Abstract
Aim:
To investigate the effects of the naturally occurring stilbenoid pinosylvin on neutrophil activity in vitro and in experimental arthritis, and to examine whether protein kinase C (PKC) activation served as an assumed target of pinosylvin action.
Methods:
Fresh human blood neutrophils were isolated. The oxidative burst of neutrophils was evaluated on the basis of enhanced chemiluminescence. Neutrophil viability was evaluated with flow cytometry, and PKC phosphorylation was assessed by Western blotting analysis. Adjuvant arthritis was induced in Lewis rats with heat-killed Mycobacterium butyricum, and the animals were administered with pinosylvin (30 mg/kg, po) daily for 21 d after arthritis induction.
Results:
In isolated human neutrophils, pinosylvin (10 and 100 μmol/L) significantly decreased the formation of oxidants, both extra- and intracellularly, and effectively inhibited PKC activation stimulated by phorbol myristate acetate (0.05 μmol/L). The inhibition was not due to neutrophil damage or increased apoptosis. In arthritic rats, the number of neutrophils in blood was dramatically increased, and whole blood chemiluminescence (spontaneous and PMA-stimulated) was markedly enhanced. Pinosylvin administration decreased the number of neutrophils (from 69 671±5588/μL to 51 293±3947/μL, P=0.0198) and significantly reduced the amount of reactive oxygen species in blood.
Conclusion:
Pinosylvin is an effective inhibitor of neutrophil activity, and is potentially useful as a complementary medicine in states associated with persistent inflammation.
Keywords: pinosylvin, neutrophils, reactive oxygen species, protein kinase C, apoptosis, adjuvant arthritis
Introduction
Neutrophils (neutrophilic polymorphonuclear leukocytes) represent the body's primary line of defense against invading pathogens. Additionally, they have recently been increasingly studied as active participants in the initiation and progression of many pathological conditions, such as ischaemia-reperfusion injury, gout, lupus, acute respiratory distress syndrome and rheumatoid arthritis. All of these conditions are generally accompanied by dysregulated, persistent and excessive activation of neutrophils, resulting in damage of adjacent tissues by neutrophil “destructive hardware”, including reactive oxygen or nitrogen species and proteolytic enzymes1,2,3,4. In rheumatoid arthritis, oxidants can induce cartilage degradation and depolymerize hyaluronan and decrease its lubricative properties; furthermore, they can reduce the protective antioxidant and antiproteinase capacity of synovial fluid and thus participate in joint erosion1,5. Moreover, neutrophils release molecules that can promote inflammation (eicosanoids, chemokines, and cytokines), and the altered recruitment and delayed apoptosis of these cells hinder the resolution of inflammation2,4.
From this perspective, novel therapeutic strategies to resolve chronic inflammation are expected to inhibit the formation of neutrophil-derived toxic substances, eg, reactive oxygen species, either directly or through enhanced apoptosis. However, inhibition would be directed against extracellularly released oxidants, rather than those formed intracellularly and involved in the initiation of constitutive apoptosis, although the mechanism of radical generation in nonactivated aging neutrophils is not clear6,7. Pharmacological interference with protein kinase C (PKC) activity represents a promising method to modulate both neutrophil activity and apoptosis. The isoforms PKCα and βII stimulate the formation of reactive oxygen species at the level of NADPH oxidase activation (by phosphorylation and translocation of p47phox from the cytosol to membranes8), while PKCα and δ are involved in antiapoptotic signaling in neutrophils9,10.
An inhibitory effect on neutrophil function was discovered for several drugs11 and natural substances12,13,14,15,16,17. The latter are particularly useful for their low toxicity and ability to control the activity of neutrophils through several mechanisms. For example, trans-resveratrol (trans-3,4′,5-trihydroxystilbene) repressed the adhesion of neutrophils to endothelial cells, the production of reactive oxygen and nitrogen species, and the liberation of elastase and β-glucuronidase, and it decreased the activities of neutrophil myeloperoxidase and 5- and 15-lipoxygenase (for review see15). Regarding the hydrophilic characteristic of resveratrol, its low bioavailability and rapid clearance from the circulation, attention has been focused on its more lipophilic derivatives18, such as pinosylvin (trans-3,5-dihydroxystilbene, Figure 1).
Figure 1.
Pinosylvin (trans-3,5-dihydroxystilbene) and its related compound trans-resveratrol (trans-3,4′,5-trihydroxystilbene).
This naturally occurring resveratrol analogue is formed constitutively and after UV irradiation or microbial attack in the wood, needles and leaves of Pinus and Alnus species19. The majority of the data available characterize antifungal, antibacterial and anticancer activities of pinosylvin20,21; however, little is known about its antioxidant and anti-inflammatory effects22,23,24. Previously, we found that pinosylvin improved the effect of methotrexate in experimental arthritis25,26, and it intensified the reduction in the number and phagocytic activity of neutrophils, hind paw volume and blood oxidant concentration. In the present paper, the impact of pinosylvin on the viability of human neutrophils and formation of reactive oxygen species was investigated, and protein kinase C activation was examined as an assumed target of pinosylvin action. Moreover, in rats with adjuvant arthritis, the efficacy of pinosylvin was assessed in neutrophils modified by inflammation.
Materials and methods
Chemicals and solutions
Pinosylvin (trans-3,5-dihydroxystilbene, 98%) was synthetized and analyzed at the Institute of Organic Chemistry and Biochemistry of the Academy of Sciences of the Czech Republic; details are provided elsewhere27. Luminol, isoluminol, PMA (4β-phorbol-12β-myristate-13α-acetate), the Ca2+ ionophore A23187, superoxide dismutase, dextran (average MW 464 kDa), zymosan (zymosan A from Saccharomyces cerevisiae), firefly luciferase from Photinus pyralis and D-luciferin sodium salt were purchased from Sigma-Aldrich Chemie (Deisenhofen, Germany); HRP (horseradish peroxidase) and catalase were purchased from Merck (Darmstadt, Germany); and lymphoprep (density 1.077 g/mL) was purchased from Nycomed Pharma AS (Oslo, Norway). Propidium iodide and rh Annexin V-FITC (produced in E coli and conjugated with fluorescein isothiocyanate, FITC) was purchased from Bender MedSystems GmbH (Vienna, Austria). M butyricum in incomplete Freund's adjuvant, which was used for the induction of adjuvant arthritis, was obtained from Difco Laboratories (Detroit, MI, USA).
Pinosylvin administered to arthritic rats was dissolved in sunflower oil at 30 g/L. For the in vitro studies, pinosylvin (1.06 mg) was dissolved in a mixture of 20 μL of 1 mol/L NaOH and 980 μL of Tyrode's solution. The stock solution (5 mmol/L) was further diluted with Tyrode's solution to give pinosylvin sample concentrations of 0.1–100 μmol/L. The corresponding final concentrations of NaOH were 0.4–400 μmol/L; at these concentrations, the solvent agent alone did not reduce the activity or viability of neutrophils. Phosphate-buffered saline (PBS) contained 136.9 mmol/L NaCl, 2.7 mmol/L KCl, 8.1 mmol/L Na2HPO4, 1.5 mmol/L KH2PO4, 1.8 mmol/L CaCl2, and 0.5 mmol/L MgCl2, pH 7.4. Tyrode's solution consisted of 136.9 mmol/L NaCl, 2.7 mmol/L KCl, 11.9 mmol/L NaHCO3, 0.4 mmol/L NaH2PO4·2H2O, 1 mmol/L MgCl2·6H2O, and 5.6 mmol/L glucose, pH 7.4. Binding buffer used in flow cytometric analyses contained 10 mmol/L HEPES, 140 mmol/L NaCl, and 2.5 mmol/L CaCl2, pH 7.4.
Whole blood, buffy coat, and neutrophil isolation
Fresh human blood was obtained at the blood bank by venipuncture from healthy male donors (20–50 years) who had not received any medication for at least 7 d. The samples were anticoagulated with 3.8% trisodium citrate (blood to citrate ratio=9:1). Subsequently, the blood was gently mixed with dextran solution (1% final concentration), and erythrocytes were allowed to sediment (1×g) at 22 °C for 25 min. A suspension of leukocytes and platelets in plasma (buffy coat) was used for flow cytometric analyses or for neutrophil isolation. For neutrophil isolation, the buffy coat was centrifuged at 500×g for 10 min, and the sediment was resuspended in PBS, layered on lymphoprep and centrifuged at 500×g for 30 min. Contaminating red blood cells were removed by hypotonic lysis (3 mL of ice-cold deionized water followed by 3 mL of 1.8% NaCl and 4 mL of PBS after 45 s). After centrifugation at 500×g for 10 min, neutrophils in PBS were counted, adjusted to a final concentration of 104 cells/μL and kept on ice. The final suspension of neutrophils contained more than 96% viable cells, as evaluated by trypan blue exclusion, and was used for a maximum of 2 h – as long as control chemiluminescence remained constant.
Neutrophil count was assessed by a Coulter Counter (Coulter Electronics, High Wycombe, England). In whole blood (diluted 500×), erythrocytes were destroyed with a lysing reagent before counting.
Formation of reactive oxygen species
The oxidative burst of neutrophils was evaluated on the basis of enhanced chemiluminescence28 in a microtiter plate computer-driven luminometer Immunotech LM-01T (Immunotech, Prague, Czech Republic). The chemiluminescence of whole human blood (diluted 250×) enhanced with luminol (250 μmol/L) was induced with PMA (0.05 μmol/L), opsonized zymosan (0.5 g/L) or the Ca2+ ionophore A23187 (1 μmol/L). The chemiluminescence of isolated human neutrophils (5×105) was initiated by PMA (0.05 μmol/L) and enhanced with either 5 μmol/L isoluminol (extracellular) or 5 μmol/L luminol in the presence of extracellular scavengers, 100 U/mL superoxide dismutase and 2000 U/mL catalase (intracellular). The formation of oxidants was evaluated on the basis of integrated values of chemiluminescence over 1800 s (isolated neutrophils and A23187-stimulated whole blood) and over 3600 s (whole blood chemiluminescence initiated with PMA or zymosan).
Western blot analysis
The phosphorylation of protein kinase C (PKC) isoenzymes α and βII was detected as previously described29. Isolated human neutrophils (5×106) were incubated at 37 °C with pinosylvin for 1 min, stimulated with PMA (0.15 μmol/L, 1 min) and rapidly lysed by the addition of solubilization buffer (20 mmol/L Tris-HCl, 5 mmol/L EDTA, 1% Triton, 10% glycerol, 10 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate, 200 μmol/L PMSF (phenylmethylsulfonylfluoride), 2 μg/mL pepstatin, 2 μg/mL leupeptin, and 2 μg/mL aprotinin, pH 7.4). Then, the samples were sonicated on ice and centrifuged (14 000×g, 5 min, 4 °C) to remove unbroken cells. The supernatant was boiled for 5 min with sample buffer (50 mmol/L Tris-HCl, 2% SDS (sodium dodecyl sulphate), 7.5% glycerol, 2.5% mercaptoethanol, and 0.01% bromophenol blue, pH 6.8) and loaded on 9.8% SDS polyacrylamide gels. Proteins (20 μg per lane) were subsequently separated by electrophoresis and immediately transferred electrophoretically to an Immobilon-P Transfer Membrane (Millipore Corp, Badford, MA, USA). Two strips of each membrane were obtained: one for the detection of PKC (area between 60 and 100 kDa) and one for the detection of β-actin (30–60 kDa). β-Actin was used as an internal control to confirm that equal amounts of cellular protein were present in each lane of the gel. Membrane strips were blocked for 60 min with 1% bovine serum albumin in Tris-buffered saline (TBS, 20 mmol/L Tris-HCl, 154 mmol/L NaCl and 0.05% Tween-20, pH 7.5) and subsequently incubated for 60 min in the presence of the following primary antibodies: phospho-PKC alpha/beta II (Thr638/641) antibody (rabbit anti-human, 1:8000, Cell Signaling Technology, Danvers, MA, USA) or β-actin antibody (rabbit anti-human, 1:4000, Cell Signaling Technology, Danvers, MA, USA). The membranes were subsequently washed six times (overall time 40 min) with TBS and incubated for 60 min with the secondary antibody conjugated to horseradish peroxidase (anti-rabbit from donkey, 1:10 000, GE Healthcare Life Sciences, Little Chalfont, UK). After washing, the activity of horseradish peroxidase was visualized using Enhanced Chemiluminescence Western Blotting Detection Reagents (GE Healthcare Life Sciences, Little Chalfont, UK) followed by autoradiography. The bands on the autoradiogram were quantified using the ImageJ program, and the optical density of each PKC band was corrected by the optical density of the corresponding β-actin band.
Measurement of ATP liberation
Pinosylvin cytotoxicity was evaluated on the basis of ATP (adenosine triphosphate) liberation measured by the luciferin-luciferase chemiluminescence method29. A suspension of isolated neutrophils (30 000/sample, 30 μL) and 20 μL of Tyrode's solution were incubated with 50 μL of pinosylvin (1–100 μmol/L) for 15 min at 37 °C. Then, 10 μL of a mixture of luciferin (1.6 μg/sample) and luciferase (45 000 U/sample) was added, and chemiluminescence was recorded for 60 s. The chemiluminescence of ATP standards (1–500 nmol/L) was measured in each experiment, and ATP concentrations in samples were calculated from the calibration curve. The total ATP content was assessed immediately after the sonication of neutrophils for 10 s.
Apoptosis assay using flow cytometry
Human plasma buffy coat (see section Whole blood, buffy coat, and neutrophil isolation) diluted with binding buffer (460 μL, 200 000 neutrophils) was incubated with 10 μL of pinosylvin (final concentration 1–100 μmol/L) for 10 min at 37 °C. Subsequently, double staining with Annexin V-FITC (5 μL) and propidium iodide (25 μL) was performed, and cells were analyzed on the flow cytometer Cytomics FC 500 (Beckman Coulter, Inc, Brea, CA, USA). From the granulocyte area, 5000 cells were gated, and the percentage of early apoptotic (Annexin positive and propidium iodide negative), late apoptotic (double positive) and viable cells (double negative) was determined according to Perečko et al14.
Effects of pinosylvin in arthritis
Adjuvant arthritis was induced in male Lewis rats (160–180 g, Breeding Farm Dobrá Voda, Slovakia) by a single intradermal injection of heat-killed M butyricum30. The study was performed in compliance with the Principles of Laboratory Animal Care and was approved by the local Ethics Committee and the State Veterinary and Food Administration of the Slovak Republic. Pinosylvin (30 mg/kg, daily, po) was administered over a period of 21 d after arthritis induction; control animals (healthy and arthritic) were treated with the solvent agent (sunflower oil). Each group consisted of 10 rats. The total production of oxidants in neutrophils (spontaneous and stimulated with 0.1 μmol/L PMA) was determined on the basis of luminol-enhanced chemiluminescence and presented as the mean integrated values over 3600 s.
Statistical analysis
All of the values were given as the mean±SEM, and the statistical significance of differences between means was established by Student's t-test. P values below 0.05 were considered to be statistically significant.
Results
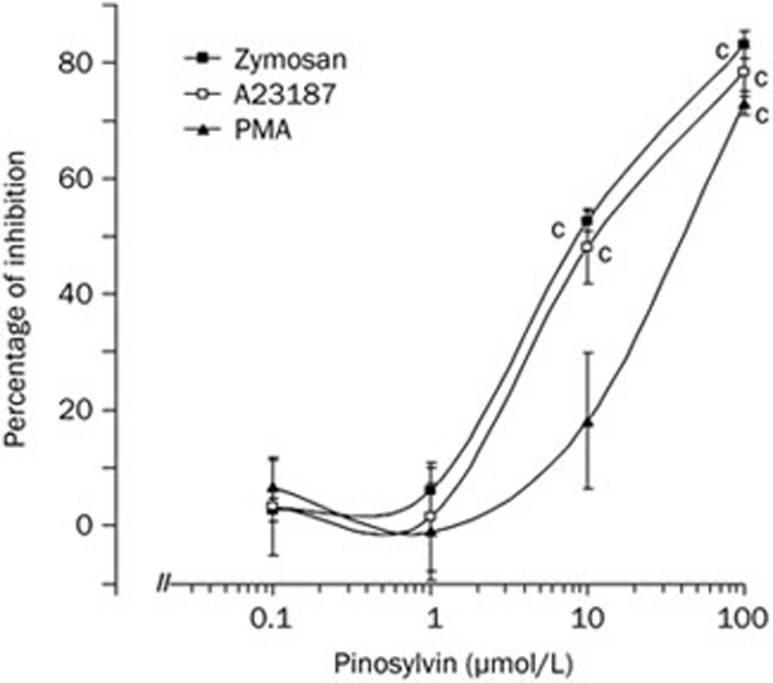
Pinosylvin reduced the oxidative burst of human neutrophils measured in whole blood (Figure 2). It inhibited chemiluminescence initiated by the activation of membrane receptors, increased calcium concentration and the stimulation of protein kinase C at the mean effective concentrations of 10.67±1.07 μmol/L (opsonized zymosan), 12.99±5.64 μmol/L (A23187) and 31.38±8.25 μmol/L (PMA). For comparison, the respective EC50 values of the related compound resveratrol were 12.80±0.97 μmol/L, 24.46±7.86 μmol/L and 3.72±0.30 μmol/L (data not shown).
Figure 2.
Dose-dependent inhibition of neutrophil chemiluminescence in the presence of pinosylvin. Chemiluminescence, measured in whole blood, was initiated with opsonized zymosan (0.5 g/L), Ca2+-ionophore A23187 (1 μmol/L) or phorbol myristate acetate (PMA, 0.05 μmol/L). Mean±SEM. n=6. cP<0.01 vs control. Mean control values of chemiluminescence, given in relative light units — RLU, were 138 375±14 776 RLU (zymosan), 26 346±3 386 RLU (A23187), and 1 303 872±173 251 RLU (PMA).
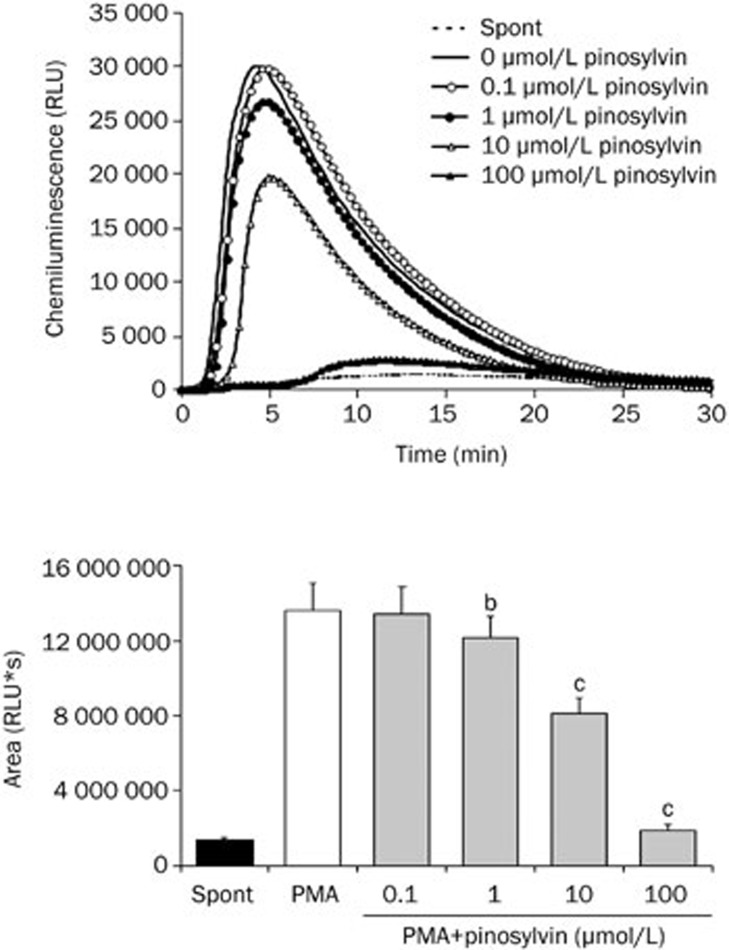
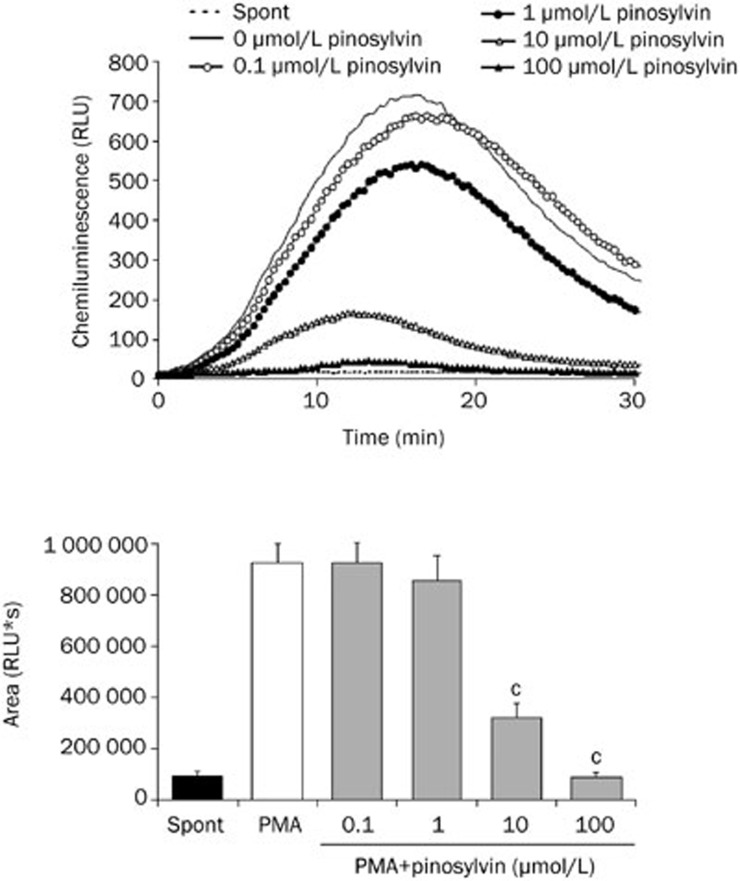
In isolated neutrophils stimulated with PMA, extra- and intracellular chemiluminescence was recorded separately. As illustrated in Figures 3 and 4, external oxidant formation was much more intensive and reached maximum values sooner than the oxidative burst arising within neutrophils. Pinosylvin decreased both the extracellular and intracellular chemiluminescence of neutrophils at the respective mean effective concentrations of 14.16±1.46 μmol/L and 5.54±1.06 μmol/L; the EC50 values assessed for resveratrol were 0.96±0.22 μmol/L and 6.00±0.57 μmol/L, respectively.
Figure 3.
Extracellular chemiluminescence of isolated human neutrophils treated with pinosylvin and stimulated with PMA. Kinetic curves are representative for 6 donors, columns show the mean integral values of chemiluminescence over 1800 s. Mean±SEM. n=6. bP<0.05, cP<0.01 vs PMA. Spont, spontaneous chemiluminescence.
Figure 4.
Intracellular chemiluminescence of isolated human neutrophils treated with pinosylvin and stimulated with PMA. Kinetic curves are representative for 6 donors, columns show the mean integral values of chemiluminescence over 1800 s. Mean±SEM. n=6, cP<0.01 vs PMA. Spont, spontaneous chemiluminescence.
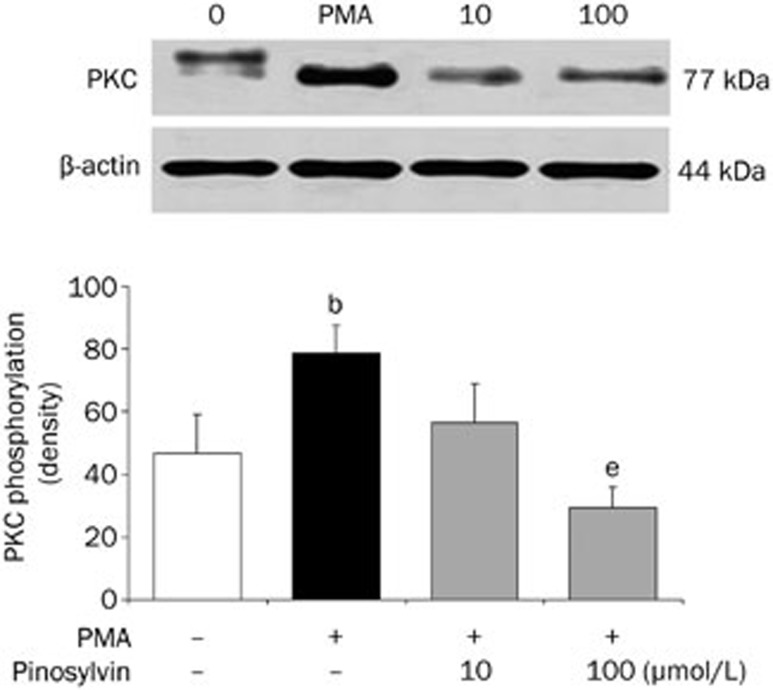
The ability of pinosylvin to inhibit chemiluminescence initiated by different mechanisms and its recorded intracellular activity indicated interference of the neutrophil activation cascade by this phytochemical, particularly with a process involved in the effect of all of the stimuli used. Therefore, the influence of pinosylvin on protein kinase C activation was tested in further experiments. The stimulation of neutrophils with PMA was accompanied by increased phosphorylation of the protein kinase C isoenzymes α and βII (Figure 5). Pinosylvin effectively reduced this increase until the values of phosphorylation were comparable with those produced by resting cells.
Figure 5.
Protein kinase C (PKC) phosphorylation in PMA stimulated human neutrophils treated with 10 and 100 μmol/L pinosylvin (PIN). The degree of phosphorylation is expressed as optical density of PKC bands corrected to β-actin content. Phosphorylated PKC isoenzymes α and βII were isolated by Western blotting and detected by phospho-PKC alpha/beta II (Thr638/641) antibody. Mean±SEM. n=8 (neutrophils from 4 donors were examined; in each sample protein separation and PKC detection were performed twice – in two separate experiments), bP<0.05 vs resting control. eP<0.05 vs PMA stimulated control. Representative blot manifests elevated phosphorylation of protein kinase C in neutrophils stimulated with PMA, as well as the effect of pinosylvin on this increase.
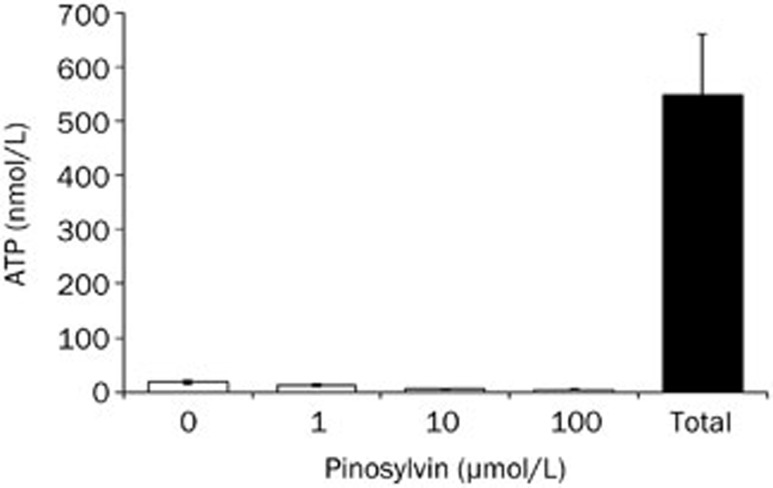
The observed inhibitory effects were not associated with neutrophil damage because in the presence of pinosylvin no increase in extracellular ATP concentration was recorded (Figure 6). Spontaneous ATP liberation from isolated neutrophils was minimal (approximately 3% of the total ATP content, as determined immediately after complete neutrophil destruction). This amount remained unchanged (or was slightly decreased) after the treatment of neutrophils with pinosylvin (1–100 μmol/L).
Figure 6.
Effect of pinosylvin on the integrity of neutrophil membranes assessed on the basis of ATP liberation. The given values represent the extracellular ATP concentration in samples containing 30 000 neutrophils. Open columns – spontaneous ATP liberation in the absence (0) and in the presence of pinosylvin (1–100 μmol/L); Total, amount of ATP determined immediately after complete neutrophil destruction. Mean±SEM. n=8.
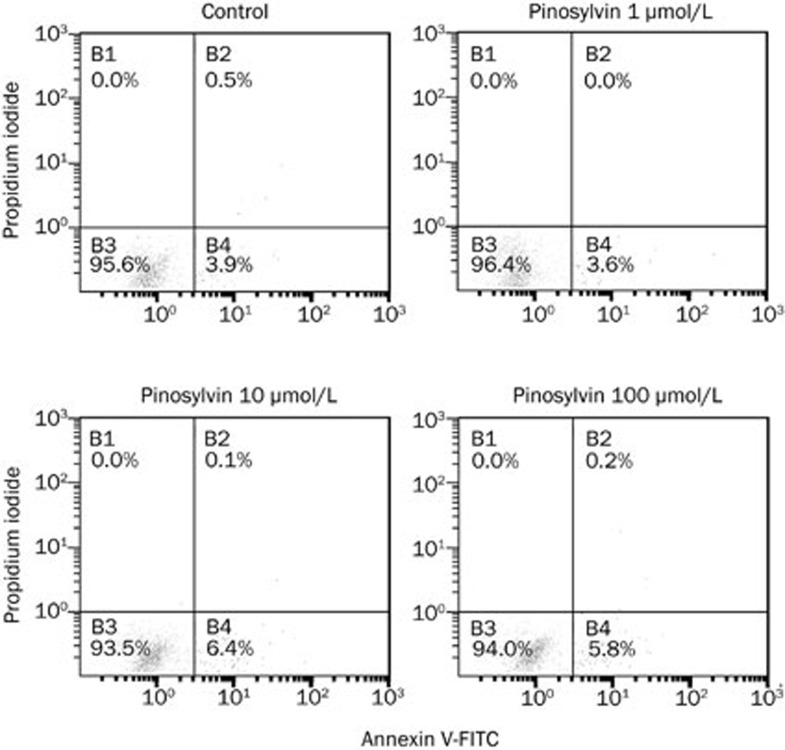
As confirmed by flow cytometry (Figure 7 and Table 1), this stilbenoid did not affect spontaneous apoptosis of human neutrophils. Compared with controls, pinosylvin (1-100 μmol/L) did not alter the percentage of viable, apoptotic or dead neutrophils.
Figure 7.
A dot plot of neutrophils stained with propidium iodide and annexin V-FITC indicates cells in three quadrants. Unstained cells are alive and are double negative; they neither express phosphatidylserine on their surface nor do they take up propidium iodide (quadrant B3). Cells stained only with annexin are apoptotic; they have begun to express phosphatidylserine on their surface but have not yet gone through the process that leads to permeabilisation of their cytoplasmic membrane (quadrant B4). Cells stained both with propidium iodide and annexin are necrotic (dead); they take up propidium iodide and also express phosphatidylserine (quadrant B2). Compared to controls, pinosylvin (1–100 μmol/L) did not alter the percentages of viable, apoptotic and dead neutrophils.
Table 1. Effect of pinosylvin (PIN) on viability and apoptosis of human neutrophils. Mean±SEM. n=8.
| Control | PIN 1 μmol/L | PIN 10 μmol/L | PIN 100 μmol/L | |
|---|---|---|---|---|
| AV−/PI− viable cells (%) | 90.1±1.6 | 91.6±1.2 | 90.1±1.0 | 85.1±2.9 |
| AV+/PI− early apoptotic cells (%) | 9.5±1.6 | 8.0±1.2 | 9.4±0.9 | 14.2±2.8 |
| AV+/PI+ late apoptotic cells (%) | 0.3±0.1 | 0.3±0.1 | 0.5±0.2 | 0.6±0.3 |
Neutrophil apoptosis was recorded by flow cytometry, using double staining with Annexin-V (A) and propidium iodide (PI).
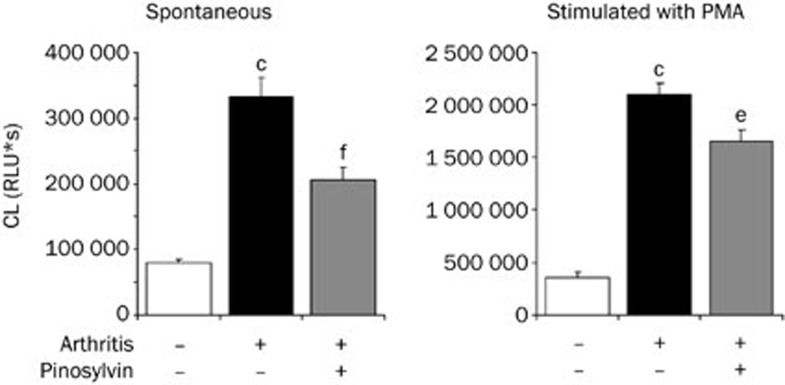
To confirm the efficacy of pinosylvin under inflammatory conditions, whole blood chemiluminescence was analyzed in arthritic rats (Figure 8). Adjuvant arthritis was accompanied by an increased number of neutrophils in the blood and by more pronounced spontaneous and PMA-stimulated chemiluminescence; all of these changes were reduced by oral administration of pinosylvin. The mean neutrophil count, assessed in 1 μL blood, was 16 040±928 (healthy controls), 69 671±5 588 (arthritic animals) and 51 293±3 947 (arthritic rats treated with pinosylvin).
Figure 8.
Effect of pinosylvin administration on the formation of reactive oxygen species in adjuvant arthritis. Whole blood chemiluminescence (spontaneous and PMA stimulated) was evaluated on the basis of integral values over 3600 s. Results from three groups of animals are compared — healthy, arthritic without any medication and arthritic animals treated with pinosylvin (30 mg/kg daily, over 21 d). Mean±SEM. n=9–10. cP<0.01 vs healthy control. eP<0.05, fP<0.01 vs arthritic control.
Discussion
The incubation of human neutrophils with pinosylvin resulted in decreased production of reactive oxygen species. Because the effect occurred in the presence of each stimulus used, the interference of a process involved in all mechanisms of chemiluminescence initiation by pinosylvin has been suggested. One of the potential candidates could be the signaling enzyme protein kinase C. Pinosylvin hindered the activation of this enzyme, as indicated by the decreased phosphorylation of protein kinase C isoenzymes α and βII on their catalytic region. Because these isoenzymes participate directly in the activation of neutrophil NADPH oxidase8, their inhibition may result in reduced oxidant formation and thus explain the decreased chemiluminescence of neutrophils treated with pinosylvin. Similar to resveratrol, a compound related to pinosylvin, protein kinase C inhibition may result from the competition for phorbol ester binding to the C1 domains of the enzyme31.
Involvement of other mechanisms in the inhibition of chemiluminescence, such as reduced activity of phosphatidylinositol 3-kinase, 5-lipoxygenase, cyclooxygenase, or myeloperoxidase15,22, cannot be excluded. However, the repressed expression of NADPH oxidase, which was observed in macrophages treated for 3–6 h with resveratrol32, seems to be unlikely with respect to the early onset of the pinosylvin effect.
Similarly, the antioxidative activity of pinosylvin may be involved to a lesser extent, because both pinosylvin hydroxyl groups are located in the meta position (with respect to the ethylene bridge of the stilbene molecule), ie, in an arrangement that is less favorable for both electron abstraction and the distribution of the unpaired electron18,33. The assessment of lipid peroxyl radical formation34 and the measurement of human low-density lipoprotein peroxidation35 confirmed pinosylvin to be a less potent scavenger than resveratrol. Nevertheless, regarding the inhibition of chemiluminescence, we found the activities of these two stilbenoids comparable (eg, in neutrophils stimulated with zymosan or the Ca2+ ionophore A23187), suggesting that radical scavenging may not be a decisive mechanism of pinosylvin action.
Because activated neutrophils form and liberate reactive oxygen species both extra- and intracellularly36, it was important to identify which part of the chemiluminescence signal was reduced in the presence of pinosylvin. This stilbenoid was found to be active in both compartments. Radicals formed within neutrophils, which are involved in the redox regulation of signal transduction in neutrophils3, were reduced more effectively than extracellular oxidants.
The decreased chemiluminescence and inhibited formation of oxidants were not associated with altered neutrophil viability. As shown by the measurement of ATP liberation and the cytometric determination of apoptosis, pinosylvin did not reduce the viability of neutrophils at concentrations up to 100 μmol/L. This result contrasted with its cytotoxic and repressive effect assessed in bacteria, fungi and cancer cells20,21 and with the initiation of apoptosis via activation of caspases, which was observed in the presence of other resveratrol derivatives37.
The efficacy of pinosylvin was further assessed in neutrophils modified by inflammation.
Adjuvant arthritis, a rat model, mimics the immunological and biochemical features of human rheumatoid arthritis. As revealed in the presented experiments, the number of neutrophils increased more than fourfold in adjuvant arthritis, and this increase was accompanied by an elevated concentration of oxidants in the blood. The assessment of chemiluminescence produced by one neutrophil confirmed that neutrophils of arthritic rats responded excessively to PMA stimulation and synthesized more radicals than neutrophils of healthy controls. A similar priming of peripheral neutrophils was observed in patients with rheumatoid arthritis38,39,40. These alterations were ascribed to the direct effect of proinflammatory cytokines on neutrophil NADPH oxidase activity, which was induced by the increased phosphorylation of p47phox, extracellular signal-regulated kinase, and p38 mitogen-activated protein kinase39,41.
Orally administered pinosylvin simultaneously decreased the concentration of oxidants and the number of neutrophils, indicating that the beneficial antioxidative effect of this stilbenoid may arise from reduced arthritic neutrophilia (and repressed inflammation) rather than its direct interference with neutrophil activity. The anti-inflammatory activity of pinosylvin, as manifested by reduced hind paw swelling42, could be ascribed to several mechanisms, such as the reduced synthesis and release of pro-inflammatory mediators, modified eicosanoid synthesis, decreased activity of immune cells and suppressed activation of nuclear factor κB22,23,24,43. Moreover, resveratrol and α-viniferin, compounds that are structurally related to pinosylvin, were found to induce apoptosis of human rheumatoid arthritis synovial cells44 and prevent tissue destruction in model arthritis45. Finally, the action of pinosylvin might involve decreased expression of inducible NO synthase and reduced formation of nitric oxide, as found in macrophages26,46. This effect may prove beneficial because nitric oxide, if transformed into highly reactive peroxynitrite, can activate proinflammatory signaling47 and contribute to the pathogenesis of arthritis48.
Conclusion
Pinosylvin decreased the concentration of oxidants released by activated neutrophils into the extracellular space and the oxidative burst occurring within neutrophils. The inhibition was accompanied by inhibited activation of protein kinase C and the formation of reactive oxygen species in neutrophils. Pinosylvin administered orally reduced the neutrophil count and decreased the concentration of oxidants in the blood of arthritic rats. The observed effects classified pinosylvin as an effective inhibitor of neutrophil activity, which may make it potentially useful as a complementary medicine in pathological states associated with persistent inflammation.
Author contribution
Viera JANČINOVÁ, Tomáš PEREČKO, and Katarína DRÁBIKOVÁ contributed to the experimental planning and design, performed the experiments, analyzed and interpreted data, and drafted the manuscript; Rado NOSÁĽ conceived the study and coordinated and supervised the experiments; Juraj HARMATHA and Jan ŠMIDRKAL participated in study conception and synthesized and analyzed pinosylvin.
Acknowledgments
We wish to thank Ing Danica MIHALOVA and Mrs Denisa KOMENDOVÁ for their kind assistance and Prof Magda KOUŘILOVá-URBANCZIK for English language correcting. The study was supported by grants APVV-0315-07, APVV-0052/10, VEGA-2/0003/10, VEGA-2/0045/11, and GACR-203/07/1227.
References
- Cascao R, Rosário HS, Fonseca JE. Neutrophils: warriors and commanders in immune mediated inflammatory diseases. Acta Reumatol Port. 2009;34:313–26. [PubMed] [Google Scholar]
- Cascao R, Rosário HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: More than simple final effectors. Autoimmun Rev. 2010;9:531–5. doi: 10.1016/j.autrev.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Rad Biol Med. 2007;42:153–64. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology. 2010;49:1618–31. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- Edwards SW, Hallett MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today. 1997;18:320–4. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
- El Kebir D, Filep JG. Role of neutrophil apoptosis in the resolution of inflammation. ScientificWorldJournal. 2010;10:1731–48. doi: 10.1100/tsw.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo HR, Loison F. Constitutive neutrophil apoptosis: Mechanisms and regulation. Am J Hematol. 2008;83:288–95. doi: 10.1002/ajh.21078. [DOI] [PubMed] [Google Scholar]
- Fontayne A, Dang PMC, Gougerot-Pocidalo MA, El Benna J. Phosphorylation of p47phox sites by PKC α, βII, δ, and ζ: Effect on binding to p22phox and on NADPH oxidase activation. Biochmistry. 2002;41:7743–50. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- Webb PR, Wang KQ, Scheel-Toellner D, Pongracz J, Salmon M, Lord JM. Regulation of neutrophil apoptosis: A role for protein kinase C and phosphatidylinositol-3-kinase. Apoptosis. 2000;5:451–8. doi: 10.1023/a:1009601220552. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LE, Sun S, Mackie DM, Baik F, Li H, Korchak HM. Regulation of TNF mediated antiapoptotic signaling in human neutrophils: role of δ-PKC and ERK1/2. J Leukoc Biol. 2006;80:1512–21. doi: 10.1189/jlb.0406284. [DOI] [PubMed] [Google Scholar]
- Burgos RA, Hidalgo MA, Figueroa CD, Conejeros I, Hancke JL. New potential targets to modulate neutrophil function in inflammation. Mini-Rev Med Chem. 2009;9:153–68. doi: 10.2174/138955709787316092. [DOI] [PubMed] [Google Scholar]
- Drábiková K, Perečko T, Nosáľ R, Bauerová K, Poništ S, Mihalová D, et al. Glucomannan reduces neutrophil free radical production in vitro and in rats with adjuvant arthritis. Pharmacol Res. 2009;59:399–403. doi: 10.1016/j.phrs.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Nosáľ R, Perečko T, Jančinová V, Drábiková K, Harmatha J, Sviteková K. Naturally appearing N-feruloylserotonin isomers suppress oxidative burst of human neutrophils at the protein kinase C level. Pharmacol Rep. 2011;63:790–8. doi: 10.1016/s1734-1140(11)70591-6. [DOI] [PubMed] [Google Scholar]
- Perečko T, Drábiková K, Račková L, Číž M, Podborská M, Lojek A, et al. Molecular targets of the natural antioxidant pterostilbene: effect on protein kinase C, caspase-3 and apoptosis in human neutrophils in vitro. Neuro Endocrinol Lett. 2010;31:84–90. [PubMed] [Google Scholar]
- Alarcón de la Lastra C, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol Nutr Food Res. 2005;49:405–30. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- Wu PF, Zhang Z, Wang F, Chen JG. Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol Sin. 2010;31:1523–31. doi: 10.1038/aps.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei R, Fei Y, Zheng S, Gao YG, Sun HX, Zeng XL. Purified polysaccharide from Ginkgo biloba leaves inhibits P-selectin-mediated leucocyte adhesion and inflammation. Acta Pharmacol Sin. 2008;29:499–506. doi: 10.1111/j.1745-7254.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- Fan GJ, Liu XD, Qian YP, Shang YJ, Li XZ, Dai F, et al. 4,4′-Dihydroxy-trans-stilbene, a resveratrol analogue, exhibited enhanced antioxidant activity and cytotoxicity. Bioorg Med Chem. 2009;17:2360–5. doi: 10.1016/j.bmc.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Ludwiczuk A, Saha A, Kuzuhara T, Asakawa Y. Bioactivity guided isolation of anticancer constituents from leaves of Alnus sieboldiana (Betulaceae) Phytomedicine. 2011;18:491–8. doi: 10.1016/j.phymed.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Lee SK, Lee HJ, Min HY, Park EJ, Lee KM, Ahn YH, et al. Antibacterial and antifungal activity of pinosylvin, a constituent of pine. Fitoterapia. 2005;76:258–60. doi: 10.1016/j.fitote.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Roupe KA, Remsberg CM, Yáňez JA, Davies NM. Pharmacometrics of stilbenes: Seguing towards the clinic. Curr Clin Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- Adams M, Pacher T, Greger H, Bauer R. Inhibition of leukotriene biosynthesis by stilbenoids from Stemona species. J Nat Prod. 2005;68:83–5. doi: 10.1021/np0497043. [DOI] [PubMed] [Google Scholar]
- Lee J, Jung E, Lim J, Lee J, Hur S, Kim SS, et al. Involvement of nuclear factor-kappaB in the inhibition of pro-inflammatory mediators by pinosylvin. Planta Med. 2006;72:801–6. doi: 10.1055/s-2006-941545. [DOI] [PubMed] [Google Scholar]
- Park EJ, Min HY, Ahn YH, Bae CM, Pyee JH, Lee SK. Synthesis and inhibitory effects of pinosylvin derivatives on prostaglandin E2 production in lipopolysaccharide-induced mouse macrophage cells. Bioorg Med Chem Lett. 2004;14:5895–58. doi: 10.1016/j.bmcl.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Bauerová K, Poništ S, Dráfi F, Mihalová D, Paulovičová E, Jančinová V, et al. Study of combination of pinosylvin and methotrexate in the model of adjuvant arthritis: Beneficial effect on clinical and non-clinical parameters. Interdisc Toxicol. 2010;3:32. [Google Scholar]
- Jančinová V, Nosáľ R, Lojek A, Číž M, Ambrožová G, Mihalová D, et al. Formation of reactive oxygen and nitrogen species in the presence of pinosylvin — an analogue of resveratrol. Neuro Endocrinol Lett. 2010;31:79–83. [PubMed] [Google Scholar]
- Šmidrkal J, Harmatha J, Buděšínský M, Vokáč K, Zídek Z, Kmoníčková E, et al. Modified approach for preparing (E)-Stilbenes related to resveratrol, and evaluation of their potential immunobiological effects. Collect Czech Chem Commun. 2010;75:175–86. [Google Scholar]
- Číž M, Pavelková M, Gallová L, Králová J, Kubala L, Lojek A. The influence of wine polyphenols on reactive oxygen and nitrogen species production by murine macrophages RAW 264.7. Physiol Res. 2008;57:393–402. doi: 10.33549/physiolres.931088. [DOI] [PubMed] [Google Scholar]
- Jančinová V, Perečko T, Nosáľ R, Košťálová D, Bauerová K, Drábiková K. Decreased activity of neutrophils in the presence of diferuloylmethane (curcumin) involves protein kinase C inhibition. Eur J Pharmacol. 2009;612:161–6. doi: 10.1016/j.ejphar.2009.03.080. [DOI] [PubMed] [Google Scholar]
- Bauerová K, Poništ S, Mihalová D, Dráfi F, Kuncírová V. Utilization of adjuvant arthritis model for evaluation of new approaches in rheumatoid arthritis therapy focused on regulation of immune processes and oxidative stress. Interdisc Toxicol. 2011;4:33–9. doi: 10.2478/v10102-011-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater SJ, Seiz JL, Cook AC, Stagliano BA, Buzas CJ. Inhibition of protein kinase C by resveratrol. Biochim Biophys Acta. 2003;1637:59–69. doi: 10.1016/s0925-4439(02)00214-4. [DOI] [PubMed] [Google Scholar]
- Park DW, Baek K, Kim JR, Lee JJ, Ryu SH, Chin BR, et al. Resveratrol inhibits foam cell formation via NADPH oxidase 1-mediated reactive oxygen species and monocyte chemotactic protein-1. Exp Mol Med. 2009;41:171–9. doi: 10.3858/emm.2009.41.3.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz AN, Gomes BAQ, Moraes WM, Borges RS. A theoretical antioxidant pharmacophore for resveratrol. Eur J Med Chem. 2009;44:1644–9. doi: 10.1016/j.ejmech.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Stojanovic S, Sprinz H, Brede O. Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Arch Biochem Biophys. 2001;391:79–89. doi: 10.1006/abbi.2001.2388. [DOI] [PubMed] [Google Scholar]
- Cheng JC, Fang JG, Chen WF, Zhou B, Yang L, Liu ZL. Structure-activity relationship studies of resveratrol and its analogues by the reaction kinetics of low density lipoprotein peroxidation. Bioorg Chem. 2006;34:142–57. doi: 10.1016/j.bioorg.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid Redox Signal. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- Li H, Wu WK, Zheng Z, Che CT, Yu L, Li ZJ, et al. 2,3′,4,4′,5′-Pentamethoxy-trans-stilbene, a resveratrol derivative, is a potent inducer of apoptosis in colon cancer cells via targeting microtubules. Biochem Pharmacol. 2009;78:1224–32. doi: 10.1016/j.bcp.2009.06.109. [DOI] [PubMed] [Google Scholar]
- Fairhurst AM, Wallace PK, Jawad AS, Goulding NJ. Rheumatoid peripheral blood phagocytes are primed for activation but have impaired Fc-mediated generation of reactive oxygen species. Arthritis Res Ther. 2007;9:R29. doi: 10.1186/ar2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M, Takahashi T, Kumeda Y, Kato T, Hato F, Yutani Y, et al. Increased basal phosphorylation of mitogen-activated protein kinases and reduced responsiveness to inflammatory cytokines in neutrophils from patients with rheumatoid arthritis. Clin Exp Rheumatol. 2008;26:52–60. [PubMed] [Google Scholar]
- Miesel R, Hartung R, Kroeger H. Priming of NADPH oxidase by tumor necrosis factor alpha in patients with inflammatory and autoimmune rheumatic diseases. Inflammation. 1996;20:427–38. doi: 10.1007/BF01486744. [DOI] [PubMed] [Google Scholar]
- Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, et al. A specific p47phox-serine phosphorylated by convergent MAPKs mediated neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–43. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalová D, Poništ S, Bauerová K, Nosáľ R, Jančinová V. Comparative study of two stilbene derivatives in the experimental rat model of arthritis based on evaluation of clinical parameters. Interdisc Toxicol. 2010;3:67. [Google Scholar]
- Park EJ, Ahn YH, Pyee JH, Park HJ, Chung HJ, Min HY, et al. Suppressive effects of pinosylvin, a natural stilbenoid, on cyclooxygenase-2 and inducible nitric oxide synthase and the growth inhibition of cancer cells. Proc Amer Assoc Cancer Res. 2005;46:176. [Google Scholar]
- Nakayama H, Yaguchi T, Yoshiya S, Nishizaki T. Resveratrol induces apoptosis MH7A human rheumatoid arthritis synovial cells in a sirtuin 1-dependent manner. Rheumatol Int. 2012;32:151–7. doi: 10.1007/s00296-010-1598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Kim JH, Kang SS, Bae CS, Choi SH. The effects of alpha-viniferin on adjuvant-induced arthritis in rats. Am J Chin Med. 2004;32:521–30. doi: 10.1142/S0192415X04002168. [DOI] [PubMed] [Google Scholar]
- Harmatha J, Zídek Z, Kmoníčková E, Šmidrkal J. Immunobiological properties of selected natural and chemically modified phenylpropanoids. Interdisc Toxicol. 2011;4:5–10. doi: 10.2478/v10102-011-0002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. The multiple actions of NO. Eur J Physiol. 2010;459:829–39. doi: 10.1007/s00424-009-0773-9. [DOI] [PubMed] [Google Scholar]
- Abramson SB. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res Ther. 2008;10:S2. doi: 10.1186/ar2463. [DOI] [PMC free article] [PubMed] [Google Scholar]