Abstract
Tobacco produces an impressive burden of disease resulting in premature death in half of users. Despite effective smoking cessation medications (nicotine replacement therapies, bupropion and varenicline), there is a very high rate of relapse following quit attempts. The use of efficient strategies for the development of novel treatments is a necessity. A `bench to bedside strategy' was initially used to develop cannabinoid CB1 receptor antagonists for the treatment of nicotine addiction. Unfortunately, after being tested on experimental animals, what seemed to be an interesting approach for the treatment of nicotine addiction resulted in serious unwanted side effects when tested in humans. Current research is focusing again on pre-clinical models in an effort to eliminate unwanted side effects while preserving the initially observed efficacy. A `bed side to bench strategy' was used to study the role of the insula (part of the frontal cortex) in nicotine addiction. This line of research started based on clinical observations that patients suffering stroke-induced lesions to the insula showed a greater likelihood to report immediate smoking cessation without craving or relapse. Subsequently, animal models of addiction are used to explore the role of insula in addiction. Due to the inherent limitations existing in clinical versus preclinical studies, the possibility of close interaction between both models seems to be critical for the successful development of novel therapeutic strategies for nicotine dependence.
Keywords: Dependence, Nicotine, Reinstatement, Therapy, Treatment
1. Introduction
Tobacco, one of the most widely used substances, is responsible for 63% of all deaths produced by non-communicable diseases and resulting in nearly six million deaths each year, a figure expected to rise to more than eight million by 2030 (WHO, 2013). The devastating effects of tobacco are also suffered by people dying as a result of exposure to second-hand smoke, accounting for more than 600,000 deaths each year. Nicotine is the main component of tobacco responsible for its addictive properties and extensive research has been and is currently being conducted to elucidate the neurobiological mechanisms responsible for its addictive properties. However, improved research strategies are critical in view of the overwhelming increase in the death toll related to tobacco use predicted. It is also important to consider that approximately 80% of smokers worldwide are located in low/middle income countries (WHO, 2013), which makes the development of low cost treatments a necessity. Current first line medications for nicotine dependence include nicotine replacement therapies (patch, gum/lozenge, inhaler or spray), partial agonists of α4β2 subtype of the nicotinic acetylcholine receptor (varenicline), or dopamine and noradrenaline reuptake inhibitors such as bupropion (Fiore et al., 2000; Lancaster et al., 2008; Le Foll and George, 2007). Nicotine replacement therapies were the first pharmacological treatments approved by the Food and Drug Administration (FDA) for use in smoking cessation therapy, followed by bupropion and varenicline. Even if the effectiveness of nicotine replacement therapies, bupropion and varenicline appear to be high (Blum et al., 2008), doubling or tripling the smoking cessation rates in controlled studies (Le Foll and George, 2007), the real impact of these therapies has been questioned due to high rates of relapse in the long term (Alpert et al., 2013). There may be multiple reasons explaining those discrepancies such as the fact that clinical trial inclusion criteria do not always allow for generalization of results to the overall population of smokers. Thus, according to a meta-analysis recently published almost 66% of participants with nicotine dependence were excluded of clinical trials due at least one criterion (Le Strat et al., 2011). Two of the main factors for excluding participants are the lack of motivation to quit and low levels of cigarette consumption. Another reason explaining discrepancies might be the fact that intervention procedures in clinical trials do not always correspond to the way medications are used in real life. Thus, clinical trials do not always account for important factors implicated on the relapse process as the behavioral counseling or abstinence time period or do not comprehend periods of time long enough to evaluate the influence of those factors (Alpert et al., 2013). There is therefore an urgent need to develop novel and more effective treatment approaches. As progress in neurobiology continues at a fast pace, it has been suggested that translational research using a `bench to bedside' strategy may be useful to accelerate the development of novel therapeutic strategies. Here, we will illustrate how such an approach can be used either from `bench to bedside' or, conversely, from `bedside to bench', depending upon the particular situation. The first illustration is based on the idea of using cannabinoid CB1 receptor antagonists/inverse agonists to treat nicotine addiction. This example, which was first generated from animal observations and moved quickly into clinical testing, will illustrate how subsequently research can quickly return the pre-clinical phase in order to refine a therapeutic strategy. The second illustration provided is a clear example of reverse translational work involving the insula in drug addiction.
2. From bench to bedside and back: the role of CB1 receptors in nicotine addiction
2.1. Targeting the CB1 receptor
The involvement of the endocannabinoid system in drug addiction has been widely investigated following the pioneering work of De Vries et al. (2001). In particular, there has been great interest in targeting the endocannabinoid system for the treatment of obesity and nicotine dependence (Le Foll et al., 2008; Pacher et al., 2006). The endocannabinoid system consists of the endocannabinoids (primarily anandamide and 2-arachidonoylglycerol), the target receptors of those endocannabinoids (cannabinoid CB1, CB2 and possibly other receptors), enzymatic degradation systems (through the enzymes fatty acid amide hydrolase and monoacylglycerol lipase for anandamide and 2-arachidonoylglycerol, respectively) and a transport reuptake system (Di Marzo, 2006; Di Marzo et al., 1998, 2001; Mechoulam et al., 1998; Piomelli, 2003, 2005; Piomelli et al., 2000; Sugiura and Waku, 2002). The cannabinoid CB1 receptor, however, has received most of the attention as a target, mainly on the initial promise of Rimonabant (Acomplia® Sanofi-aventis), the first selective CB1 ligand introduced to market. This CB1 inverse agonist was shown to be efficacious as a treatment for obesity (Despres et al., 2009; Hampp et al., 2008) and for improving dyslipidemias, diabetes, and the metabolic syndrome (Despres et al., 2005; Scheen, 2008; Van Gaal et al., 2005). Rimonabant was in fact previously approved as an obesity treatment in more than 50 countries worldwide, including the European Union, and was also being developed for smoking cessation.
The rise of Rimonabant's potential as a therapeutic for smoking cessation began at the preclinical level. Cohen et al. (2002) were the first to demonstrate that Rimonabant pretreatment can attenuate nicotine-taking behavior under a fixed-ratio schedule and that it blocks nicotine-induced elevation of dopamine in the nucleus accumbens shell and bed nucleus of the stria terminalis (Cohen et al., 2002). We were also able to demonstrate that Rimonabant pretreatment maintains its efficacy under a progressive ratio schedule as well (Forget et al., 2009), indicating that Rimonabant decreases motivation for nicotine. By using animal models of nicotine reinstatement, which utilize either presentations of nicotine-associated cues, nicotine-priming injections or stress to induce nicotine-seeking behavior following the extinction of such behavior, we and others have demonstrated that Rimonabant also effectively decreases the reinstatement of nicotine-seeking behavior (Fig. 1) (Cohen et al., 2005a; Diergaarde et al., 2008; Forget et al., 2009). Rimonabant also seems to decrease preference for nicotine as observed using other paradigms to measure the rewarding properties of a drug such as the conditioned place preference paradigm (Forget et al., 2005; Le Foll and Goldberg, 2004). A large body of consistent preclinical studies has shown that blockade of the endocannabinoid system by Rimonabant is effective in decreasing motivation and/or relapse to drug-seeking for nicotine (Cohen et al., 2002; Cohen et al., 2005b; De Vries et al., 2005; Forget et al., 2009; Shoaib, 2008) as well as a variety of other drugs of abuse such as Δ9-tetrahydrocannabinol (Justinova et al., 2008; Tanda et al., 2000), opiates (De Vries et al., 2003; Solinas et al., 2003), psychostimulant drugs (Anggadiredja et al., 2004; De Vries et al., 2001; Tanda et al., 2000; Xi et al., 2008), and alcohol (Arnone et al., 1997; Colombo et al., 1998, 2007; Gallate and McGregor, 1999; Gallate et al., 1999, 2004; Houchi et al., 2005; Hungund et al., 2003; Naassila et al., 2004; Poncelet et al., 2003; Rinaldi-Carmona et al., 2004; Rodriguez de Fonseca et al., 1999) (see De Vries and Schoffelmeer, 2005; Fattore et al., 2007; Le Foll and Goldberg, 2005; Le Foll et al., 2008 for reviews).
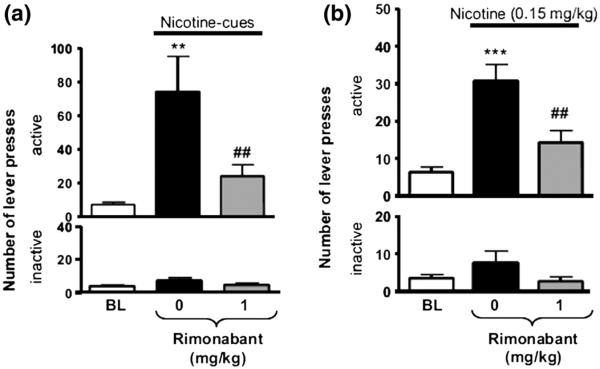
Fig. 1.
(a) Effect of Rimonabant (1 mg/kg, IP, H-60 min) on responses made on the active (top) and the inactive (below) levers during the cue-induced reinstatement of nicotine seeking tests (duration: 60min) after extinction. Data are expressed as means (±SEM) of the number of lever presses during baseline conditions (BL, no nicotine-associated cues), during sessions with vehicle pretreatment and the presence of nicotine-associated cues (0) and during sessions with Rimonabant pretreatment and the presence of nicotine-associated cues. N = 14. **p < 0.01 vs. baseline; ##p< 0.01 vs. vehicle pretreatment (0); Student–Newman–Keuls multiple comparison test after significant ANOVA for repeated measures. (b) Effect of Rimonabant (1 mg/kg, IP, H-70min) on responses made on the active (top) and the inactive (below) levers during nicotine (0.15 mg/kg, SC, H-10 min)-induced reinstatement of nicotine seeking tests (duration: 60 min) after extinction. Data are expressed as means (±SEM) of the number of active lever presses during baseline conditions (BL, no nicotine priming), during sessions with vehicle pretreatment and with nicotine priming (0) and during sessions with Rimonabant pretreatment and with nicotine priming. N=13. ***p<0.001 vs. baseline; ##p<0.001 vs. vehicle pretreatment (0); Student–Newman–Keuls multiple comparison test after significant ANOVA for repeated measures (Forget et al., 2009).
2.2. Clinical research on CB1 antagonists
With the initial promise gathered from the animal work, clinical trials investigating the efficacy of Rimonabant as a smoking cessation aid followed suit with promising results. Clinical studies using Rimonabant revealed statistically significant evidence of efficacy for the treatment of smokers by increasing the chances of quitting (abstinence rates, self-reported smoking abstinence and expired carbon monoxide concentrations verified in some of the studies by cotinine levels) (Cahill and Ussher, 2011; Le Foll et al., 2008; Rigotti et al., 2009). However, tempering this promise was a growing concern about the psychiatric safety profile of CB1 receptor inverse agonists (Christensen et al., 2007; Food and Drug Administration Endocrinologic and Metabolic Advisory, June 13, 2007; Rucker et al., 2007), notably due to increased rates of depression, anxiety and suicidal behavior related to Rimonabant use. In October of 2008, these psychiatric concerns led to a decision by the European Medicines Agency to suspend marketing of Rimonabant in the EU. Following this decision, the drug-maker Sanofi-Aventis announced on November 5th 2008 its decision to withdraw Rimonabant from the market worldwide and to discontinue its ongoing Rimonabant clinical development program for all indications (Sanofi Aventis, 2008). Around the same time, several other CB1 receptor inverse agonists not yet approved for marketing were withdrawn from clinical development by their developers (Merck, Pfizer, Solvay/Bristol-Myers Squibb) (see our commentary (Le Foll et al., 2009)).
2.3. From clinical research back to pre-clinical
Although the forward progress of Rimonabant had been halted, the potential and partial success of Rimonabant in the treatment of obesity and smoking cessation motivated the refinement and development of an alternative strategy aimed at modulating endocannabinoid transmission. The novel approach would have to be able to retain the therapeutic efficacy of CB1 inverse agonists for obesity and drug addiction, without the anxiogenic and depressive side effects in order to surpass the therapeutic potential of its predecessor. This refinement has led to the development of the novel neutral CB1 antagonist, AM4113 (Chambers et al., 2007; Cluny et al., 2011a; Sink et al., 2008, 2009a, 2009b) which exhibits 100-fold selectivity for CB1 vs CB2, with Ki = 0.89 nM and Ki = 92 nM for CB1 vs CB2, respectively (Sink et al., 2008). Preliminary preclinical studies show evidence that AM4113 retains the therapeutic efficacy of the CB1 inverse agonists AM251 and Rimonabant (Hodge et al., 2008; McLaughlin et al., 2003), as AM4113 suppresses responses for food (Sink et al., 2008) and food intake (Chambers et al., 2007), attenuates body weight gain (Cluny et al., 2011a), and alters the behavioral satiety sequence (Hodge et al., 2008).
A key determinant of neutral CB1 antagonists' success however will be based on whether it is devoid of the side effects of CB1 inverse agonists. In humans, CB1 inverse agonists increased nausea (Despres et al., 2005) and were shown to potentiate conditioned gaping in rats (McLaughlin et al., 2005) (model is thought to reflect nausea in rats, a species that cannot vomit). Unlike the inverse agonists, neutral CB1 receptor antagonists neither enhanced the nauseating effects of lithium chloride, nor produced anhedonia in a taste reactivity test (Limebeer et al., 2010) nor induced conditioned gaping in rats (Sink et al., 2008). Furthermore, CB1 inverse agonists have been shown to induce behavioral signs of anxiety (Arevalo et al., 2001; Haller et al., 2004; McGregor et al., 1996; Navarro et al., 1997; Rodgers et al., 2005), and a depression-like state in animals (Beyer et al., 2010). Evidence so far has shown AM4113 to have no effect in the elevated plus maze (Sink et al., 2010) or the forced swim test (Jutkiewicz et al., 2010), suggesting a lack of anxiety or depression-like effects.
Research related to the effects of neutral CB1 antagonists effect on nicotine – taking and seeking – in animal models is of the next highest priority, in addition to further validating its more favorable side effect profile in comparison to CB1 inverse agonists. A key emphasis in these future preclinical studies should focus on its chronic effects prior to translation into clinical studies, as this better emulates the human drug treatment experience. Chronic effects (21-days) of Rimonabant induced a depressive phenotype (Beyer et al., 2010) in animals, and whether neutral CB1 antagonists have a similar effect should be investigated. Furthermore, its efficacy on nicotine-taking during chronic treatment needs to be also studied as there is evidence that, as with Rimonabant, chronic AM4113 treatment results in a transient food intake reduction (Cluny et al., 2011b). The question that needs to be answered is whether this transient effect is limited only to food intake, or whether it is a general result seen with chronic neutral CB1 antagonist treatment. These questions need to be resolved prior to translation into clinical studies. However, if the neutral CB1 antagonists can indeed replicate the efficacy of Rimonabant without the side effects (i.e. anxiety and depression) associated with CB1 inverse agonists, it may become a promising therapeutic strategy for smoking cessation.
3. From bedside to bench and back: the role of the insula in addiction
3.1. Clinical research on the role of the insula in nicotine addiction
Although many of the neurobiological underpinnings of nicotine addiction have been identified through painstaking methodical studies in animal models and human imaging, occasionally, therapeutic targets can be identified by unconventional means. One such target is the insular cortex which, until quite recently, has been relatively overlooked in addiction literature. The seminal work of Naqvi et al. (2007), published in Science, was the first to propose that the insula may in fact play a critical role in nicotine addiction (Naqvi et al., 2007). This study took the unique approach of examining a phenomenon observed by neurologists in which stroke patients occasionally reported immediate smoking cessation without undertaking any active initiative to quit. The authors collected a sample of stroke patients who were smokers at the time of brain injury and assessed whether they demonstrated a stroke-induced disruption of smoking, exemplified by four criteria: `quitting smoking easily, immediately, without relapse, and without persistence of the urge to smoke'. It was identified that individuals with stroke-induced lesions to either the right or left insula had a significantly greater likelihood of having disruption of smoking as compared to individuals with stroke-induced lesions to non-insula areas. Though several limitations of these findings have been noted (Vorel et al., 2007), along with a study which failed to replicate them (Bienkowski et al., 2010), a recent prospective study demonstrated that stroke-induced insular lesions were a strongly associated factor in being a non-smoker at a 1-year post-stroke follow-up (Suner-Soler et al., 2012).
The insula has already been implicated in behaviors such as conscious urges, representation of interoceptive states, anxiety, pain, cognition and mood (Craig, 2002; Damasio et al., 2000; Goldman-Rakic, 1998; Hardy, 1985; Paulus and Stein, 2006; Suhara et al., 1992). The findings of Naqvi et al. (2007) described above were the first to suggest a crucial role for the insula in nicotine addiction yet the area was already known to have some involvement in addiction, particularly with regard to drug urges in human imaging studies (Naqvi and Bechara, 2009, 2010). However such studies, due to their broad observational nature, did not specifically focus on the insular cortex. Naqvi and Bechara, following their initial publication described above, conducted a review of such functional imaging studies in which the insula was observed to be activated during drug urges (Naqvi and Bechara, 2009). These studies involved cue-induced drug craving for cigarettes (Brody et al., 2002, 2007; Franklin et al., 2007; Lee et al., 2005; McBride et al., 2006; McClernon et al., 2005), cocaine (Bonson et al., 2002; Garavan et al., 2000; Kilts et al., 2004; Wang et al., 1999; Wexler et al., 2001), alcohol (Myrick et al., 2004; Tapert et al., 2004), heroin (Sell et al., 1999) and abstinence-induced craving for cigarettes (Wang et al., 2007). A more recent meta-analysis of fMRI studies on smoking cue reactivity concluded that smoking cues, as compared to neutral cues, reliably evoke larger fMRI responses in the insula (Engelmann et al., 2012). As such, more recent studies have continued to observe insular activation in a variety of addiction-relevant tasks. Left insular activation has also been correlated with cigarette craving during a smoking-related attentional bias task (Luijten et al., 2011) while right insula hyperactivity is observed in smokers during inhibition of an immediately-rewarding stimulus, in order to obtain a larger, delayed reward (Luijten et al., 2013). In other study, the smoking dependence (assessed with the Fagerström questionnaire) correlated with the magnitude of BOLD change in the right insula (Artiges et al., 2009). With regard to clinical implications for relapse, a correlation was found between smoking cue-induced insular activation and lapse during an 8-week smoking cessation in a clinical trial utilizing nicotine replacement therapy (Janes et al., 2010). These individuals also had decreased functional connectivity between the insula and inhibitory control regions such as the dorsal anterior cingulate cortex and the dorsal lateral prefrontal cortex. Similar results have been obtained in treatment-engaged, methamphetamine-dependent individuals where greater right insular activation during a simple decision making task performed during early recovery was correlated with increased likelihood of relapse at a 1 year follow-up (Paulus et al., 2005). Apart from cue-induced craving, left insular activation has been correlated with stress-induced cocaine craving and subjective distress (Sinha et al., 2005). It should be noted, in the same study, that right insular activation was inversely correlated with subjective distress suggesting differential roles between hemispheres. Interestingly, cocaine-dependent women appear to have greater stress-induced activation of the insula as compared to cocaine-dependent males (Potenza et al., 2012). In terms of clinical implications for relapse, stress-induced left posterior insular activation in treatment-engaged, abstinent cocaine-dependent individuals has been correlated with greater number of days of cocaine use reported at a 90-day follow-up (Sinha and Li, 2007).
3.2. Preclinical research on the role of the insula in addiction
The insula seems to be centrally placed to receive and integrate the information about the salience and relative value of environmental stimuli and of drug effects coming from brain structures that have been implicated in reward and addiction. Thus, neurons in the insula project their efferences to reward-related structures in rats, such as the ventral tegmental area (VTA), the medial prefrontal and cingulated cortex, and the nucleus accumbens and the amygdala (Gerfen and Clavier, 1979; Saper, 1982). It is possible that insula inactivation may modulate the function of dopamine neurons, therefore interfering with addiction processes. Since the insula also expresses high level of nicotinic receptors (nAChRs) containing the β2 subunit (Rubboli et al., 1994), the major subtype of nAChR implicated in nicotine reward (Grabus et al., 2006; Maskos et al., 2005), it is possible that activation of insula occurs during exposure to nicotine. The insula also receives a high density of terminals coming from dopaminergic neurons that arise mainly from the ventral tegmental area and substanstia nigra (Ohara et al., 2003) and contains large pyramidal neurons in layer 5 that have GABAb receptors (Margeta-Mitrovic et al., 1999). There are close appositions between dopamine fibers and GABAergic interneurons within the insula. Also GABAb receptor-bearing neurons project principally into the amygdala and nucleus accumbens (Ohara et al., 2003).
Following the seminal work of Naqvi et al. (2007), described above, several groups started using animal models of addiction to better understand the involvement of the insula in the neurobiology of addiction. Di Pietro et al. (2008) examined the role of the dopamine D1 receptor in the dorsal agranular insular subregion of the prefrontal cortex on cocaine self-administration behavior (Di Pietro et al., 2008). The D1 receptor antagonist, SCH23390, delivered intracranially directly to this area of the insula, was able to significantly decrease lever responding and cocaine intake. However, it should be noted that this behavior also resulted in significant decreases in operant food-maintained responding and overall intake, thus suggesting a non-drug specific mechanism. In another study, the conditioned preference of an amphetamine-associated environment stimulated neuronal activity in the insula while the inactivation of the granular region of the insula blocked the expression of amphetamine-induced conditioned place preference (CPP) (Contreras et al., 2007). A follow-up study also demonstrated a loss of amphetamine-induced CPP following a local injection of the protein synthesis inhibitor anisomycin into either the granular or agranular insula shortly after memory retrieval (Contreras et al., 2012). This loss, after anisomycin was injected into the agranular insula, was coupled with a decrease in zif-268 in both insular regions, but not the primary somatosensory cortex. The study also demonstrated that inactivating the granular insular cortex could facilitate the extinction of amphetamine-induced CPP. The hypocretin/orexin system has also been shown to play a role in nicotine addiction; specifically blockade of hypocretin-1 receptors in the granular insula decreases nicotine self-administration in rats (Hollander et al., 2008). The same study also demonstrated that hypocretin-containing fibers innervate the insula and that hypocretin-1 receptors are located on insular neurons. A study in mice demonstrated a role for the insula in nicotine-induced CPP but interestingly not in nicotine withdrawal-induced conditioned place aversion (CPA) (Scott and Hiroi, 2011).
The large amount of imaging data suggesting a role for the insula in drug craving, triggered by the seminal study of Naqvi et al. (2007), also stimulated interest in our laboratory to perform reverse translational work examining the role of the insula on nicotine addiction. The limitations of the findings of Naqvi et al. (2007) were primarily due to the nature of such retrospective studies. An animal model of nicotine addiction could allow for a direct validation of the implication of the insula in nicotine addiction. Hence, our first study examined the role of the insula using the intravenous self-administration paradigm in rats, as it is considered one of the best paradigms to model human addiction on experimental animals. This study involved local intracranial infusions of GABA agonists (baclofen and muscimol), in order to inactivate temporarily the granular insular cortex bilaterally for the period of the behavioral testing sessions (Forget et al., 2010). Essentially, this strategy allowed for a functional replication of the insular lesions observed by Naqvi et al. (2007). Utilizing this strategy, we were able to demonstrate that insular inactivation is capable of significantly reducing nicotine taking behavior, motivation for nicotine and nicotine-seeking behaviors reinstated by nicotine-associated cues or nicotine priming injections (Fig. 2) (Forget et al., 2010). In contrast, insula cortex inactivation did not affect lever pressing for food, motivation to get food, or food seeking behaviors, indicating that those results were selective for nicotine-seeking (Forget et al., 2010). Expanding on those results, we examined the ability of deep brain stimulation (DBS) to modulate electrical activity within the insula and thereby affect nicotine addiction. DBS is a technique that is widely used in Parkinson's disease (Bronstein et al., 2011) and a few groups have reported positive effects of DBS of the nucleus accumbens for smoking cessation (Kuhn et al., 2009; Mantione et al., 2010). Numerous psychiatric applications for DBS are being examined, particularly through the use of animal models (Hamani and Temel, 2012). Using high-frequency stimulation parameters, DBS of the bilateral insular region was able to reduce nicotine taking, motivation for nicotine and nicotine-seeking reinstated by nicotine-associated cues or nicotine priming injections (Pushparaj et al., 2013). Here again, there was no non-specific effects on responding maintained by food (Pushparaj et al., 2013). Not only were these parameters of insular DBS able to replicate the behavioral results of our previous insular inactivation study, but in vitro electrophysiology demonstrated that these parameters were capable of inactivating insular neurons. Taken together, these findings suggest that modulating the function of the insula could be a novel therapeutic strategy for treatment of nicotine dependence and our results directly confirm that the insula is a critical neural substrate in nicotine addiction.
Fig. 2.
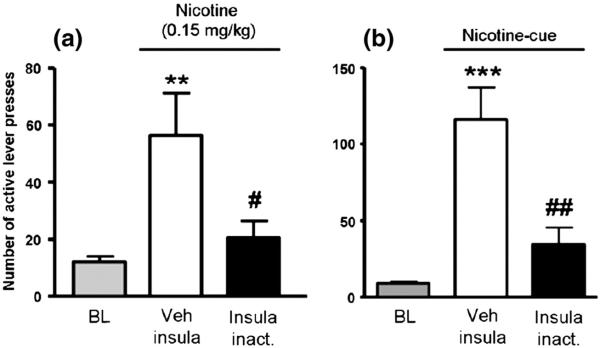
Effect of insula inactivation on (a) nicotine- (.15 mg/kg SC, 10 min before the session) or (b) cue-induced reinstatement of nicotine seeking tests after extinction (n = 8 and n = 6, respectively). Data are expressed as means (±SEM) of the number of lever presses during baseline conditions (BL) and during sessions with infusion of vehicle (Veh insula) or the Bac/Mus mix in the insula (insula inact.) 5–10min before nicotine priming. *p<.05; **p<.01; ***p< .001 versus baseline; #p<.05; ##p< .01 versus vehicle infusion (Veh insula); Student–Newman–Keuls multiple comparison test after significant analysis of variance for repeated measures.
The use of pre-clinical models to study the implication of the insula in nicotine dependence continues. Thus, Abdolahi et al. (2010) have shown enhanced protein kinase A-regulated signaling of dopamine-and cAMP-regulated phosphoprotein of 32 kDa in the insula, but not the medial prefrontal cortex, following the incubation of cue-induced nicotine-seeking behavior (Abdolahi et al., 2010) and it has been recently shown that chronic nicotine, administered through osmotic mini-pumps, also induced dendritic remodeling in the agranular insula (Ehlinger et al., 2012). However, it seems that the use of animal models has only just begun to scratch the surface with regard to establishing a place for the insula within addiction and its underlying neurobiology and neurocircuitry.
3.3. Back to clinical research strategy
More recently, human imaging work has also begun to focus on the insula and began to examine potential neural circuits through which it mediates its involvement in nicotine addiction. Reduced resting state functional connectivity of the insula, dorsal anterior cingulate cortex and striatum is correlated with increasing nicotine addiction severity, as indicated by higher Fagerstrom Test of Nicotine Dependence scores (Moran et al., 2012). In this regard, structural imaging has indicated that smokers have an increased left insular cortex gray matter density compared to non-smokers and that this increased density is correlated with a higher score on the Toronto Alexithymia Scale (TAS-20) (Zhang et al., 2011a). Smokers with higher alexithymia, a personality trait characterized by the inability to identify and describe one's emotional experiences, appear to have reduced resting-state functional connectivity between the right anterior insula and the ventromedial prefrontal cortex (Sutherland et al., 2013b). In turn, this decreased connectivity in alexithymic individuals is correlated with craving for cigarettes during withdrawal. Overnight nicotine withdrawal results in increased resting state functional connectivity of the amygdala-insula circuit and the insula-default mode network (posterior cingulate cortex, medial prefrontal cortex, parahippocampus) circuit (Sutherland et al., 2013a). These elevations are blunted by both transdermal nicotine and varenicline, suggesting that insula-related circuits are, in a likelihood, involved in the subjective symptoms of nicotine withdrawal. Interestingly, the resting state functional connectivity between the dorsal medial prefrontal cortex and the left insula was negatively correlated with smoking-cue induced activity in these regions, suggesting a possible mutual inhibition of cue reactivity (Zhang et al., 2011b).
The goal ultimately will be to target the insula as a potential therapeutic intervention for addiction. One approach to modulating electrical activity within brain regions, that is less invasive than DBS, is repetitive transcranial magnetic stimulation (rTMS). rTMS has been shown to be able to create lasting changes in brain activity and is already being explored for drug addiction (Barr et al., 2011; Bellamoli et al., 2013). The development of the H-coil for targeting deep brain regions using rTMS (Levkovitz et al., 2007; Roth et al., 2002, 2007) is already being studied for its application in psychiatric disease (Harel et al., 2011, 2012; Levkovitz et al., 2009; Rosenberg et al., 2011). The H-coil has the potential to target the insula and future work will likely examine its ability to modulate insular activity as a therapeutic for smoking cessation. Regardless, it is undeniable that the insula is a crucial brain region involved in nicotine addiction and its potential as a target is clear. Both preclinical animal and human imaging studies will continue to explore the neurobiology underlying its involvement and begin to establish its place within the neurocircuitry of nicotine addiction.
4. Conclusions
As in other fields of research, when studying drug addiction, an integrated approach combining interdisciplinary areas of research seems to offer the best results. As part of this attempt to use an integrated approach, current limitations present on recent clinical trials should be overtaken by using more flexible criteria regarding nicotine consumption while giving more specific weight to other factors showing nicotine dependence. Future approaches might benefit also from accounting for relevant factors on the relapse process as the existence of counseling or by evaluating participants for larger time's periods in order to have a whole picture of the process. On the other hand, inherent limitations of preclinical studies should be also considered when comparing or integrating with clinical research even when using the best available paradigms (i.e. intravenous self-administration, dependence models). It is clear that animal models have some limitations. Animal models allow studying different specific aspects (such as rewarding effects, or nicotine-seeking induced by different stimuli) on the addiction process and their underlying neurobiological substrates, however, in a human subject all those factors are combined and not present in isolation. Human subjects also often present dual pathology (such as concomitant medical or psychiatric conditions) and a particular history with their abused substance or are exposed to other psychotropic drugs (caffeine, illicit drugs, psychoactive medications…). All those factors are obviously very difficult to incorporate on experimental settings for animal models of addiction. Therefore, it is of critical importance to perform research in human subjects to validate the findings that are generated by animal models.
Future interactions between pre-clinical and clinical approaches are warranted in the search for novel therapeutic approaches for nicotine dependence (see (Lerman et al., 2007) for an excellent review on this topic). Specific strategies to achieve this might include developing translational centers where bench to bedside or bedside to bench and back research strategies might be easily implemented. Translational centers on a determinate area of research should group both the infrastructure and the different professionals necessary to perform both types of research on a very specific topic what might foster the process.
As illustrated by this article, improving the process for developing effective therapies will require constant communication between scientists and clinicians allowing for the continuous re-evaluation of ideas and concepts to achieve beneficial therapies with minimal side-effects.
Acknowledgments
Research presented in this project was supported by Canadian Foundation for Innovation Leader Opportunity Fund, by a CIHR grant (201203MOP-274360-BSB-CEAH-17775) and by an independent Global Research Award on Nicotine Dependence Award funded by Pfizer (WS770983) awarded to BLF. Supported in part by a 2009 NARSAD Independent Investigator Award (ID 08-269) awarded to BLF.
Abbreviations
- α4β2
ALPHA-4 beta-2 nicotinic receptor
- β2
nicotinic receptor subunit
- AM251
inverse agonist at the CB1 cannabinoid receptor
- AM4113
CB1 putative neutral antagonist
- BOLD
blood–oxygen-level dependent
- cAMP
Cyclic Adenosine Monophosphate
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- CPP
conditioned place preference
- DBS
deep brain stimulation
- EU
European Union
- FDA
Food and Drug Administration
- fMRI
functional magnetic resonance imaging
- GABA
gamma-aminobutyric acid
- GABAb
metabotropic transmembrane receptor for gamma-aminobutyric acid
- Hypocretin-1
orexin-A
- kDa
kiloDalton
- Ki
inhibition constant
- nAChRs
nicotinic receptors
- nM
nanomolar
- Rimonabant
inverse agonist at the CB1 cannabinoid receptor
- rTMS
transcranial magnetic stimulation
- SCH23390
D1 receptor antagonist
- TAS-20
Toronto Alexithymia Scale
- VTA
ventral tegmental area
- WHO
World Health Organization
- zif-268
Zinc Finger Transcription Factor
References
- Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31:733–41. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert HR, Connolly GN, Biener L. A prospective cohort study challenging the effectiveness of population-based medical intervention for smoking cessation. Tob Control. 2013;22:32–7. doi: 10.1136/tobaccocontrol-2011-050129. [DOI] [PubMed] [Google Scholar]
- Anggadiredja K, Nakamichi M, Hiranita T, Tanaka H, Shoyama Y, Watanabe S, et al. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004;29:1470–8. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- Arevalo C, de Miguel R, Hernandez-Tristan R. Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacol Biochem Behav. 2001;70:123–31. doi: 10.1016/s0091-3057(01)00578-0. [DOI] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–6. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Artiges E, Ricalens E, Berthoz S, Krebs MO, Penttila J, Trichard C, et al. Exposure to smoking cues during an emotion recognition task can modulate limbic fMRI activation in cigarette smokers. Addict Biol. 2009;14:469–77. doi: 10.1111/j.1369-1600.2009.00167.x. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB, Daskalakis ZJ. Repetitive transcranial magnetic stimulation and drug addiction. Int Rev Psychiatry. 2011;23:454–66. doi: 10.3109/09540261.2011.618827. [DOI] [PubMed] [Google Scholar]
- Bellamoli E, Manganotti P, Schwartz RP, Rimondo C, Gomma M, Serpelloni G. rTMS in the treatment of drug addiction: an update about human studies. Behav Neurol. 2013 Jul 3; doi: 10.1155/2014/815215. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer CE, Dwyer JM, Piesla MJ, Platt BJ, Shen R, Rahman Z, et al. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol Dis. 2010;39:148–55. doi: 10.1016/j.nbd.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Zatorski P, Baranowska A, Ryglewicz D, Sienkiewicz-Jarosz H. Insular lesions and smoking cessation after first-ever ischemic stroke: a 3-month follow-up. Neurosci Lett. 2010;478:161–4. doi: 10.1016/j.neulet.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen AL, Chen TJ, Braverman ER, Reinking J, Blum SH, et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model. 2008;5:24. doi: 10.1186/1742-4682-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–86. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–72. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68:165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Ussher M. Cannabinoid type 1 receptor antagonists (rimonabant) for smoking cessation. Cochrane Database Syst Rev. 2011;16:CD005353. doi: 10.1002/14651858.CD005353.pub2. http://dx.doi.org/10.1002/14651858. [CD005353.pub4. Review] [DOI] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, et al. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–93. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–13. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Chambers AP, Vemuri VK, Wood JT, Eller LK, Freni C, et al. The neutral cannabinoid CB receptor antagonist AM4113 regulates body weight through changes in energy intake in the rat. Pharmacol Biochem Behav. 2011a;97:537–43. doi: 10.1016/j.pbb.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluny NL, Chambers AP, Vemuri VK, Wood JT, Eller LK, Freni C, et al. The neutral cannabinoid CB(1) receptor antagonist AM4113 regulates body weight through changes in energy intake in the rat. Pharmacol Biochem Behav. 2011b;97:537–43. doi: 10.1016/j.pbb.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–63. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Cohen C, Kodas E, Griebel G. CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol Biochem Behav. 2005a;81:387–95. doi: 10.1016/j.pbb.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB(1)) receptor antagonist, Rimonabant (SR141716) Neuropsychopharmacology. 2005b;30:145–55. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, et al. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–30. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- Colombo G, Orru A, Lai P, Cabras C, Maccioni P, Rubio M, et al. The cannabinoid CB1 receptor antagonist, rimonabant, as a promising pharmacotherapy for alcohol dependence: preclinical evidence. Mol Neurobiol. 2007;36:102–12. doi: 10.1007/s12035-007-0017-y. [DOI] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–8. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Contreras M, Billeke P, Vicencio S, Madrid C, Perdomo G, Gonzalez M, et al. A role for the insular cortex in long-term memory for context-evoked drug craving in rats. Neuropsychopharmacology. 2012;37:2101–8. doi: 10.1038/npp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN. Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci. 2005;26:420–6. doi: 10.1016/j.tips.2005.06.002. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–4. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmeer AN. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology (Berl) 2003;168:164–9. doi: 10.1007/s00213-003-1422-1. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, de Vries W, Janssen MC, Schoffelmeer AN. Suppression of conditioned nicotine and sucrose seeking by the cannabinoid-1 receptor antagonist SR141716A. Behav Brain Res. 2005;161:164–8. doi: 10.1016/j.bbr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–34. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Despres JP, Ross R, Boka G, Almeras N, Lemieux I. Effect of rimonabant on the high-triglyceride/low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat. The ADAGIO-lipids trial. Arterioscler Thromb Vasc Biol. 2009;29:416–23. doi: 10.1161/ATVBAHA.108.176362. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2006;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–8. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Bisogno T. Endocannabinoids Part I: molecular basis of endocannabinoid formation, action and inactivation and development of selective inhibitors. Expert Opin Ther Targets. 2001;5:241–65. doi: 10.1517/14728222.5.2.241. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Mashhoon Y, Heaney C, Yager LM, Kantak KM. Role of dopamine D1 receptors in the prefrontal dorsal agranular insular cortex in mediating cocaine self-administration in rats. Psychopharmacology (Berl) 2008;200:81–91. doi: 10.1007/s00213-008-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, et al. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–8. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Ehlinger DG, Bergstrom HC, McDonald CG, Smith RF. Nicotine-induced dendritic remodeling in the insular cortex. Neurosci Lett. 2012;516:89–93. doi: 10.1016/j.neulet.2012.03.064. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–62. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Endocannabinoid regulation of relapse mechanisms. Pharmacol Res. 2007;56:418–27. doi: 10.1016/j.phrs.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER. Clinical practice guideline. U.S. Department of Health and Human Service. Public Health Service; Rockville, MD: 2000. Treating tobacco use and dependence. [Google Scholar]
- Food and Drug Administration Endocrinologic and Metabolic Advisory [(accessed March 13, 2008)];Briefing information, NDA 21-888 ZIMULTI (rimonabant)-Sanofi Aventis. 2007 Jun 13; 2007. [ http://wwwfdagov/ohrms/dockets/ac/07/briefing/2007-4306b1-fda-backgrounderpdf.
- Forget B, Hamon M, Thiebot MH. Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology (Berl) 2005;181:722–34. doi: 10.1007/s00213-005-0015-6. [DOI] [PubMed] [Google Scholar]
- Forget B, Coen KM, Le Foll B. Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration—comparison with CB(1) receptor blockade. Psychopharmacology (Berl) 2009;205:613–24. doi: 10.1007/s00213-009-1569-5. [DOI] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010;68:265–71. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–9. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Gallate JE, McGregor IS. The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacology (Berl) 1999;142:302–8. doi: 10.1007/s002130050893. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;370:233–40. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Mallet PE, McGregor IS. Combined low dose treatment with opioid and cannabinoid receptor antagonists synergistically reduces the motivation to consume alcohol in rats. Psychopharmacology (Berl) 2004;173:210–6. doi: 10.1007/s00213-003-1694-5. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Clavier RM. Neural inputs to the prefrontal agranular insular cortex in the rat: horseradish peroxidase study. Brain Res Bull. 1979;4:347–53. doi: 10.1016/s0361-9230(79)80012-x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol. 1998;42:707–11. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 2006;184:456–63. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4:142rv8. doi: 10.1126/scitranslmed.3003722. [DOI] [PubMed] [Google Scholar]
- Hampp C, Hartzema AG, Kauf TL. Cost–utility analysis of rimonabant in the treatment of obesity. Value Health. 2008;11:389–99. doi: 10.1111/j.1524-4733.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- Hardy SG. Analgesia elicited by prefrontal stimulation. Brain Res. 1985;339:281–4. doi: 10.1016/0006-8993(85)90093-9. [DOI] [PubMed] [Google Scholar]
- Harel EV, Zangen A, Roth Y, Reti I, Braw Y, Levkovitz Y. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12:119–26. doi: 10.3109/15622975.2010.510893. [DOI] [PubMed] [Google Scholar]
- Harel EV, Rabany L, Deutsch L, Bloch Y, Zangen A, Levkovitz Y. H-coil repetitive transcranial magnetic stimulation for treatment resistant major depressive disorder: an 18-week continuation safety and feasibility study. World J Biol Psychiatry. 2012 Feb 7; doi: 10.3109/15622975.2011.639802. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hodge J, Bow JP, Plyler KS, Vemuri VK, Wisniecki A, Salamone JD, et al. The cannabinoid CB1 receptor inverse agonist AM 251 and antagonist AM 4113 produce similar effects on the behavioral satiety sequence in rats. Behav Brain Res. 2008;193:298–305. doi: 10.1016/j.bbr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–5. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB(1) receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology. 2005;30:339–49. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–9. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Munzar P, Panlilio LV, Yasar S, Redhi GH, Tanda G, et al. Blockade of THC-seeking behavior and relapse in monkeys by the cannabinoid CB(1)-receptor antagonist rimonabant. Neuropsychopharmacology. 2008;33:2870–7. doi: 10.1038/npp.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EMA, Vemuri VK, Bergman J. Pro-depressant-like effects of CB1 receptor inverse agonists/antagonists in male Sprague–Dawley rats. FASEB J. 2010 Apr 24; Meeting Abstract Supplement. 581.7. http://www.fasebj.org/cgi/content/meeting_abstract/24/1_MeetingAbstracts/581.7.
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–41. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Bauer R, Pohl S, Lenartz D, Huff W, Kim EH, et al. Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. Eur Addict Res. 2009;15:196–201. doi: 10.1159/000228930. [DOI] [PubMed] [Google Scholar]
- Lancaster T, Stead L, Cahill K. An update on therapeutics for tobacco dependence. Expert Opin Pharmacother. 2008;9:15–22. doi: 10.1517/14656566.9.1.15. [DOI] [PubMed] [Google Scholar]
- Le Foll B, George TP. Treatment of tobacco dependence: integrating recent progress into practice. Can Med Assoc J. 2007;177:1373–80. doi: 10.1503/cmaj.070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport. 2004;15:2139–43. doi: 10.1097/00001756-200409150-00028. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312:875–83. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Forget B, Aubin HJ, Goldberg SR. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict Biol. 2008;13:239–52. doi: 10.1111/j.1369-1600.2008.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl) 2009;205:171–4. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Strat Y, Rehm J, Le Foll B. How generalisable to community samples are clinical trial results for treatment of nicotine dependence: a comparison of common eligibility criteria with respondents of a large representative general population survey. Tob Control. 2011;20:338–43. doi: 10.1136/tc.2010.038703. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lim Y, Wiederhold BK, Graham SJ. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Appl Psychophysiol Biofeedback. 2005;30:195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- Lerman C, Lesage MG, Perkins KA, S.O.M.S., Siegel SJ, Benowitz NL, et al. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–62. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Roth Y, Harel EV, Braw Y, Sheer A, Zangen A. A randomized controlled feasibility and safety study of deep transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:2730–44. doi: 10.1016/j.clinph.2007.09.061. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Harel EV, Roth Y, Braw Y, Most D, Katz LN, et al. Deep transcranial magnetic stimulation over the prefrontal cortex: evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimul. 2009;2:188–200. doi: 10.1016/j.brs.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Vemuri VK, Bedard H, Lang ST, Ossenkopp KP, Makriyannis A, et al. Inverse agonism of cannabinoid CB1 receptors potentiates LiCl-induced nausea in the conditioned gaping model in rats. Br J Pharmacol. 2010;161:336–49. doi: 10.1111/j.1476-5381.2010.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, van den Brink W, Hester R, Field M, Smits M, et al. Neurobiological substrate of smoking-related attentional bias. Neuroimage. 2011;54:2374–81. doi: 10.1016/j.neuroimage.2010.09.064. [DOI] [PubMed] [Google Scholar]
- Luijten M, O'Connor DA, Rossiter S, Franken IH, Hester R. Effects of reward and punishment on brain activations associated with inhibitory control in cigarette smokers. Addiction. 2013;108:1969–78. doi: 10.1111/add.12276. [DOI] [PubMed] [Google Scholar]
- Mantione M, van de Brink W, Schuurman PR, Denys D. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery. 2010;66:E218. doi: 10.1227/01.NEU.0000360570.40339.64. discussion E. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J Comp Neurol. 1999;405:299–321. doi: 10.1002/(sici)1096-9861(19990315)405:3<299::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–7. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–38. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–7. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Issakidis CN, Prior G. Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol Biochem Behav. 1996;53:657–64. doi: 10.1016/0091-3057(95)02066-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, et al. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–8. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology (Berl) 2005;180:286–93. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Moran LV, Sampath H, Stein EA, Hong LE. Insular and anterior cingulate circuits in smokers with schizophrenia. Schizophr Res. 2012;142:223–9. doi: 10.1016/j.schres.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–53. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–50. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Hernandez E, Munoz RM, del Arco I, Villanua MA, Carrera MR, et al. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8:491–6. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Granato A, Moallem TM, Wang BR, Tillet Y, Jasmin L. Dopaminergic input to GABAergic neurons in the rostral agranular insular cortex of the rat. J Neurocytol. 2003;32:131–41. doi: 10.1023/b:neur.0000005598.09647.7f. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–8. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr Opin Investig Drugs. 2005;6:672–9. [PubMed] [Google Scholar]
- Piomelli D, Giuffrida A, Calignano A, Rodriguez de Fonseca F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci. 2000;21:218–24. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- Poncelet M, Maruani J, Calassi R, Soubrie P. Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett. 2003;343:216–8. doi: 10.1016/s0304-3940(03)00397-5. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 2012;169:406–14. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushparaj A, Hamani C, Yu W, Shin DS, Kang B, Nobrega JN, et al. Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology. 2013;38:690–8. doi: 10.1038/npp.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Gonzales D, Dale LC, Lawrence D, Chang Y. A randomized controlled trial of adding the nicotine patch to rimonabant for smoking cessation: efficacy, safety and weight gain. Addiction. 2009;104:266–76. doi: 10.1111/j.1360-0443.2008.02454.x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Congy C, Martinez S, Oustric D, Perio A, et al. SR147778, a new potent and selective antagonist of the CB1 cannabinoid receptor. Biochemical and pharmacological characterization. J Pharmacol Exp Ther. 2004;310:905–14. doi: 10.1124/jpet.104.067884. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Evans PM, Murphy A. Anxiogenic profile of AM-251, a selective cannabinoid CB1 receptor antagonist, in plus-maze-naive and plus-maze-experienced mice. Behav Pharmacol. 2005;16:405–13. doi: 10.1097/00008877-200509000-00013. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR141716A decreases operant ethanol self administration in rats exposed to ethanol-vapor chambers. Zhongguo Yao Li Xue Bao. 1999;20:1109–14. [PubMed] [Google Scholar]
- Rosenberg O, Roth Y, Kotler M, Zangen A, Dannon P. Deep transcranial magnetic stimulation for the treatment of auditory hallucinations: a preliminary open-label study. Ann Gen Psychiatry. 2011;10:3. doi: 10.1186/1744-859X-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol. 2002;19:361–70. doi: 10.1097/00004691-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Roth Y, Amir A, Levkovitz Y, Zangen A. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J Clin Neurophysiol. 2007;24:31–8. doi: 10.1097/WNP.0b013e31802fa393. [DOI] [PubMed] [Google Scholar]
- Rubboli F, Court JA, Sala C, Morris C, Perry E, Clementi F. Distribution of neuronal nicotinic receptor subunits in human brain. Neurochem Int. 1994;25:69–71. doi: 10.1016/0197-0186(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–9. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanofi Aventis . Sanofi-Aventis to discontinue all clinical trials with Rimonabant. Paris: Nov 5, 2008. 2008. [Google Scholar]
- Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210:163–73. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- Scheen AJ. CB1 receptor blockade and its impact on cardiometabolic risk factors: overview of the RIO programme with rimonabant. J Neuroendocrinol. 2008;20(Suppl. 1):139–46. doi: 10.1111/j.1365-2826.2008.01681.x. [DOI] [PubMed] [Google Scholar]
- Scott D, Hiroi N. Deconstructing craving: dissociable cortical control of cue reactivity in nicotine addiction. Biol Psychiatry. 2011;69:1052–9. doi: 10.1016/j.biopsych.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell LA, Morris J, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Activation of reward circuitry in human opiate addicts. Eur J Neurosci. 1999;11:1042–8. doi: 10.1046/j.1460-9568.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- Shoaib M. The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-seeking behaviour in rats. Neuropharmacology. 2008;52:438–44. doi: 10.1016/j.neuropharm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, et al. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183:171–80. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, et al. The novel cannabinoid CB(1) receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:946–55. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Nunes EJ, Collins LE, Vemuri VK, Thakur G, et al. Intracerebroventricular administration of cannabinoid CB1 receptor antagonists AM251 and AM4113 fails to alter food-reinforced behavior in rats. Psychopharmacology (Berl) 2009a;206:223–32. doi: 10.1007/s00213-009-1602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Wood J, Makriyannis A, Salamone JD. Oral bioavailability of the novel cannabinoid CB1 antagonist AM6527: effects on food-reinforced behavior and comparisons with AM4113. Pharmacol Biochem Behav. 2009b;91:303–6. doi: 10.1016/j.pbb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Sink J, Randall PA, Collins LE, Correa M, et al. Potential anxiogenic effects of cannabinoid CB1 receptor antagonists/inverse agonists in rats: comparisons between AM4113, AM251, and the benzodiazepine inverse agonist FG-7142. Eur Neuropsychopharmacol. 2010;20:112–22. doi: 10.1016/j.euroneuro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Waku K. Cannabinoid receptors and their endogenous ligands. J Biochem. 2002;132:7–12. doi: 10.1093/oxfordjournals.jbchem.a003200. [DOI] [PubMed] [Google Scholar]
- Suhara T, Nakayama K, Inoue O, Fukuda H, Shimizu M, Mori A, et al. D1 dopamine receptor binding in mood disorders measured by positron emission tomography. Psychopharmacology (Berl) 1992;106:14–8. doi: 10.1007/BF02253582. [DOI] [PubMed] [Google Scholar]
- Suner-Soler R, Grau A, Gras ME, Font-Mayolas S, Silva Y, Davalos A, et al. Smoking cessation 1 year poststroke and damage to the insular cortex. Stroke. 2012;43:131–6. doi: 10.1161/STROKEAHA.111.630004. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013a;228:43–55. doi: 10.1016/j.biopsych.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Stein EA. Insula's functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology (Berl) 2013b;228:143–55. doi: 10.1007/s00213-013-3018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–4. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–97. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Bisaga A, McKhann G, Kleber HD. Insula damage and quitting smoking. Science. 2007;317:318–9. doi: 10.1126/science.317.5836.318c. author reply -9. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–84. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–40. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, et al. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- WHO 2013.
- Xi ZX, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, et al. Cannabinoid CB1 receptor antagonists attenuate cocaine's rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology. 2008;33:1735–45. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011a;54:42–8. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Gu H, Geng X, Yang Y, et al. Anatomical differences and network characteristics underlying smoking cue reactivity. Neuroimage. 2011b;54:131–41. doi: 10.1016/j.neuroimage.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]