Abstract
We present a case of 4-bromo-2,5-dimethoxy-N-[(2-methoxyphenyl)methyl]-benzeneethanamine (25B-NBOMe), an N-benzyl phenethylamines derivative, intoxication and a high performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS) method for detection and quantification of 25B-NBOMe.A 19-year-old male was found unresponsive with generalized grand mal seizure activity. On the second day of hospitalization, a friend admitted that the patient used ‘some unknown drug’ called 25B. Serum and urine collected were sent to the Virginia Commonwealth University Medical Center Toxicology Laboratory for analysis. An HPLC-MS/MS method for the identification and quantificationof 25B-NBOMe using 2-(2,5-dimethoxyphenyl)-N-(2-methoxybenzyl) ethanamine (25H-NBOMe)as the internal standard (ISTD)was developed. As this is a novel, single-case presentation, an assay validation was performed prior to testing to ensure the reliability of the analytical results. The serum and urine specimens were determined to contain 180 pg/ml and1900 pg/ml of 25B-NBOMe, respectively.
Keywords: designer drugs, 25B-MBOMe, HPLC-MS/MS, bath salts, overdose
Introduction
Over the past decade, a large number of new, initially non-controlled designer drugs have appeared on the Internet market sold as bath salts, plant food or fertilizer, insect repellent, pond cleaner or vacuum freshen with the disclaimer, ‘Not For Human Consumption’.[1,2] Many of these new, synthetic drugs belong to the class of beta-keto derivatives of amphetamine: methcathinone, methedrone, ethcathinone and pentedrone, as well as, the methylene dioxy ring derivatives similar to methylenedioxymethamphetamine (MDMA, ‘Ecstasy’): methylone, ethylone, butylone and pentylone.[1] In Europe and recently in the United States numerous cases of severe poisonings and fatal intoxications in young adults who have ingested or smoked these ‘bath salt’ designer drugs have been reported. [3–9] In most cases, diagnosis is presumptive since many patients are unable or refuse to provide a specific history. Additionally, timely laboratory testing to identify bath salts in biologic specimen was not available. In response to this rising epidemic of drug abuse, many European countries and the United States have made the manufacturer, distribution and sale of these drugs illegal.[9] In response to these new regulations, manufactures of bath salts have changed the composition of the products to include many newer non-regulated designer drugs.
We report a case of severe intoxication due to self-administration of a new ‘2C’ designer bath salt drug, 25B-NBOMe (2-(4-bromo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl) methyl]ethanamine) (Figure 1) with laboratory confirmatin in serum and urine by high performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS). The terminology 2Cs is an abbreviation invented by Alexander Shulgin for the perceptional distorting and/or hallucinogenic phenylethylamine derivatives he synthetized.[10–12] In 1974, he synthesized 4-bromo-2, 5-dimethoxyphenethylamine (2C-B) that became a replacement for MDMA after MDMA was scheduled in the USA. [13] 25B-NBOMe is an N-benzyl derivative of 2C-B and one of a class of N-benzyl phenylethylamine derivatives that are potent serotonin 5-HT2A receptor agonists.[14–18] The 5-HT2A receptor has been closely linked to complex behaviours including working memory and cognitive processes. It is also implicated in the pathophysiology of affective disorders such as depression and schizophrenia. Stimulation of 5-HT2A receptors is responsible for the hallucinogenic effects of recreational drugs such as lysergic acid diethylamide (LSD) and 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI).[19] While the therapeutic effects of atypical anti-psychotics are due to antagonism at 5-HT2A receptors.[20] 25B-NBOMe was first synthetized by Ralf Heim at the Free University of Berlin as one of a series of possible 5-HT2A agonist. [16–18] Recently positron emission tomography (PET) imaging of cerebral 5-HT2A receptors with carbon-11 labelled 25B-NBOMe demonstrated that 25B-NBOMe is a particularly potent agonist.[21,22]
Figure 1.
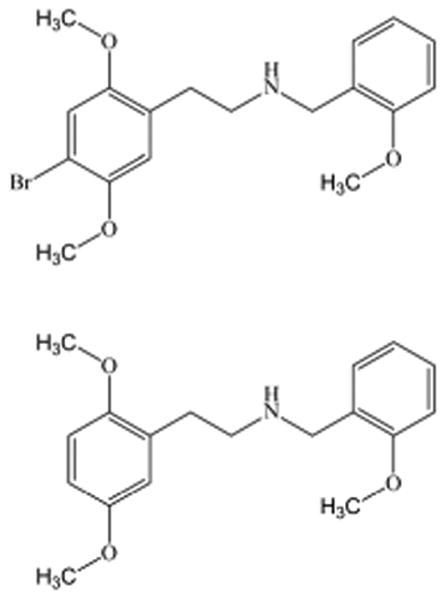
Chemical Structure of 25B-NBOMe and 25H-NBOMe.
At present there are no data in the published literature concerning the pharmacokinetics or pharmacological and toxicological effects of 25B-NBOMe in man or whole animals. To our knowledge this case is the first and at present, only report of 25B-NBOMe intoxication documented by toxicological analysis of biological specimens. As this is a novel, single-case presentation, an assay validation was performed prior to testing to ensure the reliability of the analytical results.
Case report
History and initial presentation
A 19-year-old male in prior good health was found by his roommates having ‘jerking movements’. They summoned Emergency Medical Services (EMS), who responded to find the patient unresponsive with generalized grand mall seizure activity. There was no history of drug use forthcoming by his roommates at this time. Following a transit time of approximately 25 min the patient arrived in the emergency department (ED) in status epilepticus with generalized tonic-clonic jerking movements. A rectal temperature of 104 °F was noted and the patient was immediately administered 1 mg of lorazepam and intubated with 100mg succinylcholine and etomidate.
His vital signs after intubation were: temp 103.3 °F, heart rate 152 bpm, respiratory rate 22 rpm, blood pressure 145/90 mmHg, and oxygen saturation of 97% on ventilator. The patient was placed on propofol infusion and administered pancuronium to control his agitation. Seizure-like activity ceased with these medications. He was then started on rocephin, gentamycin, and acyclovir. On physical examination, the patient was noted to be diaphoretic with facial cyanosis. His pupils were 3 mm and unresponsive to light, and he had bilateral conjunctivitis. He displayed sinus tachycardia without murmurs, rubs, or gallops. He was in respiratory distress with shallow breathing with rhonchi. Neurologic examination was noted for no focal deficits. His skin was erythematous, and a purpuric rash was on his forehead.
The patient's initial blood gas findings were as follows: pH 6.9, pCO2 89 mmHg, pO2 210 mmHg, and HCO3 19.3 mmol, with a base deficit of 13 mmol/L. Results from initial serum chemistry and hematology testing are presented in Table 1. His CT head scan showed a 3-mm hypodense focus in the high left frontoparietal region. During the CT scan, the patient sustained another seizure and required an additional 2 mg of lorazepam. A lumbar puncture (LP) displayed a cloudy fluid, testing of which yielded 55 mg/100 ml of protein and 170 mg/100 ml of glucose. No organisms were observed on smear and gram staining was negative. The patient was then loaded with 1500 mg Dilantin to assist in control of seizures. A chest x-ray confirmed that the endotracheal tube placement was acceptable and there was no evidence of infiltrates. A routine urine drug screen was positive for marijuana. The patient was subsequently transferred to the intensive care unit.
Table 1. Initial serum chemistry and hematology findings.
| Test | Patient | Normal Range |
|---|---|---|
|
| ||
| Glucose, mg/L | 286* | (136-145) |
| pCO2, mmol/L | 16* | (21-32) |
| BUN, mg/dL | 15 | (7-18) |
| Na+, mEq/L | 147* | (136-145) |
| K+, mEq/L | 5.9* | (3.6-5.2) |
| Cl−, mEq/L | 99 | (98-108) |
| Creatinine, mg/dL | 1.6* | (0.6-1.3) |
| Lactic acid, mmol/L | 20* | (0.5 - 2.2) |
| WBC, e9/L | 26.1* | (4.5 - 11.0) |
| Hemoglobin, g/dL | 15.2 | (14-17) |
| Platelets, 10e9/L | 415 | (150-450) |
Abnormal
Clinical course
The patient was administered bolus injections of saline in the ED and transferred on a continuous infusion of Lactated Ringer's at 75 cc/h. On day 2 post-admission his creatine kinase (CK) was 2862 units/L (normal, 38–174 u/L), and trended up to a peak of 11 645 on day 5 of hospitalization indicating rhabdomyelosis. In response, his Lactated Ringer's infusion was increased to150 cc/h. His urine output was noted to be acceptable, and he did not develop a worsening of his renal failure. By day 5, his serum creatinine was down to 0.4 mg/dl.
The patient required intubation, seizure control with sedation from propofol and midazolam and paralysis with vecuronium. On day 3 of hospitalization, the patient's arterial blood gas was: pH 7.366, pCO2 34.3 mmHg, pO2 155 mmHg, HCO3 19.7 mmol, with a base deficit of 6 mmol/L. The patient was then successfully extubated and switched to a nasal cannula 3 L/min. After extubated, the patient was drowsy; however, he was able to recognize his family and communicate with caregivers. Over the next day, he experienced periods of forgetfulness. By day 6, the patient was fully alert and oriented. At this time the Poison Center closed the case as the patient was markedly improving.
25B-NBOMe determination
On the patient's second day of hospitalization, a friend admitted that the patient self-administered ‘some drug’ prior to his seizures. The friend did not know exact composition of the drug only that it was called ‘25B’. He provided a sample for testing which was identified as 25B-NBOMe. As a result of this identification, serum and urine specimens were collected 39 h post-admission and were sent to the Virginia Commonwealth University Medical Center Toxicology Laboratory for analysis. The serum specimen was collected in a ‘red top tube’ of silicone-coated glass with no gel separator. These tubes are used routinely for serum chemistry determinations and other serologic testing. Their use precludes concerns about serum separator gels interfering with the assay.
HPLC-MS/MS method
Materials and methods
Reagents
The phenethylamine derivative primary reference materials for 25B-NBOMe and 2-(2,5-dimethoxyphenyl)-N-(2-methoxybenzyl) ethanamine (25H-NBOMe) (Figure 1) were purchased from Cayman Chemical Company (Ann Arbor, MI, USA) as hydrochloride salts. Acetonitrile, ammonium acetate, ethyl acetate, formic acid, hexane, methanol, sodium hydroxide and water were purchased from Fisher Scientific (Hanover Park, IL, USA). All reagents were ACS grade or better. Medical grade nitrogen was purchased from National Welders Supply Company (Richmond, VA, USA). Drug-free serum was obtained from the in-house Department of Transfusion Medicine. Liquichek™ controls were purchased from BioRad Laboratories, Inc. (Hercules, CA, USA). In-house drug-free urine was obtained from laboratory personnel who did not use tobacco products or take other prescription, over-the-counter or illicit drugs. The serum and urine were analyzed by HPLC-MS/MS for 25B-NBOMe, and screened by enzyme immunoassay and gas chromatography/mass spectrometry for drugs of abuse. All of these tests indicated no drugs were present in the drug-free serum or urine.
Calibrators and controls
A seven-point calibration curve of 25, 50, 100, 250, 500, 1000, and 2000 pg/ml was prepared fresh in duplicate before analysis of each batch of samples. The following QC serums specimens for 25B-NBOMe were prepared and analyzed with each batch of test specimens: limit of quantification quality control (LOQC),target concentration of 25 pg/ml; low control (LQC), target concentration of 75 pg/ml; medium control (MQC), target concentration of 300 pg/ml; high control (HQC), target concentration of 1500 pg/ml; and a dilution control (DQC), target concentration of 5000 pg/ml. A drug free control (negative control) with ISTD added and a double negative control containing neither 25B-NBOMe nor ISTD were also analyzed with each test batch. All QC samples were stored at −20°C until testing.
Specimen preparation
Twenty-five μl of ISTD containing 10 ng/ml 25H-NBOMe was added to 0.5 ml aliquots of calibrators, controls and patient samples; 200 μl of 0.2 M sodium hydroxide solution was added followed by 1 ml of hexane/ethyl acetate (9:1). The samples were mixed for 5 min and then centrifuged for 10 min at 3000 rpm. The upper organic layer was transferred to a clean test tube and evaporated to dryness under a gentle stream of nitrogen in a 40°C dry bath. The samples were reconstituted with 100 μl of mobile phase and placed in auto-sampler vials for analysis.
Instrumental analysis
The HPLC-MS/MS analysis was performed with an Applied Biosystems 3200 Q trap with a turbo V source for TurbolonSpray attached to a Shimadzu SCL HPLC system controlled by Analyst 1.4.2 software. The chromatographic separation was performed on a Hypersil Gold C8 100×2.1 mm, 3 μ column (Thermo Scientific, Waltham, MA, USA). The mobile phase consisted of A: Water with 10 mM ammonium acetate and 0.1% formic acid and B: Acetonitrile. The following gradient was applied: 0.00–1.10 min, 20% B, a linear gradient to 33% B at 8.00 min, hold for 6.90 min, then return to 20% B at 8.00 min. The source temperature was set at 650 °C and had a curtain gas flow rate of 30 ml/min. The ionspray voltage was 5000 V, with the ion source gases 1 and 2 at flow rates of 25 ml/min. The acquisition mode used was multiple reaction monitoring (MRM). The retention times were: 25B-NBOMe, 4.2 min; and 25H-NBOMe, 3.8 min. 25B-NBOMe had a declustering potential of 38 eV and 25H-NBOMe had a declustering potential of 19 eV. The following transition ions (m/z) were monitored in MRM mode with their corresponding collection energies (eV) in parentheses: 25B-NBOMe: 380 >121 (26) and 380 >91 (65); and 25H-NBOMe: 302 > 121 (28) and 302 > 91 (70). The chromatographic separation is presented in Figure 2. The total run time for the analytical method was 10 min.
Figure 2.
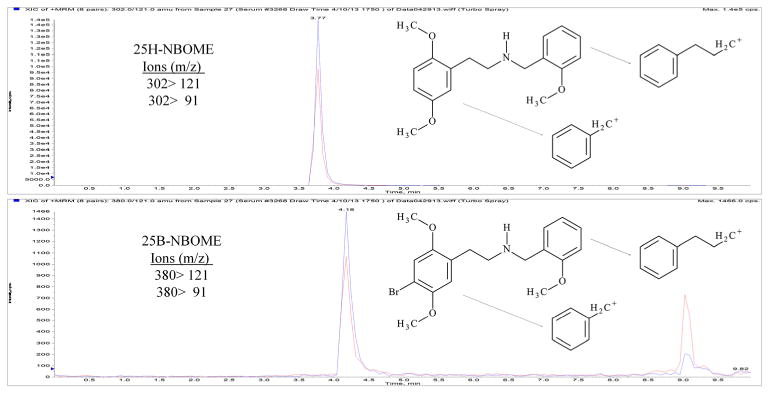
Chromatographic separation of 25B-NBOMe and 25H-NBOMe in patient serum sample.
Method validation
The evaluation of the serum assay was conducted over four separate days. Validation sample batches contained calibrators in duplicate, drug-free samples with ISTD added, drug-free samples without ISTD and replicates of the prepared LOQC, LQC, MQC, HQC, and DQC samples. The assay was validated in serum for selectivity, matrix effect, absolute recovery, linearity, precision, accuracy/bias, carryover, dilution integrity, and post-preparative stability.
Calibrations and LOQ and LOD
The linearity of the seven-point calibration in duplicate in drug-free serum was determined. The calibration curves were constructed by a linear regression plot of the ratio of the area abundance of the quantification MRM of 25B-NBOMe to the ISTD, 25H-NBOMe, versus concentration in urine. Acceptable performance for each of four days' calibration curves, was that each calibrator concentration was to be within ± 15% of its expected value except the LOQC, which could be within ± 20% of its expected value and the correlation coefficient (r2) for each curve was 0.99 or greater. The linear regression correlation coefficients (r2) for the calibration curves for 25B-NBOMe yielded a mean of 0.997 ± 0.001 (range of 0.995 – 0.998, n = 4). All of the curves used in these tests were within the excepted ranges. The lower limit of quantification (LOQ) of 25 pg/ml and the lower limit of detection (LOD) of 10 pg/ml for 25B-NBOMe were administratively set. LOQC samples were used to verify the LOQ was within ± 20% of the 25 pg/ml target value and had a response at least 10 times greater than of the response to drug-free serum. Samples prepared at the 10 pg/ml LOD were analyzed with each batch to verify that there was a response at least 5 times greater than the response to drug-free serum.
Selectivity
No interferences were observed from compounds in the following commercially available controls: Liquichek™ Immunoassay Plus Control, level 3; Liquichek™ Therapeutic Drug Monitoring, level 3; and Liquichek™ Urine Toxicology Control, Level C3. Analysis of in-house control containing commonly prescribed pain medication sand various designer drugs including 4-chloro-2,5-dimethoxyphenethyl-N-[(2-methoxyphenyl) methyl] ethanamine (2CC-NBOMe) and 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl) methyl] ethanamine (25I-NBOMe) demonstrated that these compounds did not interfere with 25B-NBOMe or the ISTD 25H-NBOMe.
Absolute recovery and ion suppression
The percent recovery was determined by first extracting drug-free serum and reconstituting it with mobile phase containing 300 pg/mL of 25B-NBOMe. The addition of the drug to the residue of extracted serum mitigated any matrix effects on recovery studies. The absolute recovery of the assay was determined by comparing the absolute peak area of the extracted aliquots of 300 pg/ml 25B-NBOMe and 500 pg/ml ISTD compared to the absolute peak area of drug-free serum reconstituted with mobile phase containing 300 pg/ml of 25B-NBOMe, multiplied by 100. The ion suppression of the assay was determined by comparing the absolute peak area of drug-free serum reconstituted with mobile phase containing 300 pg/ml of 25B-NBOMe and 500 pg/ml ISTD compared to the absolute peak area of an unextracted 300 pg/ml 25B-NBOMe and 500 pg/ml ISTD neat, multiplied by 100 and then minus 100. The absolute recovery of the assay for 25B-NBOMe at the 300 pg/ml concentration (n = 6) was 83%. The absolute recovery for the 25H-NBOMe, ISTD, at 500 pg/mL (n = 6) was 91%. The ion suppression for 25B-NBOMe at the 300 pg/ml (n = 6) and 25H-NBOMe, ISTD, at 500 pg/ml (n = 6) was 3% and 5%, respectively (Table 2).
Table 2. Recovery and ion suppression of 25B-NBOMe and 25H-NBOMe.
| Recovery & Ion Suppression (n = 3) | ||
|---|---|---|
|
| ||
| Designer Drug | Conc. (750 pg/ml) | % Mean ± % SD |
| 25B-NBOMe | Recovery (%) | 83 ± 7 |
| Suppression (%) | 3 ± 6 | |
| ISTD | Conc. (500 pg/ml) | % Mean ± % SD |
| 25H-NBOMe | Recovery (%) | 91 ± 7 |
| Suppression (%) | 5 ± 6 | |
Accuracy/bias and precision
Accuracy/bias and precision of the method were determined from analysis of three different batches of the prepared QC samples. The percent accuracy/bias of the method was calculated as the ratio of the mean 25B-NBOMe concentrations of six aliquots of each QC sample analyzed in the same batch of samples, to the target concentration of the QC samples times 100. The criteria for acceptable assay accuracy/bias were quantified 25B-NBOMe results within ±15% of the target value of the prepared QC samples (Table 3). The intra-day precision of the method was determined by quantified results of replicate analysis of four aliquots of the five different prepared QC samples. The inter-day precision was determined from quantified results of the six bias aliquots and four analyses of the prepared controls on three different days. Both intra- and inter-day accuracy and precision were determined to not exceed a 15% CV except the inter-day for the dilutions control (Table 4).
Table 3. Accuracy/bias of 25B-NBOMe.
| Accuracy/bias (n = 6) | Mean Conc. ±SD | Average | |
|---|---|---|---|
|
|
|
|
|
| Designer Drug | Control | (pg/mL) | (%) |
|
| |||
| 25B-NBOMe | LOQ (25 pg/ml) | 25 ± 2 | 100 |
| LQC (75 pg/ml) | 82 ± 7 | 109 | |
| MQC (300 pg/ml) | 261 ± 26 | 87 | |
| HQC (1500 pg/ml) | 1340 ± 717 | 89 | |
| DQC (5000 pg/ml) | 5256 ± 655 | 105 | |
Table 4. Precision of 25B-NBOMe.
| Precision | Intra-day (n = 4) | |||
|---|---|---|---|---|
|
|
|
|||
| Designer Drug | Control | Mean Conc. (pg/ml) | CV | (%) Accuracy/bias |
|
| ||||
| 25B-NBOMe | LOQ (25 pg/ml) | 27 | 8 | 108 |
| LQC (75 pg/ml) | 86 | 4 | 115 | |
| MQC (300 pg/ml) | 269 | 5 | 90 | |
| HQC (1500 pg/ml) | 1486 | 4 | 99 | |
| DOQ (5000 pg/ml) | 5613 | 7 | 112 | |
| Inter-day, 5 days (n = 14) | ||||
| Designer Drug | Control | Mean Conc. (pg/ml) | CV | (%) Accuracy/Bias |
| 25B-NBOMe | LOQ (25 pg/ml) | 26 | 9 | 104 |
| LQC (75 pg/ml) | 85 | 6 | 113 | |
| MQC (300 pg/ml) | 258 | 8 | 86 | |
| HQC (1500 pg/ml) | 1348 | 13 | 90 | |
| DOQ (5000 pg/ml) | 5020 | 19 | 100 | |
Carryover
Sample carryover was evaluated in each of the three validation batches using two different procedures. Immediately following the injection of the 2000 pg/ml 25B-NBOMe calibrator, an extract of a drug-free serum was injected. The rejection criterion for carryover was set at the detection of 25B-NBOMe at a concentration less than 20% of the 10 pg/ml LOD. 25B-NBOMe was not detected in the injected aliquot of drug-free serum. As an additional measure to evaluate possible carryover, an injection of the extracted HQC (1500 pg/ml) sample was immediately followed by injection of the LQC (75 pg/ml) sample. This procedure was routinely applied each time HQC and LQC samples were analyzed. The rejection criterion for carryover was set at a concentration with a bias of <20% of the target value of the LQC. Lack of carryover was confirmed as none of the 25B-NBOMe LQC samples demonstrated a significant quantified bias.
Dilution integrity
Dilution integrity was assessed by 5-fold dilution of four aliquots of the DQC containing 5000 pg/ml of 25B-NBOMe in each of the three validation batches. The DQC was treated as a patient specimen. The criterion for dilutions integrity was set at a 25B-NBOMe concentration of 20% of the target value of the DQC after multiplying the concentration calculated from the calibration curve multiplied by 5. The final concentrations for the diluted DQC samples were all within 20% of the of the target value of 5000 pg/ml for 25B-NBOMe for each of the validation batches.
Stability
Stability of the extracted 25B-NBOMe and the ISTD, 25H-NBOMe, was evaluated. A batch of the extracted LQC, MQC, and HQC were quantified against a freshly prepared calibration. The extracted samples were then allowed to sit in the auto-sampler for 72 h at room temperature after which they were re-injected and quantified from the initial calibration. The results of the initial analysis were compared to those of the re-injected samples. In this post-preparative study, 25B-NBOMe and the ISTD, 25H-NBOMe, were considered stable as the concentrations of the re-injected QC samples were within ± 20% of their initial concentrations.
Results and discussion
Many initial signs and symptoms displayed by the patient were consistent with those reported in cases of intoxication due to 25I-NBOMe (2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl) methyl]ethanamine) the iodine analog of 25B-NBOMe.[23–26] In 21 cases of 25I-NBOMe intoxications the average age was 20 years old with arange of 14–29 years old. Twelve of these cases included the sex of the patient, all were male.[23,25,26] Clinical features included tachycardia (95%), hypertension (71%), agitation (76%), and seizures (48%). In general these symptoms were consistent with those reported in 236 cases of bath salts use.[3] Murphy et al. found the most common symptoms were agitation(82%),combativeviolentbehavior (57%), hallucinations (40%), paranoia (36%), confusion (34%), and chest pain (17%), while common signs were tachycardia (56%), hypertension (17%), and mydriasis (13%). CPK elevations and hypokalemia, the most common lab abnormalities, were noted in 9% and 4% of cases, respectively. The identification of specific drugs in biological specimens found in these cases was unavailable. The most noticeable findings in this 25B-NBOMe intoxicated patient was persistences of seizure activity requiring continuous administration of sedatives and skeletal muscle blocking agents for almost three days.
The serum specimen was analyzed in duplicate by the presented method and was determined to contain 180 pg/ml of 25B-NBOMe. 25B-NBOMe was verified as the only NBOMe compound present in the patient serum specimen by extracting and analyzing an aliquot without any ISTD, 25H-NBOMe, added. The authors had previous analyzed serum specimens from a number of 25I-NBOMe cases, one of which was published.[25] These specimens were drawn 25–48 h after admission to the ED and ranged in concentration from 250 to 2700 pg/ml. The serum in this reported case was collected 39 h post-admission. It was anticipated this 25B-NBOMe case would have similar concentrations as these 25I-NBOMe cases. We anticipate in the future receiving specimens in a timelier manner from our ED; hence, we are likely to observe higher concentrations in future serum specimens.
The patient's urine was determined to contain 1900 pg/ml of 25B-NBOMe. The urine sample was prepared by the presented method in duplicate excepted the calibrators were prepared in drug-free urine. The expected urine concentration was based upon 25I-NBOME data from previous work. This included the urine results reported by Hill et al. that had a concentration range of 2–36 ng/ml [26] from specimen drawn 3.5 to 6h after admission to the ED. Had the urine concentration of the patient been >2 ng/ml 25B-NBOMe, the analysis would have been repeated on dilution and a urine dilution control added to the method validation. The authors are unaware of any other published report of 25B-NBOMe intoxication documented by toxicological analysis of biological specimens.
25B-NBOMe and a range of other NBOMe analogs have been known since the late 1990s/early 2000s and have served as important ligands in the investigation of the 5-HT2A receptor.[14–18] Although designer drugs such as the NBOMe analogs may enter the market at any time, it has not been until recently that these analogs have become readily available. An epidemic of 25I-NBOMe abuse in central Virginia in 2012 resulted in the Commonwealth listing 25I-NBOMe the iodine analog of 25B-NBOMe as a schedule 1 drug. As of the Scientific Advisory Committee Open Meeting held in May 2013 in Richmond, VA, the Virginia Department of Forensic Science has yet to encounter a drug seizure containing 25B-NBOMe. However, based on the authors' experience, the presence of 25B-NBOMe in this case may demonstrate how quickly designer drugs changes in response federal or state laws. The 25B-NBOMe may become a replacement designer drug for the now schedule 25I-NBOMe.
Conclusion
The patient signs and symptoms in this report of 25B-NBOMe intoxication were similar to those in previously reported cases of bath salt intoxications. However, the presented case is the first reported case attributed to 25B-NBOMe and the first description of a method to identify and/or quantify the intoxicant in biological specimens. The patient's serum and urine were determined to contain 180pg/ml and 1900 pg/ml of 25B-NBOMe, respectively. The HPLC-MS/MS method presented was applied to the identification and quantification of 25B-NBOMe in serum and urine from the patient. This assay used a simple liquid-liquid extraction procedure prior to chromatographic analysis. 25H-NBOMe was used as the internal standard as deuterated analogs were not available. Validation studies demonstrated acceptable performance of the method for such clinical cases.
Acknowledgments
This study was supported by the National Institute of Drug Abuse (NIDA) Center for Drug Abuse grant P50DA005274. The authors wish to thank Sarah Carney for proof reading this manuscript.
References
- 1.Gibbons S. ‘Legal highs’ – novel and emerging psychoactive drugs: A chemical overview for the toxicologist. Clin Toxicol (Phila) 2012;50:15. doi: 10.3109/15563650.2011.645952. [DOI] [PubMed] [Google Scholar]
- 2.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of "bath salts" and "legal highs" (synthetic cathinones) in the United States. Clin Toxicol (Phila) 2011;49:499. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 3.Murphy CM, Dulaney AR, Beuhler MC, Kacinko S. ‘Bath salts’ and ‘plant food’ products: The experience of one regional US poison center. J Med Toxicol. 2013;9:42. doi: 10.1007/s13181-012-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson JM, Hargraves TL, Hair LS, Massucci CJ, Frazee CC, III, Garg U, et al. Three fatal intoxications due to methylone. J Anal Toxicol. 2012;36:444. doi: 10.1093/jat/bks043. [DOI] [PubMed] [Google Scholar]
- 5.Cawrse BM, Levine B, Jufer RA, Fowler DR, Vorce SP, Dickson AJ, et al. Distribution of methylone in four postmortem cases. J Anal Toxicol. 2012;36:434. doi: 10.1093/jat/bks046. [DOI] [PubMed] [Google Scholar]
- 6.Cosbey SH, Peters KL, Quinn A, Bentley A. Mephedrone (methylmethcathinone) in toxicology casework: A Northern Ireland perspective. J Anal Toxicol. 2013;37:74. doi: 10.1093/jat/bks094. [DOI] [PubMed] [Google Scholar]
- 7.Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug "bath salts" containing 3,4-Methylenedioxypyro valerone (MDPV) J Med Toxicol. 2012;8:69. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, et al. Postmortem tissue distribution of MDPV following lethal intoxication by ‘bath salts’. J Anal Toxicol. 2013;37:182. doi: 10.1093/jat/bkt001. [DOI] [PubMed] [Google Scholar]
- 9.Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): Legal status and patterns of abuse. Ann Pharmacother. 2012;46:436. doi: 10.1345/aph.1Q628. [DOI] [PubMed] [Google Scholar]
- 10.Shulgin AT, Carter MF. Centrally active phenethylamines. Psychopharm Commun. 1975;1:93. [PubMed] [Google Scholar]
- 11.Shulgin A, Shulgin A. PIHKAL: A Chemical Love Story. Transform Press; Berkeley, CA: 1991. [Google Scholar]
- 12.Dean BV, Stellpflug SJ, Burnett AM, Engebretsen KM. 2C or not 2C: Phenethylamine designer drug review. J Med Toxicol. 2013;9:172. doi: 10.1007/s13181-013-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glennon RA, Kier LB, Shulgin AT. Molecular connectivity analysis of hallucinogenic mescaline analogs. J Pharm Sci. 1979;68:906. doi: 10.1002/jps.2600680733. [DOI] [PubMed] [Google Scholar]
- 14.Nichols DE, Frescas SP, Chemel BR, Rehder KS, Zhong D, Lewin AH. High specific activity tritium-labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (INBMeO): A high-affinity 5-HT2A receptor-selective agonist radioligand. Bioorgan Med Chem. 2008;16:6116. doi: 10.1016/j.bmc.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol. 2006;70:1956. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- 16.Heim R, Pertz HH, Elz S. Partial 5-HT2A-receptor agonists of the phenylethanamine series: Effect of a trifluoromethyl substituent. Arch Pharm Pharm Med Chem. 2000;333:45. abstract. [Google Scholar]
- 17.Pertz HH, Heim R, Elz S. N-Benzylated phenylethanamines are highly potent partial agonists at 5-HT2A receptors. Arch Pharm Pharm Med Chem. 2000;333:30. abstract. [Google Scholar]
- 18.Heim R. Entwicklung eines neuen Struktur-Wirkungskonzepts. Free University of Berlin; Berlin: 2003. Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. [Google Scholar]
- 19.González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Abbas A, Roth BL. Pimavanserin tartrate: A 5-HT2A inverse agonist with potential for treating various neuropsychiatric disorders. Expert Opin Pharmacother. 2008;9:3251. doi: 10.1517/14656560802532707. [DOI] [PubMed] [Google Scholar]
- 21.Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers. Eur J Nucl Med Mol Imaging. 2011;38:681. doi: 10.1007/s00259-010-1686-8. [DOI] [PubMed] [Google Scholar]
- 22.Ettrup A, Holm S, Hansen M, Wasim W, Santini MA, Palner M, et al. Pre-clinical safety assessment of the 5-HT2A receptor agonist PET radioligand [11C]Cimbi-36. Mol Imaging Biol. 2013;15:376. doi: 10.1007/s11307-012-0609-4. [DOI] [PubMed] [Google Scholar]
- 23.Kelly A, Eisenga B, Riley B, Judge B. Case series of 25I-NBOMe exposures with laboratory confirmation. Clin Toxicol (Phila) 2012;50:702. abstract. [Google Scholar]
- 24.Rose SR, Cumpston KL, Stromberg PE, Wills BK. Severe poisoning following self-reported use of 25-I, a novel substituted amphetamine. Clin Toxicol (Phila) 2012;50:707. abstract. [Google Scholar]
- 25.Rose SR, Poklis JL, Poklis A. A case of 25I-NBOMe (25-I) intoxication: A new potent 5-HT2A agonist designer drug. Clin Toxicol (Phila) 2013;51:174. doi: 10.3109/15563650.2013.772191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill SL, Doris T, Gurung S, Katebe S, Lomas A, Dunn M, et al. Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: Case series. Clin Toxicol (Phila) 2013;51:487. doi: 10.3109/15563650.2013.802795. [DOI] [PubMed] [Google Scholar]


