Abstract
Aim:
The aim of this study was to investigate the impact on expression of mRNA and protein by paradigm inducers/activators of nuclear receptors and their target genes in rat hepatic and intestinal cells. Furthermore, assess marked inter laboratory conflicting reports regarding species and tissue differences in expression to gain further insight and rationalise previously observed species differences between rodent and human based systems.
Methods:
Quantitative real time-polymerase chain reaction (QRT-PCR) and immunoblots were used to assess messenger RNA (mRNA) and protein expression for CYP2B2, CYP3A1, CYP3A2, CYP3A9, ABCB1a, ABCB1b, ABCC1, ABCC2, pregnane X receptor (PXR), farnesoid X receptor (FXR) and constituitive androstane receptor (CAR) in rat hepatoma cell line H411E, intestinal cells, Iec-6, and rat primary hepatocytes, in response to exposure for 18 h with prototypical inducers.
Results:
Dexamethasone (DEX) and pregnenolone 16α carbonitrile (PCN) significantly induced PXR, CYP3A9, ABCB1a and ABCB1b. However, when co-incubated, DEX appeared to restrict PCN-dependent induction. Chenodeoxycholic acid (CDCA) was the only ligand to induce FXR in all three cell types. Despite previously reported species differences between PCN and rifampicin (RIF), both compounds exhibited a similar profile of induction.
Conclusion:
Data presented herein may explain some of the discrepancies previously reported with respect to species differences from different laboratories and have important implications for study design.
Keywords: nuclear receptors, hepatic cells, intestinal cells, prototypical inducers, rodents, dexamethasone, pregnenolone 16α carbonitrile, cytochrome P450, pregnane X receptor, constituitive androstane receptor
Introduction
Constituitive androstane receptor (CAR), pregnane X receptor(PXR), and farnesoid X receptor (FXR) are key transcription factors regulating transcription of drug metabolising enzymes and transporters in response to prototypical inducers and activators such as PCN (rodent PXR), RIF (human PXR), PB (CAR) and CDCA (FXR). We have previously shown the impact of various prototypical inducers on expression of human isoforms of their genes1. The purpose of this study was to investigate the similarity and differences with rodent homologues using identical methodology.
Several important rodent homologues of human drug metabolising enzymes of CYP3A4/5 and CYP2B6 have been characterised in rodents: CYP3A12, 3, CYP3A24, CYP3A95 and CYP3A186, 7. These CYPs are inducible by glucocorticoids such as DEX, antibiotics such as RIF and other steroids such as PCN8, 9, 10. In addition, CYP2B2 has been studied extensively11, 12, 13, 14 and is known to be induced by many structurally divergent compounds15 incuding phenobarbital (PB).
PXR and CAR regulate overlapping sets of genes in a tissue specific fashion. In rodents, common activators of PXR and CAR upregulate ABCC2 in the liver via CAR, and in the intestine via PXR, while ABCB1a and ABCB1b are upregulated via PXR in the liver and intestine, with an additional effect via CAR in the intestine confined to ABCB1a16. A comparison of the PXR sequences from different mammalian species indicates that the PXR proteins share less than 80% amino acid identity in their ligand binding domains (LBD), while the DNA binding domain (DBD) is ∼95% similar17. The species differences in these LBDs are believed to be responsible for the selectivity in ligand binding, and thus the marked differences in the induction profiles between species18.
FXR is a ligand-activated transcription factor regulating cholesterol and fatty acid metabolism and also functions as an endogenous sensor for bile acids19, 20, 21. The relative contribution of FXR to induction of disposition genes is still unclear. However, the observation that lithocholic acid (LCA) induces CYP3A in PXR-null mice and that FXR activates human CYP3A4 promoter in vitro, implies CYP3A gene expression may also be controlled by FXR22, 23. Furthermore, ABCC2 has also been shown to be a target of FXR24, 25.
To date, analysis of gene expression and regulation of drug disposition genes utilise either immortalised or primary cell cultures. However, important differences have been reported between laboratories utilising the same strains of cells26, 27. The aim of this study was to investigate the impact of phenobarbital (PB:CAR), CDCA (FXR), PCN (rodent PXR) and RIF (human PXR) on mRNA and protein expression of ABCB1a, ABCB1b, ABCC1, ABCC2, CYP2B2, CYP3A1, CYP3A2, CYP3A9, PXR, CAR, and FXR in rat hepatic and intestinal cell lines (H411E and Iec-6) and rat primary hepatocytes.
Materials and methods
Cell culture
Rat hepatoma cell line H411E and rat intestinal cell line, Iec-6, were purchased from the American Tissue Culture Collection (ATCC, USA) and maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich company, UK) supplemented with 10% fetal bovine serum (FBS; Bio-Whittaker, Europe). Cell lines were incubated at 37 °C and 5% CO2 and subcultured every 4 days.
For isolation of fresh rat primary hepatocytes, rats were anaesthetised and the liver isolated and perfused for 10 min with wash buffer containing 10% v/v Hanks' balanced salt solution (HBSS; Sigma, UK), 0.138% w/v Hepes (Sigma, UK) and 0.5% v/v sodium hydrocarbonate (Sigma, UK) in deionised H2O. The rat liver was further perfused for 6–10 min with 500 mL of pre-warmed (37°C) digestion buffer containing wash buffer, 0.5% v/v calcium chloride, 250 mg Collagenase A (Sigma, UK) and 34 mg trypsin inhibitor (Sigma, UK). The digested rat liver was then placed into a petri dish containing 20 mg DNase and 200 mL wash buffer. The residual tissue was removed and the cells filtered through a 125 μm nylon blotting cloth and resuspended in DNase wash buffer and allowed to settle for 10 min, the supernatant was then removed and cells were washed and centrifuged at 50×g for 2 min and finally resuspended in wash buffer. Viability was determined based on Trypan blue exclusion28, 29 and found to be 85%– 90% viable.
Assessment of protein binding
Equilibrium dialysis was used to determine protein binding of PB, CDCA, PCN, and RIF (all compounds purchased from Sigma, UK) within the culture supernatant. Briefly, Dianorm dialysis membranes (GmbH, Munich, Germany) with molecular weight cut-off (MWC) of 5000 were soaked for 1 h in DMEM (Sigma-Aldrich, UK). PB, CDCA, PCN, and RIF were then individually added to DMEM containing 10% FBS (H411E, Iec-6 and primary hepatocyte media) to a final concentration of 10 μmol/L. An aliquot (1 mL) was then dispensed into one side of the dialysis block divided by the pre-soaked membrane, the other side containing control (without additions) media. The dialysis block was then sealed and rotated in a water bath for 24 h at 37 °C. A 200 μL aliquot was subsequently removed from the control side of the dialysis block and placed in quench tubes containing 200 μL of ice-cold methanol. After centrifugation at 400×g for 20 min, the supernatant was transferred into 96-well plates and analysed using liquid chromatography/mass spectrophotometry (LC-MS/MS) (AstraZeneca in-house methodology).
Treatment of cell lines for induction
H411E and Iec-6 were seeded into Nunclon™ Surface 6 well plates (Nunc A/S, Denmark) at a density of 5×106 (per well) containing DMEM and FBS (10%). Initial experiments assessed the effect of DEX on expression of CYP3A9, ABCB1a, ABCB1b, and PXR and the inducibility of CYP3A9, ABCB1a, ABCB1b, and PXR by PCN in H411E and Iec-6 cells. After 24 hours the prototypical inducers, PB, CDCA, PCN, and RIF were added to give final concentrations of 0.01, 0.1, 1.0, 10, and 100 μmol/L. The control used was the test compound vehicle, methanol for RIF or Dimethyl sulfoxide (DMSO) (0.1% v/v for both vehicles) for PB, CDCA or PCN. Cells were incubated for a further 18 hours at 37 °C and 5% CO2, total RNA isolated and cDNA constructed as described previously30.
Treatment of rat primary hepatocytes for induction
Rat primary hepatocytes were seeded at a density of 1×106 per well into 10% FBS supplemented DMEM in Nunclon™ Surface 6 well plates (Nunc A/S, Denmark) and allowed to grow for 24 h prior to treatment. Test compounds, PB, CDCA, PCN and RIF were added to give a final concentration of 1.0 μmol/L and a time course was conducted. Cells were then incubated at 37 °C and 5% CO2 and sampled at 0, 2, 4, 6, and 18 hours. DMSO treated controls were used for PB, CDCA, PCN and methanol treated controls were used for RIF (0.1% v/v for both vehicles).
Toxicity
All test compounds were assayed for toxicity by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay31 for both cell lines at final concentrations of 0.01 to 100 μmol/L. These assays were performed at 18 h to assess cell death at the point of analysis. Toxicity of test compounds was also assessed after 5 days as toxic concentrations would not necessarily be evident after 18 hours.
Quantitative real-time PCR
For H411E, Iec-6 and rat primary hepatocytes, total RNA was isolated utilising Tri-reagent (Sigma, UK) and cDNA constructed as previously described30. Real-time PCR assays were developed for quantification relative to β-actin (housekeeping gene) for each transcript in each cell set (H411E, Iec-6, and primary hepatocytes). Formation of primer dimer was optimised and eliminated out of the reaction to ensure no false positive amplification data was incorporated as a result of non-specific intercalation of picogreen (Molecular Probes, Paisley, UK). Relative expression against housekeeping gene β-actin (ΔΔCT) of transcripts were performed in an Opticon2™ Fluorescence Detector (MJ Research, Bio-Rad, Hertfordshire, UK). Amplification was performed in a final reaction volume of 25 μL utilising pico green intercalating fluorescence dye. The reaction mixture consisted of, 2.5 μL 10×Taqman Buffer II, 0.5U Taq polymerase, 1.25 μmol/L MgCl2, (Amplitaq Gold; Applied Biosystems, UK), 1.25 μmol/L dNTPs (Promega, UK), 20 ng cDNA, 0.5 μL pico green (final concentration 1:5000) and 0.03 μmol/L of forward and reverse primer, with the exception of constitutive androstane receptor and CYP3A9 which required 0.3 μmol/L (final concentration) and nuclease-free water was added to a final volume of 25 μL (Sigma-Aldrich, UK). Primer sequences and full assay conditions can be found on supplementary Table 1 and Table 2 respectively.
Immunoblotting
Western blotting analysis was performed in parallel with quantitative real time PCR using crude protein homogenates for CYPs, and for transporter proteins, crude membrane fractions which were purified as described previously32. In all cases, protein concentration was determined using the bicinchoninic acid assay (BCA)33. Samples were normalised to 5 μg/μL and stored at −80°C prior to use. Western blotting of all proteins was performed using NuPage 4%–12% Bis-Tris Gels (Invitrogen, Paisley, UK). Blotting was conducted using nitrocellulose membranes and iBlot™ Gel Transfer System (Invitrogen, UK), as per manufacturer's instructions, and membranes were blocked in 10% non-fat-dried milk (NFDM) overnight at 4 °C.
For CYP2B2, CYP3A1, CYP3A2, CYP3A9 (1:1000), mouse anti-rat CYP2B2 [sc-53242 (Santa Cruz Biotechnology, USA)], rabbit anti-rat CYP3A1 [AB1253 (Chemicon, USA)], rabbit anti-rat CYP3A2 [458223 (BD Gentest, USA)] and rabbit anti-rat CYP3A9 [AB1276 (Chemicon, USA)] were diluted in 0.1% T-TBS and 2% NFDM. For ABCB1a/b (1:4000), ABCC1 and ABCC2 (1:1000), mouse anti-rat ABCC2 [ab3373 (M2 III-6) (abcam, UK)], mouse anti rat ABCC1 [ab24102 (MRPm5) (abcam, UK)], goat anti rat P-gp [mdr(C-19) (Santa Cruz Biotechnology, USA)] were diluted in 0.05% T-TBS. β-actin loading control was used for all proteins (anti β-actin (1:5000) (Sigma, UK). All primary antibodies were incubated for 2 h at room temperature and all intermediate wash steps were performed with 0.05% T-TBS.
Horse radish peroxidise (HRP) conjugated secondary antibodies; STAR88P Donkey anti-sheep HRP conjugated (Serotec, USA), ab6701 Donkey anti-rabbit HRP conjugated (abcam, UK), P0449: rabbit anti-goat HRP conjugated (DakoCytomation, Denmark), sheep anti-mouse HRP conjugated (Amersham Biosciences, UK) were diluted 1:10000 and incubated for 1 hour at room temperature in 2% NFDM and 0.03% T-TBS. All subsequent washing steps were performed with 0.05% T-TBS, with the exception of ABCB1a/b which required 0.1% T-TBS.
Protein band visualisation was performed using enhanced chemiluminescence (ECL) technology (PerkinElmer, USA) and quantification was achieved using BioRad GS710 scanner and BioRad Quantity One™ densitometric analysis software (BioRad, USA). Optical density and relative protein expression of bands was determined against β-actin loading controls. Background saturation of ECL treated nitrocellulose membranes was corrected by subtraction of measurements taken from random sections of membrane (n=4) and subtracted from protein band density.
Statistical and data analysis
All reported data are for 18 hour incubations. The minimum concentration at which induction was observed and maximum fold change (excluding toxic concentrations) versus control are presented throughout this manuscript. Caution was taken in interpretation from data generated with concentrations of test compound shown to be toxic at 5 days for H411E and Iec-6. Specifically, concentrations that produced toxicity after 5 days are illustrated in the figures as dashed lines and statistical analysis is only presented for data at which significant induction was observed below this threshold. Therefore robust EC50 and Emax estimates were compromised and thus not used. Normality was assessed using a Shapiro-Wilk statistical test. Differences in mRNA and protein expression were assessed using a paired t-test. Logarithmic and/or linear regression was used to determine the relationship between change in mRNA and protein in cell lines.
Results
Cell line characterisation
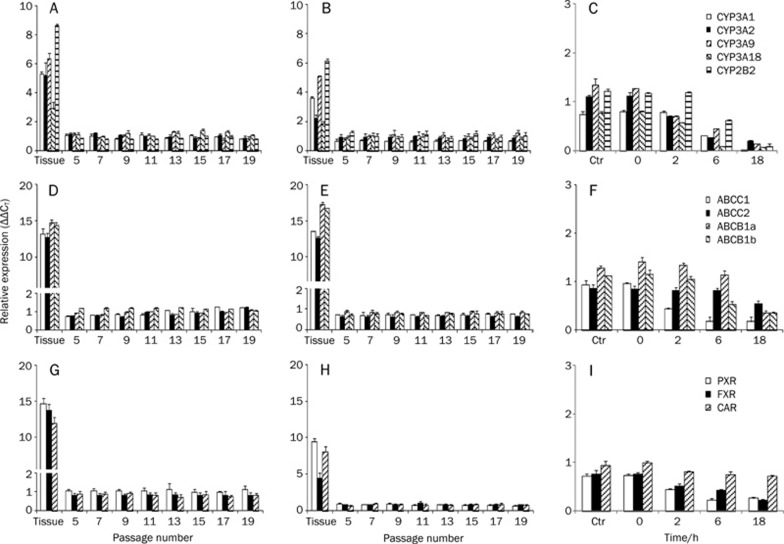
All CYPs were expressed at higher levels in liver tissue compared to intestine and primary hepatocytes (Figure 1A, 1B, and 1C), whereas transporters ABCB1a and ABCB1b were expressed at relatively higher levels in intestinal tissue compared to liver and primary hepatocytes (Figure 1D, 1E, and 1F). Lower basal levels of expression of nuclear receptors were found in the intestinal tissue compared to liver tissue and primary cells, specifically for PXR (Figure 1G, 1H, and 1I). Basal level of expression of transcripts were approximately 13 fold (NRs), 14 fold (transporters) and 6 fold (CYPs) higher in liver tissue compared to H411E and primary hepatocytes (Figure 1a, d and g respectively). 8 fold (NRs), 14 fold (transporters) and 4 fold (CYPs) higher in intestinal tissue compared Iec-6 and primary hepatocytes (Figure 1B, 1E, and 1H). Despite some minor oscillations in basal level of expression during passage, all transcripts were found to be stable from passage 5 to passage 21 after receipt from ATCC for both cell lines. However, for analysis, cell lines were only used between passage 11 and 13.
Figure 1.
Basal level of expression and stability of CYPs, transporters (ABC) and nuclear hormone receptors in rodent liver versus rodent hepatic cell line H411E (A, D, and G) and rodent intestinal tissue versus rodent intestinal cell line Iec-6 (B, E, and H) during passage. Basal level of expression and stability of CYPs, transporters and nuclear hormone receptors in rat primary hepatocytes over time. Data are the mean±SD. n=4 experiments conducted in duplicate.
Toxicity
After 18 h, there was no significant toxicity observed for any test compounds. However, after 5 days significant toxicity was detected for both H411E and Iec-6 (data not shown) implying that induction studies above certain concentrations may not be physiologically relevant ie for H411E, PB, PCN, CDCA, and RIF were all toxic at 100 μmol/L (P<0.0001). For Iec-6, CDCA, and PB were toxic at 10 μmol/L (P=0.0001 and P<0.0001 respectively) and PCN and RIF at 100 μmol/L (P=0.0027 and P<0.0001 P= 0.0027 respectively).
Protein binding
For DMEM containing 10% FBS, PB, PCN, RIF, and CDCA were determined as 98%, 97%, 98%, and 82% unbound respectively, indicating low levels of binding to FBS and a similar free drug concentration was present for incubations for all cell types.
Effects of DEX on CYP3A9, ABCB1a, ABCB1b, and PCN
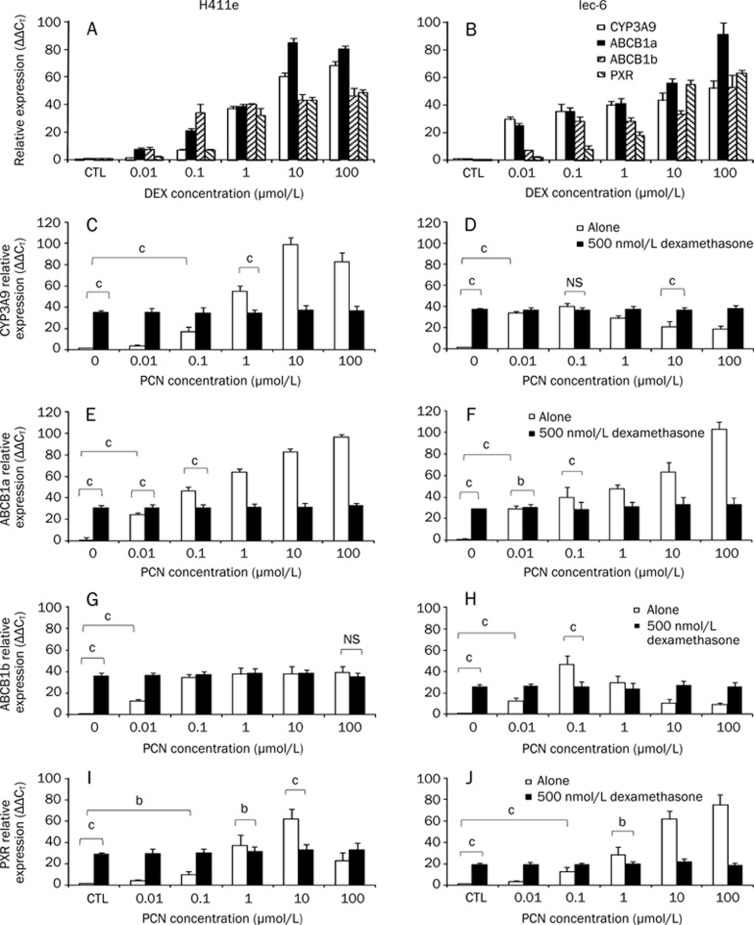
DEX was a potent inducer of all transcripts in H411E (Figure 2A) and Iec-6 (Figure 2B). Concentration dependent induction was observed for all transcripts in both cell lines, with significant induction for ABCB1a and ABCB1b in H411E and ABCB1a, ABCB1b, and CYP3A9 in Iec-6 at 0.01 μmol/L. Significant induction of PXR was detected at 0.1 μmol/L DEX in both cell lines. With the exception of ABCB1a in H411E (10 μmol/L), all transcripts were maximally induced at 100 μmol/L DEX.
Figure 2.
Implications of using dexamethasone (DEX) as a media supplement (A, B). Impact of dexamethasone (DEX) (0–100 μmol/L) on mRNA expression of CYP3A9, ABCB1a, ABCB1b, and pregnane X receptor (PXR) in H411E and Iec-6 (C–H). Impact of dexamethasone (DEX) (500 nmol/L) on inducibility of CYP3A9 (C, D), ABCB1a (E, F), ABCB1b (G, H) and pregnane X receptor (PXR) (I, J) by pregnenolone 16α-carbonitrile (PCN) in H411E and Iec-6 cells. Data are mean ±SD of four experiments conducted in duplicate. For clarity, not all statistical analyses are given: bP<0.05, cP<0.01. For each transcript, a significant increase in expression was observed at concentrations ≤1 μmol/L when cells were incubated with pregnenolone 16α-carbonitrile (PCN) alone, which would not have been observed when dexamethasone (DEX) was incorporated into the media.
In H411E and Iec-6 cells, PCN significantly induced CYP3A9 mRNA (Figures 2C and 2D), ABCB1a (Figures 2E and 2F), ABCB1b (Figures 2G and 2H) and PXR (Figures 2I and 2J) when compared to DEX-free control. However, when DEX was included in the culture media no significant induction of ABCB1b in H411E (Figure 2G) or CYP3A9 in Iec-6 (Figure 2D) was observed compared to DEX-free control.
Induction of PXR, FXR, and CAR mRNA in H411E, Iec-6, and primary hepatocytes
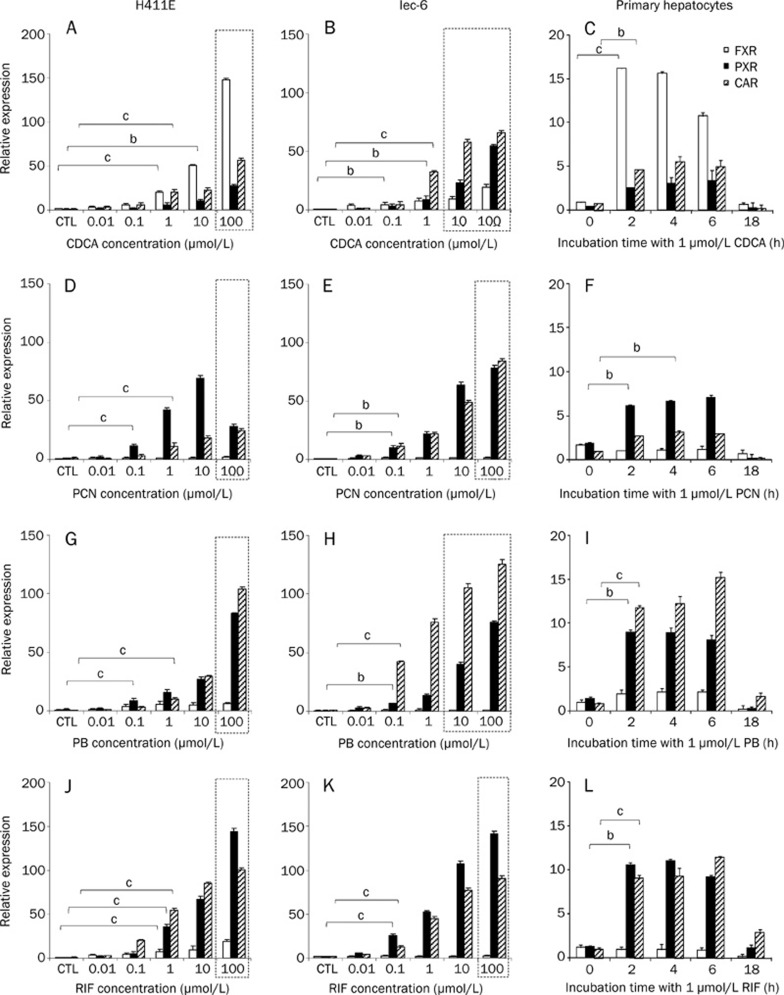
A summary of the impact on all genes of interest by prototypical inducers in H411e, Iec-6, and rat primary hepatocytes can be found in Table 1. The impact of CDCA, PCN, PB, and RIF on PXR, FXR, and CAR mRNA expression in H411E, Iec-6, and primary hepatocytes are shown in Figure 3. CDCA significantly increased expression of PXR, FXR, and CAR in H411E (Figure 3A) and Iec-6 (Figure 3B), but only for FXR and CAR in primary hepatocytes (Figure 3C). PCN caused significant induction of PXR and CAR in H411E (Figure 3D), Iec-6 (Figure 3E) in primary hepatocytes (Figure 3F). However, there was no significant increase of FXR by PCN in any cells used. For PB, there was no significant increase in mRNA expression for FXR in H411E, Iec-6 or primary hepatocytes (Figures 3G, 3H, and 3I respectively). However, significant induction of PXR and CAR was observed for all three cell types. This effect by PB was more marked with respect to magnitude of fold change for CAR in Iec-6 cells (Figure 3H) compared to H411E and primary hepatocytes. RIF upregulated FXR, PXR, and CAR in H411E (Figure 3J), PXR and CAR in Iec-6 (Figure 3K) and primary hepatocytes (Figure 3L).
Table 1. Summary of the impact of prototypical inducers (Chenodeoxycholiic acid; CDCA, Pregnane 16α-carbonitrile; PCN, Phenobarbital; PB and Rifampicin; RIF), on gene expression in H411e, Iec-6, and rat primary hepatocytes. For clarity, statistical analyses for cell lines H411e and Iec-6 are given only for the lowest concentration at which a significant difference was observed. For primary hepatocytes, the incubation time (in hours) when significant induction was detected: bP<0.05, cP<0.01.
| H411e Test compound (μmol/L) | Iec-6 Test compound (μmol/L) | Primary hepatocytes Test compound at 1 μmol/L (Incubation time in hours) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | CDCA | PCN | PB | RIF | CDCA | PCN | PB | RIF | CDCA | PCN | PB | RIF |
| CYP2B2 | 0.1c | 0.1c | 10b | NS | 1c | 0.1c | NS | NS | 2c | 2c | 2b | NS |
| CYP3A1 | 10b | 0.1c | 10b | 1c | 0.1c | 0.01c | 1c | 0.1b | 2c | 2b | 2c | 2c |
| CYP3A2 | NS | 0.1c | 10b | 1c | NS | 10c | 1b | 1c | 2c | 2c | 2c | 2c |
| CYP3A9 | 0.1c | 0.1c | 10c | 0.01b | NS | 0.01c | 0.01c | 0.1c | 2c | 2c | 2c | 2c |
| ABCC1 | 1c | 1c | NS | 1c | 1c | 1c | NS | 1b | 4b | 2c | 2b | 4b |
| ABCC2 | 1b | 1c | 10c | 1c | NS | NS | 1b | 1c | 2c | 2b | 2b | 2b |
| ABCB1a | 0.01c | 0.01c | 0.01c | 0.01c | 0.01c | 0.01c | 0.01c | 0.01c | 2b | 2c | 2c | 4c |
| ABCB1b | 0.1c | 0.01c | 0.01c | 0.1c | 1c | 0.01c | 0.01c | 0.1c | 2c | 2b | 2c | 2c |
| FXR | 1c | 0.1c | NS | 1c | 0.1b | NS | NS | NS | 2c | NS | NS | NS |
| PXR | 10b | NS | 0.1c | 1c | 1b | 0.1b | 0.1b | 0.1c | NS | 2b | 2c | 2c |
| CAR | 1c | 1c | 10c | 1c | 1c | 0.1b | 0.1c | 0.1c | 2b | 4b | 2c | 2c |
Figure 3.
Impact of typical activators on nuclear receptors. Effect of chenodeoxycholic acid (CDCA) (A–C), pregnenolone 16α-carbonitrile (PCN) (D–F), phenobarbital (PB) (G–I) and rifampicin (RIF) (J–l) in H411E, Iec-6 and primary hepatocytes. Data are mean±SD of four experiments conducted in duplicate. In cell lines, concentration dependency was investigated, whereas, in primary cells, time dependency was assessed at 1 μmol/L of each compound. Dotted lines indicate concentrations at which toxicity was observed following 5-day incubations with drug. For clarity, statistical analyses are given only for the lowest concentration at which a significant difference was observed: bP<0.05, cP<0.01.
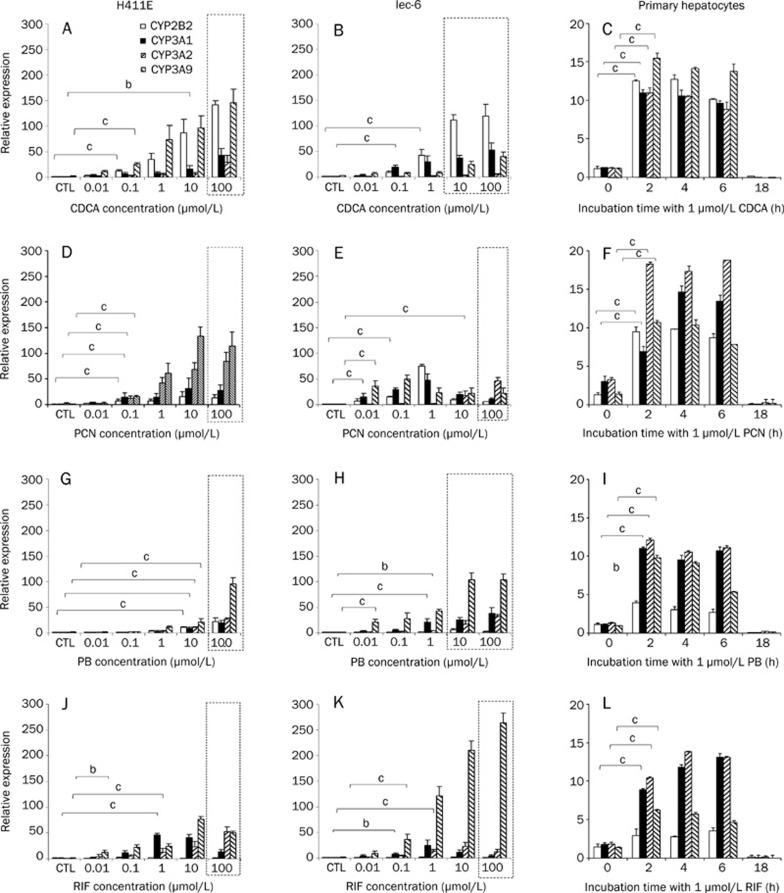
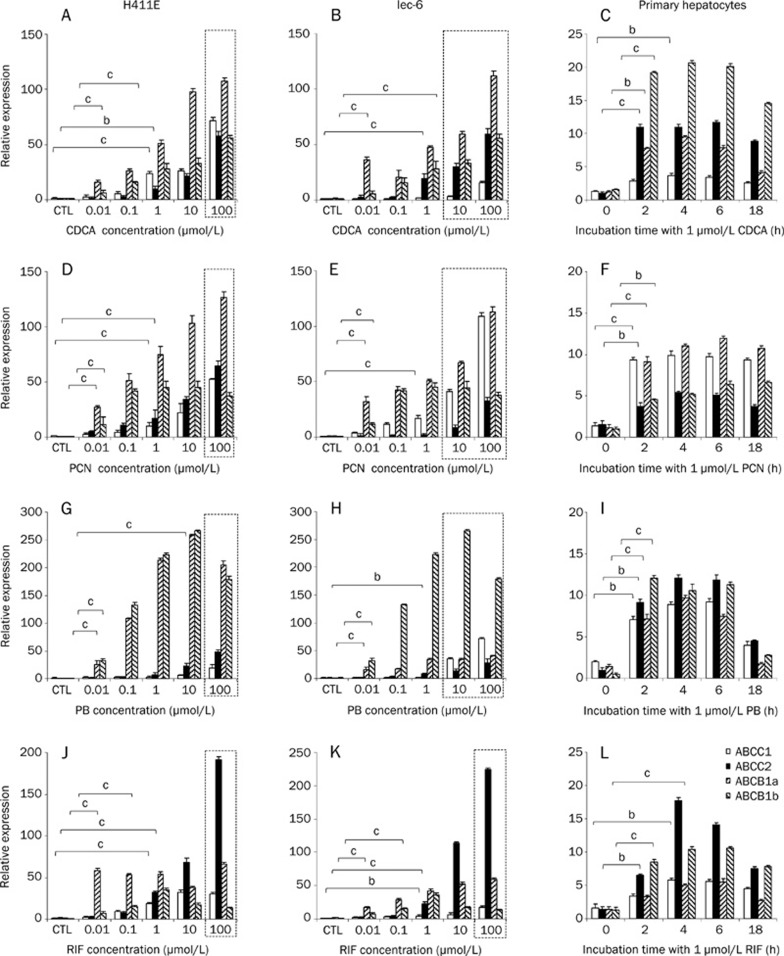
Induction of CYP2B2, CYP3A1, CYP3A2, and CYP3A9 mRNA in H411E, Iec-6, and primary hepatocytes
The effect of CDCA, PCN, PB, and RIF on mRNA expression of CYP2B2, CYP3A1, CYP3A2, and CYP3A9 can be seen in Figure 4. Statistical analysis is only given for the lowest concentration at which a significant induction was observed. CDCA significantly upregulated CYP2B2, CYP3A1, and CYP3A9 in H411E (Figure 4A), CYP2B2 and CYP3A1 in Iec-6 (Figure 4b) and CYP2B2, CYP3A1, CYP3A2, and CYP3A9 in primary hepatocytes (Figure 4C). For PCN, all CYPs were significantly increased at 0.1 μmol/L in H411E (Figure 4D), whereas in Iec-6 (Figure 4E), CYP3A1 and CYP3A9 were induced at 0.01 μmol/L, CYP2B2 at 0.1 μmol/L and CYP3A2 at 10 μmol/L PCN. In addition, all CYP transcripts were induced in primary hepatocytes (Figure 4F). With the notable exception of CYP2B2 in Iec-6 cells (Figure 4H), all CYPs were significantly induced in H411E cells (Figure 4G), Iec-6 cells (Figure 4H) and primary hepatocytes (Figure 4i) by PB. Finally, RIF had a potent effect on CYP3A1, CYP3A2, and CYP3A9 in all three cell types (Figure 4J, 4K, and 4L). This effect occurred at lower concentration in Iec-6 (Figure 4K), requiring only 0.1 μmol/L RIF to elicit a significant effect.
Figure 4.
Impact of typical activators on CYP isoforms. Effect of chenodeoxycholic acid (CDCA) (A–C), pregnenolone 16α-carbonitrile (PCN) (D–F), phenobarbital (PB) (G–I) and rifampicin (RIF) (J–I) in H411E, Iec-6 and primary hepatocytes. Data are mean±SD of four experiments conducted in duplicate. In cell lines, concentration dependency was investigated, whereas, in primary cells, time dependency was assessed at 1 μmol/L of each compound. Dotted lines indicate concentrations at which toxicity was observed following 5-day incubations with drug. For clarity, statistical analyses are given only for the lowest concentration at which a significant difference was observed: bP<0.05, cP<0.01.
Induction of ABCB1a, ABCB1b, ABCC1, and ABCC2 mRNA in H411E, Iec-6, and primary hepatocytes
The impact of CDCA, PCN, PB, and RIF on the expression of rat transporters can be seen in Figure 5. With the exception of ABCC1 in Iec-6 cells (Figure 5B), CDCA significantly upregulated all transporters in all three cell types (Figures 5A, 5B, and 5C). PCN significantly induced all transporters in H411E and primary hepatocytes (Figure 5f). ABCB1a, ABCB1b, and ABCC1 were also significantly upregulated in Iec-6 (Figure 5E) when incubated with PCN. However, there was no significant induction of ABCC2 in Iec-6 below toxic concentrations (Figure 5E). For PB, there was a significant increase observed for mRNA expression of ABCB1a, ABCB1b, and ABCC2 in H411E (Figure 5G) and Iec-6 (Figure 5H) cells and all transporters in primary hepatocytes. However, PB did not elicit a significant effect on ABCC1 in H411E or Iec-6 below toxic concentrations. RIF had a potent effect on all four transporters in all three cell lines (Figure 5J, 5K, and 5L). However, this effect was more marked with respect to magnitude of fold change for transporters in H411E (Figure 5J), significantly for ABCB1a compared to ABCB1b, ABCC1, and ABCC2.
Figure 5.
Impact of typical activators on transporters. Effect of chenodeoxycholic acid (CDCA) (A–C), pregnenolone 16α-carbonitrile (PCN) (D–F), phenobarbital (PB) (G–I) and rifampicin (RIF) (J–I) in H411E, Iec-6 and primary hepatocytes. Data are mean±SD of four experiments conducted in duplicate. In cell lines, concentration dependency was investigated, whereas, in primary cells, time dependency was assessed at 1 μmol/L of each compound. Dotted lines indicate concentrations at which toxicity was observed following 5-day incubations with drug. For clarity, statistical analyses are given only for the lowest concentration at which a significant difference was observed: bP<0.05, cP<0.01.
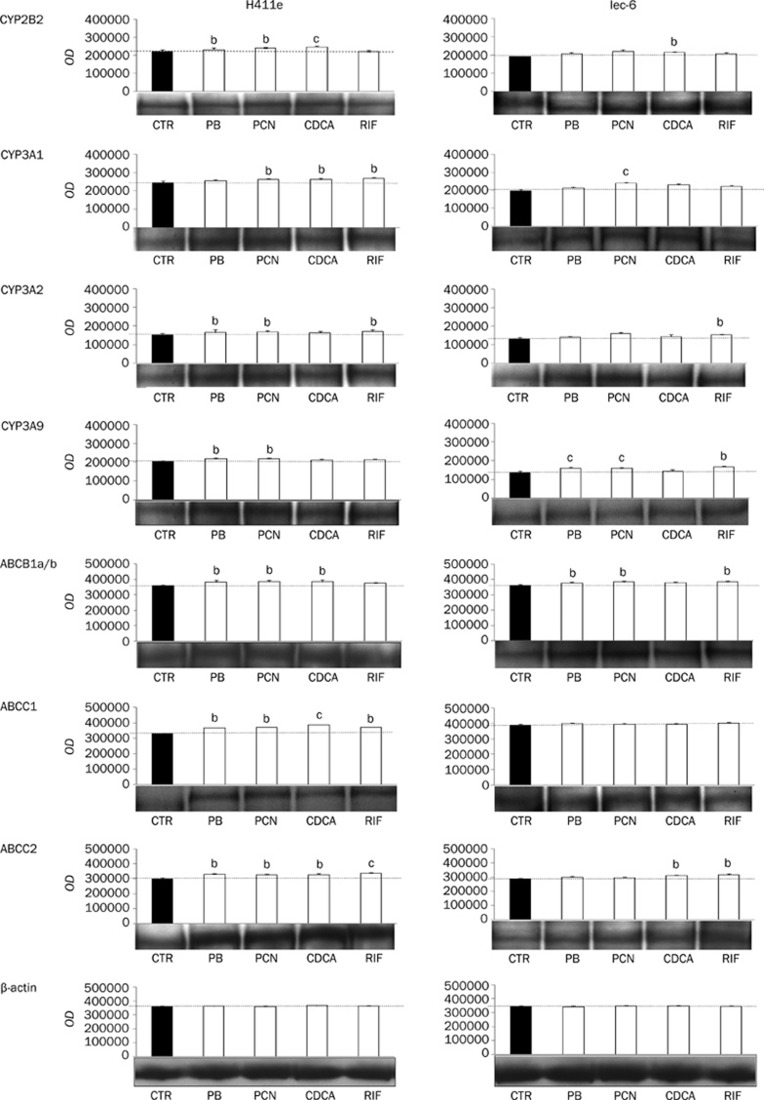
Induction of CYP2B2, CYP3A1, CYP3A2, CYP3A9, ABCB1a/b, ABCC1, and ABCC2 protein in H411E and Iec-6 cells
The effects of CDCA, PCN, PB, and RIF on protein expression of CYP2B2, CYP3A1, CYP3A2, ABCB1a/b, ABCC1, and ABCC2 in H411E and Iec-6 is shown in Figure 6. CDCA significantly upregulated CYP2B2, CYP3A1, ABCB1a/b, ABCC1, and ABCC2 in H411E and CYP2B2, CYP3A1, and ABCC2 in Iec-6 cells. With the exception of ABCC1 and ABCC2 in Iec-6, PCN significantly upregulated all proteins in both H411E and Iec-6. PB upregulated CYP2B2, CYP3A2, CYP3A9, ABCB1a/b, ABCC1, and ABCC2 in H411E cells and CYP3A9 and ABCB1a/b in Iec-6. RIF increased protein expression of CYP3A1, CYP3A2, ABCC1, and ABCC2 in H411E cells and CYP3A2, CYP3A9, ABCB1a/b, and ABCC2 in Iec-6.
Figure 6.
Effect of chenodeoxycholic acid (CDCA), pregnenolone 16α-carbonitrile (PCN), phenobarbital (PB) and rifampicin (RIF) on protein expression of CYP2B2, CYP3A1, CYP3A2, CYP3A9, ABCB1a/b, ABCC1, and ABCC2 in H411E and Iec-6. Results for β-actin are also given to illustrate equal loading. A representative western blot as well as the mean±SD optical densitometric results from four experiments conducted in duplicate are shown. bP<0.05, cP<0.01.
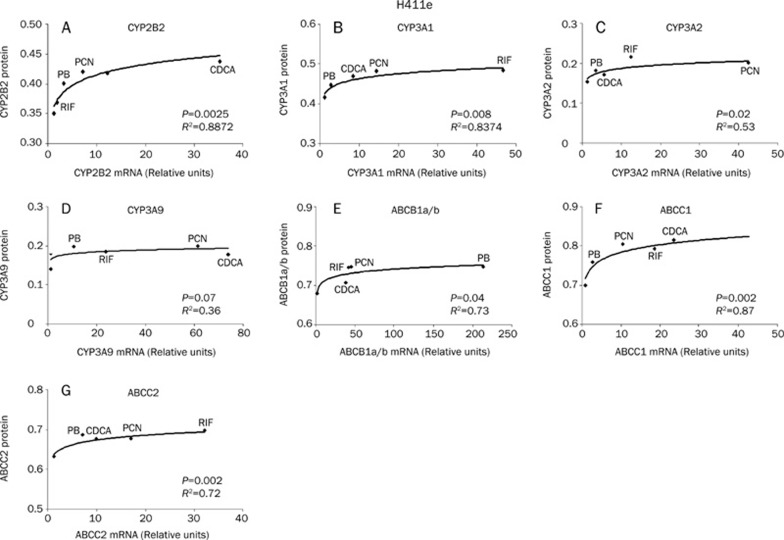
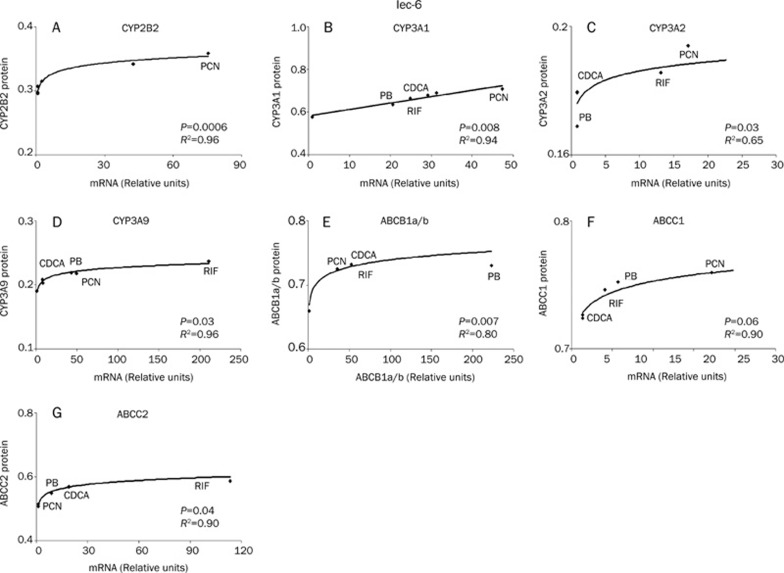
Relationship between mRNA and protein expression
The relationship between mRNA induction and protein expression for H411e and Iec-6 can be seen in Figure 7 and Figure 8 respectively. In order to assess the relationship between mRNA induction and protein expression, 1μmol/L mRNA data for each transcript were plotted against the corresponding protein data. Correlations for ABCB1a and ABCB1b were collated as no primary antibodies were available to differentiate the individual proteins. In H411E, a significant logarithmic correlation was observed for mRNA and protein for CYP2B2, CYP3A1, CYP3A2, ABCB1a/b, ABCC1, and ABCC2. For Iec-6 cells, significant logarithmic relationships were observed for CYP2B2, CYP3A2, CYP3A9, ABCB1a/b, and ABCC2. Interestingly, the relationship between mRNA and protein expression was linear for CYP3A1.
Figure 7.
Relationship between mRNA and protein expression. Correlation between mRNA and protein expression in H411e cells for CYP2B2 (A), CYP3A1 (B), CYP3A2 (C), CYP3A9 (D), ABCB1a/b (E), ABCC1 (F), and ABCC2 (G). A best fit to the data was observed by logarithmic regression for all CYPs and transporters.
Figure 8.
Relationship between mRNA and protein expression. Correlation between mRNA and protein expression in Iec-6 cells for CYP2B2 (A), CYP3A1 (B), CYP3A2 (C), CYP3A9 (D), ABCB1a/b (E), ABCC1 (F), and ABCC2 (G). A best fit to the data was observed by linear regression for all CYPs and transporters with the exception of CYP3A1, which was best described by linear regression.
Discussion
There are clear limitations associated with the use of rodent systems when extrapolating to human. For example, species differences often make it difficult to extrapolate rodent observations directly34, 35, 36 and conventional reporter based assays demonstrate that species differences are apparent for some ligands for PXR37. Importantly, PCN has been shown to activate rodent PXR but not human and the converse true for RIF17. However, other studies have reported that RIF treatment of rat hepatocytes increases CYP3A protein and PCN induces CYP3A mRNA in 50% of human cultures38 and has also been shown to activate PXR in some human systems39.
Cell lines have been reported to express decreased levels of certain transporters and CYP isoforms compared to primary cells40, 41. Previously, we have reported instability and variability of expression of some CYPs, transporters and nuclear receptors in the human hepatic (HepG2) and intestinal (Caco-2) cells over time1. Therefore, we conducted an initial screen of the studied transcripts in H411E and Iec-6 in order to assess basal levels of expression and stability of transcripts. However, unlike HepG2 and Caco-2, all CYPs, transporters and nuclear receptors were found to be stable in the rodent cell lines used here.
Isolated primary hepatocytes can be used in suspension or they can be cultured42, 43, 44. In suspension they exhibit metabolic activity for a period of 4 to 6 h45, thus remaining useful for the study of drug metabolism or toxicity46. However, gene expression changes taking place in primary hepatocyte cultures over time (0–18 h) in the absence of treatment were assessed and were consistent with previous studies involving hepatocytes in pure cultures47, 48.
The glucocorticoid DEX, is a widely used component in culture media and has been reported to exert a protective role on cell survival, prolonging cell viability, inhibiting the development of an apoptotic morphology, and stabilising expression of procaspase-3 in both human and rat hepatocytes49. However, DEX is a known inducer of the human PXR target gene CYP3A4 and has also been shown to affect p-glycoprotein (P-gp) activity50 and expression in primary rat hepatocytes51. In addition, DEX is believed to be a prerequisite for PCN inducible genes in rodent hepatocytes52. Previously we have shown that PCN upregulates expression of human CYP3A4, ABCB1 and PXR mRNA and inclusion of DEX as a media supplement resulted in a loss of ability to detect induction by PCN in human cells1. Generally, data presented herein show DEX had a similar effect in rat cells, where DEX significantly upregulated CYP3A9, ABCB1a, ABCB1b, and PXR. This is in broad agreement with similar observations in rat liver slices53. Furthermore, these data indicate a maximal response to PCN. Therefore, the addition of DEX increases the relative baseline but, as the maximum is fixed, a reduction in the potential effect is observed. This may allow competition in order to enable the biological system to prevent over-activation. Interestingly, a previous study indicated that the effects of DEX and PCN on rodent PXR target gene CYP3A1 were partially dependent upon the order these compounds were administered54.
The mechanisms controlling this PCN/DEX response currently remain unclear. However, previously it has been shown that sub-micromolar concentrations of PCN activate rodent PXR55 but antagonise the glucocorticoid receptor (GR). Furthermore, activation of GR by DEX increases PXR expression and increases CYP3A1, whereas PCN activates PXR directly54. In addition, the proposed existence of a cascade of signal transmission: GR-[PXR/CAR]-metabolising enzymes-transporters56 may explain the xenobiotic-mediated induction of CYP2B and CYP3A by prototypical activators of PXR and CAR in the presence of DEX57, 58, 59, 60. Importantly, this cascade would imply processes affecting the transcriptional activity of GR would subsequently affect the expression of PXR, CAR and FXR, and thus the expression of their target genes57. Therefore, it is not possible to assume that the induction reported here is necessarily due to discrete activation of individual nuclear receptors. However, the issues involving the DEX effect, coupled with attempts to facilitate comparisons with our human data1 led us not to include it as a supplement in subsequent experiments.
Significant induction of mRNA and protein of many transcripts in cell lines H411E and Iec-6 and primary hepatocytes in response to the nuclear receptor ligands were observed. Interestingly, for some of the genes investigated, a change in mRNA was observed with no corresponding change in protein and vice-versa. For example, mRNA was induced with no increase in protein for CYP3A9 by CDCA in H411e and CYP3A1 by PB in Iec-6, whereas increases in protein for ABCC1 by PB in H411e and ABCC2 by CDCA in Iec-6 with no corresponding increase in mRNA. However, recent work utilising microarray analysis has indicated that total mRNA levels do not always reflect protein levels61. For example, a study by Ideker et al, 2001, calculated an overall Pearson correlation coefficient between mRNA and protein expression of 0.61, although no change in protein expression was observed for almost 80% of genes reported as having a significant change in mRNA, indicating that a complex relationship exists between mRNA and protein expression. This work supporting data from a previous study62 where they observed a poor correlation between mRNA and protein for all but the most abundant proteins examined. Some similarities and important differences were seen in the profiles of induction in H411E versus Iec-6 cells. However, our data are in broad agreement with previous studies conducted in rat cells incubated with PB63, 64, PCN65, 66, and RIF 38. Where differences were observed, it is unclear whether this is due to inherent differences associated with specific tissue type (hepatic or intestinal) or whether they are due partly to the dedifferentiation process that occurs when generating and passaging continuously dividing cell systems. Specifically, because drug metabolism is a hallmark of highly differentiated, nondividing hepatocytes67.
For rat primary hepatocytes, our study conflicts with previous studies which indicated that RIF had no effect on rat PXR17. However, these investigators included DEX as a culture supplement and our observations that rat CYP3As are inducible by RIF is in agreement with another study68. Taken together these observations reinforce the probability that culturing conditions, including commonly used culture supplements i.e. antibiotics, FBS concentration, and DEX, play a major role in the variability of results between laboratories.
Several interesting observations have emerged from this study. Of note, all compounds were potent inducers of ABCB1a and ABCB1b, ABCC1 and ABCC2. Importantly, CDCA and RIF were more potent with respect to magnitude of fold change and drug concentration for ABCB1a compared to ABCB1b, generally requiring the lowest concentration (0.01 μmol/L) to elicit a significant effect. This response is consistent with observations by69, who reported preferential induction of ABCB1a versus ABCB1b. While it is difficult to directly link specific regulation of ABCB1a and ABCB1b to PXR and CAR and thus their relative contribution, from data presented herein. However, PCN and PB were more potent inducers of PXR than CAR in H411E and primary hepatocytes than for CAR in Iec-6 intestinal cells. Indeed, these observations are similar to a previous study16 where they noted CAR had an additional effect on ABCB1a. Also of note, ABCC2 mRNA levels were found to be markedly increased in primary rat hepatocytes exposed to all test compounds. In addition, a previous study also observed that ABCC2 was up-regulated at higher concentrations of PB than that needed for induction of rat CYP2B and CYP3A genes70. This confirms the PB-mediated up-regulation of ABCC271 and suggests that ABCC2 and CYPs are differentially regulated in response to PB.
CDCA had a potent effect on transporters, including ABCC2, in this study. Interestingly, it has also been reported that activation of FXR by CDCA is dependent upon the species examined, with CDCA having both an increased potency and magnitude of induction through human FXR than rodent FXR72. This is in partial agreement with this study and our previous observations1 where we found the effect of CDCA on FXR more potent for HepG2 than for H411E and a higher magnitude of induction for Caco-2 than for Iec-6. Furthermore, in both studies, human and rodent, we observed that CDCA was the only compound to significantly induce FXR mRNA.
Conclusion
In summary, induction of key transcriptional regulators PXR, CAR, and FXR by paradigm nuclear receptor ligands PB, PCN, RIF and CDCA in rat hepatic (H411E) and intestinal (Iec-6) cell lines and primary hepatocytes has been shown. In addition, a consequence of their induction is a corresponding induction of their target genes. Importantly, purported species specific PXR activators RIF (human PXR) and PCN (rodent PXR) showed comparable induction profiles of PXR and PXR target genes in all cells in the absence of DEX. Therefore, although species differences in activation of nuclear receptors are clearly evident, a similar spectrum of induction of target genes occurs in human and rat cells.
Author contribution
Philip MARTIN designed and performed all research experiments, collated all data and wrote the manuscript; Dominic WILLIAMS isolated fresh rat primary hepatocytes; Robert RILEY assisted with protein binding experiments and review of manuscript. Paul THOMPSON assisted with immunoblots and review of manuscript. David BACK assisted with writing and reviewing manuscript. Andrew OWEN performed statistical analysis for the relationship between mRNA and protein and writing manuscript.
Acknowledgments
This work was funded by AstraZeneca Charnwood.
Supplementary Information
References
- Martin P, Riley R, Back DJ, Owen A. Comparison of the induction profile for drug disposition proteins by typical nuclear receptor activators in human hepatic and intestinal cells. Br J Pharmacol. 2008;153:805–19. doi: 10.1038/sj.bjp.0707601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirita S, Matsubara T. cDNA cloning and characterization of a novel member of steroid-induced cytochrome P450 3A in rats. Arch Biochem Biophys. 1993;307:253–8. doi: 10.1006/abbi.1993.1587. [DOI] [PubMed] [Google Scholar]
- Komori M, Oda Y. A major glucocorticoid-inducible P450 in rat liver is not P450 3A1. J Biochem. 1994;116:114–20. doi: 10.1093/oxfordjournals.jbchem.a124482. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Song BJ, Hardwick JP. Pregnenolone 16 alpha-carbonitrile-inducible P-450 gene family: gene conversion and differential regulation. Mol Cell Biol. 1986;6:2969–76. doi: 10.1128/mcb.6.8.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Zgoda VG, Arison B. Almira Correia M. Structure-function relationships of rat liver CYP3A9 to its human liver orthologs: site-directed active site mutagenesis to a progesterone dihydroxylase. Arch Biochem Biophys. 2003;409:113–26. doi: 10.1016/s0003-9861(02)00582-9. [DOI] [PubMed] [Google Scholar]
- Strotkamp D, Roos PH, Hanstein WG. A novel CYP3 gene from female rats. Biochim Biophys Acta. 1995;1260:341–4. doi: 10.1016/0167-4781(94)00244-w. [DOI] [PubMed] [Google Scholar]
- Nagata K, Murayama N, Miyata M, Shimada M, Urahashi A, Yamazoe Y, et al. Isolation and characterization of a new rat P450 (CYP3A18) cDNA encoding P450(6)beta-2 catalyzing testosterone 6 beta- and 16 alpha-hydroxylations. Pharmacogenetics. 1996;6:103–11. doi: 10.1097/00008571-199602000-00009. [DOI] [PubMed] [Google Scholar]
- Deng Y, Bi HC, Zhao LZ, He F, Liu YQ, Yu JJ, et al. Induction of cytochrome P450s by terpene trilactones and flavonoids of the Ginkgo biloba extract EGb 761 in rats. Xenobiotica. 2008;38:465–81. doi: 10.1080/00498250701883233. [DOI] [PubMed] [Google Scholar]
- Garcia MC, Ma D, Dicioccio AT, Cali J. The use of a high-throughput luminescent method to assess CYP3A enzyme induction in cultured rat hepatocytes. In Vitro Cell Dev Biol Anim. 2008;44:129–34. doi: 10.1007/s11626-008-9085-1. [DOI] [PubMed] [Google Scholar]
- Hartley DP, Dai X, He YD, Carlini EJ, Wang B, Huskey SE, et al. Activators of the rat pregnane X receptor differentially modulate hepatic and intestinal gene expression. Mol Pharmacol. 2004;65:1159–71. doi: 10.1124/mol.65.5.1159. [DOI] [PubMed] [Google Scholar]
- Audet-Walsh E, Lachaud AA, Anderson A. The CYP2B2 5' flank contains a complex glucocorticoid response unit. Biochem Pharmacol. 2008;76:1298–306. doi: 10.1016/j.bcp.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Taura K, Imamura T, Yamada H, Oguri K. Induction of rat hepatic cytochrome P450 2B subfamily by azidophenobarbital, as a possible photoaffinity probe for the putative phenobarbital receptor: comparative study with modified phenobarbitals with different functional groups. Drug Metab Dispos. 1997;25:1442–6. [PubMed] [Google Scholar]
- Stoltz C, Vachon MH, Trottier E, Dubois S, Paquet Y, Anderson A. The CYP2B2 phenobarbital response unit contains an accessory factor element and a putative glucocorticoid response element essential for conferring maximal phenobarbital responsiveness. J Biol Chem. 1998;273:8528–36. doi: 10.1074/jbc.273.14.8528. [DOI] [PubMed] [Google Scholar]
- Roberge C, Beaudet MJ, Anderson A. GABA(A)/central benzodiazepine receptor and peripheral benzodiazepine receptor ligands as inducers of phenobarbital-inducible CYP2B and CYP3A. Biochem Pharmacol. 2004;68:1383–9. doi: 10.1016/j.bcp.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Azaroff L.Phenobarbital induction of cytochrome P-450 gene expression Biochem J 1992281(Pt 3): 577–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane X receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–46. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, et al. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- Quattrochi LC, Guzelian PS. CYP3A regulation: from pharmacology to nuclear receptors. Drug Metab Dispos. 2001;29:615–22. [PubMed] [Google Scholar]
- Modica S, Moschetta A. Nuclear bile acid receptor FXR as pharmacological target: are we there yet. FEBS Lett. 2006;580:5492–9. doi: 10.1016/j.febslet.2006.07.082. [DOI] [PubMed] [Google Scholar]
- Wang YD, Chen WD, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res. 2008;18:1087–95. doi: 10.1038/cr.2008.289. [DOI] [PubMed] [Google Scholar]
- Sonoda J, Pei L, Evans RM. Nuclear receptors: decoding metabolic disease. FEBS Lett. 2008;582:2–9. doi: 10.1016/j.febslet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA. 2001;98:3375–80. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, et al. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA. 2005;102:2063–8. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echchgadda I, Song CS, Roy AK, Chatterjee B. Dehydroepiandrosterone sulfotransferase is a target for transcriptional induction by the vitamin D receptor. Mol Pharmacol. 2004;65:720–9. doi: 10.1124/mol.65.3.720. [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–15. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Sambruy Y, Ferruzza S, Ranaldi G, De Angelis I. Intestinal cell culture models: applications in toxicology and pharmacology. Cell Biol Toxicol. 2001;17:301–17. doi: 10.1023/a:1012533316609. [DOI] [PubMed] [Google Scholar]
- Lin JH. CYP induction-mediated drug interactions: in vitro assessment and clinical implications. Pharm Res. 2006;23:1089–116. doi: 10.1007/s11095-006-0277-7. [DOI] [PubMed] [Google Scholar]
- Loretz LJ, Li AP, Flye MW, Wilson AG. Optimization of cryopreservation procedures for rat and human hepatocytes. Xenobiotica. 1989;19:489–98. doi: 10.3109/00498258909042288. [DOI] [PubMed] [Google Scholar]
- Li AP, Lu C, Brent JA, Pham C, Fackett A, Ruegg CE, et al. Cryopreserved human hepatocytes: characterization of drug-metabolizing enzyme activities and applications in higher throughput screening assays for hepatotoxicity, metabolic stability, and drug-drug interaction potential. Chem Biol Interact. 1999;121:17–35. doi: 10.1016/s0009-2797(99)00088-5. [DOI] [PubMed] [Google Scholar]
- Owen A, Chandler B, Back DJ, Khoo SH. Expression of pregnane-X-receptor transcript in peripheral blood mononuclear cells and correlation with MDR1 mRNA. Antivir Ther. 2004;9:819–21. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Marshak D. Strategies for protein purification and characterization: A laboratory course manual. Plainview (NY): Cold Spring Harbor Laboratory Press. 1996.
- Stoscheck CM. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- Bogaards JJ, Bertrand M, Jackson P, Oudshoorn MJ, Weaver RJ, van Bladeren PJ, et al. Determining the best animal model for human cytochrome P450 activities: a comparison of mouse, rat, rabbit, dog, micropig, monkey and man. Xenobiotica. 2000;30:1131–52. doi: 10.1080/00498250010021684. [DOI] [PubMed] [Google Scholar]
- Barton HA, Pastoor TP, Baetcke K, Chambers JE, Diliberto J, Doerrer NG, et al. The acquisition and application of absorption, distribution, metabolism, and excretion (ADME) data in agricultural chemical safety assessments. Crit Rev Toxicol. 2006;36:9–35. doi: 10.1080/10408440500534362. [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Sinal CJ. Mice as clinically relevant models for the study of cytochrome P450-dependent metabolism. Clin Pharmacol Ther. 2008;83:818–28. doi: 10.1038/clpt.2008.50. [DOI] [PubMed] [Google Scholar]
- Poso A, Honkakoski P. Ligand recognition by drug-activated nuclear receptors PXR and CAR: structural, site-directed mutagenesis and molecular modeling studies. Mini Rev Med Chem. 2006;6:937–47. doi: 10.2174/138955706777935008. [DOI] [PubMed] [Google Scholar]
- Kocarek TA, Schuetz EG, Strom SC, Fisher RA, Guzelian PS. Comparative analysis of cytochrome P4503A induction in primary cultures of rat, rabbit, and human hepatocytes. Drug Metab Dispos. 1995;23:415–21. [PubMed] [Google Scholar]
- El-Sankary W, Gibson GG, Ayrton A, Plant N. Use of a reporter gene assay to predict and rank the potency and efficacy of CYP3A4 inducers. Drug Metab Dispos. 2001;29:1499–504. [PubMed] [Google Scholar]
- Nakamura T, Sakaeda T, Ohmoto N, Tamura T, Aoyama N, Shirakawa T, et al. Real-time quantitative polymerase chain reaction for MDR1, MRP1, MRP2, and CYP3A-mRNA levels in Caco-2 cell lines, human duodenal enterocytes, normal colorectal tissues, and colorectal adenocarcinomas. Drug Metab Dispos. 2002;30:4–6. doi: 10.1124/dmd.30.1.4. [DOI] [PubMed] [Google Scholar]
- Wilkening S, Stahl F, Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab Dispos. 2003;31:1035–42. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
- Waring JF, Ciurlionis R, Jolly RA, Heindel M, Gagne G, Fagerland JA, et al. Isolated human hepatocytes in culture display markedly different gene expression patterns depending on attachment status. Toxicol In Vitro. 2003;17:693–701. doi: 10.1016/s0887-2333(03)00102-4. [DOI] [PubMed] [Google Scholar]
- Houle R, Raoul J, Levesque JF, Pang KS, Nicoll-Griffith DA, Silva JM. Retention of transporter activities in cryopreserved, isolated rat hepatocytes. Drug Metab Dispos. 2003;31:447–51. doi: 10.1124/dmd.31.4.447. [DOI] [PubMed] [Google Scholar]
- Sosef MN, Baust JM, Sugimachi K, Fowler A, Tompkins RG, Toner M. Cryopreservation of isolated primary rat hepatocytes: enhanced survival and long-term hepatospecific function. Ann Surg. 2005;241:125–33. doi: 10.1097/01.sla.0000149303.48692.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenkova K, Belejova M, Vesely J, Chmela Z, Rypka M, Ulrichova J, et al. Cell suspensions, cell cultures, and tissue slices--important metabolic in vitro systems. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2001;145:57–60. doi: 10.5507/bp.2001.012. [DOI] [PubMed] [Google Scholar]
- Morel F, Langouet S, Maheo K, Guillouzo A. The use of primary hepatocyte cultures for the evaluation of chemoprotective agents. Cell Biol Toxicol. 1997;13:323–9. doi: 10.1023/a:1007491525955. [DOI] [PubMed] [Google Scholar]
- Tuschl G, Mueller SO. Effects of cell culture conditions on primary rat hepatocytes-cell morphology and differential gene expression. Toxicology. 2006;218:205–15. doi: 10.1016/j.tox.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Paine AJ. The maintenance of cytochrome P-450 in rat hepatocyte culture: some applications of liver cell cultures to the study of drug metabolism, toxicity and the induction of the P-450 system. Chem Biol Interact. 1990;74:1–31. doi: 10.1016/0009-2797(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Bailly-Maitre B, de Sousa G, Boulukos K, Gugenheim J, Rahmani R. Dexamethasone inhibits spontaneous apoptosis in primary cultures of human and rat hepatocytes via Bcl-2 and Bcl-xL induction. Cell Death Differ. 2001;8:279–88. doi: 10.1038/sj.cdd.4400815. [DOI] [PubMed] [Google Scholar]
- Chieli E, Santoni-Rugiu E, Cervelli F, Sabbatini A, Petrini M, Romiti N, et al. Differential modulation of P-glycoprotein expression by dexamethasone and 3-methylcholanthrene in rat hepatocyte primary cultures. Carcinogenesis. 1994;15:335–41. doi: 10.1093/carcin/15.2.335. [DOI] [PubMed] [Google Scholar]
- Fardel O, Lecureur V, Guillouzo A. Regulation by dexamethasone of P-glycoprotein expression in cultured rat hepatocytes. FEBS Lett. 1993;327:189–93. doi: 10.1016/0014-5793(93)80167-s. [DOI] [PubMed] [Google Scholar]
- Haag M, Fautrel A, Guillouzo A, Frossard N, Pons F. Expression of cytochromes P450 3A in mouse lung: effects of dexamethasone and pregnenolone 16alpha-carbonitrile. Arch Toxicol. 2003;77:145–9. doi: 10.1007/s00204-002-0426-7. [DOI] [PubMed] [Google Scholar]
- Meredith C, Scott MP, Renwick AB, Price RJ, Lake BG. Studies on the induction of rat hepatic CYP1A, CYP2B, CYP3A and CYP4A subfamily form mRNAs in vivo and in vitro using precision-cut rat liver slices. Xenobiotica. 2003;33:511–27. doi: 10.1080/0049825031000085960. [DOI] [PubMed] [Google Scholar]
- Hosoe TN, Inouye TY. Divergent modes of induction of rat hepatic and pulmonary CYP3A1 by dexamethasone and pregnenolone16alpha-carbonitrile. J Health Sci. 2005;51:75–9. [Google Scholar]
- Shah YM, Ma X, Morimura K, Kim I, Gonzalez FJ. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1114–22. doi: 10.1152/ajpgi.00528.2006. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Drocourt L, Assenat E, Larrey D, Pichard-Garcia L, et al. Cross-talk between xenobiotic detoxication and other signalling pathways: clinical and toxicological consequences. Xenobiotica. 2004;34:633–64. doi: 10.1080/00498250412331285454. [DOI] [PubMed] [Google Scholar]
- Duret C, Daujat-Chavanieu M, Pascussi JM, Pichard-Garcia L, Balaguer P, Fabre JM, et al. Ketoconazole and miconazole are antagonists of the human glucocorticoid receptor: consequences on the expression and function of the constitutive androstane receptor and the pregnane X receptor. Mol Pharmacol. 2006;70:329–39. doi: 10.1124/mol.105.022046. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz EG, Schmid W, Schutz G, Brimer C, Yasuda K, Kamataki T, et al. The glucocorticoid receptor is essential for induction of cytochrome P-4502B by steroids but not for drug or steroid induction of CYP3A or P-450 reductase in mouse liver. Drug Metab Dispos. 2000;28:268–78. [PubMed] [Google Scholar]
- Pierrat OA, Mikitova V, Bush MS, Browning KS, Doonan JH.Control of protein translation by phosphorylation of the mRNA 5′-cap-binding complex Biochem Soc Trans 200735(Pt 6): 1634–7. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–30. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem LG, Trottier E, Anderson A, Jefcoate CR. Phenobarbital induction of CYP2B1/2 in primary hepatocytes: endocrine regulation and evidence for a single pathway for multiple inducers. Toxicol Appl Pharmacol. 1999;155:32–42. doi: 10.1006/taap.1998.8599. [DOI] [PubMed] [Google Scholar]
- Joannard F, Galisteo M, Corcos L, Guillouzo A. Lagadic-Gossmann D. Regulation of phenobarbital-induction of CYP2B and CYP3A genes in rat cultured hepatocytes: involvement of several serine/threonine protein kinases and phosphatases. Cell Biol Toxicol. 2000;16:325–37. doi: 10.1023/a:1026702615125. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Guzelian PS. Induction of cytochrome P-450 by glucocorticoids in rat liver. II. Evidence that glucocorticoids regulate induction of cytochrome P-450 by a nonclassical receptor mechanism. J Biol Chem. 1984;259:2007–12. [PubMed] [Google Scholar]
- Debri K, Boobis AR, Davies DS, Edwards RJ. Distribution and induction of CYP3A1 and CYP3A2 in rat liver and extrahepatic tissues. Biochem Pharmacol. 1995;50:2047–56. doi: 10.1016/0006-2952(95)02107-8. [DOI] [PubMed] [Google Scholar]
- Meyer UA, Hoffmann K. Phenobarbital-mediated changes in gene expression in the liver. Drug Metab Rev. 1999;31:365–73. doi: 10.1081/dmr-100101924. [DOI] [PubMed] [Google Scholar]
- Swales K, Plant N, Ayrton A, Hood S, Gibson G. Relative receptor expression is a determinant in xenobiotic-mediated CYP3A induction in rat and human cells. Xenobiotica. 2003;33:703–16. doi: 10.1080/0049825031000121626. [DOI] [PubMed] [Google Scholar]
- Lothstein L, Hsu SI, Horwitz SB, Greenberger LM. Alternate overexpression of two P-glycoprotein [corrected] genes is associated with changes in multidrug resistance in a J774.2 cell line. J Biol Chem. 1989;264:16054–8. [PubMed] [Google Scholar]
- Courtois A, Payen L, Le Ferrec E, Scheffer GL, Trinquart Y, Guillouzo A, et al. Differential regulation of multidrug resistance-associated protein 2 (MRP2) and cytochromes P450 2B1/2 and 3A1/2 in phenobarbital-treated hepatocytes. Biochem Pharmacol. 2002;63:333–41. doi: 10.1016/s0006-2952(01)00829-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, et al. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem. 2003;278:14146–52. doi: 10.1074/jbc.M212482200. [DOI] [PubMed] [Google Scholar]
- Cui J, Heard TS, Yu J, Lo JL, Huang L, Li Y, et al. The amino acid residues asparagine 354 and isoleucine 372 of human farnesoid X receptor confer the receptor with high sensitivity to chenodeoxycholate. J Biol Chem. 2002;277:25963–9. doi: 10.1074/jbc.M200824200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.