Abstract
Aims
To determine whether transcutaneous foot stimulation combined with a lower dose tolterodine would inhibit bladder overactivity more effectively than either treatment alone.
Methods
Cystometrograms were performed on α-chloralose anesthetized cats (N = 6) by infusing 0.25% acetic acid (AA) to induce bladder overactivity. Foot stimulation (5 Hz) was applied at 2 and 4 times the threshold (T) intensity in volts (i.e., 2T or 4T) for inducing toe movement to inhibit bladder overactivity. Cumulative doses of tolterodine (0.003–0.3 mg/kg, i.v.) were also administered to determine the effect of combination treatment.
Results
AA irritation of the bladder significantly (P < 0.0001) reduced bladder capacity to 23.6±7.1% of saline control capacity. Foot stimulation alone at 2T and 4T inhibited bladder overactivity and significantly (P < 0.0001) increased bladder capacity to 50.7±6.8% and 79.0±11.6% of saline control, respectively. Tolterodine alone at 0.3 mg/kg significantly (P < 0.05) increased bladder capacity to 65.6±15.5% of saline control. However, when tolterodine at a threshold dose (0.3 mg/kg) was combined with foot stimulation, the bladder capacity was significantly (P < 0.05) increased to 86.2±6.2% and 107.9±10.6% by 2T and 4T stimulation, respectively. Complete inhibition of bladder overactivity could be achieved at a lower tolterodine dose (0.1 mg/kg) when combined with 4T stimulation (97.0±11.2% of saline control). The amplitude of micturition contraction was not changed by tolterodine treatment.
Conclusions
This study suggests a novel, efficacious, non-invasive therapy by combining foot stimulation with a lower dose tolterodine to treat bladder overactivity. It also provides the first objective evidence supporting an additive therapeutic benefit of neuromodulation and antimuscarinic combination treatment.
Keywords: overactive bladder, antimuscarinics, tolterodine, neuromodulation, cat
INTRODUCTION
Overactive bladder (OAB) affects 16–17% of the general population and over 50% of nursing home residents in the US and Europe,1,2 and costs $12.6 billion per year in the US alone.3 Antimuscarinic drugs remain the first line of therapy, but side effects and poor efficacy result in a patient dropout rate of 41% after 4 months and 77% at 1 year.4 Meta-analyses of antimuscarinic drugs revealed that 53.4% of patients experience adverse effects including dry-mouth, pruritus, constipation, and dyspepsia.5,6 Sacral and pudendal neuromodulation are effective second line therapies for refractory OAB, however these treatments require invasive surgery for implantation of the device and adverse effects can result in reoperation.7–9 Tibial neuromodulation is also a second line option for OAB, however its efficacy is similar to antimuscarinic drugs and cannot completely eliminate OAB symptoms.10 While tibial neuromodulation may be less invasive, it is inconvenient and costly because it requires frequent stimulation sessions conducted by medically trained staff to maintain its efficacy.11 Consequently, a more effective, non-invasive, and convenient form of neuromodulation therapy is needed to treat OAB.
Our previous studies in cats have revealed a novel, non-invasive method to suppress bladder overactivity by stimulating somatic afferent nerves in the foot with skin surface electrodes.12,13 Transcutaneous foot stimulation offers a more convenient OAB treatment that can be performed by the patient at home. However, like tibial neuromodulation, foot stimulation in cats was unable to completely suppress bladder overactivity.12–14 Recent clinical evidence has demonstrated a significant improvement in OAB symptoms when patients with incomplete responses to sacral or tibial neuromodulation were also treated with antimuscarinic drugs, suggesting a positive additive benefit of the combination therapies.15,16 The novel therapy produced by combining neuromodulation with an antimuscarinic drug raises the possibility of more effective OAB treatment at a lower pharmacologic dose compared to drug therapy alone. Reducing antimuscarinic dosage could lower unwanted adverse effects thus improving patient compliance. Therefore, we hypothesize that inhibition of bladder overactivity induced by non-invasive foot stimulation can be augmented with the addition of a low dose of antimuscarinic drug.
In this preclinical study using anesthetized cats, dilute (0.25%) acetic acid (AA) was used to irritate the bladder, activate nociceptive afferent C-fibers, and induce bladder overactivity. The first-line antimuscarinic drug, tolterodine, was administered with non-invasive foot stimulation to determine whether this combination treatment is more effective in inhibiting bladder overactivity than either tolterodine or foot stimulation alone.
MATERIALS AND METHODS
All protocols involving the use of animals in the present study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Experimental Setup
Experiments were conducted in a total of 6 adult cats (4 female and 2 male cats between 2.8–3.8 kg) under α-chloralose anesthesia (65 mg/kg, supplemented as necessary) after induction with isoflurane (2–3% in O2). Heart rate and blood oxygen level were monitored with a pulse oximeter (9847 V, Nonin Medical Inc., Plymouth, MN, USA) that was attached to the tongue. Systemic blood pressure was monitored via a catheter in the right carotid artery. These physiological parameters were monitored to ensure that the animal's vital functions remained relatively stable during the entire experiment. Drugs or fluids were administered through a catheter in the right cephalic vein and airway access was secured with a tracheostomy tube. Ureters were accessed through a midline abdominal incision and drained externally. The bladder was cannulated through the urethra with a double lumen catheter to infuse (1–2 ml/min) saline or 0.25% acetic acid (AA) via one lumen and measure bladder pressure via another lumen. A ligature was tied around the proximal urethra to prevent leakage. Fur was removed from the foot and two self-adhesive pad electrode (Grass FE10ND, Astro-Medical Inc., Mentor, OH, USA; diameter 1 cm) were attached to the skin at the bottom of the left hind foot. One electrode was at the front of the foot and the other was at the heel.
Stimulation Protocol and Drug Administration
Uniphasic rectangular pulses (5 Hz frequency, 0.2 ms pulse-width) were delivered to the skin electrodes on the foot. Threshold (T) stimulation intensity (3–16 V), which was defined as the minimal intensity to induce an observable toe twitch, was determined by slowly increasing the stimulation intensity at the beginning of the experiment. Our previous studies indicated that foot stimulation at 2T is required to inhibit reflex bladder contractions.13 Therefore, we chose to use intensities of 2T and 4T to suppress bladder overactivity induced by AA irritation.
The initial bladder capacity was determined during a cystometrogram (CMG) by slowly infusing the bladder with saline. Bladder capacity was defined as the bladder volume threshold to induce a bladder reflex contraction of large amplitude (>30 cm H2O) and long duration (>20 seconds). Multiple CMGs were performed to determine reproducibility of the saline control capacity. Then, repeated CMGs were performed with AA infusion to irritate the bladder, activate nociceptive bladder C-fiber afferents, and induce bladder overactivity.17 Once the irritated bladder capacity was stabilized, four CMGs were performed prior to drug administration: 1. control CMG without stimulation, 2. CMG during 2T stimulation, 3. CMG during 4T stimulation, 4. control CMG without stimulation to determine any post-stimulation effect. Increasing cumulative doses of tolterodine (tolterodine L-tartrate, Tocris Bioscience, Bristol, UK) were then administered (0.003, 0.01, 0.03, 0.1, and 0.3 mg/kg, i.v.). Ten minutes after administering each dose of tolterodine, the CMGs were performed again under the four different conditions (control, 2T stimulation, 4T stimulation, and post-stimulation control) to determine the drug effect on bladder capacity. The bladder was emptied after each CMG followed by a 3–5 min rest period to allow the distended detrusor to recover.
Data Analysis
For the repeated CMG recordings, bladder capacity was normalized to the initial saline control capacity in the same animal to allow comparisons between animals. Capacity measurements under the same conditions were averaged and reported as mean±standard error of the mean. The mean amplitude of the bladder reflex contraction was also measured during each CMG and normalized to the AA control CMG to determine the effect of tolterodine on detrusor contractility. Statistical significance (P < 0.05) was detected by ANOVA followed by Dunnett or Bonferroni post-tests.
RESULTS
Suppression of Bladder Overactivity by Foot Stimulation
AA-induced irritation of the bladder significantly (P < 0.0001) reduced bladder capacity to a mean of 23.6±7.1% (2.0±0.6 mL) of saline control capacity (8.0±1.1 mL) (Fig. 1). Prior to tolterodine administration, foot stimulation significantly (P < 0.0001) increased bladder capacity to 50.7±6.8% at 2T and 79.0±11.6% at 4T of saline control. After stimulation, bladder capacity returned to the pre-stimulation level (Fig. 1), indicating that there was no post-stimulation inhibition.
Fig. 1.
Foot inhibition of bladder overactivity caused by 0.25% acetic acid (AA). A. Cystometrogram (CMG) pressure trace during saline or AA infusion at rate of 1 ml/min with or without foot stimulation prior to tolterodine administration. Stimulation denoted by solid black bar under the pressure trace. Foot stimulation threshold (T) is defined as minimal intensity to induce observable toe twitch. T = 3 V. B. Summarized results (N = 6 cats) showing average bladder capacity normalized to saline control. Foot stimulation: 5 Hz, 0.2 ms, T = 3–16 V. *indicates significant difference compared to AA control prior to stimulation.
Dose Dependent Effect of Tolterodine alone on Bladder Overactivity
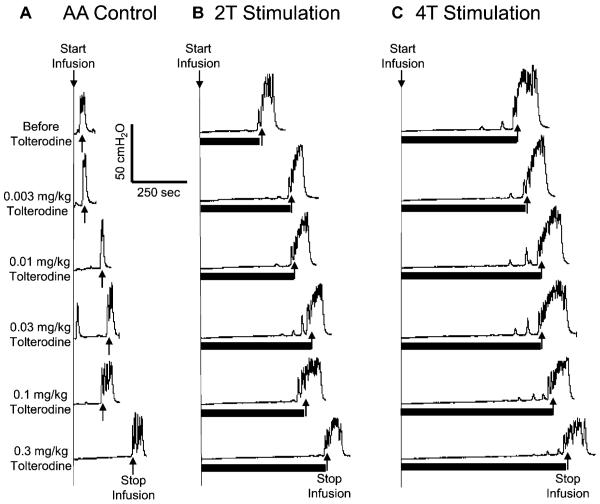
During AA infusion CMGs, cumulative doses of tolterodine (0.003–0.3 mg/kg) increased bladder capacity dose dependently in the absence of foot stimulation (Fig. 2A). However, only the largest dose of tolterodine (0.3 mg/kg) significantly (P < 0.05) increased the capacity (to 65.6±15.5% of the saline control capacity) (Fig. 3A).
Fig. 2.
Dose dependent effect of tolterodine and foot inhibition on bladder overactivity caused by acetic acid (AA) irritation. The CMGs were performed in sequence from left to right in figures A–C and from top to bottom in each figure. Duration of foot stimulation is indicated by the black bar under the bladder pressure trace. Foot stimulation threshold (T) is defined as the minimal intensity to induce an observable toe twitch. A. Control CMGs without foot stimulation after increasing doses of tolterodine. B. CMGs during 2T foot stimulation. C. CMGs during 4T foot stimulation. Stimulation: 5Hz, 0.2 ms, T=12 V. Infusion rate=1 ml/min.
Fig. 3.
Summarized results of dose dependent effect of tolterodine and foot inhibition on bladder overactivity caused by acetic acid (AA) irritation (A) and on amplitude of micturition contraction (B). Stimulation: 5 Hz, 0.2 ms, T=3–16 V. # indicates significantly different from AA control. *indicates significantly different from the bladder capacity measured before tolterodine treatment (i.e. at 0 mg/kg of tolterodine) under different conditions. Note: amplitude of micturition contraction is normalized to AA control prior to drug administration. N = 6 cats.
Combined Effect of Tolterodine and Foot Stimulation on Bladder Overactivity
When tolterodine was combined with foot stimulation, the total inhibitory effect was additive. Bladder capacity was significantly (P<0.05) increased by both 2T and 4T foot stimulation when compared to AA control at every dosage (Fig. 2 and Fig. 3A). After the 0.3 mg/kg dose of tolterodine which restored the small irritated bladder to a capacity of 65.6±15.5% of saline control, 2T or 4T foot stimulation significantly increased bladder capacity to 86.2±6.2% or 107.9±10.6%, respectively, of the saline control (Fig. 3A). A lower dose of tolterodine (0.1 mg/kg), which was not effective in significantly increasing control bladder capacity in the absence of stimulation, significantly (P<0.05) increased bladder capacity to 97.0±11.2% of saline control when combined with 4T foot stimulation. Thus, combination therapy completely restored the irritated bladder to the capacity equivalent to the saline control (see the dashed line in Fig. 3A). After 2T and 4T stimulation, the bladder capacity returned to the pre-stimulation level at every dose of tolterodine, i.e. no post-stimulation effect was observed.
Tolterodine (0.003–0.3 mg/kg) did not alter the amplitude of bladder reflex contractions during control, 2T or 4T foot stimulation CMGs (Fig. 2 and Fig. 3B). Foot stimulation at 4T significantly (P<0.05) increased the contraction amplitude only at 0 mg/kg and 0.01 mg/kg tolterodine (Fig. 3B). The threshold pressure (5.0±1.3 cm H2O) for inducing a micturition reflex was significantly (P<0.05) increased by foot stimulation (7.1±0.9 cm H2O at 2T; 8.5±1.0 cm H2O at 4T) and by tolterodine treatment (8.1±1.4 cm H2O at 0.3 mg/kg).
DISCUSSION
Our study shows that the combination of tolterodine and foot stimulation elicits a greater inhibition of bladder overactivity induced by AA irritation when compared to tolterodine or foot stimulation alone (Fig. 2 and Fig. 3A). Tolterodine alone dose dependently increased bladder capacity but only restored the small irritated bladder to a capacity about 60–70% of saline control at the maximal 0.3 mg/kg dose (Fig. 3A). Meanwhile, foot stimulation without tolterodine could only restore the capacity to 50–80% of saline control (Fig. 1B). However, by combining foot stimulation with a sub-threshold dose of tolterodine (0.1 mg/kg) a complete inhibition of bladder overactivity (return to 100% of saline control capacity) was achieved (Fig. 3A). Thus, the combined treatment offers the possibility of a non-invasive and effective treatment for OAB while limiting antimuscarinic adverse effects.
The concept of neuromodulation therapy combined with antimuscarinic treatment is a novel idea that initial studies have shown to reduce OAB symptoms in patients refractory to either sacral or tibial neuromodulation.15,16 A study in women demonstrated that tolterodine combined with tibial neuromodulation significantly decreased frequency, urgency, and incontinence episodes and increased the quality of life when compared to tolterodine alone.16 Given that tibial neuromodulation alone has been shown clinically to be equally effective to tolterodine,10 the additive benefit of dual therapy was significant. Additionally, a retrospective review of 88 patients who had a suboptimal response to sacral neuromodulation revealed that 84% of those patients had a subjective improved therapeutic response when started on antimuscarinic therapy.15 Our results provide more objective data and urodynamic evidence to support these clinical observations of combination therapy.
Our results also demonstrate that combination therapy allows the possibility of achieving higher treatment efficacy at a lower dose of tolterodine. While it may be difficult to directly compare tolterodine dosages between cats and humans, our results in cats show that the tolterodine dosage when combined with foot stimulation can be decreased (0.1 mg/kg) while producing greater inhibition than the minimum effective dose of tolterodine alone (0.3 mg/kg). While antimuscarinic drugs are the first line of therapy, discontinuation rates among patients are high due to a poor efficacy and significant adverse effects.4,5 Tolterodine is a first line antimuscarinic drug with a slightly better adverse effect profile due to an eightfold lower affinity for parotid gland tissue when compared to oxybutynin, thus significantly reducing the incidence of mouth dryness while maintaining its efficacy.18,19 Patient compliance, however, remains a major issue as the median time to discontinuation of tolterodine was determined to be only 3 months.20 If a lower dose of antimuscarinic drug can be used, the adverse effects and thus patient compliance would be greatly improved.
Utilizing foot stimulation for combination therapy also has its advantages over sacral and tibial neuromodulation. Sacral neuromodulation requires invasive surgery procedures to implant the electrode and stimulator.9 Patients also frequently experience adverse events such as loss of efficacy, pain at implant site, infection, hematoma/seroma, migration, and device malfunction which result in re-operation for 16.1–39.5% of patients.9,21 Tibial neuromodulation, a less invasive but less effective form of therapy, still requires a percutaneous needle electrode to be inserted by medically trained staff and stimulated on a weekly schedule for initial 12 weeks followed by maintenance stimulation once a month, which is very inconvenient for patients.10 Unlike tibial neuromodulation, foot stimulation uses skin surface electrodes to stimulate the nerves that innervate the foot.13 Because foot stimulation is non-invasive and tolterodine is a first-line FDA approved antimuscarinic drug, a clinical trial to test this combination treatment for OAB at a low dose of tolterodine would be safe and feasible.
Originally, it was believed that antimuscarinic drugs treat OAB through competitive inhibition of the muscarinic receptors in the detrusor, which reduce the contractility of the bladder. This belief has largely been dismissed.22 Evidence now points to antimuscarinic drugs targeting muscarinic receptors in the bladder urothelium23 and suburothelium,24 which leads to a suppression of C-fiber and Aδ-fiber afferent activity during bladder filling.25–27 Neuromodulation, however, involves electrical stimulation of somatic afferent nerves, which inhibits bladder overactivity through central interaction with the spinal and supraspinal micturition reflex.13,14 Therefore, the additive benefit of foot stimulation and tolterodine combination treatment is probably derived from simultaneous inhibition of both central and peripheral bladder afferent pathways. Our results also support the conclusion that antimuscarinic drugs do not significantly affect the efferent pathway of the micturition reflex at the therapeutic dosages22 because the amplitude of micturition contractions was not changed by the cumulative doses (0.003–0.3 mg/kg) of tolterodine (Fig. 3B). The increase in contraction amplitude caused by 4T foot stimulation (Fig. 3B) was probably due to a relatively large bladder volume when compared to the AA control. A larger bladder volume could generate a stronger afferent input and thereby a stronger micturition reflex because the bladder contracts against the closed urethral outlet in this study.28–31 Other studies also suggest that central muscarinic receptors may be involved in micturition.32,33 However, these receptors are primarily inhibitory to the bladder and are not tonically active.32,33 Furthermore, our results also indicate that muscarinic receptors are not involved in the mechanism of foot inhibition of bladder overactivity because the inhibition induced by foot stimulation remained across all doses of tolterodine (Fig. 3).
Our current study shows foot stimulation alone, or when combined with tolterodine, does not produce a post-stimulation inhibition of bladder overactivity induced by AA irritation. Tibial neuromodulation is clinically effective in treating OAB because prolonged stimulation induces a lasting inhibitory effect on the micturition reflex allowing intermittent stimulation to maintain efficacy.10 Without a lasting post-stimulation effect, foot stimulation would need to be applied continuously or more frequently to be efficacious. While continuous foot stimulation might interfere with patient daily activity, a small portable stimulator could be turned on/off by the patient as often as possible at convenient times during the day. Our previous studies in cats have also shown that opioid receptors are involved in foot inhibition of bladder overactivity34 and a long-lasting (>2 hours) post-stimulation inhibition of bladder overactivity can be induced when foot stimulation is combined with tramadol14 whose metabolite is a strong opioid agonist. Therefore, a further study of the tramadol effect on the combination treatment of foot stimulation and tolterodine is warranted.
In this preclinical study using anesthetized cats, dilute (0.25%) AA was used to irritate the bladder, activate nociceptive afferent C-fibers, and induce bladder overactivity. Although this animal model might not fully replicate the human OAB conditions, it produced bladder overactivity by activation of the bladder nociceptive afferent C-fibers that are known to play an important role in OAB symptoms. Since the pathology of human OAB is currently unknown, it is not possible to produce an animal model that can fully replicate the human OAB conditions. We believe that the bladder overactivity induced by activation of nociceptive afferent C-fibers in anesthetized cats is very useful for preclinical studies aimed at development of new therapeutic strategies for human OAB. Certainly, results of any preclinical studies will ultimately have to be verified by clinical trials using OAB patients.
This study proposes a novel and efficacious treatment strategy for OAB by combining non-invasive foot stimulation with low-dose tolterodine. Combination therapy would potentially limit adverse effects of antimuscarinic drugs and increase patient compliance. Additionally, this study provides the first urodynamic evidence to support the additive benefit observed clinically by the combination of neuromodulation and anti-muscarinic drug.15,16 If shown to be clinically efficacious, foot stimulation combined with low dose tolterodine could significantly improve the treatment for OAB.
ACKNOWLEDGEMENT
This study is supported by the National Institutes of Health under Grants DK094905, DK-068566, DK-090006 and DK-091253.
REFERENCES
- 1.Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–6. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–36. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 3.Onukwugha E, Zuckerman IH, McNally D, Coyne KS, Vats V, Mullins CD. The total economic burden of overactive bladder in the United States: a disease-specific approach. Am J Manag Care. 2009;15(Suppl S90–7) [PubMed] [Google Scholar]
- 4.Gopal M, Haynes K, Bellamy SL, Arya LA. Discontinuation rates of anticholinergic medications used for the treatment of lower urinary tract symptoms. Obstet Gynecol. 2008;112:1311–8. doi: 10.1097/AOG.0b013e31818e8aa4. [DOI] [PubMed] [Google Scholar]
- 5.Buser N, Ivic S, Kessler TM, Kessels AG, Bachmann LM. Efficacy and adverse events of antimuscarinics for treating overactive bladder: network meta-analyses. Eur Urol. 2012;62:1040–60. doi: 10.1016/j.eururo.2012.08.060. [DOI] [PubMed] [Google Scholar]
- 6.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol. 2008;54:543–62. doi: 10.1016/j.eururo.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 7.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn. 2005;24:643–7. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- 8.Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int. 2007;100:835–9. doi: 10.1111/j.1464-410X.2007.07082.x. [DOI] [PubMed] [Google Scholar]
- 9.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama a, Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 2007. 178:2029–34. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol. 2009;182:1055–61. doi: 10.1016/j.juro.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 11.Chen HW, Bercik RS, Werner EF, Thung SF. Cost-effectiveness of percutaneous tibial nerve stimulation versus extended release tolterodine for overactive bladder. J Urol. 2012;187:178–84. doi: 10.1016/j.juro.2011.09.052. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Larson JA, Ogagan PD, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Post-stimulation inhibitory effect on reflex bladder activity induced by activation of somatic afferent nerves in the foot. J Urol. 2012;187:338–43. doi: 10.1016/j.juro.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai C, Shen B, Chen M, Wang J, Liu H, Roppolo JR, de Groat WC. Suppression of bladder overactivity by activation of somatic afferent nerves in the foot. BJU Int. 2011;107:303–9. doi: 10.1111/j.1464-410X.2010.09358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mally AD, Zhang F, Matsuta Y, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Combination of foot stimulation and tramadol treatment reverses irritation induced bladder overactivity in cats. J Urol. 2012;188:2426–32. doi: 10.1016/j.juro.2012.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George E, Lane F, Noblett K. Use of combined anticholinergic medication and sacral neuromodulation in the treatment of refractory overactive bladder. Female Pelvic Med Reconstruct Surg. 2011;17:97–9. doi: 10.1097/SPV.0b013e31820e5cf3. [DOI] [PubMed] [Google Scholar]
- 16.Sancaktar M, Ceyhan ST, Akyol I, Muhcu M, Alanbay I, Mutlu Ercan C, Atay V. The outcome of adding peripheral neuromodulation (Stoller afferent neuro-stimulation) to anti-muscarinic therapy in women with severe overactive bladder. Gynecol Endocrinol. 2010;26:729–32. doi: 10.3109/09513591003649815. [DOI] [PubMed] [Google Scholar]
- 17.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–66. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilvebrant L, Andersson KE, Gillberg PG, Stahl M, Sparf B. Tolterodine–a new bladder-selective antimuscarinic agent. Eur J Pharmacol. 1997;327:195–207. doi: 10.1016/s0014-2999(97)89661-6. [DOI] [PubMed] [Google Scholar]
- 19.Nilvebrant L, Hallen B, Larsson G. Tolterodine–a new bladder selective muscarinic receptor antagonist: preclinical pharmacological and clinical data. Life Sci. 1997;60:1129–36. doi: 10.1016/s0024-3205(97)00057-x. [DOI] [PubMed] [Google Scholar]
- 20.Basra RK, Wagg A, Chapple C, Cardozo L, Castro-Diaz D, Pons ME, Kirby M, Milsom I, Vierhout M, Van Kerrebroeck P, Kelleher C. A review of adherence to drug therapy in patients with overactive bladder. BJU Int. 2008;102:774–9. doi: 10.1111/j.1464-410X.2008.07769.x. [DOI] [PubMed] [Google Scholar]
- 21.Hijaz A, Vasavada SP, Daneshgari F, Frinjari H, Goldman H, Rackley R. Complications and troubleshooting of two-stage sacral neuromodulation therapy: a single-institution experience. Urology. 2006;68:533–7. doi: 10.1016/j.urology.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Andersson KE, Yoshida M. Antimuscarinics and the overactive detrusor–which is the main mechanism of action? Eur Urol. 2003;43:1–5. doi: 10.1016/s0302-2838(02)00540-7. [DOI] [PubMed] [Google Scholar]
- 23.Kullmann F, Artim D, Beckel J, Barrick S, de Groat WC, Birder L. Heterogeneity of muscarinic receptor mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol - Renal Physiol. 2008;294:F971–81. doi: 10.1152/ajprenal.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kullmann F, Artim D, Birder L, de Groat WC. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci. 2008;28:1977–87. doi: 10.1523/JNEUROSCI.4694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedlund P, Streng T, Lee T, Andersson KE. Effects of tolterodine on afferent neurotransmission in normal and resiniferatoxin treated conscious rats. J Urol. 2007;178:326–31. doi: 10.1016/j.juro.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Iijima K, De Wachter S, Wyndaele JJ. Effects of the M3 receptor selective muscarinic antagonist darifenacin on bladder afferent activity of the rat pelvic nerve. Eur Urol. 2007;52:842–7. doi: 10.1016/j.eururo.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama O, Yusup A, Miwa Y, Oyama N, Aoki Y, Akino H. Effects of tolterodine on an overactive bladder depend on suppression of C-fiber bladder afferent activity in rats. J Urol. 2005;174:2032–6. doi: 10.1097/01.ju.0000176793.50410.9e. [DOI] [PubMed] [Google Scholar]
- 28.Habler HJ, Janig W, Koltzenberg M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J Physiol. 1993;463:449–60. doi: 10.1113/jphysiol.1993.sp019604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurons concerned with micturition in the cat. J Physiol. 1969;200:87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallory B, Steers WD, de Groat WC. Electrophysiological study of micturition reflexes in rats. Am J Physiol. 1989;257:R410–21. doi: 10.1152/ajpregu.1989.257.2.R410. [DOI] [PubMed] [Google Scholar]
- 31.de Groat WC, Saum WR. Synaptic transmission in parasympathetic ganglia in the urinary bladder of the cat. J Physiol. 1976;256:137–158. doi: 10.1113/jphysiol.1976.sp011316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishiura Y, Yoshiyama M, Yokoyama O, Namiki M, de Groat WC. Central muscarinic mechanisms regulating voiding in rats. J Pharmacol Exp Therap. 2001;297:933–9. [PubMed] [Google Scholar]
- 33.Masuda H, Ichiyanagi N, Yokoyama M, Sakai Y, Kihara K, Chancellor MB, de Groat WC, Yoshimura N. Muscarinic receptor activation in the lumbosacral spinal cord ameliorates bladder irritation in rat cystitis models. BJU Int. 2009;104:1531–7. doi: 10.1111/j.1464-410X.2009.08617.x. [DOI] [PubMed] [Google Scholar]
- 34.Tai C, Ogagan PD, Chen G, Larson JA, Shen B, Wang J, Roppolo JR, de Groat WC. Involvement of opioid receptors in inhibition of bladder overactivity induced by foot stimulation in cats. J Urol. 2012;188:1012–6. doi: 10.1016/j.juro.2012.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]