Abstract
Aim:
Filamin binding LIM protein 1, also known as migfilin, is a skeleton organization protein that binds to mitogen-inducible gene 2 at cell-extracellular matrix adhesions. The aim of this study was to investigate the role of migfilin in cisplatin-induced apoptosis in human glioma cells, to determine the functional domains of migfilin, and to elucidate the molecular mechanisms underlying the regulation of cisplatin-related chemosensitivity.
Methods:
The human glioma cell lines Hs683, H4, and U-87 MG were transfected with pEGFP-C2-migfilin to elevate the expression level of migfilin. RNA interference was used to reduce the expression of migfilin. To determine the functional domains of migfilin, U-87 MG cells were transfected with plasmids of migfilin deletion mutants. After treatment with cisplatin (40 μmol/L) for 24 h, the cell viability was assessed using the MTS assay, and the cell apoptotic was examined using the DAPI staining assay and TUNEL analysis. Expression levels of apoptosis-related proteins were detected by Western blot analysis.
Results:
Overexpression of migfilin significantly enhanced cisplatin-induced apoptosis in Hs683, H4, and U-87 MG cells, whereas downregulation of migfilin expression inhibited the chemosensitivity of these cell lines. The N-terminal region of migfilin alone was able to enhance the cisplatin-induced apoptosis. However, despite the existence of the N-terminal region, mutants of migfilin with any one of three LIM domains deleted led to a function loss. Furthermore, apoptotic proteins (PARP and caspase-3) and the anti-apoptotic protein Bcl-xL were modulated by the expression level of migfilin in combination with cisplatin.
Conclusion:
The LIM1-3 domains of migfilin play a key role in sensitizing glioma cells to cisplatin-induced apoptosis through regulation of apoptosis-related proteins.
Keywords: filamin binding LIM protein 1 (migfilin), glioma, cisplatin, apoptosis, PARP, caspase-3, Bcl-xL
Introduction
Glioma, the most common type of brain tumor, originates from glial cells of the central nervous system1. Glioblastoma multiforme (GBM) is the grade IV glioma that represents the most invasive form of malignant brain tumors. The growth of glioblastoma cells exhibits infiltration into contiguous tissue throughout the brain, which challenges the complete resection of gliomas and might be partially responsible for the high rates of disease recurrence. Thus, in spite of extensively applied multimodal therapies, glioblastoma patients commonly receive a poor prognosis with median survival duration of less than 14 months1,2,3. However, in recent years, targeted therapy has provided novel therapeutic insights into improving the sensitivity of malignant cells to systemic radiotherapy or chemotherapy2.
The connection between the extracellular matrix (ECM) and adjacent cells plays a significant role in cell fate determination, including differentiation, proliferation, migration and apoptosis4. Filamin binding LIM protein 1 (FBLIM1), also known as migfilin, is characterized as a skeleton organization protein that binds to mitogen-inducible gene 2 (Mig-2) at cell-ECM adhesions5,6,7. It has been elucidated that migfilin colocalizes with Mig-2 and binds to Mig-2 through its C-terminal domain, which is functionally required for the morphological maintenance of cells5. Moreover, the N-terminus of migfilin mediates integrin activation through competitively binding to the site of filamin immunoglobulin-like domain IgLFN21, disaggregating the filamin/integrin complex and promoting talin/integrin interaction8,9. Notably, migfilin consists of three structural parts, which possess disparate functions and act as indispensable biding domains, including an N-terminal region, a central proline-rich region and a C-terminal region. The C-terminal region has three sequential LIM domains8. In addition, two types of migfilin isoforms have been differentiated as migfilin(s) and FBLP-14,8. Migfilin(s) is a splicing variant with a proline-rich region deleted, resulting in the loss of the VASP binding site4,5. FBLP-1 is missing the third LIM domain, which most likely interferes with the interaction of Mig-24. Thus, it appears that the functional domains of migfilin, partially presented in migfilin variants, are responsible for distinct downstream signaling through binding to corresponding proteins.
Currently, the DNA-alkylating agent temozolomide is widely used to treat glioblastoma patients, showing a quite modest survival benefit10,11. Unfortunately, no effective second-line chemotherapy drug can be utilized for glioblastoma patients when chemoresistance to temozolomide occurs12. Meanwhile, the mechanism of chemoresistance to regular chemotherapeutic agents is poorly understood10,12. Cisplatin is an anti-cancer drug that specifically causes DNA damage, including DNA interstrand and intrastrand crosslinks, and is widely applied for the treatment of malignant solid tumors13,14. Overcoming the ineffectiveness of cisplatin in gliomas and possibly providing an alternative therapeutic strategy for glioma patients would be beneficial. In this study, we report that migfilin plays an important role in sensitizing glioblastoma cells to cisplatin-induced apoptosis, which might be a novel therapeutic target for gliomas.
Materials and methods
Cell lines and culture conditions
As previously described, the human glioma cell lines Hs683, H4, and U-87 MG (purchased from Cell Resource Centre, IBMS, CAMS/PUMC), were cultured in DMEM (GIBCO) culture medium containing 10% fetal bovine serum (GIBCO)15. Cells were incubated at 37 °C in a humidified atmosphere with 5% CO2.
Reagents
Mouse monoclonal anti-migfilin antibody was produced as previously described5. Antibodies specific to Bcl-xL, Bcl-2, Caspase-3, and PARP were purchased from Cell Signaling Technology Inc. Anti-p53 antibody was purchased from Santa Cruz Biotechnology Inc. Anti-beta-actin antibody was obtained from Sigma. Goat anti-mouse IgG and goat anti-rabbit IgG were purchased from Promega Inc. The anti-cancer drug cisplatin was diluted to the concentration of 1 mg/mL (obtained from Cancer Hospital, Chinese Academy of Medical Sciences).
Plasmids, siRNAs and transient transfections
The plasmids containing different types of cDNAs of migfilin deletion mutants, which were inserted into pEGFP-C2 vectors encoding green fluorescent protein (GFP), were generated by Clontech16. The siRNAs utilized were as follows: migfilin siRNA (5′-AGGGGCAUCCACAGACAUCTT-3′) and control siRNA (5′-UUCUCCGAACGUGUCACGU-3′). U-87 MG cells were seeded and cultured without antibiotics at 37 °C overnight. Cells were then transfected with plasmids or siRNAs using Lipofectamine 2000 (Invitrogen) in serum-free DMEM. After a 4-h incubation, the serum-free culture medium was changed to DMEM with 10% FBS. Cells were then incubated for 24–48 h in preparation for subsequent assays.
MTS assay
Cells were seeded into 96-well culture plates at 5×103 cells per well, incubated overnight, and transfected with plasmids or siRNAs. At 24 h post-transfection, cisplatin was supplemented in the DMEM medium at a final concentration of 40 umol/L, and cells were maintained for 24 h. Twenty microliters of MTS (CellTiter 96 aqueous one solution reagent, Promega) diluted in 100 μL of culture medium was added to each well of the 96-well culture plates. Cells were incubated for 2 h, and then the absorbance of each well at 490 nm was recorded using a 96-well plate reader (Bio-Rad Inc). All of the experiments were performed in triplicate and repeated three times.
DAPI staining
Cells were cultured on glass coverslips in 6-well plates for 24 h, transfected with plasmids or siRNAs and then treated with or without cisplatin (following the drug treatment described above). Cells subsequently were fixed with ice-cold 100% methanol overnight at 4 °C. After triple washes in phosphate-buffered saline (PBS) for 5 min each, cells were incubated with 100 nmol/L DAPI (Sigma) for 5 min at room temperature in the dark. Following triple rinses in PBS for 5 min each, coverslips were placed on slides and the edges sealed. Slides were viewed using upright fluorescence microscopy (BX51, Olympus) and pictures were captured by the Image-Pro Discovery software. Values were calculated in at least 9 different views and presented as percentage of positive apoptotic cells stained.
TUNEL apoptosis assay
TdT-mediated dUTP-biotin nick-end labeling (TUNEL) is a method used for apoptosis detection by determining the exposure end of the fragmentation of nuclear chromatin, which is one of the criteria of late stage apoptosis. The in situ cell death detection kit POD (Roche) was used to determine early stage apoptotic cells.
Western blotting
Eighty micrograms of protein extracts was loaded and separated by 10% SDS-PAGE gel (100 V, 1 h) and then transferred to PVDF membrane (12 V, 2 h). After blocking with 2% bovine serum albumin (BSA) at room temperature for 30 min, the membrane was incubated overnight with specific primary apoptosis-related antibodies, such as anti-migfilin (1:1000), anti-Bcl-xL (1:500), anti-Bcl-2 (1:500), anti-PARP (1:500), anti-Caspase-3 (1:500), and anti-p53 (1:1000). Mouse anti-beta-actin (1:5000) monoclonal antibody was used as control with a 1 h incubation. Next, goat-anti-mouse IgG (1:2000) and goat-anti-rabbit IgG (1:3000) conjugated with horseradish peroxidase (HRP) were used to probe the membrane at room temperature for 1 h. The membrane was rinsed in triplicate in PBS/Tween 0.1% for 5 min each. The chemiluminescent substrate was then added to the membrane. Photographs were taken by Image Reader LAS-4000 (LAS-4000, Fujifilm Inc.) and analyzed by Multi Gauge V3.2 software.
Statistic analysis
Data were presented as the mean±SD. For the comparison of two groups, we used the Student's t-test to determine statistically significant differences. P values less than 0.05 were considered to be statistically significant.
Results
Migfilin sensitizes cisplatin-induced apoptosis in glioma cells
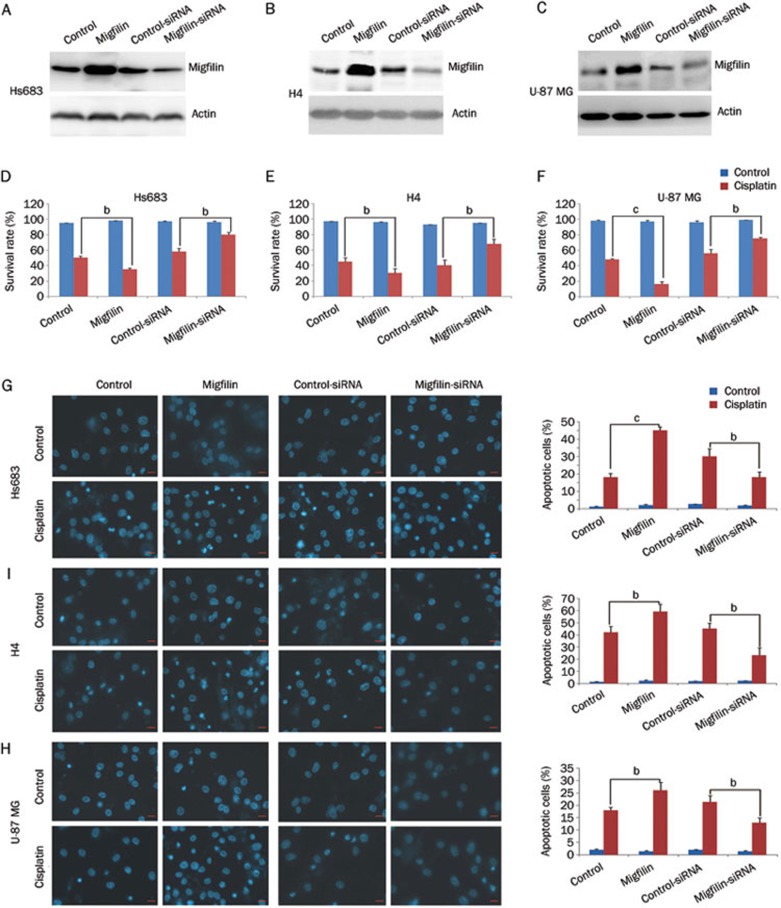
We first investigated whether migfilin played an important role in the cisplatin-induced apoptosis in gliomas. The human glioma cell lines Hs683, H4, and U-87MG were transiently transfected with the pEGFP-C2-migfilin plasmid, causing migfilin overexpression, and also with migfilin siRNA, leading to the knockdown of migfilin (Figures 1A, 1B, and 1C). Using dose-response experiments, we determined that the final treatment concentrations of cisplatin at 35, 50, and 40 μmol/L caused 50% cell death in Hs683, H4, and U-87MG cells, respectively.
Figure 1.
Regulation of migfilin sensitizes cisplatin-induced apoptosis. (A) Hs683 cells, (B) H4 cells, and (C) U-87 MG cells were examined for expression levels of migfilin by Western blotting after transfection of control vector, pEGFP-C2-migfilin vector, control siRNA, and migfilin siRNA, respectively. (D) Hs683 cells, (E) H4 cells, and (F) U-87 MG cells were examined for the effects of migfilin expression on cell viability and were analyzed by the MTS viability assay. Cells were treated with cisplatin for 24 h. (G) Hs683 cells, (H) H4 cells, and (I) U-87 MG cells were analyzed by DAPI staining for cisplatin-sensitivity. Apoptotic cells were stained with light blue (400×objective). The scale bars stand for 10 μm. Mean±SD. n=3. bP<0.05, cP<0.01 vs control.
With the treatment of cisplatin, cell viability rates of the cell lines Hs683, H4, and U-87 MG were significantly reduced when migfilin expression levels were upregulated (P<0.05, P<0.05, and P<0.01, respectively) (Figures 1D, 1E, and 1F). The viability rates of Hs683, H4, and U-87 MG cells were markedly increased with reduced expression levels of migfilin after cisplatin treatment (P<0.05, P<0.05, and P<0.05, respectively) (Figure 1D, 1E, and 1F).
Morphological evaluation using DAPI staining was performed to analyze the rates of apoptotic cells showing nuclear condensation and fragmentation. Significant increases in cisplatin-induced apoptotic cells in the cell lines Hs683, H4, and U-87 MG were detected when expression levels of migfilin were elevated (P<0.01, P<0.05, and P<0.05, respectively) (Figures 1G, 1H, and 1I). However, apoptotic cell levels decreased significantly in migfilin-knockdown groups in Hs683, H4, and U-87 MG cells, in contrast to respective cell line control groups (P<0.05, P<0.05, and P<0.05, respectively) (Figures 1G, 1H, and 1I).
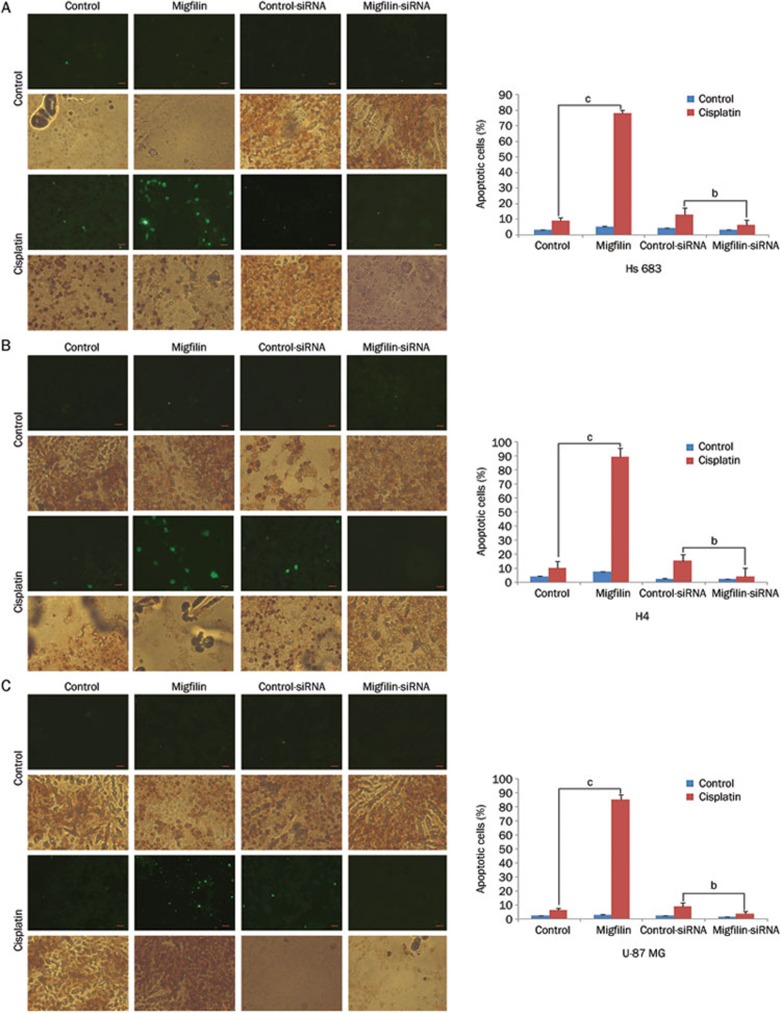
In addition, we observed similar results using the TUNEL staining assay. After exposure to cisplatin, the level of early-stage-apoptotic Hs683, H4, and U-87 MG cells was positively correlated with migfilin expression levels (Figures 2A, 2B, and 2C). However, the modulation of migfilin did not directly influence the apoptotic rates of the glioma cells without cisplatin supplement. Therefore, migfilin can sensitize cisplatin-induced apoptosis, whereas downregulation of migfilin can inhibit cisplatin-induced apoptosis in glioma cells.
Figure 2.
Regulation of migfilin sensitizes cisplatin-induced apoptosis. (A) Hs683 cells, (B) H4 cells, and (C) U-87 MG cells were analyzed by the TdT-mediated dUTP-biotin nick-end labeling (TUNEL) assay for the detection of early-stage-apoptotic cells. Apoptotic cells were determined by both DAB-stained positive areas (brown areas) and fluorescein-stained areas (green areas) (400×objective). The scale bars stand for 20 um. Apoptotic cell rates were calculated under 9 different views. Mean±SD. n=3. bP<0.05, cP<0.01 vs control.
The LIM domain of migfilin plays a key role in cisplatin-induced apoptosis
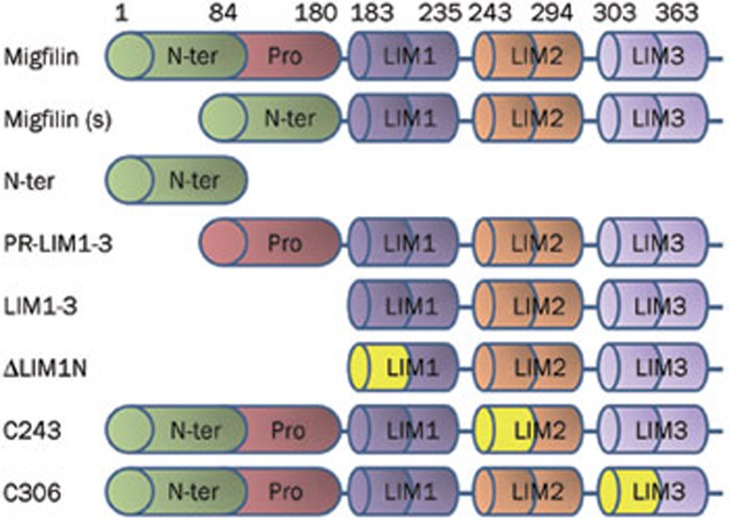
To assess the functional domains of migfilin that affect the chemosensitivity of glioma cells, we transfected cells with various deletion-mutants of migfilin. Specifically, plasmids encoding different types of GFP-tagged migfilin mutants16 were transfected to express mutant forms of migfilin in the U-87 MG cell line. As shown in Figure 3, migfilin(s), a splicing variant of migfilin, lacks a proline-rich region. PR-LIM1-3 lacks the N-terminal region, whereas the vector N-ter only expresses the N-terminal residues of migfilin. LIM1-3 lacks of the N-terminus and the proline-rich regions, whereas ΔLIM1N possesses a mutant LIM1 domain in addition to the mutations of the LIM1-3 vector. The C243 and C306 vectors possess the LIM2 domain mutant and the LIM3 domain mutant, respectively.
Figure 3.
Schematic structure of the wild-type migfilin and different deletion mutants of migfilin.
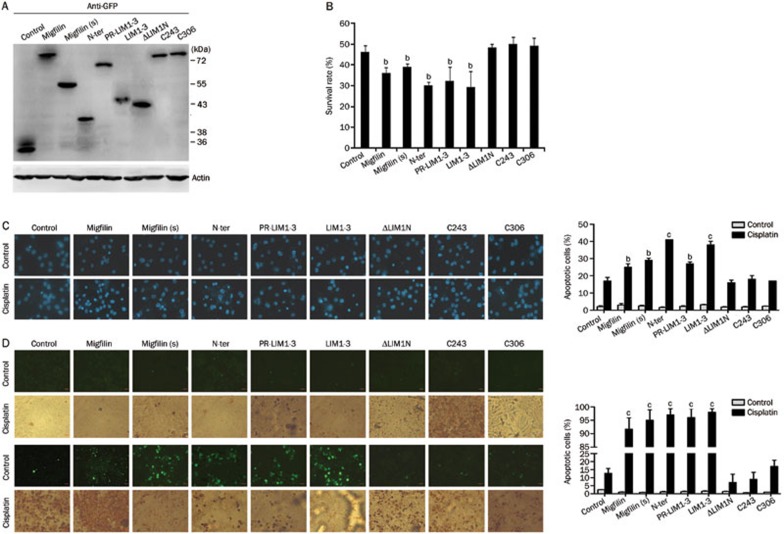
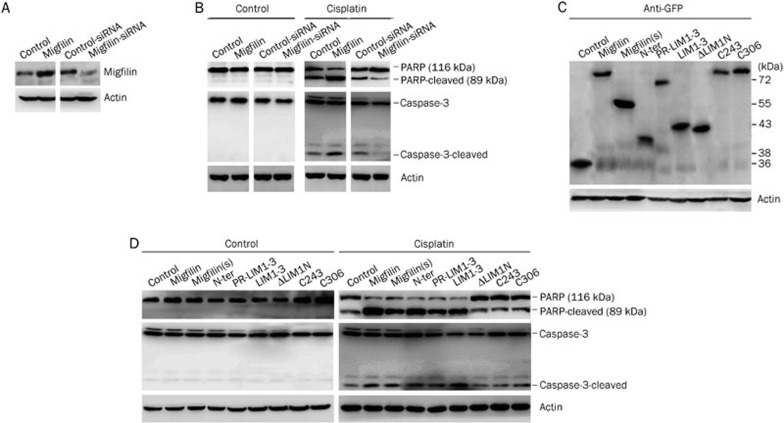
Western blotting was conducted to detect GFP protein expression levels in migfilin-mutant transfectants to assess the efficacy of the transient transfections (Figure 4A). As with wild-type migfilin, U-87 MG cells transfected with migfilin(s), N-ter, PR-LIM1-3, and LIM1-3 demonstrated decreased survival rates when treated with cisplatin (P<0.05) (Figure 4B). Moreover, migfilin(s), N-ter, PR-LIM1-3, and LIM1-3 vector transfectants showed increased apoptotic cell rates when treated with cisplatin, compared to treated control vector transfectants (approximately 1.71-fold, 2.41-fold, 1.59-fold, and 2.24-fold changes, respectively; P<0.05). In contrast, ΔLIM1N, C243 and C306 vector transfectants did not significantly differ from the control group (P>0.05) (Figure 4C). TUNEL-stained apoptotic areas were significantly increased in cisplatin-treated cells transfected with migfilin(s), N-ter, PR-LIM1-3, and LIM1-3 vectors (P<0.05), and apoptotic areas were relatively static in cells transfected with the ΔLIM1N, C243, and C306 vectors (P>0.05) (Figure 4D). Therefore, we observed that the N-terminus alone affects cisplatin-induced apoptosis in U-87 MG cells. In addition, cells transfected with PR-LIM1-3 and LIM1-3 vectors containing a deleted N-terminal region were also sensitive to cisplatin-induced apoptosis. Furthermore, we found that cell transfection with the C243 and C306 vectors, which possess the entire length of the N-terminus but contain mutated LIM domains, led to a loss of protein function, thus demonstrating that the C-terminal LIM domains may play a key role in wild-type migfilin and migfilin(s) protein activities. Furthermore, mutants of migfilin with any one of LIM domain deletions also resulted in function loss, indicating the importance of the integrity of all three LIM domains.
Figure 4.
Functional domains of migfilin were determined in U-87 MG cells. (A) Cellular extracts of transfectants of migfilin mutant vectors with the GFP flag were detected for expression levels of GFP by Western blotting. (B) The MTS viability assay was performed to demonstrate the cell viability of transfectants of migfilin, migfilin(s), N-ter, PR-LIM1-3, LIM1-3, ΔLIM1N, C243, and C306 with cisplatin treatment (n=3. Mean±SD). (C) Apoptotic cell rates of migfilin-mutant transfectants with or without cisplatin treatment were examined by DAPI staining. Apoptotic cells were stained with light blue (400×objective; n=3. Mean±SD). (D) Using the TUNEL staining assay, apoptotic cells of migfilin-mutant transfectants with or without cisplatin treatment were determined by both DAB-stained positive areas (brown areas) and fluorescein-stained areas (green areas) (400×objective). Apoptotic cell rates were calculated under at least 9 different views. Mean±SD. n=3. bP<0.05, cP<0.01 vs control.
Migfilin enhances cisplatin-induced apoptosis through the regulation of Bcl-xL and Caspase-3 expression
To investigate the underlying molecular mechanisms involved in the cisplatin-related sensitivity of U-87 MG cells, we detected expression levels of apoptosis-associated proteins in the cellular extracts of migfilin transfectants. With strict efficacy control of transient transfection with vectors and siRNA (Figure 5A and 5C), we found that without cisplatin treatment, there were no alterations in the expression of apoptotic proteins such as PARP and Caspase-3 in migfilin transfectants (Figure 5B). However, with cisplatin treatment, regulation of migfilin was positively related to the expression of cleaved PARP and cleaved Caspase-3 (Figure 5B).
Figure 5.
Expression of proapoptotic proteins in U-87 MG transfectants. (A) Migfilin expression levels were examined by Western blotting to determine the efficacy of transient transfections of migfilin vectors and siRNAs. (B) Expression levels of the proapoptotic proteins PARP and Caspase-3 were determined by Western blotting in migfilin-overexpression and migfilin-knockdown transfectants with or without cisplatin treatment. (C) Cellular extracts of transfectants of U-87 MG-migfilin mutants were examined for the expression levels of GFP protein by Western blotting. (D) Expression levels of PARP and Caspase-3 were detected by Western blotting in migfilin-mutant transfectants with or without cisplatin treatment.
Moreover, we observed that after exposure to cisplatin, the migfilin-mutant transfectants, including migfilin(s), N-ter, PR-LIM1-3, and LIM1-3, showed increased expression of cleaved PARP and cleaved Caspase-3, compared to the control group, whereas ΔLIM1N, C243, and C306 transfectants had no significant impact on the expression of apoptotic proteins (Figure 5D).
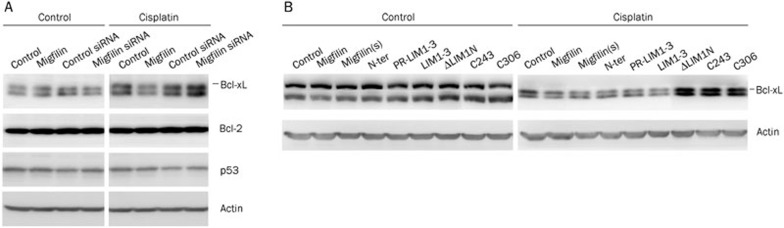
We further investigated upstream apoptosis-related proteins. Expression levels of Bcl-2 and p53 showed no differences in migfilin-overexpression and migfilin-knockdown transfectants with or without cisplatin treatment (Figure 6A). With cisplatin treatment, expression of the anti-apoptotic protein Bcl-xL was negatively associated with the expression level of migfilin (Figure 6A). Transfectants of functional migfilin-mutant vectors, including migfilin(s), N-ter, PR-LIM1-3, and LIM1-3, consistently resulted in the downregulation of Bcl-xL (Figure 6B). Thus, it has been suggested that migfilin plays an important role in the chemosensitivity of glioma cells through modulating Bcl-xL and Caspase-3 expression.
Figure 6.
Expression of apoptosis-related proteins in U-87 MG transfectants. (A) Expression levels of the apoptosis-related proteins Bcl-xL, Bcl-2, and p53 were determined by Western blotting in migfilin-overexpression and migfilin-knockdown transfectants with or without cisplatin treatment. (B) Expression levels of Bcl-xL were detected by Western blotting in migfilin-mutant transfectants with or without cisplatin treatment.
Discussion
Programmed cell death (PCD) has been regarded as a key point in development, and PCD disorders play a role in a wide range of diseases, such as neuro-degeneration, autoimmune diseases, cardiovascular diseases, and cancer17. Apoptosis-resistance, growth signal autonomy and insensitivity to anti-growth signals are major factors in the malignant transformation of cells, ultimately leading to unlimited cell proliferation18. In this study, we have demonstrated that migfilin plays a key role in regulating cisplatin-induced cellular apoptosis in glioblastoma cells. In addition, we found that U-87 MG cells transfected with N-terminus, PR-LIM1-3 and LIM1-3 vectors containing mutant migfilin were sensitized to cisplatin-induced apoptosis. However, C243 and C306 vector transfectants that contained a full length N-terminus and mutated LIM domains led to a function loss. Therefore, the LIM domain likely plays a key role in wild-type migfilin and migfilin(s) protein activities. It is also probable that the N-terminus fragment is involved in a different mechanism of regulating chemosensitization than the LIM region. We speculate that the LIM1-3 domain might function to influence the conformational space or protein-binding sites of the N-terminus to ultimately perform the regulation of cisplatin-induced apoptosis in wild-type migfilin. Moreover, in addition to interacting with Mig-25, the C-terminal LIM domains have been implicated in the localization of migfilin to cell-cell adhesions through regulation of the cadherin–β-catenin complex4,16. Previous studies have reported that the regulation of cell-cell junctions by E-cadherin could influence cisplatin-based chemoresistance19,20,21. In human glioma cells, expression levels of E-cadherin, β-catenin and PARP are regulated in ZEB2-mediated cellular apoptosis22. Therefore, it seems likely that the LIM domain of migfilin participates in cisplatin-induced apoptosis via remodeling of cell-cell or cell-matrix adhesions.
In addition to acting as a focal adhesion protein involved in cell-cell and cell-ECM adhesion, migfilin is a candidate oncogene that has distinct potential in becoming a biological marker of human leiomyosarcomas (LMS)23. In human mammary epithelial cells, downregulation of migfilin directly leads to anoikis by interacting with Src24. Therefore, migfilin appears to be an anti-apoptotic candidate oncogene in addition to being a gene with the apoptotic functions described in this paper. While paradoxical, this is not the first incidence of an oncogene promoting apoptosis. Activation of the oncogene Ras sensitizes chemotherapeutic agent-induced apoptosis both in HCT116 cells25 and in embryonic stem cells26. The oncogene c-myc has also been reported to enhance cisplatin-induced apoptosis by inhibiting NF-κB signaling, which represses the expression of Bcl-2 families in mouse pancreatic cancer cells27. In addition, E1A has been proposed to be an immortalization oncoprotein that exhibits the anti-tumor properties of cell-cycle arrest and apoptosis induction25,28,29. The paradox caused by the dual function of oncogenes might derive from the functions of targeted genes downstream of the oncogenes, which could counteract the intrinsic anti-apoptotic function of oncogenes25,30,31,32. It has also been proposed that cell growth and apoptosis are closely linked and that apoptosis might trigger the early progression of tumorigenesis33,34.
Cisplatin is a strong, widely used anti-cancer therapeutic drug that causes DNA damage and programmed cell death and is activated in a p53-independent pathway35,36. Here, we have demonstrated that with the treatment of cisplatin, expression levels of cleaved Caspase-3 and PARP were elevated and accompanied by increased migfilin expression. Bcl-xL expression levels were negatively modulated by the expression level of migfilin and did not affect the expression level of p53 in glioblastoma cells. The anti-apoptotic factors Bcl-2 and Bcl-xL appear to be the most significant regulators involved in irradiation- and chemotherapy-induced apoptosis, particularly in a p53-dispensable pathway27,34,37,38. The suppression of Bcl-xL induced by intrinsic or extrinsic upstream signals can result in the activation of apoptosis37. In the nervous system, expression of endogenous Bcl-xL plays a vital role in the protection of neurons from DNA damage-induced apoptosis, while Bcl-xL deficiency causes activation of caspase-3 and neuronal cell death with or without p53-deficiency39. Essentially, the level of Bcl-xL has been associated with the chemosensitivity and regulation of apoptosis40. Suppression of Bcl-xL deamidation causes chemoresistance to cisplatin-induced apoptosis, which is maintained through suppressing the proapoptotic ability of BH3 domain-only proteins in fibroblasts41. In this study, upregulation of migfilin was found to sensitize glioblastoma cells to cisplatin-induced apoptosis through a mitochondria-dependent pathway. Migfilin regulates functional Bcl-xL expression to respond to the DNA damage caused by cisplatin. Given that a variety of tumors lack p53 activity, chemotherapeutic agent-induced apoptosis relies on p53-independent signaling41,42.
Our results establish a link between the functional domain of migfilin and cisplatin-mediated chemosensitivity in human gliomas. Moreover, migfilin might be considered a promising novel therapeutic target, through its interference with chemotherapy-induced cytotoxicity in glioblastomas, and could serve as an important molecular target for drug discovery.
Author contribution
Yong-mei SONG and Chun-jiang YU designed this research; Yong-mei SONG and Qi-min ZHAN supervised the research; Yun-wei OU and Jing FAN performed the research; Yun-wei OU analyzed the data; Jing FAN wrote the paper; Chuan-yue WU contributed plasmids and anti-migfilin antibody.
Acknowledgments
This work is supported by funding from the 973 National Key Fundamental Research Program of China (2009CB521801) and the National Natural Science Foundation of China (81071633 and 81021061).
References
- Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci. 2011;12:495–508. doi: 10.1038/nrn3060. [DOI] [PubMed] [Google Scholar]
- Wick W, Weller M, Weiler M, Batchelor T, Yung AW, Platten M. Pathway inhibition: emerging molecular targets for treating glioblastoma. Neuro Oncol. 2011;13:566–79. doi: 10.1093/neuonc/nor039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick G, Fine HA. Integration and analysis of genome-scale data from gliomas. Nat Rev Neurol. 2011;7:439–50. doi: 10.1038/nrneurol.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Migfilin and its binding partners: from cell biology to human diseases. J Cell Sci. 2005;118:659–64. doi: 10.1242/jcs.01639. [DOI] [PubMed] [Google Scholar]
- Tu Y, Wu S, Shi X, Chen K, Wu C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell. 2003;113:37–47. doi: 10.1016/s0092-8674(03)00163-6. [DOI] [PubMed] [Google Scholar]
- Takafuta T, Saeki M, Fujimoto TT, Fujimura K, Shapiro SS. A new member of the LIM protein family binds to filamin B and localizes at stress fibers. J Biol Chem. 2003;278:12175–81. doi: 10.1074/jbc.M209339200. [DOI] [PubMed] [Google Scholar]
- Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda K, et al. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem. 2007;282:20455–66. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- Ithychanda SS, Das M, Ma YQ, Ding K, Wang X, Gupta S, et al. Migfilin, a molecular switch in regulation of integrin activation. J Biol Chem. 2009;284:4713–22. doi: 10.1074/jbc.M807719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, Wegener KL, et al. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell. 2006;2:337–47. doi: 10.1016/j.molcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Gong X, An Z, Wang Y, Guan L, Fang W, Strömblad S, et al. Kindlin-2 controls sensitivity of prostate cancer cells to cisplatin-induced cell death. Cancer Letters. 2010;299:54–62. doi: 10.1016/j.canlet.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Baldwin RM, Garratt-Lalonde M, Parolin DA, Krzyzanowski PM, Andrade MA, Lorimer IA. Protection of glioblastoma cells from cisplatin cytotoxicity via protein kinase Ci-mediated attenuation of p38 MAP kinase signaling. Oncogene. 2006;25:2909–19. doi: 10.1038/sj.onc.1209312. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wan Y, Pan T, Gu X, Qian C, Sun G, et al. MicroRNA-21 inhibitor sensitizes human glioblastoma U251 stem cells to chemotherapeutic drug temozolomide. J Mol Neurosci. 2012;47:346–56. doi: 10.1007/s12031-012-9759-8. [DOI] [PubMed] [Google Scholar]
- Eastman A. Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells. 1990;2:275–80. [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Micallef J, Taccone M, Mukherjee J, Croul S, Busby J, Moran MF, et al. Epidermal growth factor receptor variant III-induced glioma invasion is mediated through myristoylated alanine-rich protein kinase C substrate overexpression. Cancer Res. 2009;69:7548–56. doi: 10.1158/0008-5472.CAN-08-4783. [DOI] [PubMed] [Google Scholar]
- Gkretsi V, Zhang Y, Tu Y, Chen K, Stolz DB, Yang Y, et al. Physical and functional association of migfilin with cell-cell adhesions. J Cell Sci. 2005;118:697–710. doi: 10.1242/jcs.01638. [DOI] [PubMed] [Google Scholar]
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–58. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Livshits G, Kobielak A, Fuchs E. Governing epidermal homeostasis by coupling cell–cell adhesion to integrin and growth factor signaling, proliferation, and apoptosis. Proc Natl Acad Sci U S A. 2012;109:4886–91. doi: 10.1073/pnas.1202120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M, Hermannstädter C, Hutzler P, Häcker G, Haller F, Höfler H, et al. Deletion of exon 8 increases cisplatin-induced E-cadherin cleavage. Exp Cell Res. 2008;314:153–63. doi: 10.1016/j.yexcr.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Kato Y, Fuji H, Horiuchi T, Chiba Y, Tanaka K. E-cadherin-dependent intercellular adhesion enhances chemoresistance. Int J Mol Med. 2003;12:693–700. [PubMed] [Google Scholar]
- Qi S, Song Y, Peng Y, Wang H, Long H, Yu X, et al. ZEB2 mediates multiple pathways regulating cell proliferation, migration, invasion, and apoptosis in Glioma. PLoS One. 2012;7:e38842. doi: 10.1371/journal.pone.0038842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristou DJ, Gkretsi V, Tu Y, Shi X, Chen K, Larjava H, et al. Increased cytoplasmic level of migfilin is associated with higher grades of human leiomyosarcoma. Histopathology. 2007;51:499–508. doi: 10.1111/j.1365-2559.2007.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang Y, Ithychanda SS, Tu Y, Chen K, Qin J, et al. Migfilin interacts with Src and contributes to cell-matrix adhesion-mediated survival signaling. J Biol Chem. 2009;284:34308–20. doi: 10.1074/jbc.M109.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Oncogenic Ras promotes butyrate-induced apoptosis through inhibition of gelsolin expression. J Biol Chem. 2004;279:36680–8. doi: 10.1074/jbc.M405197200. [DOI] [PubMed] [Google Scholar]
- Brooks DG, James RM, Patek CE, Williamson J, Arends MJ. Mutant K-ras enhances apoptosis in embryonic stem cells in combination with DNA damage and is associated with increased levels of p19(ARF) Oncogene. 2001;20:2144–52. doi: 10.1038/sj.onc.1204309. [DOI] [PubMed] [Google Scholar]
- El-Kady A, Sun Y, Li YX, Liao DJ. Cyclin D1 inhibits whereas c-Myc enhances the cytotoxicity of cisplatin in mouse pancreatic cancer cells via regulation of several members of the NF-kappaB and Bcl-2 families. J Carcinog. 2011;10:24. doi: 10.4103/1477-3163.90437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Mymryk JS. Adenovirus-5 E1A: paradox and paradigm. Nat Rev Mol Cell Biol. 2002;3:441–52. doi: 10.1038/nrm827. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Chen CT, Chou CK, Pal A, Bornmann W, Hortobagyi GN, et al. Adenovirus 5 E1A enhances histone deacetylase inhibitors-induced apoptosis through Egr-1-mediated Bim upregulation. Oncogene. 2010;29:5619–29. doi: 10.1038/onc.2010.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EA, Fanidi A, Evan GI. Oncogenes and cell death. Curr Opin Genet Dev. 1994;4:120–9. doi: 10.1016/0959-437x(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Fanidi A, Harrington EA, Evan GI. Cooperative interaction between c-myc and Bcl-2 proto-oncogenes. Nature. 1992;359:554–6. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Evan G, Harrington E, Fanidi A, Land H, Amati B, Bennett M. Integrated control of cell proliferation and cell death by the c-myc oncogene. Philos Trans R Soc Lond B Biol Sci. 1994;345:269–75. doi: 10.1098/rstb.1994.0105. [DOI] [PubMed] [Google Scholar]
- Tang D, Lotze MT, Kang R, Zeh HJ. Apoptosis promotes early tumorigenesis. Oncogene. 2011;30:1851–4. doi: 10.1038/onc.2010.573. [DOI] [PubMed] [Google Scholar]
- Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–34. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- Tomasini R, Seux M, Nowak J, Bontemps C, Carrier A, Dagorn JC, et al. TP53INP1 is a novel p73 target gene that induces cell cycle arrest and cell death by modulating p73 transcriptional activity. Oncogene. 2005;24:8093–104. doi: 10.1038/sj.onc.1208951. [DOI] [PubMed] [Google Scholar]
- Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin WG, Jr, Levrero M, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–9. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- Maclean KH, Keller UB, Rodriguez-Galindo C, Nilsson JA, Cleveland JL. c-Myc augments gamma irradiation-induced apoptosis by suppressing Bcl-XL. Mol Cell Biol. 2003;23:7256–70. doi: 10.1128/MCB.23.20.7256-7270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhao J, Kang S, Yi M, You S, Shin DS, et al. A novel cromakalim analogue induces cell cycle arrest and apoptosis in human cervical carcinoma HeLa cells through the caspase- and mitochondria-dependent pathway. Int J Oncol. 2011;39:1609–17. doi: 10.3892/ijo.2011.1153. [DOI] [PubMed] [Google Scholar]
- Klocke BJ, Latham CB, D'Sa C, Roth KA. p53 deficiency fails to prevent increased programmed cell death in the Bcl-X(L)-deficient nervous system. Cell Death Differ. 2002;9:1063–8. doi: 10.1038/sj.cdd.4401067. [DOI] [PubMed] [Google Scholar]
- Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–10. [PubMed] [Google Scholar]
- Deverman BE, Cook BL, Manson SR, Niederhoff RA, Langer EM, Rosová I, et al. Bcl-xL deamidation is a critical switch in the regulation of the response to DNA damage. Cell. 2002;111:51–62. doi: 10.1016/s0092-8674(02)00972-8. [DOI] [PubMed] [Google Scholar]
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]