Abstract
Genetic variation alone may not account for common chronic disease susceptibility. Rather, an interaction between genetic and environmental factors may clarify the underlying disease mechanism. Hence, we tested whether BMI modified the genetic association of the Apolipoprotein E (APOE) ε4 allele with cognitive decline. The data came from a longitudinal population-based sample of 4,055 participants interviewed at 3-year intervals from 1993 to 2012. Cognitive function was assessed using a standardized global cognitive score and BMI was assessed at baseline and classified as normal, overweight, and obese. There were 1,374 (34%) participants with the ε4 allele. In normal BMI participants, cognitive decline was 0.048-unit per without the ε4 allele, and increased by an additional 0.031-unit per year with the ε4 allele. In overweight participants, cognitive decline was 0.038-unit per year without the ε4 allele, and increased by an additional 0.026-unit per year with the ε4 allele. Finally, in obese participants, cognitive decline was 0.038-unit per year without the ε4 allele, and increased by an additional 0.014-unit per year with the ε4 allele. The association of ε4 allele with cognitive decline was significantly lower in obese participants compared to normal BMI participants (p=0.003), thereby suggesting significant gene-environment interaction on cognitive decline.
Keywords: Cognitive decline, Apolipoprotein E, Body mass index, gene environment interaction, Cohort studies
INTRODUCTION
Cognitive decline and Alzheimer’s disease (AD) are major causes of morbidity and mortality in older adults.1–4 As life expectancy increases, the risk of late-life AD and the associated health care burden concomitantly rises. Thus, it is imperative to identify risk factors associated with cognitive decline that may also alter the risk of late-life AD. Among such risk factors is the Apolipoprotein E (APOE) ε4 allele found on Chromosome 19 that has been shown to be associated with increased risk for cognitive decline, cognitive impairment, and late-life AD.5–9 The APOE gene encodes a protein that helps carry cholesterol and other types of lipids in blood. Even though the molecular pathogenesis of APOE and late-life AD is not completely understood, it has been postulated that insufficient fat and cholesterol transported to the brain may reduce the clearance of β-amyloid (Aβ) from the brain, thereby increasing the risk of late-life AD.10
In the general population, about 25 to 30% carry the APOE ε4 allele, of whom only about 40% develop late-life AD, underscoring the non-deterministic nature of this genetic variation.11 This suggests that genetic variation alone likely cannot account for late-life AD susceptibility without the inclusion of environmental factors. One such environmental factor that has been shown to have a differential association on cognitive decline, especially during late-life, is body mass index (BMI). A lower level of BMI in older age has been shown to be associated with increased risk of late-life AD.12,13 However, the association of low BMI with cognitive function and subsequent decline are also presumed to be markers of pathological and neurological deterioration. On the other hand, there is conflicting evidence for the association of higher BMI with cognitive function and subsequent decline. For example, Cournot et al. found that higher BMI was associated with lower baseline and decline in cognitive function measured by word-list learning in middle-aged men and women.14 Similarly, Yoon et al. showed that higher BMI was associated with lower cognitive function in a cross-sectional study of 250 subjects.15 However, Doruk et al. reported that being overweight or obese was associated with lower risk of cognitive decline in a sample of older adults.16 In addition, Sturman et al. found that BMI was not associated with cognitive decline in a population-based sample.17 These differences in findings might be explained by the underlying interaction of the genetic lipid transport mechanism with metabolic syndrome in older adults. Therefore, one may postulate that cognitive decline may accelerate or slow, depending on significant APOE and BMI interaction in older age.
To better understand the underlying mechanism, we examined the interaction of APOE ε4 allele status with BMI on cognitive decline in older adults, while controlling for stroke, hypertension, heart disease, and diabetes and demographic variables such as age, gender, education, and race. We examined these associations in our sample of 4,055 participants who were followed for up to 18.7 years (average follow-up of 9.2 years). Hence, we tested the hypothesis that BMI modifies the association of the APOE ε4 allele and cognitive decline in older adults.
METHODS
Participants
The Chicago Health and Aging Project (CHAP) is a longitudinal, population-based study of Alzheimer’s disease and other common health conditions among adults 65 years or older performed from 1993–2012. The CHAP study has been described in greater detail by Evans et al.18 and Bienias et al.19 Briefly, baseline data were collected beginning in 1993, where about 79% of residents 65 years and older provided consent to participate in the study (original cohort). The CHAP study used a rolling enrollment scheme, where community residents who attained the age of 65 years were also enrolled as successive cohorts. Of the total of 10,802 subjects enrolled in CHAP, 6,158 were enrolled as members of the original cohort and 4,644 as members of successive cohorts.
Study participants who provided data for at least two cycles of data collection were included in this investigation. From the original CHAP study of 10,802 participants, we excluded subjects who died without a follow-up (2,796), participants who declined follow-up participation (171), participants who completed two or more cognitive tests but had no DNA extracted (3,594), participants who did not provide sufficient information for calculating cognitive test results (78), and participants who did not provide baseline BMI or covariate data (33). Thus, the study sample consisted of 4,130 participants. However, our analysis removed 75 participants with low BMI, generally considered as a marker of end of life, resulting in a final analytical sample of 4,055 participants.
Data collection and measures
BMI was collected at the baseline interview for the study participants. Cognitive function was collected at baseline and all subsequent follow-ups starting from Cycle 1 through Cycle 6, each three years apart, as part of the in-home population interview. Cognitive function was collected for six cycles for the original cohort and two to five cycles for successive cohorts. At the end of the Cycle 2 population interview, DNA samples were collected from a stratified random sample of the population, about one-sixth of all participants. Towards the end of Cycle 5 and for the entire Cycle 6, DNA samples were collected from all subjects during their population interviews. Thus, APOE genotype was ascertained through a combination of stratified random sample and population sample of participants from the original and successive cohorts.
As a sample description problem, we compared those who died without providing a follow-up interview to those who provided two or more follow-up interviews, and found that those who died had significantly lower cognitive function at baseline assessment (p<0.0001). BMI was also significantly lower in participants who had died compared to those who provided two or more cognitive function data (p<0.0001). We also compared those who completed two or more cognitive function tests without DNA samples to those with two or more cognitive tests with DNA samples. We found that those who provided DNA samples had significantly higher cognitive function (p<0.0001) and showed slower cognitive decline (p<0.0001) compared to those who were not chosen for DNA extraction. However, the patterns of cognitive decline in normal, overweight, and obese participants were similar between those included and excluded from the analysis.
Cognitive function
Cognitive function was evaluated using a battery of four tests including two tests of episodic memory (immediate and delayed recall) derived from the East Boston Test,20,21 a test of perceptual speed (the Symbol Digits Modalities Test),22 and a test of general orientation and global cognition (the Mini-Mental State Examination).23 Because tests loaded on a single factor that accounted for about 75% of the variance in a factor analysis,24 we constructed a composite measure of global cognitive function based on all four tests. This measure combines variables with different ranges and floor-ceiling effects by averaging the four tests together after centering and scaling each to their baseline mean and standard deviation (SD). Thus, a participant whose composite performance matches the average participant at baseline has a composite cognitive score of 0, and a person who performs one SD better than average on every test has a composite cognitive score of +1. To provide some prospective of our global composite score, participants with a positive diagnosis of Alzheimer’s disease (AD) in our sample had average cognitive function of −0.579 SD units at the population interview (using NINCDS-ADRDA Criteria).25 The diagnosis of AD and mild cognitive impairment (MCI) was performed at the end of Cycle 2; hence, a diagnosis for AD or MCI was unknown at baseline. However, in a sensitivity analysis, we removed participants below the 5th, 10th, and 15th percentile of baseline cognitive function from our analysis.
Body Mass Index
Body mass index (BMI) was assessed at baseline during the in-home interview by measuring the participants’ weight in pounds and asking about height, measured in feet and inches, via the question: “What is your height?” All measurements were converted to the standard metric system for weight to kilograms and height to meters. Then, BMI was computed as kg/m2 and categorized as underweight (<18.5 kg/m2), normal (18.5 to <25 kg/m2), overweight (25 to <30 kg/m2), and obese (≥30 kg/m2), based on the classification by the World Health Organization (WHO).26 We created indicator variables for overweight and obese with normal as the reference group, since prior literature suggests a U-shaped or J-shaped relationship between BMI and cognitive decline. We performed descriptive analysis on 75 underweight participants, but removed them from our analysis, since these participants were significantly older with poor health conditions, and more importantly, about 47% of them were diagnosed with AD.
Apolipoprotein E Allele
Apolipoprotein E genotyping was done using the methods described by Hixson and Vernier27 based on the primers described by Wenham et al.28 APOEε4 genotypes were determined using two single nucleotide polymorphisms (SNPs): rs7412 and rs429358. These SNPs were genotyped in each subject using the hME Sequenom MassARRAY® platform. The genotyping success rate was 100% for SNP rs7412 and 99.8% for SNP rs429358. Both SNPs were in Hardy-Weinberg equilibrium with p-values of 0.0833 and 0.7925 respectively. APOE isoforms for ε2ε2, ε2ε3, ε2ε4, ε3ε3, ε3ε4, and ε4ε4 were created based on these two SNPs. An indicator variable was created for participants who had one or more ε4 allele.
The Institutional Review Board of Rush University Medical Center approved the study and all participants provided written, informed consent.
Covariates
Our analyses adjusted for age, sex, race (blacks vs. whites), and education (measured in number of years of schooling completed), and time-varying measures of four chronic health conditions that might mitigate the association of BMI and APOE ε4 on cognitive decline: heart disease (heart attack or coronary thrombosis or coronary occlusion or myocardial infarction), hypertension, stroke, and diabetes.
Statistical analysis
Descriptive statistics were computed using weighted sample means and standard deviations for continuous variables and percentages for categorical variables stratified by BMI groups after adjusting for sample weights (design-based adjustment). In our sample of underweight participants, higher levels of neurodegenerative disease and chronic health conditions were observed, and hence, removed from our descriptive tests and regression models. To better understand the interaction of BMI and APOE on cognitive function, we also performed a descriptive analysis of blood biomarkers stratified by BMI groups. We compared baseline characteristics using an analysis of variance F-tests for continuous measures and a chi-squared test statistic for categorical measures.
In terms of modeling, we used weighted linear mixed-effects regression model to examine cognitive decline stratified by BMI subgroups.29 From our regression models, we estimated coefficients of interest and their associated variances using linear contrasts. We tested cognitive decline between normal and overweight participants, and normal and obese participants, using two additional models with three-way interaction of overweight and obese indicators with time and APOE ε4 allele status. In a separate analysis, we tested whether the association of APOE ε4 allele and cognitive decline in normal, overweight, and obese participants was different between non-Hispanic blacks and whites. For this analysis, we used three-way interactions of APOE, BMI categories, and time for each of the ethnic groups. We used four-way interactions of indicator for race/ethnicity, APOE BMI categories, and time to test whether the association of BMI categories, APOE and time with cognitive decline was different between blacks and whites.
Our estimation process was complicated by mixed sampling scheme of stratified and population samples. Of the 4,055 included in our regression model, 2,198 were selected using a stratified random sample from the population, and the remaining 1,857 were part of the population sample. In order to account for this sampling structure, we computed subject-specific sample weights for participants from the stratified random sample, while setting those from the population to be equal to 1. Given that sample weights were design features, the parameter estimates from a weighted regression model would be unbiased for this sampling scheme, but the variance estimates may be biased. To remedy this situation, we estimated bootstrap sampling weights for mixture of two-stage sampling and population sampling schemes.30,31 We estimated variances for parameter estimates in the BMI-specific and combined models using this resampling procedure. All models were fitted using R version 2.15.3 using the lmer function in the lme4 package, and bootstrap variances were estimated with coding program using the sampling package.32
RESULTS
The study sample consisted of 4,130 (70% blacks and 63% females) participants with a genotyped APOE allele and BMI data and two or more cognitive function measurements. Of the 4,130 participants, 75 (2%) reported underweight, 1,091 (26%) reported normal, 1,531 (37%) reported overweight, and 1,433 (35%) reported obese BMI levels. The demographic and health characteristics of the sample are shown in Table 1. Underweight participants had significant adverse health conditions including diagnosis of AD and subsequently were removed from our analysis. Of the remaining 4,055 participants, about 34% of the participants had at least one APOE ε4 allele and the percentage of participants with APOE ε4 allele was not significantly different across the BMI groups (p=0.07). At baseline, global cognitive function, MMSE, symbol digits tests, and delayed and immediate recall tests were not significantly different between the three BMI groups (p>0.05). Obese participants were mostly younger black females with higher rates of hypertension and diabetes and a lower prevalence of diagnosed AD.
Table 1.
Demographic and health characteristics of participants stratified by BMI categories (N=4,130)
| Characteristics | Underweight Mean (SE) N=75 |
Normal Mean (SE) N=1,091 |
Overweight Mean (SE) N=1,531 |
Obese Mean (SE) N=1,433 |
p-valuea |
|---|---|---|---|---|---|
| Follow-up time (years) | 7.9 (.48) | 9.3 (.13) | 9.4 (.11) | 9.0 (.12) | 0.23 |
| Age (years) | 74.5 (1.9) | 72.4 (.38) | 71.4 (.27) | 69.9 (.21) | <0.0001 |
| Education (years) | 11.8 (.51) | 12.4 (.24) | 12.5 (.18) | 12.4 (.26) | 0.92 |
| Cognitive function | −0.169 (.200) | 0.302 (.035) | 0.397 (.025) | 0.394 (.031) | 0.07 |
| MMSE | 24.2 (1.09) | 26.8 (.22) | 27.3 (.14) | 27.3 (.15) | 0.08 |
| Symbol digits test | 25.2 (2.87) | 31.1 (.81) | 32.9 (.69) | 32.1 (.79) | 0.51 |
| Delayed recall | 7.0 (.86) | 8.3 (.14) | 8.5 (.10) | 8.6 (.13) | 0.13 |
| Immediate recall | 7.1 (.54) | 8.8 (.13) | 9.0 (.11) | 9.1 (.12) | 0.06 |
| Body mass index | 17.0 (.21) | 22.6 (.10) | 27.2 (.08) | 34.8 (.26) | <0.0001 |
| Males, % | 17, 23% | 443, 41% | 656, 43% | 420, 29% | <0.0001 |
| Blacks, % | 43, 57% | 602, 55% | 460, 70% | 1161, 81% | <0.0001 |
| APOE e4 allele, % | 23, 31% | 345, 32% | 513, 34% | 516, 36% | 0.07 |
| Stroke, % | 10, 13% | 86, 8% | 97, 6% | 111, 8% | 0.21 |
| Heart disease, % | 12, 16% | 113, 10% | 149, 10% | 161, 11% | 0.41 |
| Hypertension, % | 28, 38% | 413, 38% | 804, 53% | 902, 63% | <0.0001 |
| Diabetes, % | 3, 4% | 37, 3% | 100, 7% | 142, 10% | <0.0001 |
| Alzheimer’s disease, %b | 27, 47% | 192, 27% | 202, 24% | 137, 22% | 0.07 |
p-values are based on F-test for continuous data and chi-square test for discrete data; compares normal, overweight and obese BMI groups
Alzheimer’s disease was diagnosed in a subset of 2,245 participants
From Table 2, age, gender, race, and education were associated with baseline cognitive function. Older age was associated with lower levels of cognition and faster cognitive decline over the duration of follow-up. In addition, normal BMI participants with the APOE ε4 allele had significantly lower baseline levels of cognitive function compared to those with no ε4 allele. However, no such baseline association was observed in overweight and obese participants.
Table 2.
Average levels of cognitive function and annual cognitive decline stratified by BMI categories in a population-based sample of 4,055 older adults
| Model terms | Normala Coefficient (SE) N=1,091 |
Overweighta Coefficient (SE) N=1,531 |
Obesea Coefficient (SE) N=1,433 |
|---|---|---|---|
| Intercept | 0.520 (.026)1 | 0.502 (.023)1 | 0.503 (.026)1 |
| Age | −0.034 (.002)1 | −0.030 (.002)1 | −0.028 (.002)1 |
| Males | −0.119 (.027)2 | −0.129 (.019)1 | −0.076 (.020)2 |
| Blacks | −0.350 (.031)1 | −0.259 (.024)1 | −0.252 (.027)1 |
| Education | 0.074 (.004)1 | 0.054 (.003)1 | 0.066 (.003)1 |
| APOE ε4 allele | −0.118 (.029)2 | −0.021 (.021) | −0.034 (.021) |
| Time since baseline | −0.048 (.003)1 | −0.038 (.002)1 | −0.038 (.003)1 |
| Age × Time | −0.004 (.000)1 | −0.004 (.000)1 | −0.003 (.000)1 |
| Black × Time | 0.001 (.004) | −0.008 (.003)3 | −0.007 (.004) |
| APOE ε4 allele × Time | −0.031 (.004)1 | −0.026 (.003)1 | −0.014 (.003)1 |
Note:
p<.0001;
p<.005;
p<.05
Models were also adjusted for stroke, myocardial infarction, hypertension and diabetes
In terms of cognitive decline over time, cognitive function declined by 0.048-units per year (SE=0.003; p<0.0001) among normal BMI participants without the APOE ε4 allele. Cognitive decline increased by additional 0.031-units per year (SE=0.005; p<0.0001) in normal BMI participants with the APOE ε4 allele. Using a linear contrast, participants with normal BMI and the APOE ε4 allele had a cognitive decline of 0.079-units per year (adding 0.048 and 0.031) (SE=0.005). In an effort to provide a better interpretation of these cognitive decline effects, we estimated the number of hypothetical years of cognitive decline that equates to average cognitive function among those with incident AD diagnosis in our sample. Using our regression estimates, normal BMI participants without the APOE ε4 allele would hypothetically have 9.8 years to reach average cognitive function similar to participants diagnosed AD, and 5.9 years for normal BMI participants with the APOE ε4 allele.
Cognitive function declined by 0.038-units per year (SE=0.002; p<0.0001) for overweight participants without the APOE ε4 allele. Cognitive decline increased by additional 0.026-units per year (SE=0.003; p<0.0001) in overweight participants with the APOE ε4 allele. Using a linear contrast, cognitive function declined by 0.064-units per year (adding 0.038 and 0.025) (SE=0.003) in overweight participants with the APOE ε4 allele. Using a combined model, cognitive decline was slower by 0.010-units per year (SE=0.004; p=0.021; data not shown) among overweight participants without the APOE ε4 allele and 0.016-units per year (SE=0.005; p=0.004; data not shown) among overweight participants with the APOE ε4 allele compared to normal BMI participants. Even though cognitive decline in overweight participants was slower than normal participants, we did not find a significant difference in the association of the APOE ε4 allele between normal and overweight participants (p=0.27; data not shown). On an average, overweight participants without the APOE ε4 allele would hypothetically have 16.8 years to reach average cognitive function similar to participants diagnosed with AD, and 8.8 years for overweight participants with the APOE ε4 allele.
In obese BMI participants, cognitive function declined by 0.038-units per year (SE=0.003; p<0.0001) among those without the APOE ε4 allele, which increased by 0.014-units per year (SE=0.003; p<0.0001) among those with the APOE ε4 allele. Using a linear contrast, obese participants with the APOE ε4 allele had cognitive decline of 0.052-units per year (adding 0.038 and 0.014) (SE=0.003). Using a combined model for normal and obese participants, cognitive decline in obese participants was significantly slower by 0.010-units per year (SE=0.005; p=0.031; data not shown) among participants without the APOE ε4 allele and 0.025-units per year (SE=0.005; p<0.0001; data not shown) among obese participants with the APOE ε4 allele compared to normal BMI participants. Additionally, the association of the APOE ε4 allele was significantly different between normal and overweight participants (p=0.003; data not shown). Hypothetically, obese BMI participants without the APOE ε4 allele would have 15.1 years to reach average cognitive function similar to participants diagnosed with AD, and 10.8 years for obese participants with the APOE ε4 allele.
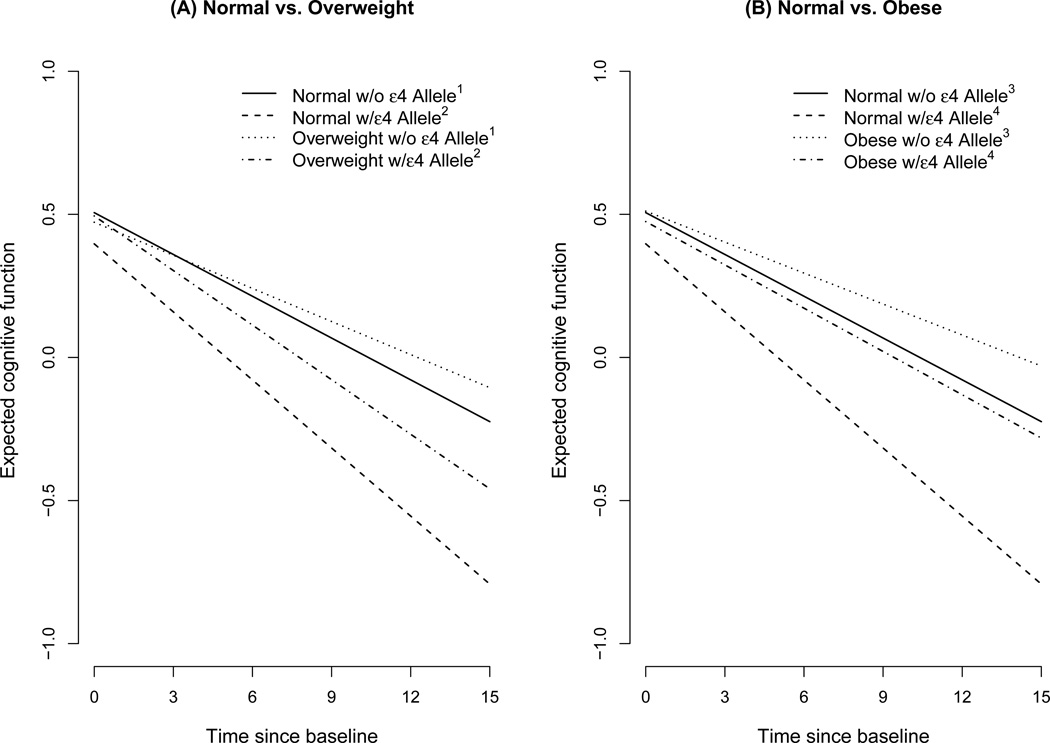
To illustrate these associations, we computed cognitive decline estimates for BMI groups among those without and with the APOE ε4 allele. Figure 1(A) compares the trajectories of normal and overweight BMI participants. The decline in normal participants without the APOE ε4 allele (solid line) was faster than overweight participants without the APOE ε4 allele (dotted line). In addition, cognitive function in overweight participants with the APOE ε4 allele (dotted and dashed line) was significantly slower than normal BMI participants with the APOE ε4 allele (dashed line). Figure 1(B) compares obese participants to normal BMI participants. Cognitive decline in obese participants with (dotted and dashed line) was slower than normal participants without (solid line) and with (dashed line) the APOE ε4 allele.
Figure 1.
Cognitive decline comparing normal vs. overweight and normal vs. obese participants with and without the APOE ε4 allele
1p=0.021; 2p=0.27; 3p=0.031; 4p=0.003
As a secondary analysis, we wanted to examine whether the presence of the APOE ε4 allele has a dose response to cognitive decline in obese participants. Hence, we compared cognitive decline among obese participants with no copy of the ε4 allele to 1 copy of the ε4 allele and 2 copies of the ε4 alleles. Cognitive function declined by 0.036-units per year among those with no copy of the ε4 allele, 0.049-units per year among those with 1 copy of the APOE ε4 allele, and 0.057-units per year among those with 2 copies of the ε4 allele. The rate of cognitive decline from 0 copy to 1 copy of the APOE ε4 allele was significant (p<0.0001; data not shown), but the change from 1 copy to 2 copies was not significant (p=0.54; data not shown), suggesting no dose response increase in risk of cognitive decline in obese participants.
As a sensitivity analysis, we removed participants in the lowest 5th, 10th, and 15th percentile of baseline cognitive function from our analyses. From those models, cognitive decline ranged from 0.044-units per year (5th percentile) to 0.049-units per year (15th percentile) among normal BMI participants without the APOE ε4 allele. Cognitive decline increased by 0.034-units per year (5th percentile) to 0.032-units per year (15th percentile) among normal BMI participants with the APOE ε4 allele. For overweight participants, cognitive decline ranged from 0.040-units per year (5th percentile) to 0.043-units per year (15th percentile) among those without the APOE ε4 allele. Cognitive decline increased by 0.023-units per year (5th percentile) to 0.022-units per year (15th percentile) among overweight BMI participants with the APOE ε4 allele. Among obese participants, cognitive decline ranged from 0.040-units per year (5th percentile) to 0.039-units per year (15th percentile) among those without the APOE ε4 allele. Cognitive decline increased by 0.014-units per year (5th percentile) to 0.013-units per year (15th percentile) among obese participants with the APOE ε4 allele. This sensitivity analysis showed that the association of the APOE ε4 allele with cognitive decline in the BMI subgroups was robust and largely unchanged after removing participants in the lowest percentiles of baseline cognitive function. In another sensitivity analysis, we estimated the association of APOE and BMI categories on cognitive decline while accounting for selection bias due to mortality. We found that our estimates of cognitive decline in normal BMI participants with and without APOE ε4 allele was somewhat higher in our mortality adjusted model compared to core models presented in Table 2. However, the estimates in the overweight and obese groups were mostly unchanged, thereby providing further evidence for robustness of our findings, and reducing the possibility of selection bias impacting our findings.
In a separate analysis, we tested whether BMI also modified the association of the APOE ε4 allele with cognitive decline differently between blacks and whites (Table 3). In normal BMI participants, cognitive decline was 0.044-units per year in black participants without the APOE ε4 allele, and increased by 0.031-units per year among those with the APOE ε4 allele. Among overweight and obese black participants without the APOE ε4 allele, BMI was not associated with an increase in cognitive decline relative to normal BMI participants. However, using a linear contrast, overweight and obese black participants with the APOE ε4 allele showed significantly slower change in cognitive decline by 0.012-units per year and 0.016-units per year, respectively. In white participants with normal BMI, cognitive decline was 0.052-units per year among those without the APOE ε4 allele and increased by 0.040-units per year among those with the APOE ε4 allele. Among overweight and obese white participants without the APOE ε4 allele, BMI was not associated with increased cognitive decline. Using a linear contrast, overweight and obese white participants showed significantly slower increase in cognitive decline by 0.017-units per year and 0.027-units per year, respectively. Using a combined model, blacks and whites did not show a significantly different increase in cognitive functions overweight (p=0.68; data not shown) and obese participants (p=0.17; data not shown).
Table 3.
Average levels of cognitive function and annual cognitive decline stratified by race in a population-based sample of 4,055 older adults
| Model terms | Black Subjects Onlya N=2,834 |
White Subjects Onlya N=1,221 |
|---|---|---|
| Intercept | 0.109 (.020)1 | 0.604 (.020)1 |
| Age | −0.035 (.001)1 | −0.027 (.001)1 |
| Males | −0.078 (.017)1 | −0.182 (.022)1 |
| Education | 0.076 (.002)1 | 0.041 (.003)1 |
| ε4 allele | −0.030 (.016)3 | −0.054 (.024)3 |
| Overweight | 0.111 (.021)1 | 0.018 (.025) |
| Obese | 0.102 (.022)1 | 0.015 (.029) |
| Time since baseline | −0.044 (.003)1 | −0.052 (.004)1 |
| Age × Time | −0.003 (.000)1 | −0.005 (.000)1 |
| Males × Time | 0.004 (.003) | 0.010 (.004)3 |
| Education × Time | −0.0008 (.004)3 | 0.000 (.001) |
| ε4 allele × Time | −0.031 (.005)1 | −0.040 (.006)1 |
| Overweight × Time | −0.002 (.003) | 0.008 (.005) |
| Obese × Time | −0.001 (.003) | 0.001 (.006) |
| Overweight × ε4 allele × Time | 0.014 (.005)3 | 0.009 (.009) |
| Obese × ε4 allele × Time | 0.017 (.005)3 | 0.026 (.011)3 |
Note:
p<.0001;
p<.005;
p<.05
Models were also adjusted for stroke, myocardial infarction, hypertension and diabetes
DISCUSSION
Cognitive decline in older age was differentially associated with BMI and APOE ε4 allele status. The association of the APOE ε4 allele with cognitive decline was lower in obese participants compared to normal BMI participants. In addition, cognitive decline in obese participants with the APOE ε4 allele was not significantly different from normal BMI participants without the APOE ε4 allele. We also did not find a significant difference in the association of BMI and APOE ε4 allele in blacks and whites.
Our results contradict some of the earlier findings on the association of higher BMI and faster cognitive decline. The reasons for such disparate findings may be due to differences in the following: the cognitive testing instruments used; the measurement of BMI-discrete vs. continuous; adjustment for important confounding variables; cross-sectional vs. longitudinal designs, and smaller sample sizes. In these analyses, we controlled for important demographic and health variables from a well-designed longitudinal study with validated measures of cognitive testing in older adults. We used a priori BMI categorization based on the WHO recommendation rather than a continuous BMI measure which has the potential to show a non-linear relationship with BMI as evidenced by prior studies.
The results of this study provide some evidence for gene-environment interaction models. Specifically, the association of cognitive decline with genetic risk factors, such as APOE may significantly vary by BMI classifications, providing evidence for reduced genetic risk of the APOE ε4 allele among subgroups of participants. . The findings from this study also suggest that the direct association of obesity through the dysfunction in the lipid transport mechanism on cognitive function is not as high among obese participants. However, there may be indirect associations that may increase the risk of cognitive decline in obese participants through other pathways that have not been investigated here. Nonetheless, we observed a positive association between participants with obese BMI and cognitive decline. As obesity is known to increase the risk of diabetes, heart disease, hypertension, and stroke, which also independently increase the risk of cognitive decline in older adults, we adjusted for several time-dependent chronic health conditions, such as, stroke, heart disease, hypertension, and diabetes. An analysis not adjusting for chronic health conditions provided slightly large cognitive declines, but obese participants still showed significantly slower decline than normal BMI participants. In an attempt to see if slower cognitive decline in obese participants extrapolated to lower risk of AD, we found that age, gender, and race-adjusted cognitive decline may help slow diagnosis of AD by about 5 years in those with APOE ε4 allele.
The primary strength of this study is that it is one of the few large population-based studies with relevant data that has the requisite power to detect longitudinal change in cognitive function based on genetic and environmental risk factors. Our cognitive function tests were conducted every three years using the same instrument, providing a reliable estimate of long-term cognitive decline. Our analytical strategy accounted for complex sampling schemes, providing unbiased parameter estimates and their variances. We also adjusted for several demographic and chronic health conditions that may impact the association between BMI and cognitive function. Furthermore, our population was comprised of a large number of blacks from a population-based sample, and our secondary analysis suggested no significant difference in the association of the APOE ε4 allele with AD between blacks and whites in any of the three BMI groups.
There are limitations to be noted. First, we used a 3-group classification of BMI that may not be most appropriate in a biological sense. Even though these BMI classifications were based on the WHO recommendations, BMI may be classified in other ways, such as N-point data-driven cutoffs that might provide a different relationship. However, using data-driven cutoffs might be harder to interpret, and might be driven by both empirical evidence and unknown latent factors, rendering the results harder to interpret. Second, we had a small number of underweight participants who were excluded from our analyses because they had a higher number of chronic health conditions. We found that the underweight participants had the fastest cognitive decline consistent with prior literature demonstrating poorer neurological functions and adverse health outcomes. Third, DNA samples were collected in about 40% of participants from the population-based sample which could give rise to selective attrition, especially among participants with higher levels of BMI; however, we did not find any significant differential attrition in the BMI groups for cognitive decline.
Even though our analyses were age, race, and gender adjusted, younger black participants might have slower decline than the rest of the population. However, our secondary analysis suggested no significant differences in the association of the APOE ε4 allele and cognitive decline between blacks and whites, especially in the obese BMI group. It is also possible that this race effect might be explained by some other unobserved or unadjusted variable that has high correlation with BMI and needs to be further investigated.
We expect that our study serves three purposes: 1) to understand the APOE mechanism from the perspective of metabolic activity in late life, 2) to provide evidence for important gene-environment interactions that may be replicated in other large population-based studies, and 3) to provide direction for future intervention studies to address the genetic factors of obese participants in an attempt to reduce cognitive decline in older age.
In conclusion, we found that BMI was associated with cognitive decline among those with and without the APOE ε4 allele; that is, the APOE ε4 allele behaves differently in substrates of BMI. Specifically, we observed that obese participants with the APOE ε4 allele had a slower cognitive decline than normal BMI participants with the APOE ε4 allele. Despite the potential moderating association of obesity on slowing cognitive decline, one has to evaluate the deleterious effect of the obesity on other health related problems in older age before making any public health recommendations. However, our study has shown significant gene-environment interplay with the risk of cognitive decline in a subgroup of APOE ε4 allele carriers.
Acknowledgments
Funding: This work was supported by grants from the National Institutes for Health (R01-AG-09966).
REFERENCES
- 1.Daviglus ML, Bell CC, Berrettini W, et al. National Institutes of Health State-of-the-Science Conference Statement: Preventing Alzheimer’s Disease and Cognitive Decline. NIH Consens State Sci Statements. 2010;27:1–30. [PubMed] [Google Scholar]
- 2.Anstey KJ, Mack HA, von Sanden C. The relationship between cognition and mortality in patients with stroke, coronary heart disease or cancer. European Psychol. 2006;11:182–195. [Google Scholar]
- 3.Berg S. Aging, behavior, and terminal decline. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 4th ed. New York, NY: Academic Press; 1996. pp. 323–337. [Google Scholar]
- 4.Dixon RA, de Frias CM. The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating changes in memory compensation. Aging Neuropsych Cog. 2004;11:346–376. [Google Scholar]
- 5.Staehelin HB, Perrig-Chiello P, Mitrache C, et al. Apolipoprotein E genotypes and cognitive functions in healthy elderly persons. Acta Neurol Scand. 1999;100:53–60. doi: 10.1111/j.1600-0404.1999.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 6.Shadlen MF, Larson EB, Wang L, et al. Education modifies the effect of apolipoprotein epsilon 4 on cognitive decline. Neurobiol Aging. 2005;26:17–24. doi: 10.1016/j.neurobiolaging.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Knopman DS, Mosley TH, Catellier DJ, et al. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement. 2009;5:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Packard C, Westendorp R, Stott D, et al. Association between apolipoprotein E4 and cognitive decline in elderly adults. J Am Geriatr Soc. 2007;55:1777–1785. doi: 10.1111/j.1532-5415.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- 9.Carmelli D, Swan GE, Reed T, et al. Midlife cardiovascular risk factors, ApoE, and cognitive decline in elderly male twins. Neurology. 1998;50(6):1580–1585. doi: 10.1212/wnl.50.6.1580. [DOI] [PubMed] [Google Scholar]
- 10.Deane R, Sagare A, Hamm K, et al. ApoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;11:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alzheimer’s disease and referral center. National Institute for Aging. Alzheimer’s disease genetics: fact sheet. NIH Publication. 2011;11:6424. [Google Scholar]
- 12.Buchman AS, Wilson RS, Bienias JL, et al. Change in body mass index and incident Alzheimer’s disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 13.Cronk BB, Johnson DK, Burns JM. Body mass index and cognitive decline in mild cognitive impairment. Alzheimer Dis Assoc Disord. 2010;24:126–130. doi: 10.1097/WAD.0b013e3181a6bf3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cournot M, Marquié JC, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 15.Yoon DH, Choi SH, Yu JH, et al. The relationship between visceral adiposity and cognitive performance in older adults. Age Aging. 2012;41:456–461. doi: 10.1093/ageing/afs018. [DOI] [PubMed] [Google Scholar]
- 16.Doruk J, Naharci MI, Bozoglu E, et al. The relationship between body mass index and incidental mild cognitive impairment, Alzheimer’s disease and vascular dementia in elderly. J Nutrition Health & Aging. 2010;10:834–838. doi: 10.1007/s12603-010-0113-y. [DOI] [PubMed] [Google Scholar]
- 17.Sturman MT, Mendes de Leon CF, Bienias JL, et al. Body mass index and cognitive decline in a biracial community population. Neurology. 2008;70:360–367. doi: 10.1212/01.wnl.0000285081.04409.bb. [DOI] [PubMed] [Google Scholar]
- 18.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer's disease in a community population higher than previously reported. JAMA. 1989;262:2251–2256. [PubMed] [Google Scholar]
- 19.Bienias JL, Beckett LA, Bennett DA, et al. Design of the Chicago Health and Aging Project. J Alz Dis. 2003;5:349–355. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 20.Albert M, Smith LA, Scherr PA, et al. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J of Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RS, Bennette DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older adults. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 22.Smith A. Western Psychological Services. Los Angeles: 1982. Symbol Digits Modalities Test. [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh TR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Wilson RS, Benette DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54:155–160. doi: 10.1093/geronb/54b.3.p155. [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachmann D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease. Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.WHO Consultation on Obesity. Obesity: preventing and managing the global epidemic: report of a WHO consultation. (WHO technical report series) Geneva, Switzerland: 1999. [PubMed] [Google Scholar]
- 27.Hixson J, Vernier DO. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with Hha l. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 28.Wenham PR, Price WH, Blundell G. Apolipoprotein E genotyping by one-stage PCR. Lancet. 1991;337:1158–1159. doi: 10.1016/0140-6736(91)92823-k. [DOI] [PubMed] [Google Scholar]
- 29.Diggle PJ, Heagerty P, Liang KY, et al. Analysis of longitudinal data. 2nd ed. New York, NY: Oxford University Press Inc; 2004. [Google Scholar]
- 30.Canty AJ, Davison AC. Resampling-based variance estimation for labor force surveys. The Statistician. 1999;48:379–391. [Google Scholar]
- 31.Preston J. Rescaled bootstrap for stratified multistage sampling. Survey Methodology. 2009;35(2):227–234. [Google Scholar]
- 32.R: A language and environment for statistical computing. Version 2.15.2. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]