Abstract
Chemokines and their receptors are important mediators of leukocyte trafficking and recruitment and sometimes work as modulators of T-cell responses during infections and inflammation. Modulating the biological activity of chemokines has been found to influence the course of diseases. However, little is known about the role of chemokine responses during chlamydial lung infections. We therefore analyzed the dynamics of multiple chemokines, which are frequently associated with type 1 (Th1) T cell immune responses, and their receptors for their expression in the lungs during Chlamydia muridarum (Cm) infections. We also examined the relationship between chemokine responses and the development of Th1 responses as well as the clearance of infection. Our results showed that in parallel with the high levels of gamma interferon (IFN-γ) and IL-12 production in the lungs and draining lymph nodes, and the expansion of IFN-γ-producing CD4 and CD8+ T cells, the production of the cell-related chemokines RANTES, IFN-γ-inducible protein-10 (IP-10) and macrophage inflammatory protein-1α (MIP-1α) and their receptor CCR1 was elevated in the lung tissues after infection. Interestingly, in a later phase of infection, the expression of RANTES and IP-10 remained elevated but the expression of MIP-1α and CCR1 decreased to a low level, which suggests a closer association with the pattern of Th1 cytokine responses in the process of infection. These results suggest a close association between the MIP-1α response and the Th1-type T-cell responses in chlamydial lung infections.
Keywords: Chlamydial pneumonia, MIP-1α, RANTES, Th1 response
Introduction
Chlamydia is an obligate intracellular bacterial pathogen that is estimated to cause at least one infection during the lifetime of nearly every person.1 Although most infections are mild or subclinical, Chlamydia is a common cause of community-acquired pneumonia, bronchitis, pharyngitis and sinusitis.2 It has also been reported to be associated with chronic diseases, including asthma, chronic obstructive pulmonary disease and multiple sclerosis.3, 4, 5 It causes significant human morbidity despite the availability of effective antimicrobial treatments.6, 7
Recent studies have clearly shown that clearance of chlamydial infections requires high levels of cell-mediated immune responses, especially CD4+ T helper 1 (Th1) cells and CD8+ cytotoxic T cells, which produce gamma interferon (IFN-γ).8, 9 In animals lacking IFN-γ signaling, bacterial loads are higher and the clearance of organisms is greatly hampered.10 Moreover, in the absence of CD4+ or CD8+ T cells, Chlamydia-infected mice show increased bacterial burdens and disease severity.11 Current vaccine candidates only show limited protection against Chlamydial infections because they lack the ability to induce and retain a Th1 response and cannot foster long-term protective immunity in the host. The proper selection and application of chemokines, which can be used as possible mucosal adjuvants that promote lymphocyte activation and recruitment,12, 13 are expected to enhance protective T-cell responses and minimize the pathological changes caused by Chlamydial infections.
Chemokines are members of a large superfamily of small, secretory proteins that are structurally and functionally related. Based on the spacing of the first two conserved cysteine residues at the N-terminus, chemokines are subdivided into the CXC, CC, CX3C and C chemokine subfamilies.14 As a group, these proteins modulate multiple aspects of the host inflammatory response against infection, such as leukocyte adhesion and migration and inflammatory cell activation, and even contribute to the balance of type 1 and type 2 T-cell responses. However, little is known about their response in chlamydial lung infections. Some recent reports suggest that IFN-γ-inducible protein-10 (IP-10) (CXC chemokine), RANTES and macrophage inflammatory protein-1α (MIP-1α) (CC chemokine) are associated with the regulation of T-cell differentiation and polarize more for Th1 than for Th2 responses.15, 16, 17 It is important to note, however, that inconsistent findings have also been reported.18, 19 Therefore, we propose that these chemokines contribute to the Th1 responses needed for the clearance of Chlamydial infection.
To determine whether the chemokines IP-10, RANTES and MIP-1α and the receptor CCR1 are produced during the development of Chlamydial pneumonia and to examine the relationships of these proteins with Th1 Chlamydia-specific immune responses and the disease process, we analyzed the expression of these chemokines in the lungs of mice exposed to Chlamydia muridarum (Cm) at different times post-infection (p.i.). Our results demonstrate that the expression of all of the tested chemokines, RANTES, IP-10 and MIP-1α, and their receptor CCR1 were upregulated during the early phase of Chlamydial lung infection in conjunction with the enhancement of Th1-related cytokine responses. However, we found that the expression of MIP-1α and CCR1 are better associated with the Th1-related T-cell responses because in the later phase of infection, MIP-1α and CCR1 were decreased in parallel with the decrease of Th1 responses, while the levels of IP-10 and RANTES remained elevated. These data suggest that MIP-1α is likely an important chemokine for the protective Th1 responses in Chlamydial lung infection.
Materials and methods
Organism and infection of mice
Cm, formally called mouse pneumonitis biovar of Chlamydia trachomatis (MoPn), was grown in HeLa 229 cells and purified by discontinuous density gradient centrifugation as described previously.17 The purified organisms were resuspended in sucrose–phosphate–glutamic acid buffer and stored at −80 °C until use. Female C57BL/6 mice, 6–8 weeks old, were purchased from the Beijing Academy of Military Medical Sciences Center for Laboratory Animals (Beijing, China) and maintained at a pathogen-free animal care facility at Tianjin Medical University (Tianjin, China). Mice were inoculated intranasally with 3×103 inclusion-forming units (IFUs) Cm in a volume of 40 μl and monitored daily for body weight changes and viability following infection.
The in vivo growth of the organism
To determine the in vivo growth of the organism, the mice were killed at designed days p.i. The lung tissues were homogenized in 3 ml sucrose–phosphate–glutamic acid buffer. After a brief centrifugation, the tissue supernatants were kept at −80 °C until use. Chlamydial infectivity in the lung homogenates was assayed by infection of HeLa 229 cell monolayers for 48 h followed by enumeration of inclusions that were stained by Chlamydia-specific murine monoclonal antibody and horseradish peroxidase-conjugated goat antimouse immunoglobulin G secondary antibodies as described previously.20 The lung tissue supernatant was also used for determining the levels of MIP-1α by ELISA.
Reverse transcription-PCR
Lung tissues were frozen immediately in liquid nitrogen and stored at −80 °C until use. Total lung RNA was isolated using TRIzol Reagent (Invitrogen, San Diego, CA, USA) according to the manufacturer's protocol. Reverse transcription of 1 μg total RNA was performed using Moloney murine leukemia virus reverse transcriptase (TaKaRa, Dalian, China). The cDNA products were used as a template for PCR. The expression of chemokine and cytokine mRNA is presented as a percentage of β-actin. The primers used in the PCR analysis are as followed: RANTES, 270 bp, P1 5′-GAA GAT CTC TGC AGC TGC CCT-3′, P2 5′-GCT CAT CTC CAA ATA GTT GA-3′ IP-10, 431 bp, P1 5′-CCT ATC CTG CCC ACG TGT TG-3′, P2 5′-CGC ACC TCC ACA TAG CTT ACA-3′ MIP-1α, 257 bp, P1 5′-GCC CTT GCT GTT CTT CTC TGT-3′ P2 5′-GGC ATT CAG TTC CAG GTC AGT-3′ IFN-γ, 380 bp, P1 5′-AAC GCT ACA CAC TGC ATC T-3′ P2 5′-TGC TC ATT GTA ATG CT TGG-3′ IL-12, 411 bp, P1 5′-CTC ACC TGT GAC ACG CCT GA-3′ P2 5′-CAG GAC ACT GAA TAC TTC TC-3′ β-actin, 550 bp, P1 5′-GTG GGG CGC CCC AGG CAC CA-3′ P2 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′. PCR products were run on 1.2–1.8% agarose gels, and the bands were analyzed for density on scion image software.
Isolation of splenocytes and lymph node cells and cytokine analysis
Spleens and mediastinal and hilar lymph nodes were harvested from the mice at selected times after infection and processed into single-cell suspensions. Briefly, the spleens and mediastinal and hilar lymph nodes were digested in 2 mg/ml collagenase D (Roche Diagnostics, Meylan, France) in RPMI-1640 at 37 °C for 30 min. After lysis of red blood cells by adding Red Blood Cell Lysis Buffer (150 mM NH4Cl, 10 mM KHCO3 and 0.1 mM EDTA), the single-cell suspensions of spleen and lymph node cells were cultured at 7.5×106 and 5×106 cells/well, respectively, with complete RPMI medium in the presence or absence of UV-inactivated Cm (1×105 IFUs/ml) in 48 well plates at 37 °C in 5% CO2 for 72 h. Culture supernatants were assayed to determine the levels of IFN-γ and IL-12 by ELISA as previously described.21
Intracellular cytokine analysis
For intracellular cytokine staining, splenocytes were stimulated with phorbyl myristate acetate (50 ng/ml; BD PharMingen, San Jose, CA, USA) and ionomycin (1 μg/ml; BD PharMingen) and incubated for 6 h in complete RPMI-1640 medium with 10% fetal bovine serum at 37 °C. Monensin (eBioscence, San Diego, CA, USA) was added to accumulate cytokines intracellularly during the last 3 h of incubation. The cells were then stained with FITC-antimouse CD4 and FITC-antimouse CD8. After fixation and permeabilization, intracellular cytokine staining was performed using phycoerythrin-antimouse-IFN-γ. The cells were washed and analyzed by flow cytometry as described previously.20
Histopathological analysis of the lung
The lung tissues from the naive and infected mice were fixed in 10% formalin, and tissue sections were stained with hematoxylin and eosin. Histological changes were observed under light microscopy as previously described.20
Statistical analysis
The Student's t-test was performed to determine statistical differences between the groups. One-way ANOVA analysis was used for analyzing data for multiple groups. Values were considered to be significantly different when the P value was <0.05.
Results
Cm infection induced Th1 cytokine production in local tissue and peripheral immune organs
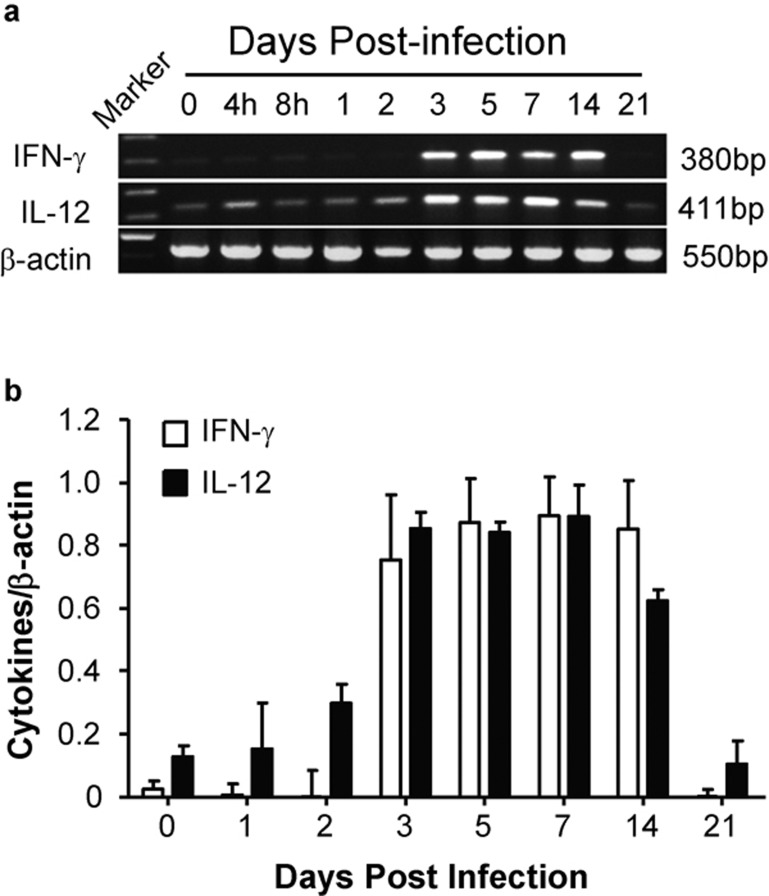
Th1 cytokine production is thought to be the major mechanism for the clearance of Chlamydial infections. We therefore investigated the gene expression and protein production of the Th1 cytokine IFN-γ and the Th1-related cytokine IL-12 in the lung tissues and peripheral immune organs at selected time points p.i. Both IFN-γ and IL-12 mRNA expressions were significantly increased following infection, but the increase of IL-12 appeared earlier (day 2 p.i.) than that of IFN-γ (day 3 p.i.; Figure 1). The mRNA for the Th2 cytokines IL-4 and IL-5 was not detectable in the lungs at the tested time points during Chlamydial infection (data not shown).
Figure 1.
The mRNA expression of IFN-γ and IL-12 in the lungs during Cm infection. Mice were inoculated intranasally with 3×103 IFUs Cm. Total RNA was extracted from fresh lung tissues and assayed for IFN-γ and IL-12 mRNA expression by RT-PCR using the specific primers described in the ‘Materials and methods' section. (a) Representative image of three independent experiments with similar results; (b) the ratios of IFN-γ/β-actin and IL-12/β-actin band densities are shown. Cm, Chlamydia muridarum; IFN-γ, gamma interferon; IFU, inclusion-forming unit; RT, reverse transcription.
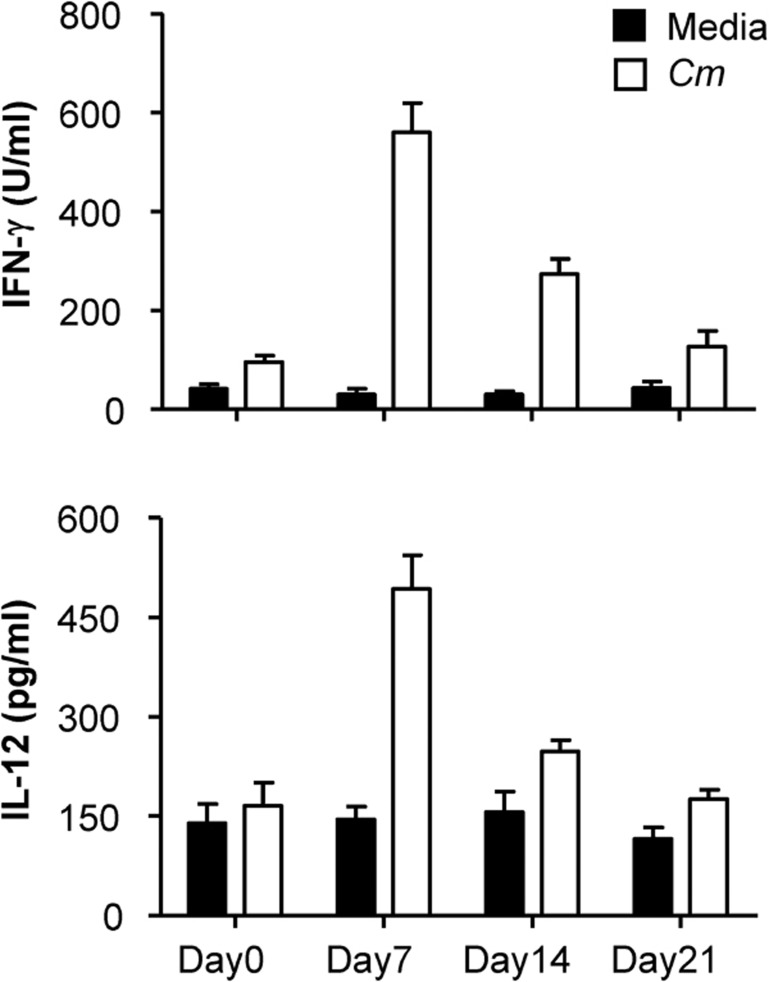
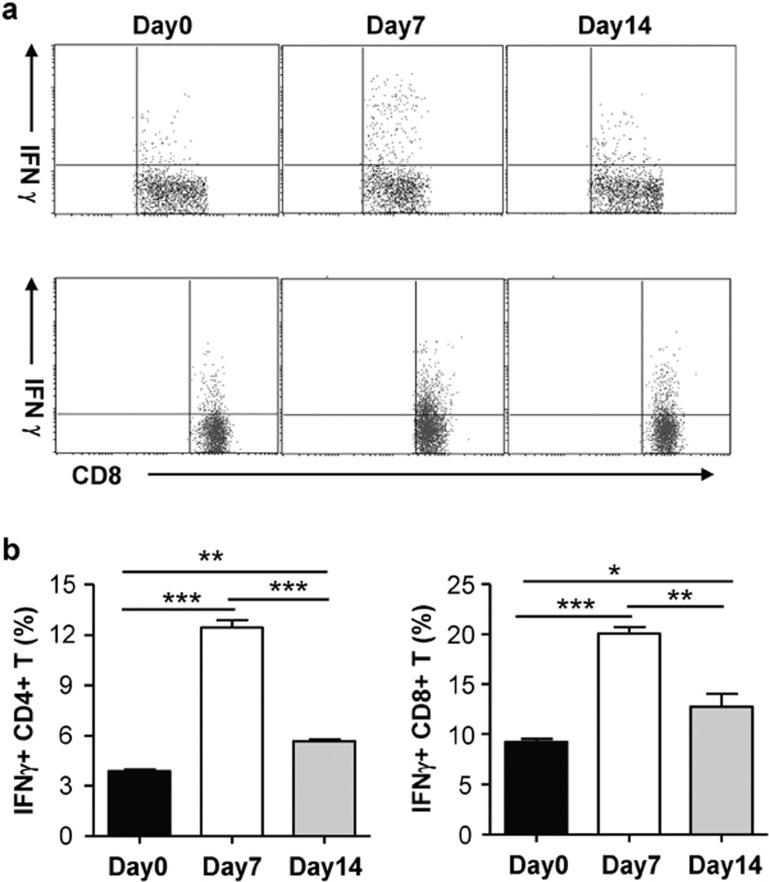
The antigen-driven production of IFN-γ and IL-12 by the mediastinal and hilar lymph nodes was further analyzed by in vitro culture. The results showed that lymph node cells cultured with UV-inactivated Chlamydial antigen produced much higher levels of IFN-γ and IL-12 p.i. compared to control media (Figure 2). Intracellular cytokine staining analyses also showed significantly increased IFN-γ-producing CD4+ and CD8+ T cells in the spleen at days 7 and 14 p.i. compared to that in the naive control mice (day 0; Figure 3a and b). Both the in vitro culture and flow cytometry tests showed that production of Th1 cytokines was higher at day 7 p.i. than at day 14 p.i. These results demonstrated that mice exposed to Chlamydia through the airway induced a characteristic Th1-type immune response, which has been shown to be critical for protection against Chlamydial infection.
Figure 2.
Production of the Th1-related cytokines IFN-γ and IL-12 in the mediastinal and hilar lymph node cells during Cm infection. Mice were infected intranasally with Cm as described in the legend for Figure 1 and killed at day 7, 14 or 21 post-infection. Mediastinal and hilar lymph node cells were cultured with UV-inactivated Cm or control media. IFN-γ and IL-12 levels in 48-h culture supernatants were measured by ELISA as described in the ‘Materials and methods' section. Statistical analysis was performed using the Student's t-test. Data represent mean±SD from three independent experiments with four mice per group. Cm, Chlamydia muridarum; IFN-γ, gamma interferon; Th1, T helper 1.
Figure 3.
IFN-γ-producing CD4 and CD8+ T cells in the spleen after intranasal Cm infection. Mice were infected with Cm as described in the legend for Figure 1. Spleen cells obtained at days 7 and 14 post-infection were analyzed for IFN-γ production by intracellular cytokine staining as described in the ‘Materials and methods' section. (a) IFN-γ-producing CD4 and CD8+ T cells; (b) summary graphs representing IFN-γ production by CD4+ and CD8+ T cells, respectively. Data represent mean±SD from three independent experiments with four mice per group. Statistical analysis was performed using the Student's t-test. **P<0.05, ***P<0.01, comparing the infected and control mice. Cm, Chlamydia muridarum; IFN-γ, gamma interferon.
Cm infection-induced production of chemokines and chemokine receptors in the lung
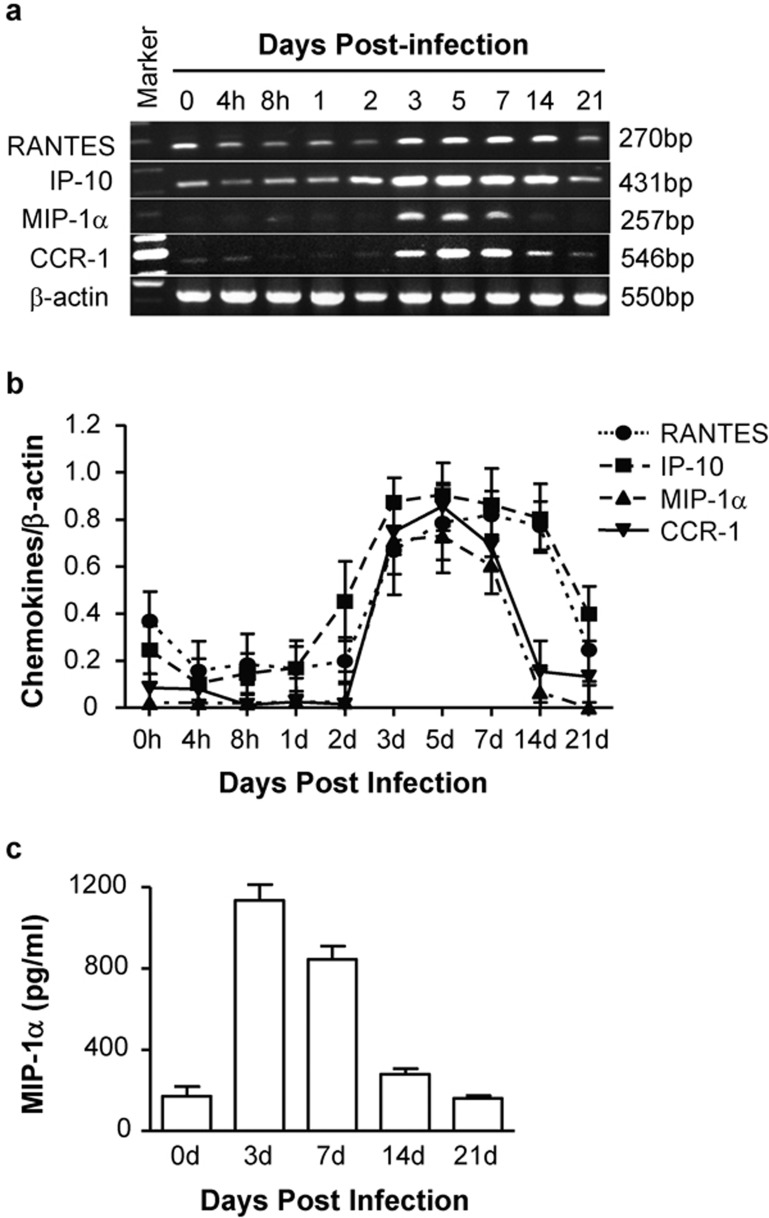
The mRNA expression levels of several chemokines, RANTES, IP-10 and MIP-1α, and the receptor CCR1 in the lungs were further analyzed following intranasal Cm infection. As shown in Figure 4a and b, the expression of RANTES, IP-10, MIP-1α and CCR1 were significantly upregulated beginning at day 3 p.i. after intranasal inoculation with Cm, which correlated with the elevation of Th1 responses. Interestingly, the expression of MIP-1α and CCR1 decreased to a low level at day 14 p.i., in parallel with the production pattern of IFN-γ and IL-12, while the expression of RANTES and IP-10 remained high at day 14 p.i. In contrast with MIP-1α and CCR1, there was obvious gene expression of RANTES and IP-10 in normal lung tissue (day 0) and at day 21 after infection, suggesting constitutive expression of these chemokines in the lung tissues. The protein secretion of the chief chemokine MIP-1α in the lung tissue supernatants showed the same dynamics as its mRNA expression (Figure 4c). These results suggest that MIP-1α expression has a closer association with the type 1 T-cell response in chlamydial lung infections than RANTES and IP-10. Therefore, the combination of the expression of MIP-1α and its receptor in the T cells is likely a more significant mechanism of attracting Th1 cells to infection sites for the clearance of Chlamydial lung infections.
Figure 4.
Chemokines and chemokine receptor production in the lungs during Cm infections. Mice were inoculated with Cm as described in the legend for Figure 1. Fresh lung tissues were collected at selected times post-infection (four mice per group at each time point), and total cellular RNA was isolated. The expression levels of RANTES, IP-10, MIP-1α and CCR1 were evaluated by RT-PCR. The lung tissue supernatants were collected at days 3, 7, 14 and 21 post-infection and analyzed for MIP-1α production by ELISA. (a) Representative image of three independent experiments with similar results; (b) the ratios of chemokines/β-actin and receptor/β-actin band densities are shown; (c) MIP-1α levels in lung supernatants were determined by ELISA. Cm, Chlamydia muridarum; IP-10, IFN-γ-inducible protein-10; MIP-1α, macrophage inflammatory protein-1α RT, reverse transcription.
Clearance of chlamydial infection was associated with Th1-type cytokine and chemokine responses
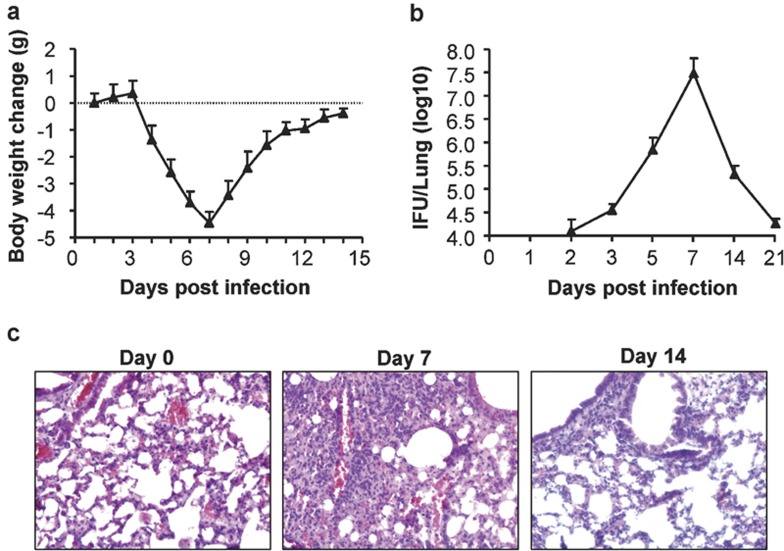
To elucidate the relationship between cytokine and chemokine production and the clearance of chlamydial infections, we assessed body weight changes and chlamydial growth and performed histological analyses of the lungs following intranasal Cm infection. Body weight loss started from day 3 p.i., decreased to the lowest level on day 7 and gradually recovered to the original weight by day 14 (Figure 5a). Tests of the in vivo growth of the Chlamydia in the lungs showed that Cm growth was detected from day 3 p.i., peaked at day 7 and became hardly detectable at day 21 p.i. (Figure 5b). Histopathological examination of the lungs showed significant tissue damage and pathological changes at day 7 in Cm-infected mice relative to naive control mice, and these effects gradually decreased to lower levels by day 14 (Figure 5c). The course of the Chlamydial infection was correlated with the kinetics of the Th1-type cytokine and chemokine response, especially with MIP-1α expression.
Figure 5.
Body weight changes and lung clearance of Cm in C57 mice after intranasal infection. Mice were inoculated with Cm as described in the legend for Figure 1. (a) Mice were monitored daily for body weight changes. (b) Mice were killed on day 3, 5, 7, 14 or 21 post-infection, and the lungs were analyzed for in vivo chlamydial growth as described in the ‘Materials and methods' section. (c) Lung sections were stained by H&E for histological analysis at ×400 magnification under light microscopy at days 0, 7 and 14 post-infection. Cm, Chlamydia muridarum; H&E, hematoxylin and eosin; IFU, inclusion-forming unit.
Discussion
Previous studies of chlamydial airway infections in mice by both our group and others have shown that the type 1 CD4 and CD8 T-cell immune responses are associated with protection against chlamydial infection.6, 21, 22, 23, 24, 25 These studies were primarily done by analyzing spleen cells. Our data in this study further confirm this conclusion by detecting type 1 immune responses in both local infected tissues and draining lymph nodes during respiratory tract Cm infections and the relationship between Cm clearance from the lung and Th1 immune responses in mice. Our results show that the expression of IL-12 and IFN-γ in the lungs was significantly upregulated starting at day 3 following Cm infection. The earlier expression of Th1 cytokines in the lungs is likely of great significance because Cm was reported to be largely restricted to the epithelium mucosa.26 Cm respiratory infections induced high levels of both IFN-γ and IL-12 production in the lungs and in draining lymph nodes. More importantly, the kinetics of Cm growth in the lungs and the body weight loss following infection were highly correlated with the pattern of Th1 responses. These results suggest that the Th1 response plays a critical role in the protective immunity against Cm infection.
The expression of chemokines is reported to be inducible and upregulated in inflammatory lesions, thus playing an important role in attracting effector T cells.27 Chemokines also play a role in directing different types of immune response following infection. Some recent studies suggested that the chemokines RANTES, MIP-1α and IP-10, and one of their receptors, CCR1, are responsible for recruiting the relevant Th1 cells and that MIP-1α is a specific promoter of the Th1 response.28 During Chlamydial genital tract infections, increased MIP-1α levels are associated with a stronger Th1 response.29 Upregulation of MIP-1α mRNA in dendritic cells pulsed with non-viable Chlamydia generated a potent protective Th1 immune response following adoptive transfer.30 We show here an enhancement of the chemokines RANTES, MIP-1α and IP-10 and one of their receptors, CCR1, in local tissues following chlamydial lung infection and that this enhancement is associated with the development of Th1 responses.
Chlamydia, as intracellular bacteria, differs from extracellular bacteria in that it can survive and replicate within cells. It may therefore take more time for the infected cells to respond. As shown in Figure 4, the expression of the chemokines MIP-1α, RENTES and IP-10 following chlamydial infection was increased at day 3 p.i., which corresponds with the amount of time needed for Chlamydia to finish its first intracellular life cycle. In contrast, cytokine and chemokine secretion following infection with Salmonella or invasive bacteria increases within 90 min after infection, reaching maximal levels at 3 h p.i. and decreasing rapidly thereafter,31 suggesting that different mechanisms are involved. Moreover, all of the tested chemokines (MIP-1α, RENTES and IP-10) are highly expressed in parallel with the increased Th1 responses, which begin to increase at day 3 p.i. However, in the late stage of infection (day 14), the expression of RANTES and IP-10 remained high while the expression of MIP-1α and CCR1 dramatically decreased to much lower levels as the Th1 responses were reduced and the Chlamydia was cleared. These results demonstrate a close association between MIP-1α and Th1 cell trafficking during Cm lung infections. In contrast, IP-10 and RANTES showed less association with Th1 responses, particularly in the late stage of infection. This is consistent with previous reports that showed inconsistent correlations of these chemokines with T-cell responses.18, 19 Aside from Th1-driven protection, RANTES can also induce Th2-mediated immunopathology.18 Furthermore, IP-10 has been shown to be upregulated in pulmonary allergic inflammations, which contributes to airway hyper-reactivity and Th2-type inflammations in asthma models.19
Although our data suggest a close relationship between MIP-1α and Th1 responses in Chlamydial infections, the potential mechanisms for the effects of MIP-1α on Th1 cells are unknown. Previous studies have suggested that MIP-1α is able to polarize T cells toward a Th1 response by reducing the number of IL-4- and IL-10-producing CD4 T cells32 and that it enhances the type 1 cytokine production of CD8+ T cells.33 Moreover, it has been reported that MIP-1α can drive T-cell antigen receptor-transgenic Th0 cells to differentiate into Th1 cells in vitro,34 and mice lacking MIP-1α are unable to generate a protective Th1 response.35 Therefore, MIP-1α may promote Th1 responses through direct and indirect means.
In summary, our data show that Cm lung infections induce a series of chemokine responses that are associated with the development of Th1 responses and the clearance of local infection. Moreover, we found that although the expression levels of all of the tested chemokines (RANTES, MIP-1α and IP-10) were associated with Th1 responses in the early stage of Cm infection, the expression levels of MIP-1α showed a better correlation with the Th1 cytokine response, which suggests the critical importance of MIP-1α in the host defense against chlamydial lung infections.
Acknowledgments
This work was supported by a fund from the Tianjin Municipal Science and Technology Commission (07JCYBJC10600).
References
- Grayston JT. Background and current knowledge of Chlamydial pneumoniae and atherosclerosis. J Infect Dis. 2000;181:402–410. doi: 10.1086/315596. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Jackson LA, Campbell LA, Grayston JT. Chlamydial pneumoniae (TWAR) Clin Microbiol Rev. 1995;8 4:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydial pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991;266 2:225–230. [PubMed] [Google Scholar]
- von Hertzen L, Alakarppa H, Koskinen R, Liippo K, Surcel HM, Leinonen M, et al. Chlamydial pneumoniae infection in patients with chronic obstructive pulmonary disease. Epidemiol Infect. 1997;118 2:155–164. doi: 10.1017/s095026889600725x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram S, Stratton CW, Yao S, Tharp A, Ding L, Bannan JD, et al. Chlamydial pneumoniae infection of the central nervous system in multiple sclerosis. Ann Neurol. 1999;46 1:6–14. [PubMed] [Google Scholar]
- Brunham RC, Rey-Ladino J. Immunology of Chlamydial infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5 2:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70 6:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telyatnikova N, Hill Gaston JS. Prior exposure to infection with Chlamydial pneumoniae can influence the T-cell-mediated response to Chlamydia trachomatis. . FEMS Immunol Med Microbiol. 2006;47 2:190–198. doi: 10.1111/j.1574-695X.2006.00080.x. [DOI] [PubMed] [Google Scholar]
- Mygind T, Vandahl B, Pedersen AS, Christiansen G, Hollsberg P, Birkelund S. Identification of an in vivo CD4+ T cell-mediated response to polymorphic membrane proteins of Chlamydial pneumoniae during experimental infection. FEMS Immunol Med Microbiol. 2004;40 2:129–137. doi: 10.1016/S0928-8244(03)00300-6. [DOI] [PubMed] [Google Scholar]
- Rottenberg ME, Gigliotti Rothfuchs A, Gigliotti D, Ceausu M, Une C, Levitsky V, et al. Regulation and role of IFN-gamma in the innate resistance to infection with Chlamydial pneumoniae. . J Immunol. 2000;164 9:4812–4818. doi: 10.4049/jimmunol.164.9.4812. [DOI] [PubMed] [Google Scholar]
- Penttila JM, Anttila M, Varkila K, Puolakkainen M, Sarvas M, Makela PH, et al. Depletion of CD8+ cells abolishes memory in acquired immunity against Chlamydial pneumoniae in BALB/c mice. Immunology. 1999;97 3:490–496. doi: 10.1046/j.1365-2567.1999.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillard JW, Singh UP, Boyaka PN, Singh S, Taub DD, McGhee JR. MIP-1alpha and MIP-1beta differentially mediate mucosal and systemic adaptive immunity. Blood. 2003;101:807–814. doi: 10.1182/blood-2002-07-2305. [DOI] [PubMed] [Google Scholar]
- Lillard JW, Boyaka PN, Taub DD, McGhee JR. RANTES potentiates antigen-specific mucosal immune responses. J Immunol. 2001;166:162–169. doi: 10.4049/jimmunol.166.1.162. [DOI] [PubMed] [Google Scholar]
- Sadek MI, Sada E, Toossi Z, Schwander SK, Rich EA. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol. 1998;19 3:513–521. doi: 10.1165/ajrcmb.19.3.2815. [DOI] [PubMed] [Google Scholar]
- Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2 2:102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168 7:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- Darville T, Andrews CW, Jr, Sikes JD, Fraley PL, Braswell L, Rank RG. Mouse strain-dependent chemokine regulation of the genital tract T helper cell type 1 immune response. Infect Immun. 2001;69 12:7419–7424. doi: 10.1128/IAI.69.12.7419-7424.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley FJ, Pennycook AM, Tregoning JS, Dodd JS, Walzl G, Wells TN, et al. Role of CCL5 (RANTES) in viral lung disease. J Virol. 2006;80 16:8151–8157. doi: 10.1128/JVI.00496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff BD, Sauty A, Tager AM, Maclean JA, Smith RN, Mathew A, et al. IFN-γ-inducible protein 10 (CXCL10) contributes to airway hyperreactivity and airway inflammation in a mouse model of asthma. J Immunol. 2002;168:5278–5286. doi: 10.4049/jimmunol.168.10.5278. [DOI] [PubMed] [Google Scholar]
- Joyee AG, Qiu H, Wang S, Fan Y, Bilenki L, Yang X. Distinct NKT cell subsets are induced by different Chlamydia species leading to differential adaptive immunity and host resistance to the infections. J Immunol. 2007;178 2:1048–1058. doi: 10.4049/jimmunol.178.2.1048. [DOI] [PubMed] [Google Scholar]
- Yang X, HayGlass KT, Brunham RC. Genetically determined differences in IL-10 and IFN-gamma responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156 11:4338–4344. [PubMed] [Google Scholar]
- Barron AL, White HJ, Rank RG, Soloff BL, Moses EB. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981;143 1:63–66. doi: 10.1093/infdis/143.1.63. [DOI] [PubMed] [Google Scholar]
- Wang S, Fan Y, Brunham RC, Yang X. IFN-gamma knockout mice show Th2-associated delayed-type hypersensitivity and the inflammatory cells fail to localize and control chlamydial infection. Eur J Immunol. 1999;29 11:3782–3792. doi: 10.1002/(SICI)1521-4141(199911)29:11<3782::AID-IMMU3782>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Yang X, Gartner J, Zhu L, Wang S, Brunham RC. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydial trachomatis lung infection. J Immunol. 1999;162 2:1010–1017. [PubMed] [Google Scholar]
- Geng Y, Berencsi K, Gyulai Z, Valyi-Nagy T, Gonczol E, Trinchieri G. Roles of interleukin-12 and gamma interferon in murine Chlamydial pneumoniae infection. Infect Immun. 2000;68 4:2245–2253. doi: 10.1128/iai.68.4.2245-2253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZP, Kuo CC, Grayston JT. A mouse model of Chlamydial pneumoniae strain TWAR pneumonitis. Infect Immun. 1993;61 5:2037–2040. doi: 10.1128/iai.61.5.2037-2040.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siveke JT, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160 2:550–554. [PubMed] [Google Scholar]
- Schrum S, Probst P, Fleischer B, Zipfel PF. Synthesis of the CC-chemokines MIP-1alpha, MIP-1beta, and RANTES is associated with a type 1 immune response. J Immunol. 1996;157 8:3598–3604. [PubMed] [Google Scholar]
- Darville T, Andrews CW, Jr, Sikes JD, Fraley PL, Braswell L, Rank RG. Mouse strain-dependent chemokine regulation of the genital tract T helper cell type 1 immune response. Infect Immun. 2001;69 12:7419–7424. doi: 10.1128/IAI.69.12.7419-7424.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JH, Grund VR, Durling L, Caldwell HD. Expression of genes encoding Th1 cell-activating cytokines and lymphoid homing chemokines by Chlamydia-pulsed dendritic cells correlates with protective immunizing efficacy. Infect Immun. 2001;69 7:4667–4672. doi: 10.1128/IAI.69.7.4667-4672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SJ, Eckmann L, Quayle AJ, Shen L, Zhang YX, Anderson DJ, et al. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydial infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99 1:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski MA, Huffnagle GB, McDonald RA, Lindell DM, Moore BB, Cook DN, et al. The role of macrophage inflammatory protein-1 alpha/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J Immunol. 2000;165 11:6429–6436. doi: 10.4049/jimmunol.165.11.6429. [DOI] [PubMed] [Google Scholar]
- Trifilo MJ, Bergmann CC, Kuziel WA, Lane TE. CC chemokine ligand 3 (CCL3) regulates CD8+-T-cell effector function and migration following viral infection. J Virol. 2003;77 7:4004–4014. doi: 10.1128/JVI.77.7.4004-4014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski MA, Huffinagle GB, McDonald RA, Toews GB. The role of macrophage inflam-matory protein-1α/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J Immunol. 2000;165:6429–6436. doi: 10.4049/jimmunol.165.11.6429. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Kennedy KJ. MIP-1a and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J Leukocyte Biol. 1997;62:681. [PubMed] [Google Scholar]