Abstract
Aim:
Lactoferrin (LF), an 80-kDa iron-binding glycoprotein, is a pleiotropic factor found in colostrum, milk, saliva and epithelial cells of the exocrine glands. The aim of this study was to evaluate the effects of LF on the bones in ovariectomized (Ovx) rats and to identify the pathways that mediate the anabolic action of LF on the bones.
Methods:
Female Sprague-Dawley rats (6-month-old) underwent ovariectomy, and were treated with different doses of LF (10, 100, 1000, and 2000 mg·kg−1·d−1, po) or with 7β-estradiol (0.1 mg·kg−1, im, each week) as the positive control. By the end of 6 month-treatments, the bone mass and microstructure in the rats were scanned by micro-computed tomography (micro-CT), and the bone metabolism was evaluated with specific markers, and the mRNA levels of osteoprotegerin (OPG) and the receptor-activator of nuclear factor κB ligand (RANKL) in femur were measured using qRT-PCR.
Results:
LF treatment dose-dependently elevated the bone volume (BV/TV), trabecular thickness (TbTh) and trabecular number (TbN), and reduced the trabecular separation (TbSp) in Ovx rats. Furthermore, higher doses of LF (1000 and 2000 mg·kg−1·d−1) significantly increased the bone mineral density (BMD) compared with the untreated Ovx rats. The higher doses of LF also significantly increased the serum levels of OC and BALP, and decreased the serum levels of β-CTx and NTX. LF treatment significantly increased the OPG mRNA levels, and suppressed the RANKL mRNA levels, and the RANKL/OPG mRNA ratio in Ovx rats.
Conclusion:
Oral administration of LF preserves the bone mass and improves the bone microarchitecture. LF enhances bone formation, reduces bone resorption, and decreases bone mass loss, possibly through the regulation of OPG/RANKL/RANK pathway.
Keywords: lactoferrin, bone, bone mineral density, OPG/RANKL/RANK pathway, bone turnover
Introduction
Osteoporosis is a common disease that is characterized by reduced bone mass and altered bone microarchitecture, which results in fragility fractures1. The bone loss is caused by an imbalance between the activities of osteoclasts and osteoblasts, and it leads to the uncoupling of bone resorption and bone formation2. The bone loss found in postmenopausal osteoporosis (PMOP) cases is caused by estrogen deficiency that increases osteoclast activity and bone remodeling — a predominance of bone resorption over bone formation3. The increased rate of bone turnover as observed from markers, such as β-CrossLaps (β-CTx) and collagen type I N-telopeptide (NTX), may be an additional risk factor for fractures by exacerbating bone loss. Furthermore, the identification of additional bone formation markers, such as osteocalcin (OC) and bone alkaline phosphatase (BALP), may serve to reduce the risk of osteoporosis and lessen the probability of fractures.
Lactoferrin (LF), an 80-kDa iron-binding glycoprotein that belongs to the transferrin family, is a pleiotropic factor found in colostrum, milk, saliva, and epithelial cells of the exocrine glands4,5. Many studies have demonstrated that lactoferrin has antimicrobial activity and functions as a regulator of immune response6,7. In recent years, several studies have shown that LF acts as a growth factor and has both in vitro and in vivo anabolic activity in bone8,9,10,11,12. Lactoferrin induces the proliferation and differentiation of osteoblast-like cells and inhibits osteoclastogenesis in vitro10,13. LF has also been shown to have an inhibitory effect on osteoclast formation11. Locally injecting LF above the hemicalvaria in adult mice for 5 successive days resulted in increased bone formation and bone area compared with the controls8. In addition, oral LF administered to Ovx rats for 3 months protected against Ovx-induced decreases in bone volume and mineral density and led to increased mechanical strength parameters14. In a clinical study, 38 healthy, postmenopausal women randomly received either oral RNAse-enriched lactoferrin or a placebo, resulting in an obvious reduction in osteoblast and bone resorption markers in the lactoferrin-treated group15.
The pathways that are activated by lactoferrin are unknown. Osteoprotegerin (OPG) and the receptor activator of nuclear factor-kB ligand (RANKL) are cytokines that are necessary for regulating bone formation and bone resorption. The RANKL/OPG ratio is the key element regulating the balance between bone formation and bone resorption, and the shortage of estrogen leads to an imbalance in this ratio. OPG is a naturally occurring inhibitor of RANKL; it binds RANKL with high affinity and prevents its interaction with RANK on osteoclasts16. Therefore, OPG negatively regulates osteoclast formation and prevents osteoporosis17. Studies have shown that excessive osteoclast activity in OPG-knockout mice (OPG−/−) results in high bone turnover rate, disorganized matrix and impaired attachment of new bone to old bone in the cement lines18. The inability of RANK to bind RANKL leads to the inhibition of osteoclast differentiation and maturation, thereby causing a deficiency of mature osteoclasts at the bone surface19. In addition, other cytokines that mediate bone remodeling, such as monocyte-macrophage colony stimulating factor (M-CSF), are also known to promote osteoclastogenesis20.
In a number of recent studies, osteoblast cultures treated with LF showed positive effects on bone in vitro. It is important to study the effects of oral LF in an animal osteoporosis model as observed on bone mass and bone microarchitecture. Moreover, to utilize LF as a therapeutic agent for treating bone loss, a better understanding has to be obtained of the pathways involved in LF's anabolic effects in the bone and its effects on the biochemical markers of bone turnover. Herein, we explored in further details the pathways by which lactoferrin stimulates osteoblast growth.
Materials and methods
Materials
The bovine LF (95% purity with 20% iron saturation) used in this study was produced in Australia. Prior to use, the LF was diluted in distilled water, and the 17β-estradiol (E2; Shanghai General Pharmaceutical Co, LTD; batch number: 090801) was dissolved in corn oil. All of the rats were treated in accordance with “Fujian experimental animal management measures” and with the approval of the Fujian Provincial Hospital, Fuzhou, China.
Ovx rat model
A total of seventy 6-month-old virgin female Sprague-Dawley rats weighing 300±20 g were purchased from Shanghai SCXK Experimental Animals Co, LTD (qualified number: SCXK (Shanghai) 2007-0005). The rats were housed in individual stainless-steel cages with a controlled temperature of 20±3 °C, a relative humidity of 40%–60% and a 12-h light-dark cycle. All of the rats were given free access to a standard pellet diet and water during the 6-month feeding period. After 1 week of adaptability feeding, 10 rats were randomly assigned to the sham-operated (Sham) group, and the remaining 60 rats were assigned to the ovariectomized (Ovx) group. The Ovx rats were anesthetized with ketamine (100 mg/kg), and both ovaries were removed. The Sham rats had surgery that only resected the fat tissue near the ovaries. After surgery, penicillin was administered intramuscularly at 50 000 U per day, with each dose lasting for three days. One week after the surgery, the Ovx rats were randomly divided into six groups of 10 rats each, as follows: Ovx untreated (Con); Ovx+17β-estradiol 0.1 mg·kg−1·week−1, the positive control (E2); Ovx+LF 10 mg·kg−1·d−1 (LF1); Ovx+LF 100 mg·kg−1·d−1 (LF2); Ovx+LF 1.0 g·kg−1·d−1(LF3); and Ovx+LF 2.0 g·kg−1·d−1 (LF4). The doses of LF were chosen according to previous studies in the literature21.
Treatment
In the Sham and Con groups, the rats were orally intubated with 0.9% physiological saline at a dose of 2 mL/kg. For the Ovx+E2, the E2 was dissolved in corn oil and injected into muscle at a dose of 0.1 mg/kg body weight on a weekly basis. The LF was dissolved in distilled water and given orally every day at the appropriate dose. The doses were readjusted every week to accommodate changes in body weight.
Sample collection
After 6 months of treatment, each rat was euthanized, its uterus and subcutaneous fat were removed and weighed, and the femur and the L2-4 vertebrae of each rat were separated from the surrounding muscle. Venous blood was drawn into a Vacutainer (BD Biosciences, NJ, USA) and the serum was separated by centrifugation. The serum specimens were stored at −80 °C until analysis. The right femur was collected in a tube, frozen in liquid nitrogen and stored at −80 °C until analysis. The left femur and L2-4 vertebrae were preserved in 4% paraformaldehyde for the micro-computed tomography scans.
Micro-computed tomography (micro-CT)
Scanning parameters
Each specimen consisted of the proximal femur and L2-4 vertebrae and was evaluated using a microfocus CT system (ZKKS-SHARP Institute of Chinese Science Research). Each sample was scanned at 10-mm intervals at a voltage of 70 kVP, amplitude of 200 mA, scanning resolution of 50 μm, rotating angle of 360 degrees, power of 40 W, frame average of 6 and pixel combination of 1×1.
Three dimensional (3D) reconstruction and bone parameter analysis
The trabecular bone microarchitecture of the proximal femur and L2-4 vertebrae was analyzed with mCT at a 50-μm isotropic image resolution. The trabecular region was isolated from the cortical region in each 2D image by a manual contouring analysis. Model-independent 3D measurement methods were used to reconstruct and define the trabecular bone volume of interest (VOI) for the cancellous bone, and the following parameters were calculated: trabecular bone volume fraction (BV/TV, %), trabecular thickness (TbTh, mm), trabecular number (TbN, mm−1), trabecular separation (TbSp, mm), and bone mineral density (BMD).
Serum E2 level and biochemical markers of bone turnover
Total estradiol (E2), osteocalcin (OC), bone alkaline phosphatase (BALP), β-CrossLaps (β-CTx), and collagen type I N-telopeptide (NTX) levels were determined in serum samples obtained at the time of bone biopsy. E2, OC, BALP, and β-CTx were measured using an immunoradiometric assay autoanalyzer (R&D, USA). NTX levels were determined by electrochemiluminescence (ECL) assay (Roche Elecsys 2010, Switzerland). The sensitivities of these assays were 1 pg/mL and 50 pg/mL, and their inter-assay variation coefficients were less than 10%.
Quantitative real-time reverse transcription-PCR (qRT-PCR)
The local expression of the RANKL and OPG genes in the trabecular bone of the femur was analyzed by qRT-PCR. A small cube of trabecular bone (proximal femur) was homogenized, and the total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA)22. The extracted RNA was dissolved in RNAse-free distilled water. The quality and quantity of the RNA samples were determined by spectrophotometry, with the ratios of absorbance at 260 nm and 280 nm ranging from 1.8 to 2.0. Next, 5 mg of total RNA was first reverse transcribed into cDNA using a High-Capacity cDNA Kit (DRR037, TaKaRa, Japan) according to the manufacturer's instructions. The quantitative PCR for the local RANKL and OPG gene expression was performed in 96-well plates; the reaction volume was 25 mL/well, which included the SYBR PremixEx Taq II (2×) (DRR081A, TaKaRa, Japan), diluted gene primers, and cDNA. The primer sequences are listed in Table 1. The qRT-PCR was performed using a Thermal Cycler DiceTM Real Time (TP800, TaKaRa). The standard curve method was used to calculate the relative expression levels, with the levels of each gene normalized to β-actin. The data from the qRT-PCR analyses are presented in relative mRNA units. The RANKL/OPG mRNA ratio was calculated from the RANKL/actin and OPG/actin mRNA values.
Table 1. The primer sequences for quantitative real-time PCR.
| Genes | GenBank numbers | Primer sequence | Product size (bp) |
|---|---|---|---|
| OPG | NM-012870 | Forward 5′-ACAATGAACAAGTGGCTGTGCTG-3′ Reverse 5′-CGGTTTCTGGGTCATAATGCAAG-3′ | 109 |
| RANKL | NM-057149 | Forward 5′-GCAGCATCGCTCTGTTCCTGTA-3′ Reverse 5′-GCATGAGTCAGGTAGTGCTTCTGTG-3′ | 164 |
| β-Actin | NM-031144.2 | Forward 5′-GGAGATTACTGCCCTGGCTCCTA-3′ Reverse 5′-GACTCATCGTACTCCTGCTTGCTG-3′ | 150 |
Statistical analysis
The results are reported as the mean±standard deviation (SD). The data analyses were performed using the Statistical Package for the Social Sciences (SPSS version 16.0) software package. The differences among the 7 groups were analyzed using a one-way ANOVA model followed by post-hoc tests. The changes in body weight within a group over time were compared using the paired-samples t-test. The variables with unequal variances were analyzed using the Kruskal-Wallis H test. Differences were considered significant at P<0.05.
Results
General index changes
The body weights of the rats were not significantly different from each other before the treatment (P>0.05, Table 2). By the end of the 6-month experiment, all of the rats had increased body weight. The Con rats tended to gain more weight than the Sham rats (P<0.05). The final body weights of the LF-treated rats tended to be lower than those of the Con group; however, these differences were not statistically significant (P>0.05, Table 2). In addition, estrogen treatment completely prevented the Ovx-induced weight gain.
Table 2. The body, uterus, and subcutaneous fat weights in the Con, Sham, E2, and LF groups.
| Weights |
Numbers |
Body (g) |
Uterus (g) |
Subcutaneous fat (g) |
||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Final | Final | |
| Con |
10 |
7 |
289.9±19.2 |
434.1±46e |
0.15±0.09 |
20.1±1.3 |
| Sham |
10 |
7 |
298.0±18.0 |
358.9±40.5be |
0.91±0.85 |
12.3±0.6 |
| E2 |
10 |
8 |
295.6±13.8 |
370±42.4be |
0.85±0.78 |
13.5±0.7 |
| LF1 |
10 |
7 |
300.9±14.4 |
426.1±43.3e |
0.36±0.55 |
18.8±1.5 |
| LF2 |
10 |
10 |
290.1±15.0 |
393.6±29.4e |
0.45±0.49 |
17.5±1.0 |
| LF3 |
10 |
9 |
294.5±13.8 |
393.1±35.2e |
0.60±0.51 |
15.3±0.8 |
| LF4 | 10 | 8 | 297.02±18.8 | 395.3±288e | 0.86±0.59 | 13.8±0.7 |
Con: ovariectomized untreated; Sham: sham-operated; E2: Ovx+E2 0.1 mg·kg−1·week−1; LF1: Ovx+LF 10 mg·kg−1·d−1; LF2: Ovx+LF 100 mg·kg−1·d−1; LF3: Ovx+LF 1.0 g·kg−1·d−1; LF4: Ovx+LF 2.0 g·kg−1·d−1. Mean±SD. bP<0.05, cP<0.01 compared with the Con group; eP<0.05 compared with the initial body weight.
The Con rats had uterine tissue atrophy, indicating the success of the surgical procedure (Table 2). The estrogen partly prevented the Ovx-induced uterine atrophy, whereas the LF had no detectable effect on the uterus. The subcutaneous fat weight of the Con rats was significantly greater than that of the Sham rats, but this difference was not statistically significant (P>0.05, Table 2).
Serum E2 level
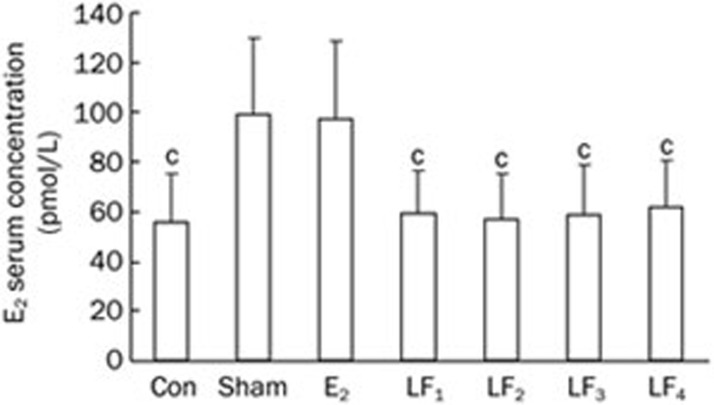
At the completion of the experiment, the levels of serum estradiol were comparable between the E2 and the Sham groups (P>0.05), whereas the Con and the different LF groups had decreased estradiol levels as compared to the Sham group (P<0.01) (Figure 1).
Figure 1.
The serum E2 level in the Con, Sham, E2, and LF groups at the end of the experiment. Mean±SD. cP<0.01 compared with the Sham group.
Micro-computed tomography (micro-CT)
Microstructural parameters of the trabecular bone
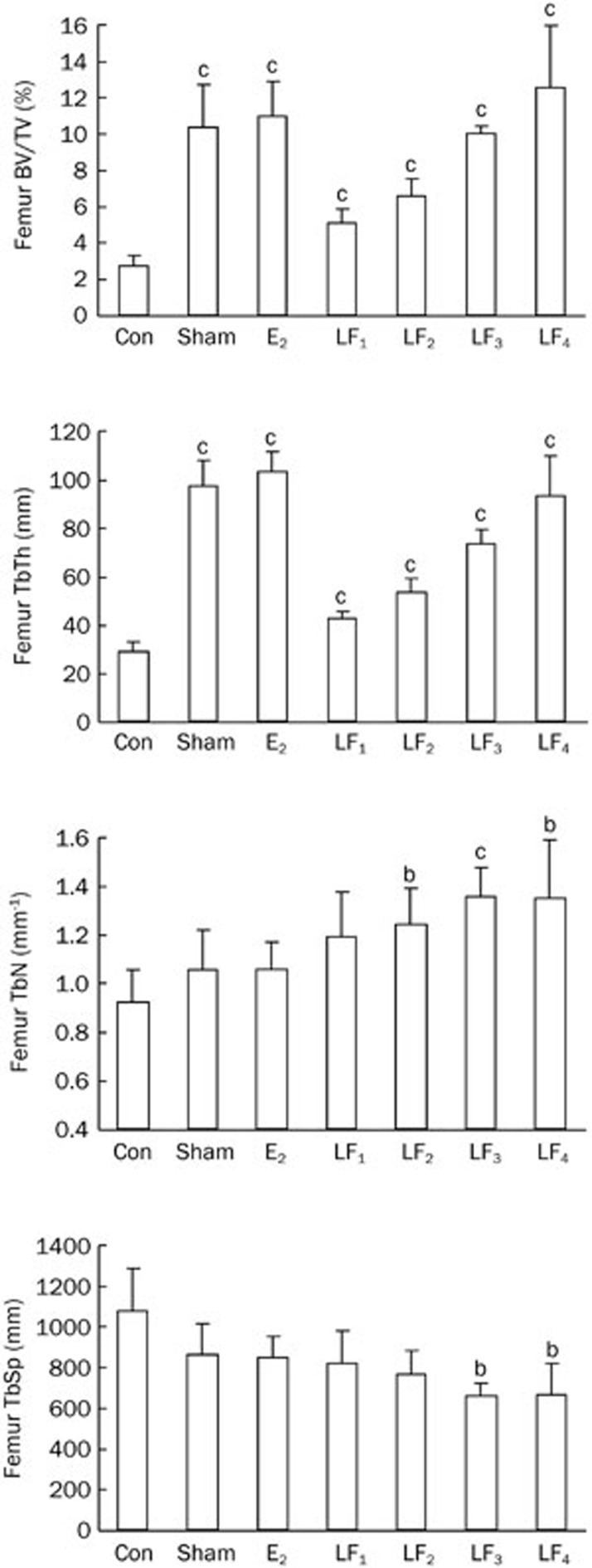
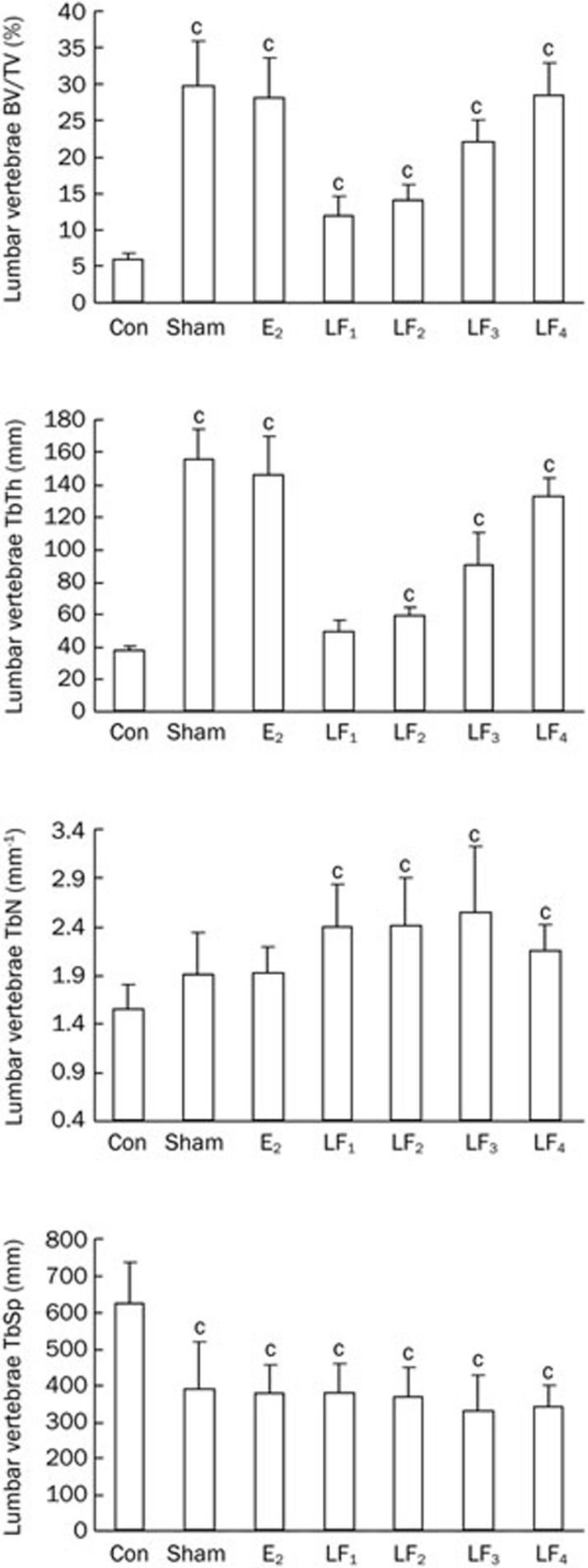
As shown in Figures 2 and 3, the ovariectomies significantly reduced BV/TV, TbTh, TbN values and increased the TbSp values in both femur and L2-4 vertebrae of the rats. Treatment with LF or estrogen protected the rats from the Ovx-induced effects on the above biomarker levels.
Figure 2.
The trabecular bone microstructural parameters of the femurs in the Con, Sham, E2, and LF groups at the end of the experiment. bP<0.05, cP<0.01 compared with the Con group. BV=bone volume; TV=tissue volume; TbTh=trabecular thickness; TbN=trabecular number; TbSp=trabecular separation.
Figure 3.
The trabecular bone microstructure parameters of the L2-4 vertebrae in the Con, Sham, E2, and LF groups at the end of the experiment. bP<0.05, cP<0.01 compared with Con group.
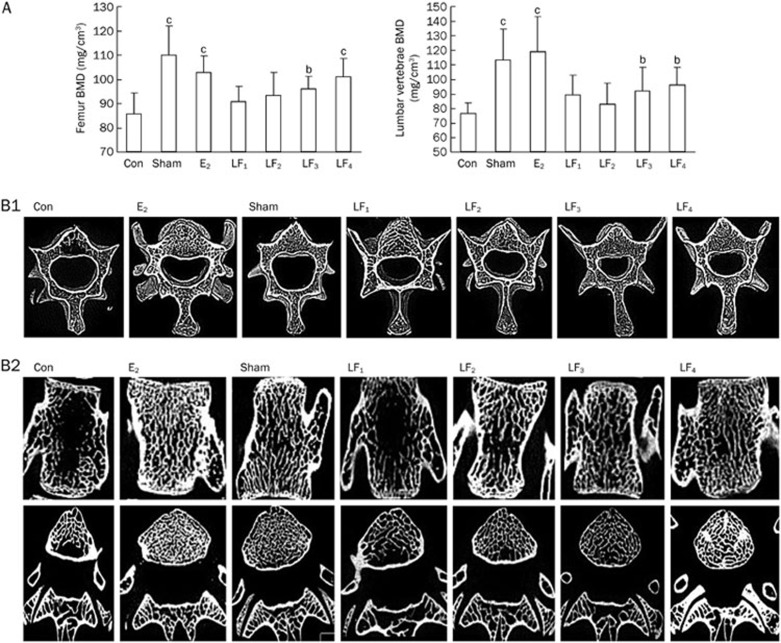
Bone mineral density (BMD)
At the end of the experiment, the Con rats had markedly reduced BMD in both their femurs and L2-4 vertebrae compared with the Sham and E2 groups (P<0.01). The BMD values in the rats treated with 1.0 g·kg−1·d−1 and 2.0 g·kg−1·d−1 LF were greater than those in the Ovx control (P<0.05 and P<0.01). However, the lower LF doses (10 mg·kg−1·d−1 and 100 mg·kg−1·d−1) did not significantly alter the BMD (P>0.05, Figure 4). The BMD differences among the groups followed a similar pattern for both L2-4 lumbar vertebrae and femur.
Figure 4.
(A) The BMD of the femurs and L2-4 vertebrae in the Con, Sham, E2, and LF groups at the end of the experiment. bP<0.05, cP<0.01 compared with Con group. (B1) The trabecular bone microstructure of the L3 vertebrae in the Con, Sham, E2, and LF groups at the end of the experiment. B1. A representative 3D reconstruction of a cross-section of the bone microstructure in the L3 vertebrae. (B2) Representative 2D images of a coronal section and a cross-section of the bone microstructure in the L3 vertebrae separately from the micro-CT scans. These pictures show that the Con group has severe osteopenia, main bone loss with trabecular thinning, and fewer areas of trabecular bone, which were highly disconnected and partly fractured. However, the LF groups display orderly trabecular arrangements, with more areas of trabecular bone and better connectivity.
Bone turnover markers
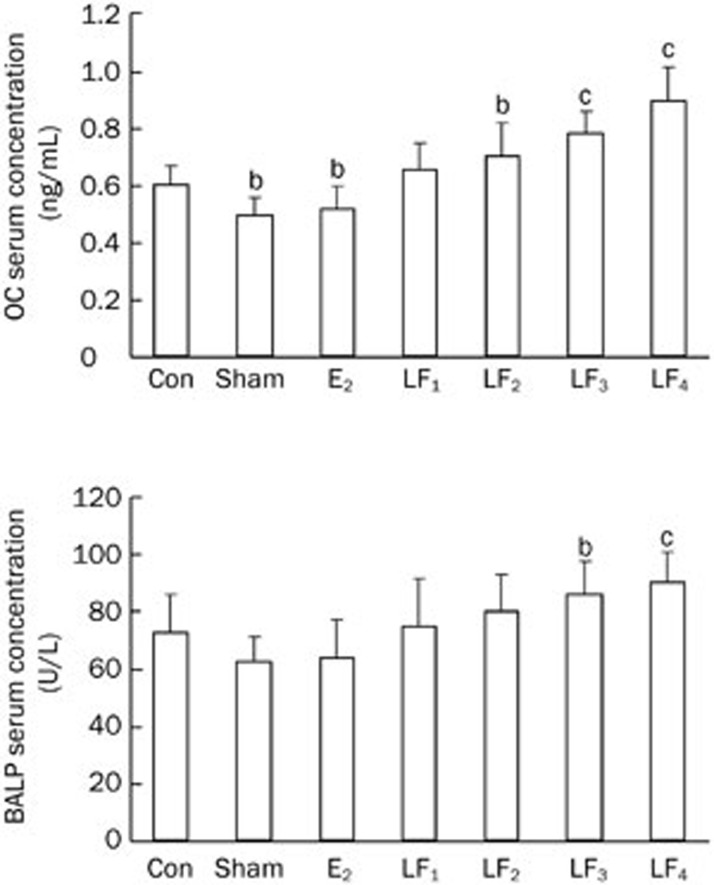
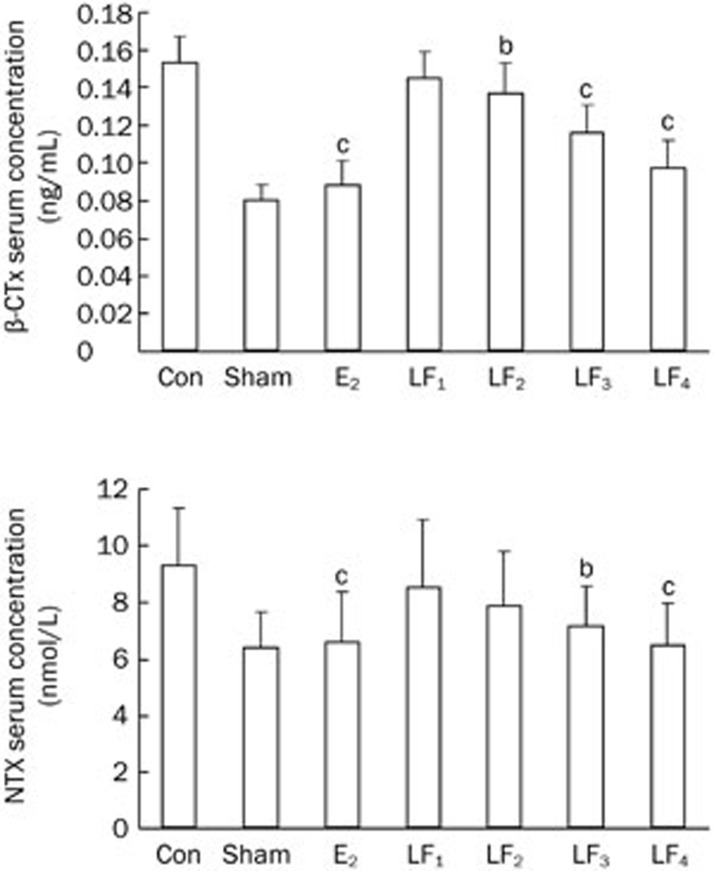
The serum levels of OC, BALP, β-CTx, and NTX in the Con group were higher compared with those in the Sham group (P<0.01), with the levels of β-CTx and NTX being noticeably higher than those of OC and BALP. The serum levels of OC, BALP, β-CTx, NTX increased by 17.9%, 14.0%, 47.7%, and 31.7%, respectively, in the Con group compared with the Sham. Although the serum levels of OC and BALP in the different LF groups were higher compared with the Con group, these increases were only statistically significant for LF3 (1 g·kg−1·d−1 LF treatment) and LF4 (2 g·kg−1·d−1 LF treatment) groups. Moreover, the serum levels of β-CTx and NTX were lower in the different LF groups compared with the Con group, and these decreases were significant for the LF3 and LF4 groups. The serum levels of OC, BALP, β-CTx, and NTX were similar between the E2 and Sham groups (P>0.05, Figures 5 and 6).
Figure 5.
The bone formation makers of serum in the Con, Sham, E2, and LF groups at the end of the experiment. bP<0.05, cP<0.01 compared with the Con group. OC=Osteocalcin; BALP=bone alkaline phosphatase.
Figure 6.
The bone absorption makers of serum in the Con, Sham, E2, and LF groups at the end of the experiment. bP<0.05, cP<0.01 compared with the Con group. β-CTx=β-CrossLaps; NTX=collagen type I N-telopeptide.
The local expression of RANKL and OPG genes in femur
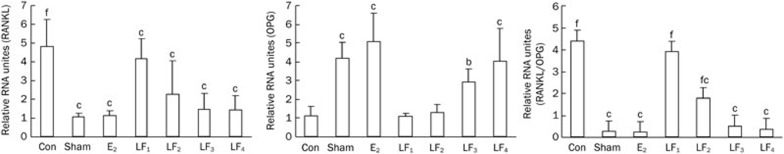
As shown in Figure 7, the local expression of the RANKL gene was higher in the Con group than in the Sham and E2 groups (P<0.01). The groups with 1 g·kg−1·d−1 and 2 g·kg−1·d−1 LF treatments had significantly lower RANKL mRNA levels in the proximal femur compared with the Con group (P<0.01 and P<0.05). However, the groups with 10 mg·kg−1·d−1 and 100 mg·kg−1·d−1 LF treatments did not show significant alterations of the RANKL mRNA levels (P>0.05). The local OPG mRNA expression increased in the Sham and E2 groups compared with the Con group (P<0.01). The groups with 1 g·kg−1·d−1 and 2 g·kg−1·d−1 LF treatment had significantly higher OPG levels compared with the Con group (P<0.01 and P<0.05); however, the two lower doses of LF did not show significant effects (P>0.05). The ratio of RANKL/OPG mRNA in the Con group was significantly higher than that in the Sham and E2 groups (P<0.01). Moreover, there was a significant decrease (P<0.01) in the RANKL/OPG mRNA ratio in the proximal femur following treatment with 100 mg·kg−1·d−1, 1 g·kg−1·d−1, and 2 g·kg−1·d−1 LF compared with the Con group; however, 10 mg·kg−1·d−1 LF treatment did not show any significant change (P>0.05) for the same.
Figure 7.
The effects of LF on the local expression of RANKL and OPG mRNA and the RANKL/OPG mRNA ratio in the bone trabecula of the proximal femur. The RANKL and OPG mRNA expression in the proximal femur was determined by qRT-PCR, and the RANKL/OPG mRNA ratio was calculated. Mean±SD. bP<0.05, cP<0.01 compared with the Con group; fP<0.01 compared with the E2 group..
Discussion
Previous studies have shown that lactoferrin has an anabolic effect on osteoblasts and osteoclasts in vitro8,9,11. The aim of this study was to evaluate the changes induced by lactoferrin in the Ovx rat model and to identify the pathways that mediate the anabolic action of lactoferrin in bone. We found that LF at concentrations of 1 g·kg−1·d−1 and 2 g·kg−1·d−1 significantly increased the bone mass and microarchitecture compared with the untreated Ovx rats that suffered from increasing bone loss, severe osteopenia and increased bone resorption. Compared with the untreated Ovx rats, the LF treatment resulted in increased bone formation in the femur and lumbar vertebrae, and these changes were associated with higher bone volume (BV/TV) and trabecular number (TbN). LF also improved the trabecular microarchitecture, which is consistent with the increase in bone mineral density (BMD). Moreover, LF seemed to partially restore trabecular connectivity by increasing trabecular thickness (TbTh) while reducing trabecular separation (TbSp), suggesting that LF could prevent the loss of bone mass after Ovx. These results were confirmed in a series of experiments on a mixed primary culture of murine bone cells. In our study, significant microarchitectural differences were observed between the rats given the LF treatment and the untreated Ovx controls. We need to pay attention to that what in the Guo's study, LF could increase bone mineral density at 8.5- and 85- mg/kg doses14. However, similar increases did not occur at the 10 mg·kg−1·d−1 and 100 mg·kg−1·d−1 LF doses that were used in our study. A possible explanation could be the differences in density and purity of different LF preparations in these studies. As a new anti-osteoporosis agent, LF has been reported to exert beneficial effects on osteoblast proliferation and differentiation as well as inhibit osteoclast activity in bone cell cultures9,10.
Bone mass depends on the balance between bone resorption and bone formation within a remodeling unit, and it varies with the number of remodeling units that are activated within a given period of time in a defined area of bone. Osteoblasts and osteoclasts are specialized cells responsible for bone formation and resorption, respectively. Biochemical markers of bone turnover are indicative of events occurring during the bone remodeling cycle. These biochemical markers are divided into two categories: markers of bone formation and markers of bone resorption. These markers reflect the metabolic conversion of bone, predict osteoporosis and indicate the risk of bone fractures. The levels of biochemical markers of bone turnover, both for formation and resorption, continue to increase with age following menopause23. In this study, all of the markers displayed this tendency. The serum levels of OC, BALP, β-CTx, and NTX in the Con group were increased after ovariectomy compared with the Sham group. In addition, β-CTx and NTX levels demonstrated stronger changes than OC and BALP levels, suggesting that at 6-months post-ovariectomy, there is enhanced bone resorption resulting in bone loss. However, LF prevented the Ovx-induced bone loss by increasing bone formation over bone resorption.
We also observed that treatment with 1 g·kg−1·d−1 and 2 g·kg−1·d−1 LF increased the local OPG gene expression in the proximal femur. These elevations in OPG can contribute to a reduced local RANKL/OPG ratio, suggesting that LF may decrease osteoclast differentiation.
Previous studies have shown that osteogenesis is determined by the duration of ovariectomy and diet, factors that regulate the expression of genes involved in bone formation. This regulation specifically allows bone loss to occur following ovariectomy24,25 until the end of the osteogenic process. Therefore, the ovariectomy procedure induced negative long-term regulation of bone formation results in the predominance of bone resorption over bone formation. An increase in bone resorption is a general characteristic of osteoporosis and is primarily regulated by the relative balance between RANKL and OPG levels. The LF treatment induced a bone formation marker expression pattern similar to that observed in the untreated ovariectomized rats. The LF induced a decline in RANKL gene expression in the trabecular bone of the Ovx rats, suggesting that LF prevents bone loss by reducing the expression of RANKL in osteoblasts. The LF treatment did not cause differentiation of the bone marrow stromal cells to an osteoblastic lineage in this ovariectomized rat model. According to previous studies3, however, the inhibition of bone resorption markers by oral LF suggests that LF may prevent Ovx-induced bone loss via a preferential anti-osteoclastogenic pathway.
RANKL has been shown to have a significant role in the generation, activity, and survival of osteoclasts26,27. OPG is an endogenous antagonist of RANKL and inhibits RANKL action by acting as a soluble decoy receptor, thus balancing bone turnover28. In bone, RANKL is expressed most abundantly in MSCs, osteoblasts and T cells29. Despite OPG being a known inhibitor of RANKL, its biological effect may depend on the molar ratio between RANKL and OPG30. In this study of ovariectomized rats, we demonstrated that although LF treatment seems to have an inhibitory effect on RANKL, it enhances OPG. The RANKL/OPG ratio may be a major mechanism for the increased bone formation and decreased bone resorption observed in the LF treated rats, and this finding suggests that LF decreases osteoclast differentiation.
Nevertheless, this study has several limitations worth discussing. First, the number of rats in this study was small, and there was much variability in the measurements; therefore, additional studies should be conducted to confirm these results. Second, we did not assess the biochemical markers of mineral homeostasis (estrogen, calcium, phosphate and parathyroid hormone); therefore, we cannot evaluate their impact in this study. Finally, this study did not include information on mechanical bone properties or histological data; thus, we cannot determine whether the routine of daily LF treatment impaired the osteoid mineralization rate or individual osteoblast function.
In conclusion, this study used an ovariectomy-induced osteoporosis rat model to show that orally administered LF preserves bone mass and bone microarchitecture and prevents bone loss in both the femoral and lumbar vertebrae. We also demonstrated that LF has dual effects on both osteoblasts and osteoclasts as well as on the OPG/RANKL/RANK pathway. In summary, LF may be a safe and effective treatment for estrogen-dependent bone loss, and it is possible that OPG/RANKL/RANK signaling may be involved in the LF-induced antiresorptive effect. Further investigations need be performed on the relevance of LF treatment strategy for postmenopausal bone loss in humans with implications on the anabolic effects of LF on bone metabolism.
Author contribution
Jian-ming HOU, Ying XUE, and Qing-ming LIN designed the research project; Jian-ming HOU supervised the project; Ying XUE performed the animal assays, serum assays, analyzed the data and wrote the manuscript; and Jian-ming HOU,Ying XUE, and Qing-ming LIN revised the manuscript.
Acknowledgments
This study was supported by Fujian Science & Technology Major Special Project (No 2010Y0015).
References
- Garnero P, Delmas PD, Bilezikian JP. Evaluation of risk for osteoporosis fractures. Principles of Bone Biology. 2002. pp. 1291–301.
- Naot D, Grey A, Reid IR, Cornish J. Lactoferrin — a novel bone growth factor. Clin Med Res. 2005;5:93–101. doi: 10.3121/cmr.3.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omi N, Ezawa I. The effect of ovariectomy on bone metabolism in rats. Bone. 1995;17:163S–168S. doi: 10.1016/8756-3282(95)00329-c. [DOI] [PubMed] [Google Scholar]
- Metz-Boutigue MH, Jollès J, Mazurier J, Schoentgen F, Legrand D, Spik G, et al. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem. 1984;145:659–76. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- Ward PP, Paz E, Conneely OM. Multifunctional roles of lactoferrin: a critical overview. Cell Mol Life Sci. 2005;62:2540–8. doi: 10.1007/s00018-005-5369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M, Wakabayashi H, Shin K, Yamauchi K, Yaeshima T, Iwatsuki K. Twenty-five years of research on bovine lactoferrin applications. Biochimie. 2009;91:52–7. doi: 10.1016/j.biochi.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Cornish J, Callon KE, Naot D, Palmano KP, Banovic T, Bava U, et al. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology. 2004;145:4366–74. doi: 10.1210/en.2003-1307. [DOI] [PubMed] [Google Scholar]
- Grey A, Banovic T, Zhu Q, Watson M, Callon K, Palmano K, et al. The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol Endocrinol. 2004;18:2268–78. doi: 10.1210/me.2003-0456. [DOI] [PubMed] [Google Scholar]
- Grey A, Zhu Q, Watson M, Callon K, Cornish J. Lactoferrin potently inhibits osteoblast apoptosis, via an LRP1-independent pathway. Mol Cell Endocrinol. 2006;251:96–102. doi: 10.1016/j.mce.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Lorget F, Clough J, Oliveira M, Daury MC, Sabokbar A, Offord E. Lactoferrin reduces in vitro osteoclast differentiation and resorbing activity. Biochem Biophys Res Commun. 2002;296:261–6. doi: 10.1016/s0006-291x(02)00849-5. [DOI] [PubMed] [Google Scholar]
- Takayama Y, Mizumachi K. Effect of lactoferrin-embedded collagen membrane on osteogenic differentiation of human osteoblast-like cells. J Biosci Bioeng. 2009;107:191–5. doi: 10.1016/j.jbiosc.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Naot D, Grey A, Reid IR, Cornish J. Lactoferrin: a novel bone growth factor. Clin Med Res. 2005;3:93–101. doi: 10.3121/cmr.3.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HY, Jiang L, Ibrahim SA, Zhang L, Zhang H, Zhang M, et al. Orally administered lactoferrin preserves bone mass and microarchitecture in ovariectomized rats. J Nutr. 2009;139:958–64. doi: 10.3945/jn.108.100586. [DOI] [PubMed] [Google Scholar]
- Bharadwaj S, Naidu AG, Betageri GV, Prasadarao NV, Naidu AS. Milk ribonuclease-enriched-lactoferrin induces positive effects on bone turnovermarkers in postmenopausal women. Osteoporos Int. 2009;20:1603–11. doi: 10.1007/s00198-009-0839-8. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Mitchner NA, Harris ST. Current and emerging therapies for osteoporosis. J Fam Pract. 2009;58:S45–9. [PubMed] [Google Scholar]
- Amizuka N, Shimomura J, Li M, Seki Y, Oda K, Henderson JE, et al. Defective bone remodelling in osteoprotegerin-deficien mice. J Electron Microsc (Tokyo) 2003;52:503–13. doi: 10.1093/jmicro/52.6.503. [DOI] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–24. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagos SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–11. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- Blais A, Malet A, Mikogami T, Martin-Rouas C, Tomé D. Oral bovine lactoferrin improves bone status of ovariectomized mice. Am J Physiol Endocrinol Metab. 2009;296:E1281–8. doi: 10.1152/ajpendo.90938.2008. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Melton LJ, 3rd, Khosla S, Atkinson EJ, O'Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res. 1997;12:1083–91. doi: 10.1359/jbmr.1997.12.7.1083. [DOI] [PubMed] [Google Scholar]
- Schroeder TM, Jensen ED, Westendorf JJ. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today. 2005;75:213–25. doi: 10.1002/bdrc.20043. [DOI] [PubMed] [Google Scholar]
- Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98–105. doi: 10.1016/j.abb.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Rauner M, Sipos W, Pietschmann P. Osteoimmunology. Int Arch Allergy Immunol. 2007;143:31–48. doi: 10.1159/000098223. [DOI] [PubMed] [Google Scholar]
- Sandberg WJ, Yndestad A, Øie E, Smith C, Ueland T, Ovchinnikova O, et al. Enhanced t-cell expression of rank ligand in acute coronary syndrome: possible role in plaque destabilization. Arterioscler Thromb Vasc Biol. 2006;26:857–63. doi: 10.1161/01.ATV.0000204334.48195.6a. [DOI] [PubMed] [Google Scholar]