was “no evidence of a systematic reduction of function associated with perlite exposure” (Cooper, 1980). Elmes (1987) concluded that the Cooper studies of perlite-exposed workers confirmed that “prolonged exposure produces little if any X-ray change or loss of lung function”.
Weill (1990, 1994) wrote reports (unpublished in the peer-reviewed literature) that described continued studies (respiratory symptoms, pulmonary function, and chest X-rays) of workers exposed to perlite. The 1990 study included plants in New Mexico, Colorado, and an expanding facility in Illinois. Pulmonary function results were available for 132 workers (no exposure duration reported) that indicated normal, very mild, or mild abnormality in 131 workers and moderate abnormality in 1 worker. This study included an analysis of chest films in 147 workers, some of which Weill regarded as being of poor technical quality. Nonetheless, considering the available evidence, Weill concluded that there was no evidence of pneumoconiosis and “The working population is healthy, from a respiratory standpoint, not exhibiting undue respiratory symptoms, and having, on average, normal lung function”. The 1994 study included seven expanding plants and 89 workers with an average duration of 7 years with some exposed as long as 26 years. Average FEV1 and FVC (% of predicted) among male workers (95% of population), including smokers, were 100.6 and 104.2% predicted, respectively. Results for female workers (5% of total), including smokers, were 99.3 and 106.8% predicted for FEV1 and FVC, respectively. Although this cohort was relatively young with limited exposure duration, the author concluded “This survey provides substantial reassurance that the currently employed workforce has, to date, been free of any evidence of a silicosis risk, or, indeed, any measurable adverse respiratory effects of perlite exposure”.
Studies have also been conducted of workers occupationally exposed to perlite in plants in Turkey (Çalişir et al., 1989; Çok et al., 2003; Polatli et al., 1994, 2001; Uçan et al., 1986). One of the difficulties of evaluating studies of perlite workers in Turkey is the high percentage of smokers in the population. Cigarette smoking is associated with several adverse effects in the lungs including chronic inflammation typical of chronic bronchitis, structural damage as seen with emphysema, functional impairment resulting in obstructive lung disease, radiographic abnormalities including irregular opacities, and lung cancer. There is some controversy whether smoking is an independent risk factor for the presence of small irregular opacities and pneumoconiosis (see e.g. Blanc & Gamsu, 1988; Hessel et al., 2003; Weiss, 1991), but it is clear (and not surprising) that cigarette smoking is a confounding factor in any analysis of the effects of dust exposure.
The Uçan et al. (1986) study included only 27 workers exposed to perlite for only 4 years (see Polatli et al., 2001) and is of limited relevance.
The Çalişir et al. (1989) study included 53 perlite workers and noted that small airway obstruction was found; nonetheless, they concluded that the decrease in flow rate was associated with smoking because 70% of the workers had smoking histories up to 17.9 pack-years. 4
Polatli et al. (1994) studied 65 perlite workers and 13 office workers. In this study, results of PFTs were negatively correlated with cigarette smoking, but not with perlite exposure.
Polatli et al. (2001) studied 36 perlite exposed and 22 unexposed (office) workers (all smokers) at a perlite plant in Menderes near Izmir (activities not stated). They noted that respirable dust levels exceeded the OEL in the years under study, but because workers were rotated in the facility there was no method for estimating cumulative dust exposures. Four of the perlite-exposed workers were from the Cappadocia and Anatolia regions and might have been environmentally exposed to asbestos. Polatli et al. (2001) found that among non-smoking perlite-exposed workers all radiographs were normal. Among 27 perlite-exposed smokers, chest X-rays of three workers were interpreted as showing small rounded opacities (0/1 = doubtful) and 7 workers exhibited abnormalities (tuberculosis in three, diaphragmatic pleural calcification in two, and probable asbestosis in two, which the authors believed was due to environmental asbestos exposure). The authors concluded that 12-year perlite exposure did not lead to a decrease in mean pulmonary function tests nor exhibit any correlation between PFTs and duration of work in perlite areas. However, they noted that there was “a tendency to decline in the transfer factor for carbon monoxide (TL, CO or diffusion capacity) in the 4-year study period, which might be due to high perlite dust levels”. The study is of interest in suggesting that measurements of the transfer coefficient might provide improved detection of the early effects of perlite exposure. The fact that dust levels exceeded the OEL makes it difficult to assess the likely health effects in a well-controlled workplace. This study was evaluated by the Health Council of the Netherlands (2003), which found no reason to revise the perlite OEL.
Çok et al. (2003) studied 99 workers in a perlite processing facility in Izmir, Turkey. Among 22 workers those with a lowered transfer coefficient, there was evidence of reduced FVC and FEV1, but all were smokers with a mean 22.1 (±9.3) pack-years. The 22 workers with reduced transfer coefficient had a longer tenure (16.6 ± 6.6 years compared to 11.2 ± 4.8 years), but no estimates of cumulative exposure were reported.
As of this writing there are no other published long-term studies of the effects of occupational exposure to perlite. The above studies of workers occupationally exposed to both perlite ore and expanded perlite, including some that were exposed to perlite dust at levels above the present OEL provide strong evidence that the health effects of occupational exposure to perlite dust are relatively minimal; little (if any) pneumoconiosis, decrease in lung function, or respiratory symptoms.
Du et al. (2010) examined the acute effects of perlite exposure on workers in a plant located in Taiwan following an accidental spill and explosion, which released perlite insulation. Among 24 exposed workers followed for more than 6 months, three developed persistent respiratory symptoms compatible with reactive airway dysfunction syndrome (RADS). During a simulation experiment designed to replicate possible perlite exposures following the accident, the average air concentration was estimated to be in the range of 191 to 4150 mg/m3. Although the exposure was brief, the level was orders of magnitude higher than any applicable OEL.
Studies of populations environmentally exposed to perlite
Sampatakakis et al. (2013) conducted a mortality and morbidity study of the permanent resident population of the island of Milos, Greece. The island has a long history of mining for various minerals including barite, bentonite, kaolin, manganese, obsidian, perlite, pozzolan, sulfur and zeolites (Economopoulos, 1998; Tzintzos, 2013). Today, mines on this island are major sources of bentonite, perlite and pozzolan. The authors sought to determine whether or not environmental exposure to various mining dusts (particularly, but not exclusively bentonite and perlite) might affect the respiratory health of residents of this island.
Mortality study results
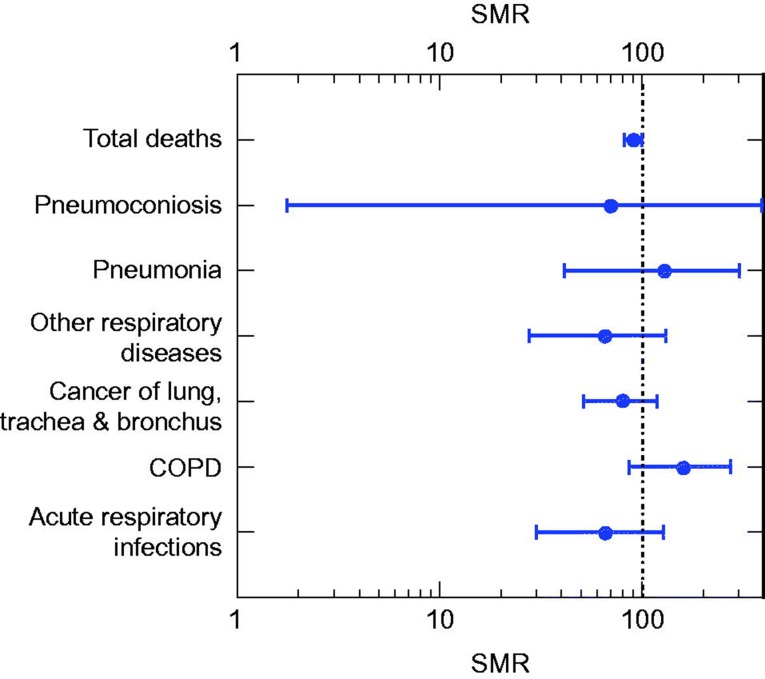
Deaths (in total, and associated with acute respiratory infections, pneumonia, chronic obstructive pulmonary disease (COPD), pneumoconiosis, cancer of the lung, trachea, and bronchus, and other diseases of the respiratory system) on Milos over the period 1999–2009 were compared to deaths from these same causes in the Cyclades Prefecture (a former administrative prefecture of Greece in which the island of Milos was located). Standard mortality ratios (SMRs) and associated confidence intervals were computed. Figure 2 shows the estimated SMRs and 95% confidence intervals by cause of death for men and women combined for the period 1999–2009.
Figure 2.
Estimated SMRs and 95% confidence intervals by cause of death for men and women of Milos, Greece, combined for the period 1999–2009. Source: Data presented in Sampatakakis et al., 2013.
Over the period 1999–2009 there were deficits (i.e. SMRs <100) in mortality for total deaths, and those due to acute respiratory infections, pneumoconiosis, cancer of the lung, trachea, and bronchus, and other diseases of the respiratory system. SMRs for pneumonia and COPD exceeded 100, but were not statistically significant. Moreover, the strength of the association for these two causes of death with elevated SMRs would be termed “weak” compared to the threshold suggested by Wynder (1987). 5
The investigators also calculated SMRs for the total of respiratory deaths for other time periods and observed an excess for the period 1989–1998. Then for 1999–2009, on a year-by-year basis, there was a decreasing trend in the SMR for total respiratory deaths, which the authors claimed might be related to improvements in the mining-extraction process 6 or to an easier access to health care services. (Statistics from the Greek Mining Association indicate that production of both bentonite and perlite increased substantially over the years from 1990 to 2008). In any event, the SMR for total respiratory deaths in recent years (1999 forward) has not been significantly elevated.
Morbidity study results
For the morbidity study the authors selected (sampling procedure critical but not specified) a sample of 269 persons residing on the island of Milos along with 1811 persons in the municipality of Oinofita for comparison. Oinofita is described by the authors as an industrial region housing chemical production, detergents, pesticides, pharmaceuticals, leather production, aluminum, and food production. Whether this municipality is appropriate for comparison is questionable; other studies (e.g. Linos et al., 2011) indicate that there is elevated cancer of several sites due to oral ingestion of contaminated (hexavalent chromium) drinking water.
The study team administered a questionnaire to residents of Milos and Oinofita supplemented with face-to-face interviews that reportedly covered such topics as demographic and socioeconomic characteristics, smoking habits, and whether or not the respondent had ever been diagnosed by a medical doctor with specific system diseases (ICD9 7 range: 460–519) or lung cancer (ICD9 162). Based upon responses to the questionnaire, the authors computed odds ratios (ORs), both raw and corrected for gender, age, and smoking using logistic regression. Corrected ORs for allergic rhinitis (2.24, 95% CI [1.2, 4.39]), pneumonia (5.47, [2.73, 10.97]), and COPD (2.28, [1.11, 4.66]) were elevated and statistically significant. ORs for asthma, respiratory failure, and cancer of the lung, trachea, and bronchus, were not significantly elevated. The significance of the results of the mortality and morbidity studies is addressed in the discussion section.
Another study
Bania et al. (2013) also reported results of a health surveillance study of patients in the Milos (Greece) Health Centre. The authors collected data (demographics, risk factors, possible occupational exposure, COPD, and FVC and FEV1) on 181 patients. This study concluded that 5.5% of patients had COPD. Among these 77.9% had no occupational exposure of perlite, bentonite, kaolin, or asbestos. The authors also concluded that the incidence of COPD was independent of occupational exposure to minerals among the 22.1% occupationally exposed.
Results of the mortality study of residents of Milos, Greece, did not reveal any significantly elevated SMRs. Results of a companion morbidity study of residents of Milos, Greece, are suggestive and cautionary, but it is not possible to ascribe the observed effects to specific mining activities or emissions (such as those related to perlite production) without further research. Moreover, these results are inconsistent with another recent study as discussed below.
Animal study results
The toxicity of perlite has been investigated in studies of mice, rats, and guinea pigs exposed via intratracheal administration and inhalation. Table 3 summarizes the available studies by pathway and date. The Health Council of the Netherlands (2003) reviewed these studies and concluded:
“Based on the above data, the committee takes the NOAEL of 226 mg/m3 in rats or guinea pigs (18-month inhalation study) as a starting point in deriving a HBROEL. For extrapolation to a HBROEL, an overall assessment factor of 9 is established. This factor covers the following aspects: intra- and interspecies variation. Applying this factor and the preferred value approach would lead to a health-based occupational exposure limit of 20 mg/m3, which is still higher than the usual occupational exposure limit for nuisance dust (10 mg/m3)”.
Table 3.
A summary of animal studies conducted with perlite.
| Pathway | Species | Results | Source |
|---|---|---|---|
| Inhalation | Guinea pig & rat | In an 18-month inhalation study, guinea pigs and rats (numbers, strain, or sex not given) were exposed to perlite dust at a concentration of 226 mg/m3. No significant pulmonary reaction, including fibrosis, was observed. No further details were given. The authors concluded that perlite acted as an inert or nuisance dust. | Vorwald, 1953, cited in Cooper, 1975 and Health Council of the Netherlands, 2003. |
| Inhalation | Guinea pig | Inhalation exposure of 284 mg/m3, 8 h/d, 5.5 d/wk. for six months “appeared to stimulate the progression of experimental tuberculosis”. When exposure ceased the tuberculosis infection progressed for about eight months and then began to heal and ultimately was arrested. | Schepers, 1955 as reported in Cooper, 1975. |
| Inhalation | Guinea pig | Guinea pigs were exposed to perlite dust at mean airborne concentrations of 0 or 6.6 mg/ml for 30 min/d, 5 d/wk, for 24 wk. Another group was exposed to fir bark dust. Perlite mass median aerodynamic diameters (MMADs) ranged from 1.7 to 1.9 µm. No pulmonary fibrosis or extensive destruction of parenchymal tissues was observed, but the authors con noted “moderate to severe” changes in the lungs for both dusts. The authors concluded that perlite particles were more than just a nuisance dust. | McMichael et al., 1978, 1983. The reported dose might be a misprint, but is extremely high. Health Council of the Netherlands converted this to 6,600 mg/m3 and noted it was unrealistically high. |
| Intratracheal injection | Guinea pig | Nine perlite products were tested in guinea pigs, by weekly intratracheal injection of 0.5 ml of a 5% perlite suspension in saline for 3 wk. At 4 to 12 months after the last injection, no evidence of pulmonary fibrosis was shown. | Vorwald, 1953, cited in Cooper 1975. |
| Intratracheal instillation | Rat | A single intratracheal instillation of a 75 mg/ml saline dose of perlite (18–30% quartz) produced a “foreign body reaction” in white male rats, but no pulmonary fibrosis was observed (post observation period not reported). | Timar et al., 1965 as cited in Health Council of the Netherlands, 2003. |
| Intratracheal instillation | Rat | A single 50 mg suspension of crude or expanded was administered intratracheally to albino rats. Nine months later, it was found that expanded perlite caused more lung fibrosis than crude perlite, but the degree of fibrosis was not reported. | Borshchevkii et al., 1967 as cited in Health Council of the Netherlands, 2003. |
| Intratracheal instillation | Rat | Rats given a single intratracheal instillation of 50 mg perlite dust showed pulmonary fibrosis at 12 to 18 months following administration. The perlite composition was not specified. | Liu et al., 1988, as cited in Health Council of the Netherlands, 2003. |
| Intratracheal instillation | Rat | Intratracheal infusing (instillation) of 5 mg perlite dust in 5% alcohol did not result in a strong pulmonary fibrogenic reaction in rats, at 12 wk after administration. No more details were provided. | Ueda et al., 1978 as cited in Health Council of the Netherlands, 2003. |
| Acute oral toxicity | Rat | Acute oral toxicity tests in rats ingesting perlite were performed by the Rosner-Hixon Laboratories in 1977. The results of the tests showed that the acute oral LD50 of perlites for rats is greater than the largest dose administered in the test (10 g/kg body weight). | Schundler Company, 2002, http://www.schundler.com/perlitehealth.htm. |
| Acute oral toxicity | Rat | In 1982, the WIL Research Laboratories conducted studies to examine the acute oral toxicity, if any, of agglomerated filter aid in albino rats. The study showed no signs of systemic toxicity, and concluded that the LD50 was greater than the highest dose level administered to the rats (10 g/kg body weight.) | Schundler Company, 2002, http://www.schundler.com/perlitehealth.htm. |
| Oral ingestion | Mouse | Ohkuma et al. (1972) and Itoh et al. (1981) performed acute toxicity studies using dd/y and C3H/He mice. The results showed no acute toxicity despite large doses. The estimated LD50 was 12 960 mg/kg or more in each case. | Cited in Sakai & Nagao, 1985. |
| Oral ingestion | Mouse | Groups of male and female mice were given diets containing 0%, 1%, 10%, 20% perlite for 28 wk. Appearance, behavior, and food consumption of mice of treated groups were not affected during the experimental period. | Sakai & Nagao, 1985. |
The inhalation pathway is most relevant biologically. Among these, the Vorwald (1953) and Schepers (1955) studies are most relevant. The Vorwald (1953) study did not result in any significant pulmonary reaction, including fibrosis. The Schepers (1955) study resulted in apparently reversible effects. The McMichael et al. (1978, 1983) studies failed to disclose any fibrosis or “extensive destruction of parenchymal tissues”, but did result in “moderate to severe” changes in the lungs of guinea pigs exposed to perlite. However, the doses used in the McMichael studies were exceptionally high and well above any OEL. Significantly the Health Council of the Netherlands (2003) did not base any risk calculations on the McMichael studies.
Reproductive toxicity
We could find no published studies on reproductive toxicity of perlite. However, in view of the likely routes of exposure, no such effect is likely.
Mutagenicity and genotoxicity
We could find no published studies on perlite mutagenicity or genotoxicity.
Irritation and sensitization
The Health Council of the Netherlands (2003) found no published studies on irritation or sensitization associated with perlite exposure. More recently, Dracheva et al. (2012) examined the effects of dermal contact of “perlite sand” on male Sprague–Dawley rats. Exposed rats developed skin irritation, which prompted the study authors to recommend that workers protect themselves from this hazard. Most safety data sheets (SDS) indicate that skin and eye irritation are possible as is respiratory tract irritation.
Discussion
Occupationally exposed populations
As noted above, there are a substantial number of well-conducted studies of workers occupationally exposed to both perlite ore and expanded perlite, including some that were exposed to perlite dust at levels above the present OEL. Collectively, these provide strong evidence that the health effects of occupational exposure to perlite dust are relatively minimal; little (if any) pneumoconiosis, decrease in lung function, or respiratory symptoms. To be sure, these occupational studies have limitations, such as the confounding effects of cigarette smoking (particularly in the Turkish studies), small sample sizes, inadequate exposure assessment, and limited exposure durations in other studies. Nonetheless, these studies are consistent with the conclusion that perlite should be regarded as a particulate not elsewhere regulated.
Environmentally exposed population
The mortality study of residents of Milos, Greece over the period from 1999 to 2009 is properly viewed as negative, with only weak and not statistically significant increases in SMR for pneumonia and COPD and deficits (i.e. SMRs <100) in mortality from other causes including total deaths, acute respiratory infections, pneumonociosis, cancer of the lung, trachea, and bronchus, and other diseases of the respiratory system.
Results of the morbidity study of residents of Milos, Greece, are suggestive and cautionary, but it is not possible to ascribe the observed effects to specific mining activities or emissions (such as those related to perlite production) without further research:
Over the years, residents of Milos were exposed to dusts from several different mining operations as well as geothermal sources of SO2 and H2S and the study did not attempt to quantify exposure to various mineral dusts or other agents that might cause or contribute to respiratory distress. No monitoring data are presented by Sampatakakis et al. (2013) perhaps because no ambient monitoring data were available, a significant limitation.
Several of the endpoints measured in the morbidity study may have many other causes or contributing factors. For example, allergic rhinitis (one of the endpoints with an elevated OR) is an allergic inflammation of the nasal airways that occurs when an allergen, such as pollen, dust or animal dander (particles of shed skin and hair) is inhaled by an individual with a sensitized immune system. Mineral dusts could cause or exacerbate allergic rhinitis, but so too could tree or grass pollen, mold spores, house mites, and work as a grape farmer (see e.g. Chatzi et al., 2005; Gioulekas et al., 2004; Papageorgiou, 1999). Though Milos is relatively barren, orange, olive, cypress, tamarisk, juniper, and arbutus trees grow on the island and a vineyard is also located there.
It is known that there are issues with interpreting weak effects from epidemiological studies (see e.g. Boffetta, 2010; Boffetta et al., 2008; Grimes & Schultz, 2002). Only the OR for pneumonia exceeded the threshold for a weak effect.
Although several bentonite and perlite mines and associated facilities are located on Milos, so too are other mineral mines, such as pozzolan mines. Kaolin mining has taken place episodically on Milos although production quantities have been small in comparison to bentonite or perlite. A World Health Organization (2005) study on various clay materials offered this conclusion regarding kaolin: “Kaolin produces a specific pneumoconiosis, known as kaolinosis. Its fibrogenic potential is considered to be at least an order of magnitude less than that of quartz. Specific exposure limits should be set, and kaolin should not be considered an inert (nuisance) dust”. Thus, it is possible that coexposures to kaolin might have contributed to the morbidity results. WHO did not evaluate perlite, but offered the following conclusion regarding bentonite: “With regard to bentonite, a comparable montmorillonite pneumoconiosis has not been consistently reported. Based on its surface chemistry, lack of fibrogenicity in experimental systems, and limited human findings, inhaled bentonite is likely to be less dangerous to humans than kaolin”.
Moreover, there is exposure to yet other agents on Milos. D’Alessandro et al. (2013) measured H2S concentrations at Adamas, Fyriplaka, and Paleochori (geothermal areas on Milos) with results ranging from 1.3 to 49 µg/m3. Although these concentrations are well beneath OELs for this chemical, the USEPA Toxicological Review of Hydrogen Sulfide (USEPA, 2003) notes “the daily inhalation exposure [of H2S] to the human population that is likely to be without an appreciable risk of deleterious effects during a lifetime, the RfC, has been determined to be 0.002 mg/m3 [2 µg/m3] or 1.4 ppb”, which is lower than several of the measured H2S concentrations. [Material in square brackets inserted for clarity.] D’Alessandro et al. (2013) concluded that the H2S risk to tourists on Milos was negligible, but the exposure duration for tourists is very much lower 8 than that for residents. The RfC determined by EPA was based on a study of Sprague–Dawley rats leading to nasal lesions of the olfactory mucosa, but the respiratory system (along with the neurological system) has been reported to be a target organ by several researchers and impairments of lung function have been reported in studies of humans exposed to H2S.
We do not claim that any adverse pulmonary effects in permanent residents of Milos were caused by bentonite, H2S, kaolin, or any other of the materials to which this population was coexposed. Rather, it is important to note that (lacking additional data) it is not possible to ascribe any of the effects to any single agent or to use the results of the morbidity study to derive a risk-based exposure limit for perlite.
Finally, the reported results of the morbidity study are inconsistent with the negative results of several studies of workers occupationally exposed to perlite. Although environmental exposure occurs 24 h per day for 7 d per week whereas occupational exposure occurs only during work hours, the mining dust concentrations in the workplace are probably significantly higher than environmental exposures. However, the occupational morbidity studies examined X-rays and pulmonary mechanics while the environmental study looked at symptoms and clinical outcomes. The morbidity study speaks to a need for ongoing surveillance of the worker population.
Animal studies
The animal studies are relatively limited in terms of assessment of the potential toxicity of perlite. Most are short-term and/or feature not physiologically relevant routes of exposure (e.g. intratracheal instillation rather than inhalation). As noted above, the Health Council of the Netherlands (2003) concluded that that the 18-month inhalation study of rats and guinea pigs was appropriate for deriving an NOAEL and associated HBROEL, although this study is relatively old and was not published in a peer-reviewed journal.
Exposure data
There is relatively little published exposure data. It is likely that mines and expanding plants collect such data, but these data have not been published in the peer-reviewed literature, a notable gap.
Concluding comments
On balance, we conclude that the available toxicology and epidemiology data indicate that occupational exposure to perlite entails risks comparable to exposure to other inert insoluble (“nuisance”) dusts. Companies should continue to use available means (engineering controls, workplace practices, and personal protective equipment) to ensure that workplace exposures are in compliance with applicable OELs. Continued medical surveillance of occupationally exposed cohorts is also a reasonable precaution. Special attention needs to be paid to monitor and control any exposures to respirable crystalline silica associated with any minerals mined. This review also underscores the benefits of smoking cessation programs for perlite workers.
There is only limited information on the possible effects of no occupational exposure to perlite. A mortality study of residents of Milos, Greece was negative. A companion morbidity study showed elevated ORs for allergic rhinitis, pneumonia, and COPD, but residents were co-exposed to other mining dusts and no environmental monitoring data were provided, so it is not possible to use this study to estimate possible risks from perlite exposure. Another recent morbidity study of patients of the Milos Health Centre found no correlation between occupational exposure to minerals and COPD.
Acknowledgements
We appreciate the insightful comments of Dr. Mark J. Utell (University of Rochester School of Medicine and Dentistry, Rochester, New York, NY) on an earlier draft of this article. We also appreciate the assistance of Bill Mihalopoulos in providing copies of some of the unpublished studies referenced in this article and Michalis Stefanakis for providing the Bania et al. reference and for useful comments on perlite production technology. It is also appropriate to acknowledge the constructive comments of the anonymous reviewers of this manuscript in draft. Their comments were useful and improved the quality of this work. The conclusions are those of the authors and do not necessarily represent the views of S&B Minerals or subsidiary companies.
Footnotes
1Parting is separation along a structural plane in minerals. In the case of onionskin parting, the mineral fractures along rounded “shells” that resemble onion skin peels.
2This is the rock or soil overlying the mineral deposit.
3This is the overall range among all grades, not the range for any particular grade.
4A pack-year is equivalent to smoking one pack of cigarettes per day for 1 year. This is a conventional measure used in epidemiological studies of the amount a person has smoked over a long period.
5Wynder (1987) wrote “The important point to note, however, is that the closer the risk of some association comes to unity (i.e. an SMR of 100), the more likely it is that choice of the comparison standard, bias, confounding, or inappropriate analysis may explain it and the greater the need for thorough understanding of the underlying biological mechanisms”.
6We were unable to find published data on occupational or ambient exposures to mining dusts at Milos. There is evidence that occupational exposures to nuisance dusts have decreased over the years in some countries (see e.g. Creely et al., 2007), but no data for Milos.
7International Classification of Diseases, 9th revision.
8We could not find data specific to Milos, but according to the UNCTAD Handbook of Statistics, the average length of stay of tourists in Greece in 2007 was 5.37 d (see http://www.nationsencyclopedia.com/WorldStats/UNCTAD-average-length-stay-visitors.html).
Declaration of interest
This work was sponsored by S&B Minerals Inc., which is a major producer of several industrial minerals including perlite. Among many other mining activities, S&B operates bentonite and perlite mines on the island of Milos. Drs Maxim and McConnell are external members of a product stewardship committee for S&B.
References
- Aminifard S, Jamalzadeh H, Biazar E, Fouladi M. Effect of temperature and pH in adsorption of Pb+2 ions by porous perlite clay. Oriental J Chem. 2011;27:1397–401. [Google Scholar]
- Austin GS, Barker JM. (1998). Commercial perlite deposits of New Mexico and North America. New Mexico geological society guidebook, 49th field conference, Las Cruces Country, II, 271–8.
- Baker JM, Santini K. (2006). Perlite. In: Kogel JE, Trivedi NC, Barker JM, Krukowski ST. (eds.) Industrial minerals and rocks, 7th ed. Littleton, CO: Society for Mining, Metallurgy, and Exploration, 685–702.
- Bania E, Apostolopoulos I, Kyritsi Z, et al. (2013). Prevalence of chronic obstructive pulmonary disease and correlation with occupational exposure on Milos Island. Presentation at the 22nd Hellenic Conference of the Hellenic Thoracic Society; 2013 Dec 5–7; Athens, Greece.
- Blanc PD, Gamsu G. The effect of cigarette smoking on the detection of small radiographic opacities in inorganic dust diseases. J Thorac Imaging. 1988;3:51–16. doi: 10.1097/00005382-198810000-00008. [DOI] [PubMed] [Google Scholar]
- Boffetta P. Causations in the presence of weak associations. Crit Rev Food Sci Nutr. 2010;50:13–6. [Google Scholar]
- Boffetta P, McLaughlin JK, Vecchia CL, et al. False-positive results in cancer epidemiology: a plea for epistemological modesty. J Natl Cancer Inst. 2008;100:988–95. doi: 10.1093/jnci/djn191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen W. (2012). Perlite (Advanced Release). U.S. Geological Survey 2012 Minerals Yearbook. Washington, DC: USGS, 55.0–55.4.
- Bolen W. (2013). Perlite. U.S. Geological Survey, Mineral Commodity Summaries, January 2013. Washington, DC: USGS, 116–17.
- Borshchevkii YM, Oksova EE, Retnev VM. Investigation into fibrogenic properties of the dust of silicate-containing materials as related to the technology of their manufacture. Article in Russian. Gig Tr Prof Zabol. 1967;11:27–30. [PubMed] [Google Scholar]
- Breum NO, Schneider T, Jørgensen O, et al. Cellulosic building insulation versus mineral wool, fiberglass or perlite: installer’s exposure by inhalation of fibers, dust, endotoxin and fire-retardant additives. Ann Occup Hyg. 2003;47:653–69. doi: 10.1093/annhyg/meg090. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Shaw RA, Bide T, et al. British Geological Survey. (2013). World Mineral production 2007–2011. Nottingham: Keyworth, 85 p.
- Çalişir HC, Tufan M, Erdinç E, et al. Perlit tozuna maruz kalan işçilerde solunum fonksiyon testleri ve silikozis riski. Solunum. 1989;14:173–80. [Google Scholar]
- Chatzi L, Prokopakis E, Tzanakis N, et al. Allergic rhinitis, asthma, and atopy among grape farmers in a rural population in Crete, Greece. Chest. 2005;127:372–8. doi: 10.1378/chest.127.1.372. [DOI] [PubMed] [Google Scholar]
- Çok G, Erdinç E, Erdinç E. Mesleki perlit maruziyetinde bronkoalveoler lavaj sıvısı ve difüzyon kapasitesi sonuçları. Solanum Hastaliklari. 2003;14:165–8. [Google Scholar]
- Collins LZ, Naeeni M, Schäfer F, et al. The effect of a calcium carbonate/perlite toothpaste on the removal of extrinsic tooth stain in two weeks. Int Dental J. 2005;55:179–82. doi: 10.1111/j.1875-595x.2005.tb00056.x. [DOI] [PubMed] [Google Scholar]
- Coombs HA. (1952). Spherulitic breccias in a dome near Wenatchee, Washington. Am Minralog 37:197–206. Available from: http://www.minsocam.org/ammin/am37/am37_197.pdf. [Last accessed: 26 Nov 2013]
- Cooper WC. Radiograph survey of perlite workers. J Occup Med. 1975;17:304–9. [PubMed] [Google Scholar]
- Cooper WC. Pulmonary function of perlite workers. J Occup Med. 1976;18:723–9. doi: 10.1097/00043764-197611000-00006. [DOI] [PubMed] [Google Scholar]
- Cooper WC. (1980). Health considerations in the perlite industry, a chapter in USEPA (1980), Office of Pesticides and Toxic Substances, Proceedings of the National Workshop on Substitutes for Asbestos, EPA 560/3-80-001, Washington, DC.
- Cooper WC, Sargent EN. Study of chest radiographs and pulmonary ventilatory function in perlite workers. J Occup Med. 1986;28:199–206. [PubMed] [Google Scholar]
- Creely KS, Cowie H, Van Tongeren M, et al. Trends in inhalation exposure – review of the data in the published scientific literature. Ann Occup Hyg. 2007;8:665–78. doi: 10.1093/annhyg/mem050. [DOI] [PubMed] [Google Scholar]
- Cros CJ, Morrison GC, Siegel JA, Corsi RL. Long-term performance of passive materials for removal of ozone from indoor air. Indoor Air. 2011;22:43–53. doi: 10.1111/j.1600-0668.2011.00734.x. [DOI] [PubMed] [Google Scholar]
- D’Alessandro W, Aiuppa W, Bellomo S, et al. Sulphur-gas concentrations in volcanic and geothermal areas in Italy and Greece: characterizing potential human exposures and risks. J Geochem Explor. 2013;131:1–13. [Google Scholar]
- Doğan M, Alkan M. Some physicochemical properties of perlite as an absorbent. Fres Environ Bull. 2004;13:251–7. [Google Scholar]
- Dracheva EE, Iatsyna IV, Lapina NE, et al. (2012). Influence of perlite sand on the skin in experiment. Met Tr Prom Ekal 3:30–4. [PubMed]
- Du C-L, Wand J-D, Chu P-C, Leon YL. Acute expanded perlite exposure with persistent reactive airway dysfunction syndrome. Ind Health. 2010;49:119–22. doi: 10.2486/indhealth.48.119. [DOI] [PubMed] [Google Scholar]
- Duchstein S. (1982). Casting perlite before the swine, US Patent 4310552 A.
- Economopoulos JN. (1998). The mining history of the island of Milos. Online document published by the Milos Mining Museum, Milos, Greece, 4 p. Available from: http://www.milosminingmuseum.com/wp-content/uploads/2011/11/mining_history_en1.pdf. [Last accessed: 25 Nov 2013]
- Elmes PC. Perlite and other ‘nuisance’ dusts. J R Soc Med. 1987;80:403–4. doi: 10.1177/014107688708000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis DJ. (2011). Perlite mining and reclamation in the no aqua peaks, Taos County, New Mexico. New Mexico Geological Society Guidebook, 62nd Field Conference, Geology of the Tusas Mountains – Ojo Caliente, 409–18.
- Gioulekas D, Papkosta D, Damialis A, et al. Allergenic pollen records (15 years) and sensitization in patients with respirator allergy in Thessaloniki, Greece. Allergy. 2004;59:174–84. doi: 10.1046/j.1398-9995.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- Gironás J, Adriasola JM, Fernández B. Experimental analysis and modeling of a stormwater perlite filter. Water Environ Res. 2008;80:524–39. doi: 10.2175/193864708x267432. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Schultz KF. Bias and causal associations in observational research. LANCET. 2002;359:248–52. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- Health Council of the Netherlands. (2003). Perlite; health-based reassessment of administrative occupational exposure limits. Committee on Updating of Occupational Exposure Limits. The Hague: Health Council of the Netherlands, 2000/15OSH/086. Available from: http://www.gezondheidsraad.nl/en/publications/healthy-working-conditions/health-based-reassessment-administrative-occupational-exp-13. [Last accessed: 26 Nov 2013]
- Hessel PA, Gamble JF, Nocolich M. Relationship between silicosis and smoking. Scand J Work Environ Health. 2003;29:329–36. doi: 10.5271/sjweh.739. [DOI] [PubMed] [Google Scholar]
- Huntting MT. (1949). Report of Investigations, No. 17, Perlite and other volcanic glass occurrences in Washington. State of Washington Department of Conservation and Development, Division of Mines and Geology. Olympia, Washington, 78 p. Available from: http://www.dnr.wa.gov/publications/ger_ri17_perlite_volcanic_glass.pdf. [Last accessed: 26 Nov 2013]
- Huwig A, Freimund S, Käppeli O, Dutler H. Mycotoxin detoxification of animal feed by different adsorbents. Toxicol Lett. 2001;122:179–88. doi: 10.1016/s0378-4274(01)00360-5. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). (2012). A review of human carcinogens: arsenic, metals, fibres, and dusts. IARC Monograph 100 C. Lyon, France: IARC, 355–406. Available from: http://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C.pdf. [Last accessed: 26 Nov 2013]
- Itoh S, Matsuzawa E, Ohno T, et al. An experimental study of perlite 1. The result of acute toxicity study for water of perlite (turbid water with powder) in mice. Bull Coll Kokusai Gakuin. 1981;2:1–7. [Google Scholar]
- Jaster MC. Perlite resources of the United States. A contribution to economic geology. Geological Survey Bulleting 1027-1. Washington, DC: United States Government Printing Office; 1956. p. 33. [Google Scholar]
- Johannsen A. A descriptive petrography of igneous rocks. Chicago, IL: University of Chicago Press; 1939. p. 315. [Google Scholar]
- Kabra S, Katara S, Rani A. Characterization and study of Turkish perlite. Int J Innov Res Sci Eng Tech. 2013;2:4319–26. [Google Scholar]
- Kadey Jr. FL. (1983). Perlite. In: Lefond SJ (ed.) Industrial minerals and rocks, 5th ed. Littleton, CO: Society for Mining, Metallurgy, and Exploration, 571–7.
- Liang Y, Su Z, Wu W, et al. New trends in the development of occupational exposure limits for airborne chemicals in China. Reg Toxicol Pharm. 2003;38:112–23. doi: 10.1016/s0273-2300(03)00077-1. [DOI] [PubMed] [Google Scholar]
- Linos A, Petralias A, Christophi CA, et al. Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece – an ecological study. Environ Health. 2011;10:50–8. doi: 10.1186/1476-069X-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xiao X, Fang Y. (1988). Problems of labour health on preventing dust hazard in township industries. Proceedings of the VIIth International Pneumoconiosis Conference, Part II. Pittsburgh, Pennsylvania. Poster Session II, Abstracts, 1288–89. Available from: http://www.cdc.gov/niosh/pdfs/90-1082s.pdf. [Last accessed: 26 Nov 2013]
- Malakootian M, Jaafarzadeh N, Hossaini H. Efficiency of perlite as a low cost adsorbent applied to removal of Pb and Cd from paint industry effluent. Desalin Water Treat. 2011;26:243–9. [Google Scholar]
- McMichael RF, DiPalma JR, Blumenstein R, et al. A small animal model study of perlite and fir bark dust on guinea pig lungs. J Pharmacol Methods. 1983;9:209–18. doi: 10.1016/0160-5402(83)90040-2. [DOI] [PubMed] [Google Scholar]
- McMichael RF, DiPalma JR, Amenta PS. Comparative effects of perlite and fir bark dust on guinea pig lungs. Fed Proc. 1978;37:712. doi: 10.1016/0160-5402(83)90040-2. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH). (2002). Health effects of occupational exposure to respirable crystalline silica. Department of Health and Human Services, Centers for Disease Control Prevention, National Institute for Occupational Safety and Health, Atlanta, Georgia, 145 p.
- Noh JH, Boles JR. Diagenetic alternation of perlite in the guryongp area, Republic of Korea. Clays Clay Min. 1989;37:47–58. [Google Scholar]
- Ohkuma S, Fukuda Y, Tamaki N, et al. (1972). An acute toxicity study of water of perlite (turbid water with powder) in mice. Unpublished report dated 1972 (cited in Sakai & Nagao, 1985)
- Ozel U, Akdemir A, Ergun ON. Utilization of natural zeolite and perlite as landfill liners for in situ leachate treatment in landfills. Int J Environ Res Public Health. 2012;9:1581–92. doi: 10.3390/ijerph9051581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos AM. State of the art in thermal insulation materials and aims for future developments. Energ Build. 2005;37:77–86. [Google Scholar]
- Papageorgiou PS. Particularities of pollen allergies in Greece. Pediatr Pulmonol Suppl. 1999;18:168–71. [PubMed] [Google Scholar]
- Perlite Institute. (2009). Applications of Perlite, the versatile mineral. Available from: http://www.perlite.org/library-perlite-info/PerliteWheel.pdf. See also Sustainability fact sheet http://www.perlite.com/Sustainability_Fact_Sheet.pdf. [Last accessed: 27 Nov 2013]
- Polatli M, Sayiner A, Erdinç M, et al. Perlit işçilerinde solunum fonksiyon testleri. Solunum. 1994;19:899–904. [Google Scholar]
- Polatli M, Erdinç M, Erdinç E, Okyay E. Perlite exposure and 4-year change in lung function. Environ Res. 2001;86:238–43. doi: 10.1006/enrs.2001.4268. [DOI] [PubMed] [Google Scholar]
- Rotella M, Simandl G. (1995). Marilla perlite – volcanic glass occurrence, British Columbia. Geological Fieldwork, Paper 2003-1, 165–73. Available from: http://www.empr.gov.bc.ca/Mining/Geoscience/PublicationsCatalogue/Fieldwork/Documents/2002/13_GSp165-174.pdf. [Last accessed: 26 Nov 2013]
- Sa’ad Z, Al-Mashaikie AK, Al-Hawbanie AM. Petrography and geochemical study of the perlite rocks from Bait Al-Qeyarie, Kawlan Area, Yemen. JAKU: Earth Sci. 2010;21:195–217. [Google Scholar]
- Sakai T, Nagao S. Twenty-eight week toxicity study of perlite powder in mice. J Toxicol Sci. 1985;10:83–93. doi: 10.2131/jts.10.83. [DOI] [PubMed] [Google Scholar]
- Sampatakakis S, Linos A, Papadimitriou E, et al. Respiratory disease related mortality and morbidity on an island of Greece exposed to perlite and bentonite mining dust. Int J Environ Res Public Health. 2013;10:4982–95. doi: 10.3390/ijerph10104982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers GWH. (1955). The biotoxicity of perlite. Report to the Perlite Institute by the Saranac Laboratory, March 19, 1955.
- Schundler Company. (2002). Perlite health issues: studies and effects. Website sponsored by The Schundler Company. Available from: http://www.schundler.com/perlitehealth.htm. Revised May 29, 2002. [Last accessed: 26 Nov 2013]
- Select Committee on GRAS Substances (SCOGS). (1979). Opinion: Silicon dioxides. 1 p. Available from: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm261095.htm. [Last accessed: 26 Nov 2013]
- Shackley D, Allen MJ. (1992). Perlite and the perlite industry. Miner Indus Int 1008:13–22. London: IMM.
- Simandi GJ, Church BN, Hodgson W. (1995). “Perlite” from Terrace mountain, vernon area: possible industrial applications. Geological Fieldwork, Paper 1996-1, 223–26. Available from: http://www.empr.gov.bc.ca/Mining/Geoscience/IndustrialMinerals/Documents/223-226-simandl.pdf. [Last accessed: 26 Nov 2013]
- Stamm JW. Multi-function toothpastes for better oral health: a behavioural perspective. Int Dental J. 2007;57:1–13. [Google Scholar]
- Tatar A, Boldaji F, Dastar B, et al. Effects of dietary supplementation with perlite and zeolite on performance, litter quality, and carcass characteristics of broilers from 7-42 days of age. Int Res J App Basic Sci. 2012;3:1148–54. [Google Scholar]
- Timar M, Kendry G, Juhasz Z. The effect of mineral dusts on the pulmonary tissue. Article in Hungarian. Egeszsegtudomany. 1965;9:183–8. [Google Scholar]
- Torab-Mostaedi M, Ghassabzadeh H, Ghannadi-Maragheh M, et al. Removal of cadmium and nickel from aqueous solution using expanded perlite. Brazil J Chem Eng. 2010;27:299–308. [Google Scholar]
- Tzintzos S. (2013). Milos island: a sustainable case of mining and tourism. Presentation prepared by S. Tzintzos of S&B industrial Minerals for the 6th International Conference of Sustainable Development in the Minerals Industry (SDIMI) 20 June–13 July, 2013, Milos Island, Greece. Presentation Available from: http://www.sdimi.org/papers_2013/SDIMI_A1/2nd%20day/Milos%20island_A%20sustainable%20case%20of%20mining%20&%20tourism.pdf. [Last accessed: 25 Nov 2013]
- Uçan ES, Erdinç E, Erdinç S, Yalgun F. Perlit tozuna maruz kalan işçilerde solunum fonksiyonlari ve silikozis riski. Ege Üniversitesi Tip Fakültesi Dergisi. 1986;25:452–5. [Google Scholar]
- Ueda A, Ueda T, Nomura S. Experimental study on fibrogenicity [sic] of oil absorbent particles made of perlite dust. Japanese Sangyo Igaku. 1978;20:367–73. cited as occurring in ACGIH, 1999 within DECOS 2003. [PubMed] [Google Scholar]
- United States Environmental Protection Agency (USEPA). (1980). Source category survey: Perlite industry. Report EPA-450/3-80-005, Contract No. 68-02-3064, Emission Standards and Engineering Division, USEPA, Washington, D.C. 76 p. Available from: http://www.nepis.epa.gov.
- United States Environmental Protection Agency (USEPA). (1995a). Section 11.30 Perlite processing. In: AP-42, Compilation of air pollutant emission factors, 5th ed, Vol. I, Chapter 11: Mineral products industry. Washington, DC: USEPA, 5 p. Available from: http://www.epa.gov/ttn/chief/ap42/ch11/final/c11s30.pdf. [Last accessed: 26 Nov 2013]
- United States Environmental Protection Agency (USEPA). (1995b). Emission factor documentation for AP-42 Section 8.17: perlite processing. Perlite processing. In: AP-42, Compilation of Air Pollutant Emission Factors, 5th ed, Vol. I, Chapter 11: Mineral products industry. Washington, DC: USEPA, 22 p. Available from: http://www.epa.gov/ttnchie1/ap42/ch11/bgdocs/b11s30.pdf. [Last accessed 26 Nov 2013]
- United States Environmental Protection Agency (USEPA). (2003). Toxicological Review of Hydrogen Sulfide (CAS No. 7783-06-4). Report EPA/635/R-03/005 in support of summary information on the Integrated Risk Information System (IRIS). June 2003, Washington, DC: USEPA, 74 p. Available from: http://www.epa.gov/iris/toxreviews/0061tr.pdf. [Last accessed: 30 Jan 2014]
- United States Food and Drug Administration (FDA). (1979). Select committee on GRAS substances (SCOGS) opinion: perlite (filter aid). Available from: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm260952.htm. [Last accessed: 27 Nov 2013]
- Vorwald AJ. (1953). Perlite dust investigation, Final report: the effect of inhaled perlite dust upon lungs of normal animals. Saranac Lake, NY: Trudeau Foundation, Saranac Laboratory; 1953. Cited in Cooper, 1975, 1976; and Copper and Sargent, 1986.
- Weber RH. (1963). Geologic features of the Socorro perlite deposit. In: Kuellmer FJ (ed.). Guidebook of the Socorro region, New Mexico, 14th Field Conference, New Mexico Geological Society.
- Weill H. (1990). Summary Report on Perlite Worker Survey. Tulane University, April 26, 1990 (Unpublished)
- Weill H. (1994). Perlite Worker Study. Tulane University, October, 1994 (Unpublished)
- Weiss W. Cigarette smoking and small irregular opacities. Br J Ind Med. 1991;48:841–4. doi: 10.1136/oem.48.12.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2005). Environmental Health Criteria 231. Bentonite, kaolin, and selected clay minerals. First draft prepared by Dr Zoltán Adamis, József Fodor National Center for Public Health, National Institute of Chemical Safety, Budapest, Hungary; and Dr Richard B. Williams, US Environmental Protection Agency, Washington, DC, and Regional Office for the Americas of the World Health Organization. World Health Organization, Geneva, 196 p. Available from: http://whqlibdoc.who.int/ehc/WHO_EHC_231.pdf. [Last accessed: 1 Dec 2013]
- Wynder EL. Workshop on guidelines to the epidemiology of weak associations – introduction. Prevent Med. 1987;16:139–41. doi: 10.1016/0091-7435(87)90078-8. [DOI] [PubMed] [Google Scholar]
- Yaneva Z, Koumanova B, Georgieva N. Study of the mechanism of nitrophenols sorption on expanded perlite – equilibrium and kinetics modelling. Macedonian J Chem Eng. 2012;31:101–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.