Abstract
T-cell tolerance is an important mechanism for tumor escape, but the molecular pathways involved in T-cell tolerance remain poorly understood. It remains unknown whether the inhibitory immunoreceptor programmed death-1 (PD-1) plays a role in conditions of human non-small cell lung cancer (NSCLC). In this study, we detected PD-1 expression on CD8+ T cells from healthy control peripheral blood mononuclear cells (PBMCs) and the PBMCs of NSCLC patients as well as NSCLC tissues. Results showed that tumor-infiltrating CD8+ T cells had increased PD-1 expression and impaired immune function, including reducing cytokine production capability and impairing capacity to proliferate. Blockade of the PD-1/PD-L1 pathway by the PD-L1-specific antibody partially restored cytokine production and cell proliferation. These data provide direct evidence that the PD-1/PD-L1 pathway is involved in CD8+ T-cell dysfunction in NSCLC patients. Moreover, blocking this pathway provides a potential therapy target in lung cancer.
Keywords: lung cancer, programmed death-1, tumor-infiltrating lymphocyte
Introduction
Lung cancer remains the leading cause of cancer death worldwide.1 Despite advances in early detection and standard treatment, lung cancer is often diagnosed at an advanced stage and has a poor prognosis, particularly for cases of non-small cell lung cancer (NSCLC). The needs of lung cancer treatment and prevention are largely unmet, but hopefully that can be improved through a better understanding of the molecular mechanisms of tumor immune escape.
According to the two-signal model for T-cell activation,2 CD8+ T cells, which have been shown to be potent mediators of antitumor immunity, require two signals to become fully activated. The first signal, which gives specificity to the immune response, is provided by the major histocompatability complex antigenic peptide's interaction with the T-cell receptor. The second, antigen-independent costimulatory signal, which provides positive and negative second signals to antigen-experienced effector T cells, is delivered to T cells by antigen-presenting cells to promote T-cell clonal expansion, cytokine secretion and effector function. In the absence of the second signal, antigen-specific lymphocytes fail to respond effectively and are functionally inactivated, or anergic, and resistant to subsequent antigen activation.
In the process of tumor immune escape, tumor-infiltrating lymphocytes become functionally impaired, which is indicated by their poor proliferation and decreased interferon-γ (IFN-γ) production. In the past, special attention was given to tumor microenvironments and their involvement in regulating T-cell function,3 including tumor antigen loss or major histocompatability complex molecule downregulation. This is often accompanied by the presence of both CD4+CD25+ regulatory T cells and CD1d-restricted T cells which suppress antitumor immunity and activation of T cells in the absence of appropriate costimulation, resulting in anergy and tumor expression of soluble suppressive factors such as transforming growth factor-β, vascular endothelial growth factor and IL-10. There is little information about whether potential inhibitory pathways serve functions in regulating T-cell tumor-setting dysfunction.
Programmed death-1 (PD-1), an inhibitory receptor in the CD28 ‘superfamily',4 and its ligand PD-L1 is reported to play an important role for CD8+ T cell exhaustion (loss of function) during chronic viral infection.5 A blockade of the PD-1/PD-L pathway in vivo increases virus-specific CD8+-T cell responses, enhances ‘per-cell' function and decreases the viral load.5 Increasing evidence demonstrates that upregulation of the PD-1 inhibitory receptor mediates HIV-specific CD8+ T-cell functional exhaustion and CD8+ T cell is apoptosis-sensitive, resulting in an impairment of CD8+ T cell's ability to control virus replication.6, 7, 8, 9 Involvement of the PD-1 pathway has also been shown during hepatitis B and C virus infection10, 11, 12, 13 with PD-L1 expression demonstrated in situ on a wide variety of solid tumors including pancreas, lung, ovarian and bladder tumors.14, 15, 16, 17, 18 Studies relating PD-L1 expression on tumors to disease outcome show that PD-L1 expression strongly correlates with unfavorable prognosis in kidney, bladder, gastric and pancreatic cancer.16, 17, 18 Such studies indicate that the PD-1/PD-L1 pathway may also play a role in tumor immunity. Although PD-1 expression is upregulated on tumor-infiltrating lymphocytes for patients with renal cell carcinoma and lung cancer,17, 19 PD-1 expression has not yet been linked to impairment of host antitumor immunity, particularly in NSCLC patients.
In this study, we show that in patients with NSCLC, high expression of PD-1 on tumor-infiltrating CD8+ T cells correlates with impaired T-cell function and we also demonstrate that blocking the PD-1/PD-L1 pathway could increase T-cell proliferation and cytokine production.
Materials and methods
Study subjects
We examined 21 patients with histologically confirmed NSCLC who underwent surgery at the department of cardiothoracic surgery at Changhai Hospital, the Second Military Medical University (Shanghai, China), between November 2007 and July 2008. The median patient age was 63 years, with a range of 46–73 years. Peripheral blood CD8+ T cells were obtained from the healthy controls without a prior history of cancer matched to cases by age and sex. In 16 patients, fresh lung cancer tissues were also obtained. The study protocol was approved by the Human and Animal Ethics Review Committee of the Second Military Medical University, China.
PD-1 expression and phenotypic analysis
Peripheral blood mononuclear cells (PBMCs) were isolated from freshly heparinized blood through centrifugation by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) and were resuspended at approximately 5×106 cells in 100 µl phosphate-buffered solution (PBS). We then added CD8-allophycocyanin (APC) and anti-PD-1-phycoerythrin at 0.3 µg per 1×106 cells and incubated the cells at room temperature for 15 min, followed by two washes and resuspention in 200 µl PBS followed by analysis on a FACScalibur (Becton Dickinson, San Jose, CA, USA). For tumor tissue specimens, fresh tumor tissues were dissected and digested with 125 U/ml collagenase type IV, 60 U/ml DNase1 and 450 U/ml collagenase type I (all enzymes were obtained from Sigma-Aldrich, St Louis, MO, USA) in PBS containing 20 mM HEPES at 37 °C for 1 h. A cell suspension was obtained by mashing the digested specimen through a 70 µm strainer, and expression of PD-1 was detected as above.
By the same methods mentioned above, the following antibodies were used for phenotypic analysis of CD8+ T cells: CD4-fluorescein isothiocyanate (FITC), CD8-APC, CD25-APC, CD27-FITC, CD127-FITC, CD45RA-FITC and CD28-phycoerythrin.
CD8+ T-cell proliferation
Freshly isolated peripheral lymphocytes or freshly thawed lymphocytes were resuspended at 1×106/ml in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (R10; Invitrogen, Grand Isle, NY, USA) and stimulated with 1 µg/ml anti-CD3 and 0.5 µg/ml anti-CD28 (ebioscience) antibodies. CD8+ T-cell proliferation assays were performed as described previously.20 Cells resuspended in exactly 300 µl PBS were briefly doubl stained with anti-CD8-FITC and 7-amino-actinomycin D, and cellular data were acquired for 60 s with the flow cytometer (1×105 phycoerythrin-labeled beads of 3 µm in diameter were added to each well as an internal control before antibody labeling). The numbers of CD8-positive and 7-amino-actinomycin D-negative live cells were acquired for analysis, and the total cells in each well calculated according to the formula: Number total=(Number live/Number beads)×105.
CD8+ T lymphocyte purification
PBMCs were separated by Ficoll-Hypaque centrifugation from buffy coats obtained from patients with lung cancer and from healthy blood donors. CD8+ T lymphocytes were purified by immunomagnetic cell sorting by positive selection using a human CD8+ T Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Sort purities were consistently greater than 95%.
Cytokine stain and ELISA intracellular measurement
Supernatants of purified CD8+ T cells were collected on day 3 after being stimulated with anti-CD3 and anti-CD28, and then IL-2. IFN-γ concentration was measured by ELISA following the manufacturer's instructions (BD Biosciences, San Jose, CA, USA). To measure intracellular cytokine, approximately 1×106 cells PBMC were incubated for 6 h at 37 °C in a total volume of 200 µl medium with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 500 ng/ml ionomycin in the presence of 3 µg/ml Brefeldin A. After incubation, cells were harvested and stained with anti-CD8, fixed with 2% paraformaldehyde and were permeabilized with permeabilization buffer followed by intracellular staining with antihuman IFN-γ-APC or anti-IL-2-APC. IFN-γ+- or IL-2-positive cells were determined after gating on CD8+ cells.
Cell apoptosis assay
PBMCs or freshly digested tumor tissues were cultured at 4×105/well in plates pre-coated with 0.5 µg/ml anti-CD3 (clone HIT3a; Pharmingen, San Diego, CA, USA) or control immunoglobulin (mouse IgG2a) for 72 h to induce apoptosis in CD8+ T cells. Cells at 1×105 per sample were doubled stained with annexin V (5 µl per test) and anti-CD8-FITC for 30 min and the samples were analyzed by FACS. Apoptosis was calculated as the percentage of annexin V+ after gating on CD8+ cells.
PD-L1 and PD-L2 blockade
Freshly isolated PBMCs were pre-treated with either isotype control antibody (IgG2b clone MPC.11; 10 µg/ml), purified anti-PD-L1 (10 µg/ml) or purified anti-PD-L2 (10 µg/ml). Cells were then incubated for 3 days, stained with indicated surface antibodies and analyzed by flow cytometry.
Statistical analysis
We used unpaired and paired t-tests assuming independent samples and unknown unequal variances for the underlying populations. A paired t-test was also used to show the effect of treatment with antibodies to PD-L1 or PD-L2 on the number of CD8+ T cells. P<0.05 was considered significant.
Results
PD-1 is upregulated on tumor-infiltrating CD8+ T cells
To address the potential role of PD-1, we first analyzed the expression of PD-1 on cells from three different groups: PBMCs from 23 healthy volunteers, PBMCs from 21 NSCLC patients and tumor-infiltrating lymphocytes from 16 NSCLC patients (Figure 1). Our results showed that of the three groups, expression of PD-1 on CD8+ T cells was the highest in tumor tissues, and between the two PBMC groups, CD8+ T cells of NSCLC patients expressed much higher levels (2.08-fold) of PD-1 than that of healthy controls (P<0.01). CD8+ T cells in tumor tissues expressed the highest level of PD-1 (9.16- and 4.40-fold, respectively, all P<0.0001; Figure 1a). Figure 1b shows representative PD-1 expression levels in patients with NSCLC versus healthy controls.
Figure 1.
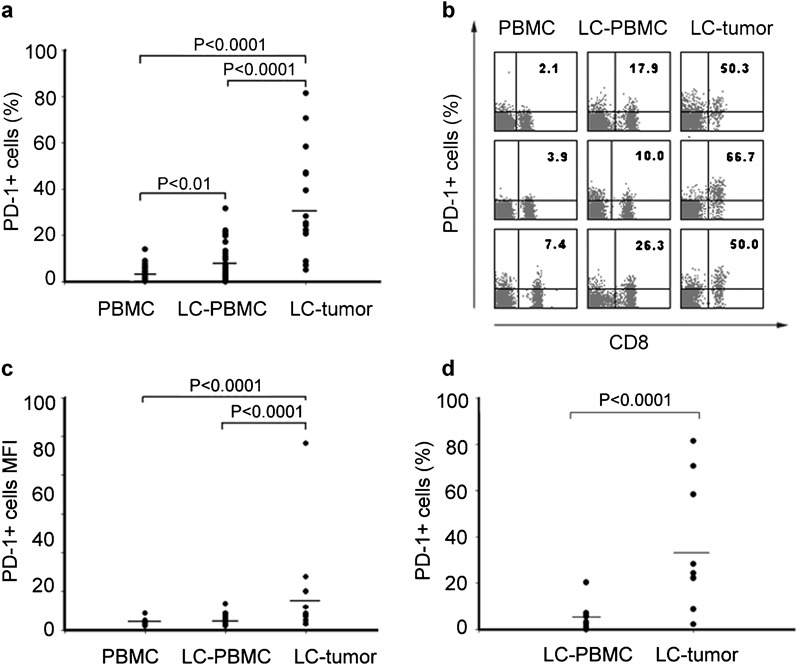
PD-1 is highly upregulated by tumor-infiltrating CD8+ T cells in patients with NSCLC. (a) Frequencies of PD-1-expressing CD8+ T cells in 58 blood samples, 16 lung tissue samples for LC patients and 23 blood samples for healthy controls. Each dot represents one individual. (b) PD-1 staining representative dot plots for one blood sample from a healthy person, as well as one blood sample and one tumor tissue sample from LC. Values in the upper right quadrant indicate the percentage of cells that express PD-1. (c) MFI of PD-1 expression on CD8+ T cells in PBMC and lung tissue samples for LC patients and healthy controls. Each dot represents one individual. (d) Positive rate of PD-1 in PBMCs and lung tissues from the same individual for 16 LC patients with. LC, lung cancer; MFI, mean fluorescence intensity; PBMC, peripheral blood mononuclear cell; PD-1, programmed death-1.
PD-1 expression was also verified by mean fluorescence intensity demonstrating that CD8+ T cells in tumor tissues expressed much higher levels of PD-1 than CD8+ T cells in PBMCs either from cancer patients or from healthy controls (P<0.001, respectively), although differences between CD8+ T mean fluorescence intensity in the two PBMC groups were not significant (Figure 1c). We also detected the expression of PD-1 on CD8+ T cells from tumor tissues and PBMCs from the same patients. We found that PD-1 expression was much higher on CD8+ T cells (6.44-fold) than on PBMCs (P<0.0001; Figure 1d). Taken together, our studies showed that PD-1 expression on tumor-infiltrating CD8+ T cells is significantly higher than that of PBMCs either from lung cancer patients or from healthy controls. Moreover, the high expression of PD-1 on tumor-infiltrating CD8+ T cells was unique as we did not detect upregulation of another important inhibitory receptor, cytotoxic T lymphocyte-associated antigen 4 expression on tumor-infiltrating CD8+ T cells (data not shown).
Phenotype and function analysis of PD-1+ tumor-infiltrating CD8+ T cells
Phenotypes of PD-1+ tumor-infiltrating CD8+ T cells were studied by detecting levels of CCR7, CD45RA, CD27 and CD127 on the cells' surface (Figure 2a). Our results showed that most PD-1+ tumor-infiltrating CD8+ T cells expressed low levels of CCR7, CD127, CD45RA and high level of CD27, which indicates that tumor-infiltrating PD-1+CD8+ T cells has a less differentiated phenotype (PD-1+CD27hiCD127loCCR7−CD45RA−) as previously reported with dysfunctional HIV-specific CD8+ T cells.7, 9
Figure 2.
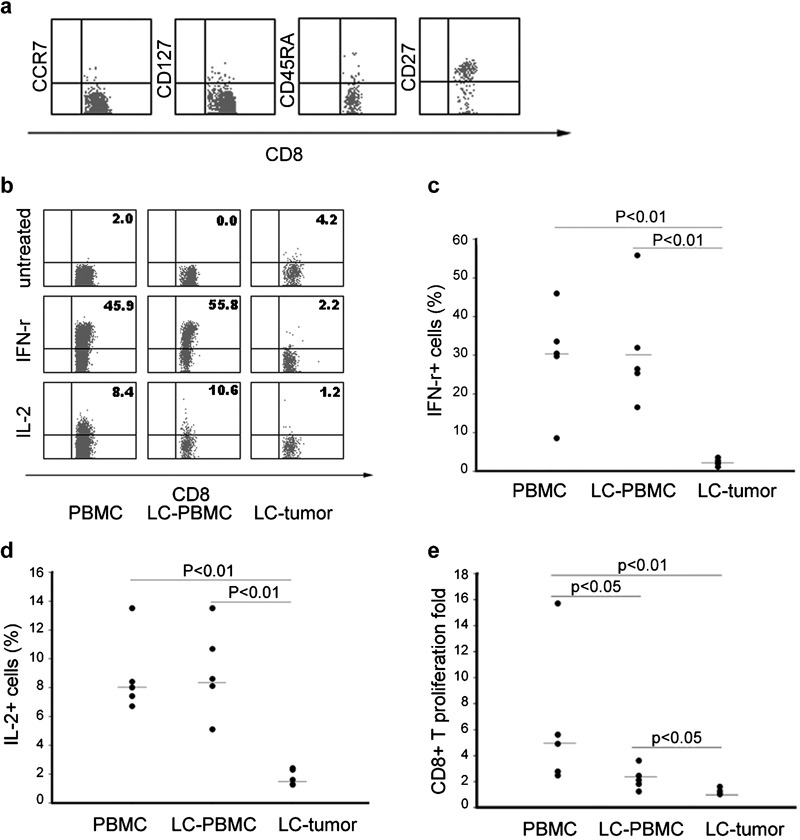
Preterminally differentiated phenotype and functional impairment of PD-1+ tumor-infiltrating CD8+ T cells. (a) Expression levels in PD-1+CD8+ T cells of CD45RA, CD127 (IL-7-receptor-α), CD27 and CCR7. (b) Representative dot plots of IFN-γ and IL-2 intracellular staining gated on CD8-positive cells for one blood sample from a healthy person, as well as one blood sample and one tumor tissue sample from LC. PBMCs and tumor tissues were stimulated with PMA and ionomycin for 6 h in the presence of 3 µg/ml of Brefeldin A and were stained with anti-CD8, fixed and permeabilized, followed by intracellular staining with antihuman IFN-γ or anti-IL-2 antibody. (c and d) Cytokine production in CD8+ T cells: IFN-γ (c) and IL-2 (d). (e) Proliferating fold of CD8-positive cells stimulated by anti-CD3 and anti-CD28. IFN, interferon; LC, lung cancer; PBMC, peripheral blood mononuclear cell; PD-1, programmed death-1; PMA, phorbol 12-myristate 13-acetate.
We then studied the function of PD-1+ tumor-infiltrating CD8+ T cells by investigating the capacity of these cells to produce IFN-γ and IL-2 upon stimulation with PMA and ionomycin. Figure 2b shows representative dot plots of IFN-γ and IL-2 intracellular staining gated on CD8+ T cells. Without PMA and ionomycin, we observed the background secretion of these cytokines. After stimulation with PMA and ionomycin, CD8+ T cells from healthy controls produced a lot of IFN-γ and IL-2 (Figure 2b). The ratio of IFN-γ- or IL-2-producing cells to tumor-infiltrating CD8+ T cells was significantly lower compared to peripheral blood CD8+ T cells from either healthy control or NSCLC patients (P<0.01, respectively), but there were no significant differences in cytokine production between peripheral blood CD8+ T cells from NSCLC patients and CD8+ T cells from healthy controls (Figure 2c and d).
We next detected the proliferation of tumor-infiltrating CD8+ T cells in response to anti-CD3 and anti-CD28. Our results showed that proliferation of tumor-infiltrating CD8+ T cells was much lower than that of peripheral blood CD8+ T cells from either healthy controls or NSCLC patients (P<0.01 and P<0.05, respectively) (Figure 2e). These data indicate that tumor-infiltrating PD-1+CD8+ T cells are functionally impaired in that they are less able to produce cytokines including IFN-γ and IL-2, and that they proliferate poorly in response to stimulation.
Blocking the PD-1/PD-L1 pathway increased cytokines production and proliferation of tumor-infiltrating CD8+ T cells
Next, we examined whether interrupting the interaction of PD-1 with its ligands PD-L1 or PD-L2 could affect the function of tumor-infiltrating CD8+ T cells. Our results showed that in healthy controls, blocking either PD-L1 or PD-L2 had no effect on proliferation of CD8+ T cells compared with isotype antibodies. In NSCLC patients' CD8+ T cells from either PBMCs or tumor tissues, however, we observed that the anti-PD-L1 but not the anti-PD-L2 antibody was able to augment CD8+ T-cell proliferation as indicated by an increase in PD-1+CD8+ T cells compared to those non-treated or treated with isotype antibodies (P<0.05; Figure 3a).
Figure 3.
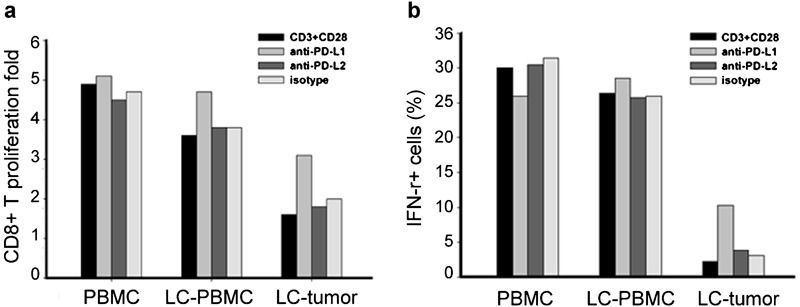
Blocking the PD-1/PD-L1 pathway increases production of effector molecules and proliferation of tumor-infiltrating CD8+ T cells. A purified CD8+ T-cell stimulation assay was performed in the presence of anti-CD3 and anti-CD28 alone and alternately with an antibody blockade of PD-L1 or PD-L2. (a) A fold of proliferating CD8-positive cells in the presence or absence of anti-PD-L1 or anti-PD-L2 antibodies. (b) Production of IFN-γ in the supernatants of 3-day cultures measured by ELISA in the presence or absence of anti-PD-L1 or anti-PD-L2. IFN, interferon; LC, lung cancer; PBMC, peripheral blood mononuclear cell; PD, programmed death.
We also determined whether blocking the PD-L1 or PD-L2 pathway would allow tumor-infiltrating CD8+ T cells to secrete more cytokines upon T-cell receptor triggering. Intracellular cytokine stain assays confirmed that in the absence of PD-L1 and PD-L2 blocked antibodies, CD8+ T cells secreted a moderate amount of IFN-γ after stimulation with anti-CD3 and anti-CD28, however, when blocking PD-L1 but not PD-L2, tumor-infiltrating CD8+ T cells significantly increased the production of IFN-γ (P<0.05; Figure 3b). These results provided further evidence that disruption of PD-1–PD-L1 interaction increases proliferation and cytokine production of tumor-infiltrating PD-1+CD8+ T cells.
Increasing apoptosis of tumor-infiltrating CD8+ T cells and blocking the PD-1/PD-L1 pathway cannot abrogate apoptosis
Because PD-1 upregulation has been reported to be more sensitive to apoptosis, resulting in a deficiency of CD8+ T cells in controlling virus replication,6, 7, 8, 9 we tested apoptosis of different CD8+ T cells induced by anti-CD3. Tumor-infiltrating CD8+ T cells exhibited much higher apoptosis rates than peripheral blood CD8+ T cells from either healthy control or patients with NSCLC (all P<0.01; Figure 4a). Increased apoptosis of tumor-infiltrating CD8+ T cells could not be significantly reversed by blocking either PD-L1 or PD-L2 (Figure 4b). Our results indicate that neither the PD-1/PD-L1 nor the PD-1/PD-L2 pathway is involved in CD3-mediated apoptosis of tumor-infiltrating PD-1+CD8+ T cells.
Figure 4.
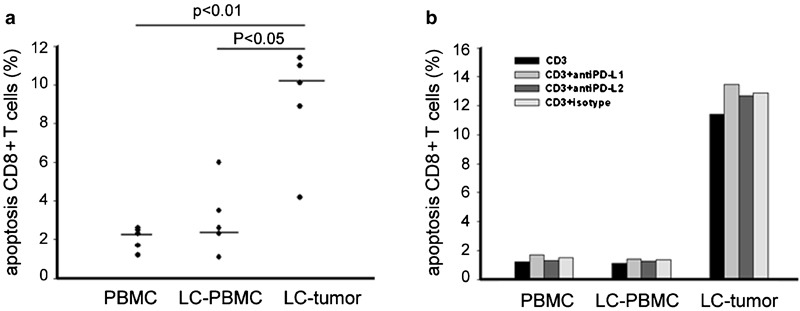
Increased tumor-infiltrating CD8+ T-cell apoptosis and blocking the PD-1/PD-L1 pathway cannot abrogate apoptosis. (a) Increased apoptosis of tumor- infiltrating CD8+ T cells upon incubation with anti-CD3 for 3 days. Cells were harvested and examined for apoptosis by double staining with annexin V+ and an antibody against CD8. Apoptosis was calculated as the percentage of annexin V+ cells in CD8+ fraction. (b) Apoptosis of tumor-infiltrating CD8+ T cells in the presence of anti-PD-L1 or anti-PD-L2. LC, lung cancer; PBMC, peripheral blood mononuclear cell; PD, programmed death.
Discussion
With the following results, we provide direct evidence that PD-1 inhibitory receptors on tumor-infiltrating CD8+ T cells are indeed involved in dysfunctional CD8+ T-cell activity in patients with NSCLC: first, PD-1 expression on CD8+ T cells, either in PBMCs or in tumor tissues, dramatically increased in NSCLC patients. Second, PD-1+CD8+ T cells exhibited impaired function, which was indicated by a reduced capacity to produce cytokines as well as a poor proliferation in response to anti-CD3 and anti-CD28. Third, blocking the PD-1/PD-L1 pathway by anti-PD-L1 antibodies increased cytokine production and proliferation of PD-1+ tumor-infiltrating CD8+ T cells. Yet the PD-1/PD-L1 pathway was not involved in the increased apoptosis of PD-1+ tumor-infiltrating CD8+ T cells induced by anti-CD3 monoclonal antibody.
Tumor-infiltrating CD8+ T cells expressed the highest level of PD-1, and its capability to proliferate and produce cytokines was the lowest. Although we found moderate upregulation of PD-1 on peripheral blood CD8+ T cells in NSCLC patients, there were no significant difference in cytokine production between peripheral blood CD8+ T cells from NSCLC and CD8+ T cells from healthy controls. Perhaps this difference in cytokine production is due to the fact that PD-1 typically has greater effects on cytokine production than on cellular proliferation, with significant effects on IFN-γ, tumor-necrosis factor-α and IL-2 production.21
Another possible explanation is that, in addition to PD-1 upregulation in CD8+ T cells, higher PD-L1 expression in tumor cells16, 17, 18 not in PBMCs, may also have contributed to impaired CD8+ T-cell function. As a result, it is possible that the PD-1/PD-L1 pathway may have greater effects on CD8+ T cells in a tumor setting than on peripheral blood CD8+ T cells. Blocking the interaction between PD-1 and PD-L1 could partially reverse the functions of tumor-infiltrating CD8+ T cells, such as restoring their ability to undergo proliferation and secrete cytokines. Meanwhile, blocking the PD-1/PD-L2 pathway had little effect on tumor-infiltrating CD8+ T cells, indicating that, at least in NLCLC, PD-1/PD-L1 inhibitory pathways play a much more important role in immune tolerance than PD-1/PD-L2 pathways. A PD-1/PD-L1 blockade only resulted in an incomplete functional restoration, however, and tumor-infiltrating CD8+ T-cell function remained defective after a PD-1 pathway blockade as previously reported.5 It is possible, therefore, that other signal pathways are also involved in tumor-infiltrating CD8+ T-cell dysfunction. Lymphocyte-activation gene-3 (LAG-3) could be a potential candidate because blocking both PD-1 and LAG-3 pathways synergistically improved T-cell response and diminished viral load in vivo during chronic viral infection.22 Alternatively, a combined blockade of PD-1/PDL pathway or the IL-10 receptor with therapeutic vaccination have shown promising results.23, 24
Studies in animal models demonstrate that PD-L1 on tumor cells inhibits T-cell activation, decreases T cells' ability to kill tumor cells, and in some cases leads to increased tumor-specific T-cell death.14, 25, 26 Indeed, in our experiment, tumor-infiltrating CD8+ T cells exhibited increased apoptosis induced by anti-CD3. But blocking the PD-1/PD-L1 or PD-1/PD-L2 pathway had little effect on tumor-infiltrating CD8+ T-cell apoptosis. There may be a few possible reasons for this: first, previous research concentrated on purified human T cells whereas we were using fresh tumor tissues in which many other types of cells may be involved in the apoptosis process. Second, PD-1 may have different ligands other than PD-L1 and PD-L2 in an NSCLC setting. All these factors indicate that the PD-1/PD-L1 pathway is not involved in apoptosis of tumor-infiltrating CD8+ T cells in our system. Other mechanisms involved in this anti-CD3-induced apoptosis need further investigation.
Consistent with recent studies in mice and humans,5, 6, 7, 8, 9, 27 our data confirm that blocking the PD-1/PD-L1 pathway has potential therapeutic value in tumor immunotherapy. At least in NSCLC conditions, blocking the PD-1/PD-L1 pathway was more effective than blocking the PD-1/PD-L2 pathway. However, functional restoration by blocking the PD-1/PD-L pathway is incomplete, and defects in CD8+ T cells remain after blocking of the PD-1 pathway occurs, as was previously reported.5 This suggests the involvement of other negative regulatory pathways in CD8+ T-cell dysfunction in NSCLC patients. More effective therapeutic interventions may be achieved by simultaneously targeting different negative regulatory pathways such as PD-1 and LAG-3 in order to combine LAG-3 blocking with PD-1/PD-L1 blocking to result in substantially better exhaustion reversal than would appear with PD-1/PD-L1 blocking alone during chronic lymphocytic choriomeningitis virus infection.21
PD-1+ tumor-infiltrating CD8+ T cells leads to decreased proliferation and downregulated IL-2 and IFN-γ production possibly by downregulating the T-cell receptor signal through phosphatase SHP-1 and SHP-2 recruitment.28 It has been reported that PD-1 may exert its effects directly by inhibiting early activation events that are positively regulated by CD28 or indirectly through IL-2.29 In our study, we did not detect upregulation of cytotoxic T lymphocyte-associated antigen 4 or downregulation of CD28 (data not shown) on tumor-infiltrating CD8+ T cells, so cytotoxic T lymphocyte-associated antigen 4 and CD28 are unlikely to be contributing to the CD8+ T-cell defects. More likely, PD-1+ tumor-infiltrating CD8+ T-cell function was impaired due to decreased IL-2 production upon PD-1 ligation since IL-2 production was indeed lower than PD-1lowCD8+ T cells in our system. Of note, under conditions of high PD-1 expression (such as tumor-infiltrating CD8+ T cells), CD8+ T cells showed a lack of IFN-γ production which differed from that of the exhausted PD-1+CD8+ T cells capable of producing IFN-γ in chronic infection,9, 30 showing differences between exhausted CD8+ T cells in chronic infection and dysfunctional CD8+ T cells in a tumor setting.
In summary, inhibitory receptor PD-1 was dramatically upregulated on tumor-infiltrating CD8+ T cells, and was involved in impaired CD8+ T-cell function. Blocking PD-1 interaction with its ligand (PD-L1) enhanced the capacity of tumor-infiltrating CD8+ T cells to proliferate and led to increased production of cytokines. These data demonstrate that the PD-1 inhibitory pathway, in addition to regulating T-cell responses to self-antigens and viral antigens, also regulates tumor-infiltrating CD8+ T-cell responses in lung cancer. PD-1 may therefore be used as a new target in designing T cell-based immunotherapy for human cancers.
Acknowledgments
This work was supported by Grant 2006AA02A247 from the National High Biotechnology Development Program of China.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Boettler T, Panther E, Bengsch B, Nazarova N, Spangenberg HC, Blum HE, et al. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol. 2006;80:3532–3540. doi: 10.1128/JVI.80.7.3532-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 2008;45:1470–1476. doi: 10.1016/j.molimm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–1937, 1937.e1–2. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


