Abstract
Aim:
Platinum-(IV)-derivative satraplatin represents a new generation of orally available anti-cancer drugs that are under development for the treatment of several cancers. Understanding the mechanisms of cell cycle modulation and apoptosis is necessary to define the mode of action of satraplatin. In this study, we investigate the ability of satraplatin to induce cell cycle perturbation, clonogenicity loss and apoptosis in colorectal cancer (CRC) cells.
Methods:
CRC cells were treated with satraplatin, and the effects of satraplatin on apoptosis and the cell cycle were evaluated by flow cytometry. Western blot analysis was used to investigate the effects of satraplatin on cell cycle and apoptosis-related proteins. RT-qPCR was used to evaluate p53-related mRNA modulation.
Results:
Satraplatin induced an accumulation of CRC cells predominantly in the G2/M phase. Increased p53 protein expression was observed in the p53 wild-type HCT116 and LoVo cells together with p21waf1/cip1 protein up-regulation. However, p21waf1/cip1 protein accumulation was not observed in the p53 mutant HCT15, HT29, and WiDr cells, even when p53 protein expression was compromised, suggesting that the cell cycle perturbation is p53-p21waf1/cip1 independent. Following a candidate approach, we found an elevated expression of 14-3-3σ protein levels in CRC cells, which was independent of the status of p53, further supporting the role of satraplatin in the perturbation of the G2/M cell cycle phase. Moreover, satraplatin treatment induced apoptosis along with Bcl-2 protein down-regulation and abrogated the clonogenic formation of CRC cells in vitro.
Conclusion:
Collectively, our data suggest that satraplatin induces apoptosis in CRC cells, which is preceded by cell cycle arrest at G2/M due to the effect of 14-3-3σ and in a p53-p21waf1/cip1–independent manner. Taken together, these findings highlight the potential use of satraplatin for CRC treatment.
Keywords: satraplatin, G2/M phase, 14-3-3σ, p53, apoptosis, colorectal cancer
Introduction
The formation of DNA adducts by platinum analogs is recognized to be the most prominent effects in cells, blocking replication and transcription and inducing tumor cell death1, 2. The nature of the DNA adducts formed by these analogs affects a number of transduction pathways and triggers apoptosis and necrosis3, thereby mediating cytotoxicity in these models. The most studied platinum analogs, cisplatin and oxaliplatin, have been used in the clinic for the treatment of various tumors4, yet the acquisition of drug resistance remains a major obstacle in cancer management. Among the numerous platinum analogs, satraplatin has been shown to counteract de novo resistance and acquired resistance in some resistant human tumors5, 6.
Satraplatin, also known as JM-216, is the first orally available active lipophilic platinum (Pt)-(IV) derivative that exhibits antiproliferative activity against various tumors5, 6. This drug is particularly attractive because of its convenience of administration, its milder toxicities (such as nephrotoxicity, neurotoxicity, and ototoxicity) and the lack of cross-resistance with cisplatin. Moreover, satraplatin demonstrates anti-cancer activity in tumors that are usually non-responsive to platinum drugs7.
Upon diverse genotoxic stresses, DNA damage and other cellular lesions usually activate cell cycle checkpoints that inhibit cell cycle progression by decreasing the activity of key regulatory proteins8, primarily including cyclin-dependent kinase (Cdk) complexes9, 10. After DNA damage signaling, the “p53 → p21waf1/cip1 pathway” is activated9, 10. Activated p53 induces the expression of its downstream targets, including p21waf1/cip1, which is a universal inhibitor of Cdks and is required to arrest cells at the G1 and G2 checkpoints11,12,13. The cells are then arrested at the G1-S or G2/M phase. We have previously reported cell cycle perturbation following satraplatin and oxaliplatin treatment in a panel of CRC cell lines that differ in their p53 gene status14. We observed that satraplatin induces cell cycle arrest at the G2/M phase and that oxaliplatin induces arrest at the G1 phase and results in apoptosis. On the basis of this observation, we proposed that satraplatin is a remarkably more potent activator of cell cycle and cell death proteins than oxaliplatin. We speculate that the perturbation of the G2/M cell cycle phase may be a prominent effect of satraplatin treatment in CRC cells. Additionally, we demonstrate that p53-null cells, which are arrested in the same manner as wild-type cells, subsequently induce apoptosis with satraplatin treatment but not with oxaliplatin treatment.
The aim of this study was to elucidate the effect of satraplatin on modulators of the G2/M cell cycle transition, apoptosis, and clonogenic formation. We show that satraplatin induces cell cycle arrest at G2/M at least in part because of the effect of 14-3-3σ and in a p53-p21waf1/cip1-independent manner. Moreover, satraplatin treatment affects DNA repair genes at the mRNA level. It also abrogated the clonogenic formation of CRC cells in vitro.
Materials and methods
Drug, chemicals, and reagents
Satraplatin was a kind gift from Agennix AG (formerly GPC Biotech AG), Fraunhoferstr, Germany. A satraplatin stock solution was prepared in 0.09% saline and stored at -20 °C. Antibodies were purchased from the following companies: anti-p53 (BD Pharmingen), anti-β-actin (Sigma), anti-Bax (Millipore), anti-Bcl-2 (Sigma), anti-p21waf1/cip1 and anti-cyclin B1 (Santa Cruz) antibodies. Anti-rabbit and anti-mouse secondary antibodies were purchased from DakoCytomation, Denmark A/S. ECL detection reagents were purchased from Perkin-Elmer.
Cell culture
The HCT116 human CRC cell lines (wt, p53−/−, and p21−/−), HT29, HCT15, WiDr, and LoVo were tested for mycoplasma infection according to UKCCCR guidelines15 and were cultured in monolayer in DMEM (HCT116, p53−/−, p21−/−, and HT29), DMEM:F12 (WiDr), RPMI-1640 (HCT15) and Ham's Nutrient mix F12 (LoVo) supplemented with 10%–20% FBS and 1% penicillin/streptomycin. No antibiotics were added prior to treatment. The cells were trypsinized and subcultured twice a week. All experiments were conducted at an early passage as previously described14.
BrdU cell proliferation assay
A BrdU (5-bromo-2′-deoxyuridine) incorporation assay (Calbiochem/Merck, Whitehouse Station, NJ, USA) was used to assess the antiproliferative effects of satraplatin according to the protocol of the manufacturer. Briefly, 10 000 cells/well were seeded in 96-well plates followed by satraplatin treatment of 0–50 μmol/L. After cells adhered to the plate BrdU was added (1:2000 dilution), and plates were incubated for different time intervals (24 h and 72 h). The plates were then washed, fixed with anti-BrdU antibody and a peroxidase goat anti-mouse IgG conjugate was added. Immunocomplex formation was measured using tetra-methylbenzidine solution, and the reaction was terminated with 2.5 mol/L sulfuric acid. The measured intensity was proportional to the amount of incorporated BrdU in the cells. Absorbance was measured at 450 nm using a Microplate Manager (Bio-Rad Laboratories, Inc) according to the protocol of the manufacturer. The absorbance results were expressed as relative to untreated control.
Cell cycle analysis and apoptosis
Cell cycle perturbations and the apoptotic fractions of untreated and treated cells following satraplatin treatment were defined by flow cytometry analysis of cells stained with propidium iodide as described previously14.
Western blot analysis
Aliquots of whole cell extracts containing 20 μg of protein were used for Western blot analysis as previously described14.
Clonogenicity assay
Approximately 300 cells were seeded into each well of a 6-well culture plate, which were treated and then incubated in 2 mL of medium for 10 d. Subsequently, the medium was removed, and the cells were fixed for 15 min in 0.5 mL of 0.5% glutaraldehyde. The fixing solution was removed, the wells were rinsed with PBS, the colonies were stained for 30 min in 0.5 mL of 1% (w/v) methylene blue solution in PBS, washed once again with water, and then photographed16.
Quantitative PCR
p53 signaling pathway qRT-PCR array
For the p53 Signaling Pathway PCR Array analysis, RNA from HCT116 (wt, p53−/−, and p21−/−) treated and untreated cells were used to analyze the fold change in expression. A total of 1 μg of total RNA was reverse transcribed, and the cDNA was analyzed on a human p53 signaling pathway RT2 Profiler PCR Array (SABioscience, Fredrick, MD, USA) using the Stratagene Real-Time PCR System (Mx3005). The human p53 signaling pathway RT2 Profiler PCR Array profiles the expression of 84 genes that are known to be involved in p53 signaling pathway regulation. A complete list of the genes assayed by the array can be viewed at http://www.sabiosciences.com/rt_pcr_product/HTML/PAHS-027A.html. PCR amplification was performed with an initial 10-min step at 95 °C, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The SYBR green fluorescent signal was detected immediately after the extension step of each cycle, and the cycle at which the product was first detectable was recorded as the cycle threshold. The data were analyzed using the comparative cycle threshold method with normalization of the raw data to the normalizer on the array. The results are presented as n-fold changes versus the values in the untreated control cells.
mRNA isolation, cDNA synthesis and qRT-PCR for the expression of DNA damage-related genes
Total RNA was isolated with TRIzol reagent (Invitrogen, CA, USA). The total RNA integrity was determined by 1% agarose gel electrophoresis. cDNA synthesis was performed with the Superscript III cDNA synthesis kit (Invitrogen, CA, USA) using 1 μg of total RNA as the template according to the protocol of the manufacturer. The PCR amplification was performed in a final volume of 25 μL containing 12.5 μL of SYBR green (2×), 0.5 μL of primers that detect MLH1, MSH2, PMS2 and ERCC1 as previously reported14 (10 μmol/L) and 1 μL of cDNA. The cycling conditions included preheating at 95 °C for 10 min and then 40 cycles of the following: 95 °C for 30 s, 60 °C for 30 s and 72 °C for 45 s followed by a standard dissociation curve. Values that fell below 35 cycles were considered for the 2−ΔΔCq determination.
Statistical analysis
The gene expression of ΔCq values from each sample were calculated by normalizing to ACTB and B2M, and the relative quantitation values were calculated according to the 2−ΔΔCq method. Statistical analyses were performed using the SPSS statistical software package, version 12.0 (Chicago, IL, USA). Statistically significant differences in the expression level between the control and treated samples for each target were calculated using a paired Wilcoxon test. A comparison of the mean values of proliferation and the apoptosis levels in response to satraplatin and oxaliplatin treatment was performed using the Bonferroni post hoc multiple comparisons ANOVA test. bP≤0.05 value was considered significant, and cP≤0.01 value was considered highly significant.
Results
Anti-proliferative effects of satraplatin on CRC cells
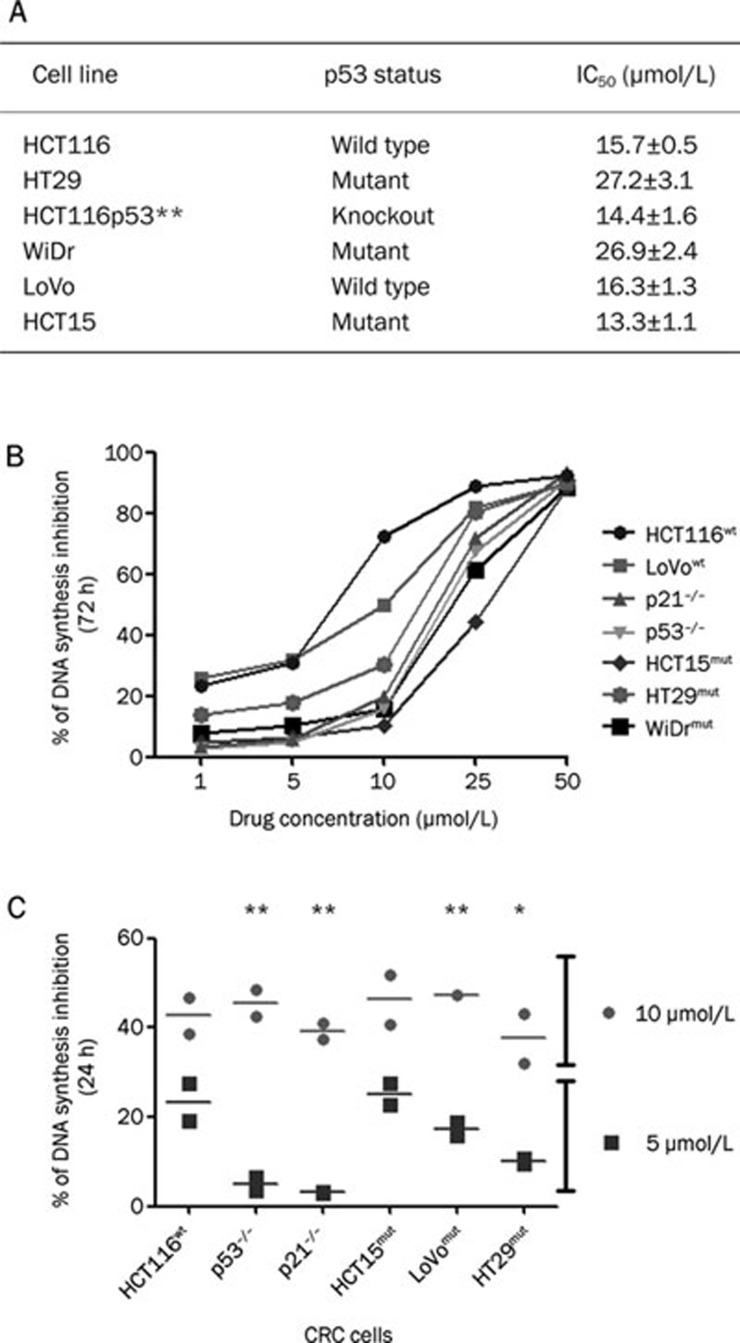
The role of p53-p21waf1/cip1 in the G2/M cell cycle phase following satraplatin treatment was investigated in a panel of CRC cell lines that differ in their p53 genotype (HCT116wt, LoVowt, HT29mut, HCT15mut, and WiDrmut) and isogenic HCT116 cells that were p53−/− and p21−/−. To assess the dose limiting effects of satraplatin on the rate of DNA synthesis, we performed a BrdU incorporation assay following satraplatin treatment for 24 and 72 h. As shown in Figure 1, we found that cells responded to satraplatin (when added below its IC50 value) in a dose- and time-dependent manner (Figure 1A). At a higher concentration (50 μmol/L), satraplatin decreased the proportion of S phase cells by about 90% in all seven cell lines tested (Figure 1B). Consistent with our previous findings on the effects of satraplatin on cell proliferation and apoptosis14, no evidence of cytotoxicity was observed when cells were exposed to lower drug doses (ie, ≤50 μmol/L). We also tested two drug concentrations, 5 and 10 μmol/L, that are lower than previously determined IC50 concentrations (Figure 1A)14. As expected, treatment with 10 μmol/L, but not 5 μmol/L, satraplatin led to 36%–47% S phase inhibition as early as 24 h (Figure 1C). For example, 10 μmol/L at 72 h demonstrated that the wildtype p53 cells (HCT116 and LoVo) were more prone to S phase inhibition (61.06%±6.61%) than the mutant p53 HCT15, HT29, and WiDR cells (19.00%±4.02%, P<0.01). In addition, when comparing the 24 h vs the 72 h analysis, we noticed that all cells were more prone to S phase inhibition after as early as 24 h of treatment (43.08%±1.6%) but later returned to normal cell cycle progression, at least in mutant p53 cells but not in p53 wild-type cells. Thus, the BrdU incorporation assay confirmed the partial accumulation of S phase cells before cells exhibited a G2/M phase perturbation.
Figure 1.
The IC50 value and the effect of satraplatin on CRC cells. (A) Table showing the IC50 value of satraplatin in a panel of CRC cell lines. (B) HCT116 (wt, p53−/−, and p21−/−), LoVowt, HT29mut, HCT15mut, and WiDrmut cell lines were treated with 0–50 μmol/L satraplatin, and the effect of satraplatin on DNA synthesis inhibition was determined by a BrdU incorporation assay at 24 and 72 h. At higher concentrations, all cells demonstrated about 90% inhibition, which indicates the optimal activity of satraplatin in CRC cells. (C) DNA synthesis was inhibited at least 50% by treatment with 10 μmol/L satraplatin, whereas a different pattern of inhibition was observed with 5 μmol/L satraplatin. The percentage of DNA synthesis inhibition was defined as the optimal density at a given drug concentration divided by the optimal density for untreated control cells multiplied by 100. Each point is the average of two independent trials; data are presented as the mean±standard deviation.
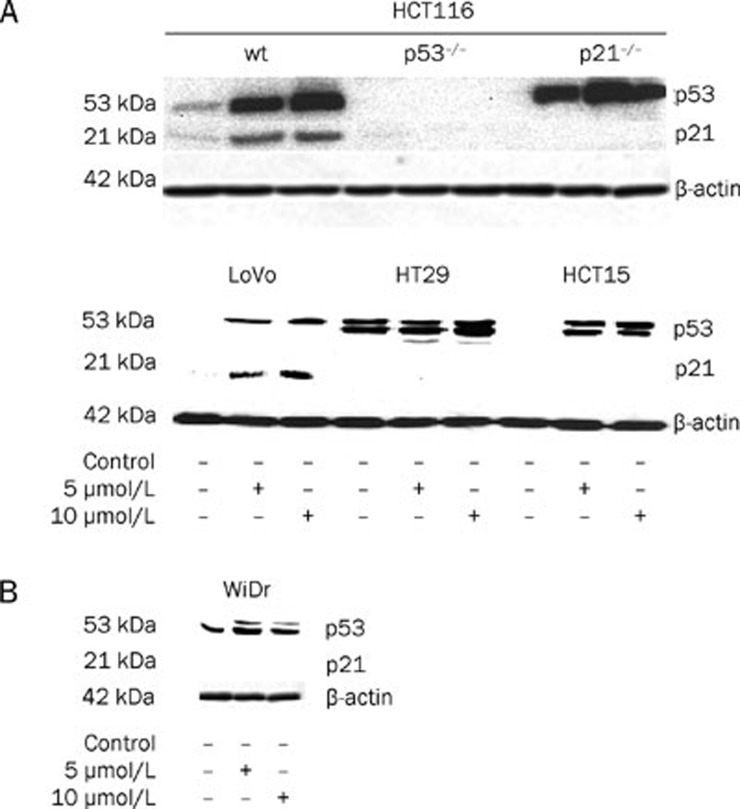
Western blot analysis of proteins from HCT116wt and LoVowt cells treated with 5 and 10 μmol/L satraplatin demonstrated a sustained increased level of p53 and p21waf1/cip1 protein, particularly at 10 μmol/L (Figure 2A). In contrast, despite an elevated p53 protein level in HCT15mut and HT29mut cells, no expression of p21waf1/cip1 was detected (Figure 2A, lower panel). To further confirm these data, we used the WiDr mutant cell line (Figure 2B) and showed no evident of p21waf1/cip1 accumulation. Furthermore, when p53−/− cells were treated with satraplatin, no evidence of p21waf1/cip1 accumulation was observed (Figure 2A, upper panel), which indicates that p21waf1/cip1 expression requires a p53-dependent mechanism. Moreover, an accumulation of p53 was observed in p21−/− HCT116 cells treated at both concentrations (Figure 2), indicating that p53-dependent expression of p21waf1/cip1 has no or only a modest effect on cell cycle perturbation.
Figure 2.
The effect of satraplatin on a clinically relevant concentration below its IC50 value on p53-p21waf1/cip1 protein expression at 48 h. Whole cell extracts were prepared from HCT116 (wt, p53−/−, and p21−/−), HCT15mut, HT29mut, and WiDrmut cells treated with 5 and 10 μmol/L satraplatin for 48 h and subjected to Western blot analysis for the presence of p53 and p21waf1/cip1 protein accumulation.
Satraplatin treatment induces G2/M perturbation
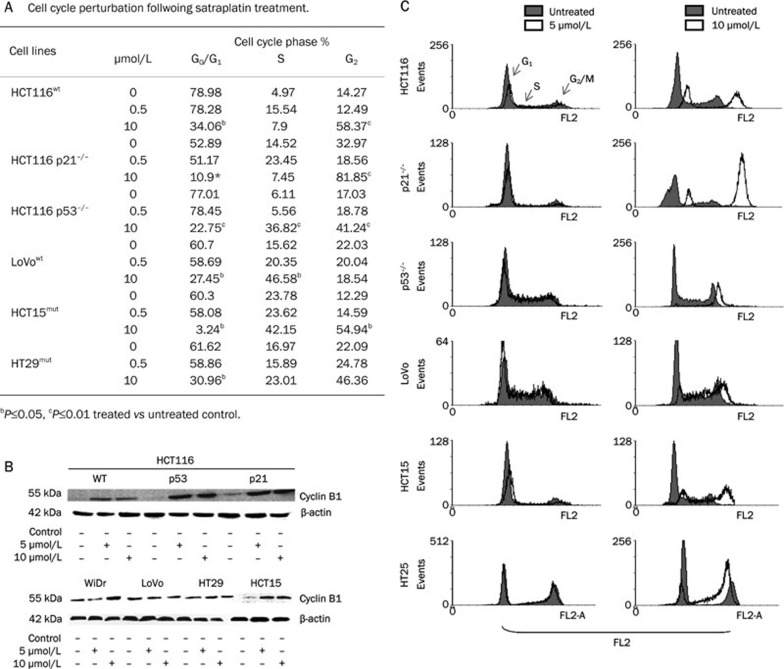
Consistent with our previous study14, we found that CRC cells treated with different satraplatin concentrations were past the initial entry into G2 prior to DNA damage and were subsequently arrested (Figure 3A and Supplementary Table 1). The expression of cyclin B1, a marker of the G2/M phase, was up-regulated following 5 and 10 μmol/L satraplatin treatment (Figure 3B). Cellular growth inhibition and cell cycle perturbation induced by satraplatin was evident at 10 μmol/L (Figure 3C, right panel) compared with 0.5 μmol/L (Figure 3C, left panel). Similar perturbations were observed in all six CRC cell lines when the cells were treated with low and high satraplatin concentrations, and this was independent of their p53 gene status (Figure 3C).
Figure 3.
The effect of satraplatin-induced cell cycle perturbation at the G2/M phase, apoptosis and cyclin-B1 expression in CRC cells at 48 h. (A) Table showing an accumulation of cells at each phase of the cell cycle following satraplatin treatment at lower and higher concentrations. (B) Cyclin B1 protein expression levels following treatment with 5 and 10 μmol/L satraplatin in CRC cells harvested at 48 h. The results shown are representative western blots of at least two independent experiments. Equal loading was confirmed by β-actin detection. (C) The effects of various satraplatin concentrations on DNA synthesis and cell cycle progression in CRC cells. Typical cytograms are presented that represent the overlaps of the percentage of cells in each phase (G0/G1, S, and G2/M) compared with untreated (control) cells (red background: untreated control cells; white background: treated cells) as determined by flow cytometry. This analysis indicates that the cells were substantially past the initial entry to G2 after DNA damage and were subsequently arrested.
Satraplatin increases 14-3-3σ expression in CRC cells
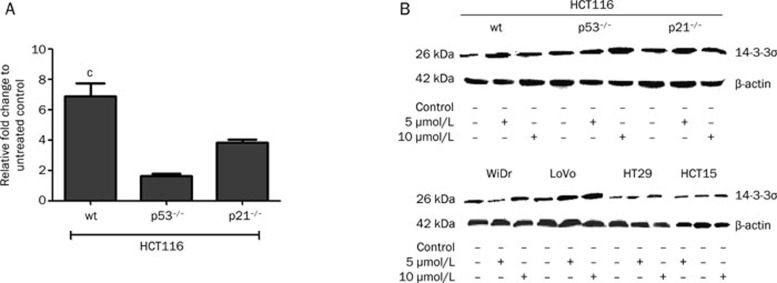
The results described above demonstrate that the presence of p21waf1/cip1 and/or p53 following satraplatin treatment played a minor role in inducing cell cycle perturbation and apoptosis. To determine which genes significantly alter the cell cycle profile at the G2/M phase, we used a candidate approach and found that 14-3-3σ mRNA and protein expression were elevated in CRC cells treated for 24 h (mRNA) and 48 h (protein) (Figure 4). We found a significant increase in 14-3-3σ mRNA expression in the wild-type p53 HCT116 cells but no significant increase in the p53−/− and p21−/− cells. At 48 h, elevated 14-3-3σ protein expression was detected after 5 and 10 μmol/L satraplatin treatment. We also observed that p53-null cells demonstrated an increase in 14-3-3σ that was independent of p53 action, particularly after treatment with 10 μmol/L satraplatin. Moreover, a similar protein expression pattern was observed in other CRC cell lines (Figure 4B, lower panel).
Figure 4.
mRNA and 14-3-3σ protein levels following satraplatin treatment. (A) 14-3-3σ mRNA expression is markedly increased in HCT116 cells compared with isogenic p53−/− and p21−/− cells. Fold changes in this experiment are based on normalizing ACTB and B2M to untreated control cells. The results shown are representative of at least two independent experiments at 24 h. (B) 14-3-3σ protein levels indicated that the cells were arrested at the G2/M phase. A marked increase in 14-3-3σ levels was also detected in p53−/− cells, indicating an activation of cell cycle arrest in a p53-idependent manner at 48 h. The results shown are representative of at least two independent experiments. Equal loading was confirmed by β-actin detection. cP<0.01.
Satraplatin induces apoptosis in CRC cells
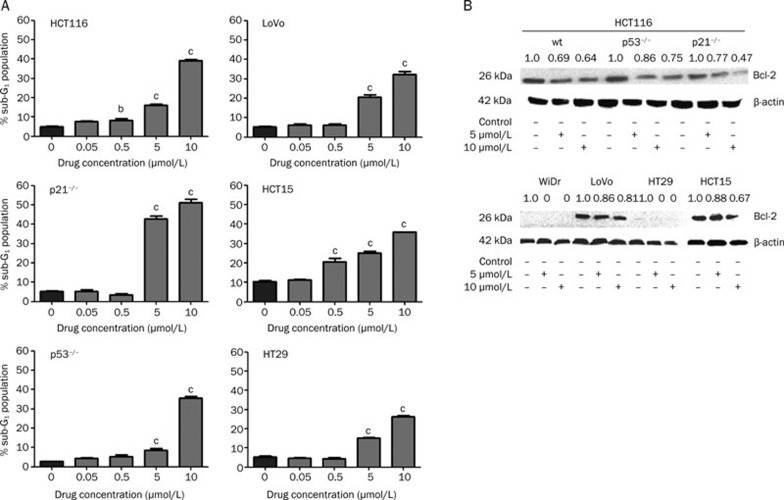
The cells that were G2/M arrested also underwent apoptosis in a dose-dependent and a p53-independent manner (Figure 2 for p53 protein expression, Figure 5A for apoptosis by flow cytometry). A higher percentage of apoptotic cells was observed at 5 and 10 μmol/L (P<0.01) than at the lower concentrations of 50 and 500 nmol/L (Figure 5A). In particular, we found that the p53−/− and p21−/− cells showed a significant down-regulation of Bcl-2 protein expression (Figure 5B) after satraplatin treatment. In HT29mut and WiDrmut cells, no Bcl-2 protein accumulation was detected (Figure 5B, upper panel), whereas significant down-regulation of Bcl-2 protein expression was observed in HCT15mut and LoVowt cells at 10 μmol/L (Figure 5B, lower panel).
Figure 5.
Dose dependent of Bcl-2 protein expression levels during satraplatin sustained treatment in CRC cells. A) Following treatment with different satraplatin concentrations, the CRC cell apoptotic fractions were measured by FACScan analysis. The bar indicates that a greater accumulation of apoptotic cells was observed with a clinically relevant satraplatin concentration vs the untreated control cells. Data points indicate the mean percentages of apoptosis fractions from three independent experiments; bars, standard deviation. B) The cellular extracts were subjected to Western blot. Satraplatin treatment resulted in markedly decreased Bcl-2 protein expression. The results shown are representative of at least two independent experiments. Equal loading was confirmed by β-actin detection. cP<0.01.
In HCT116wt cells, 5 and 10 μmol/L satraplatin induced apoptosis by at least 16% (P≤0.05) and 40% (P≤0.01), respectively, versus only 5% in the untreated control. In Lovowt cells, we observed 21% and 34% apoptotic fractions at 5 and 10 μmol/L satraplatin, respectively, versus 4.7% in the untreated control (P≤0.01). Moreover, in HT29mut cells, the same concentrations affected 15% and 27%, respectively, versus only 4% in the untreated control (P≤0.01). Finally, HCT15mut cells exhibited 26% and 36% apoptosis at 5 and 10 μmol/L satraplatin, respectively, whereas untreated cells showed just 10% (P≤0.01) apoptosis. We also found that p53−/− cells responded in the same manner following 5 and 10 μmol/L satraplatin treatment, with 9% and 34% (P≤0.01) apoptosis, respectively, versus only 3% in the untreated control. However, in p21−/− knockout cells, the percentage of apoptotic cells was 44% and 53% at 5 and 10 μmol/L satraplatin, respectively, versus only 5% in the untreated control (P≤0.01). We also observed that the cells responded in a dose- and time-dependent manner.
Satraplatin abrogated clonogenic formation in CRC cells
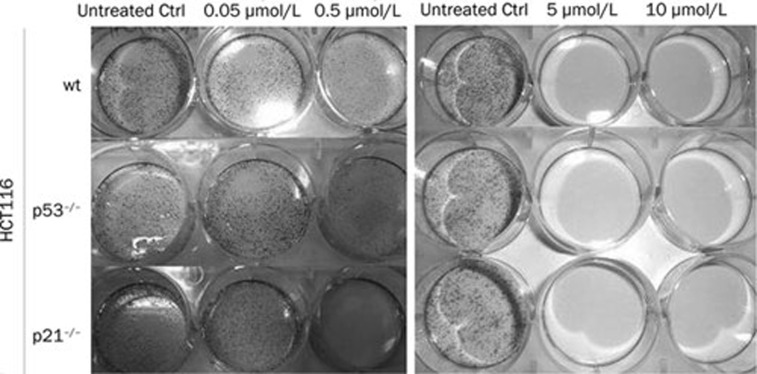
We also found that CRC cells, particularly HCT116 (wt, p53−/−, and p21−/−) (Figure 6, right panel), loss the ability to form colonies when treated with 5 or 10 μmol/L satraplatin as compared with 50 nmol/L treated cells (left panel). However, a partial clonogenicity loss was observed in all CRC cells when the cells were treated with 500 nmol/L satraplatin. This was also observed in the other CRC cell lines, including HCT15mut, LoVomut, WiDrmut, and HT29mut (data not shown).
Figure 6.
Clonogenicity loss following satraplatin induced DNA damage. Untreated- and satraplatin-treated cells were cultured in medium for two weeks and then fixed and stained with methylene blue for the clonogenicity assay. The results demonstrate that long-term satraplatin incubation leads to a loss of CRC cell clonogenicity potential.
G2/M cell cycle arrest and apoptosis occurs in a p53-independent manner
To further elucidate the ability of satraplatin to perturb the G2/M phase and subsequently apoptosis, we used a p53 signaling pathway qRT-PCR array containing 86 human p53 related genes. We compared the gene expression profiles of HCT116 (wt, p53−/−, and p21−/−) cells treated with 5 μmol/L satraplatin (Supplementary Table 2). We found that the cells responded independent of p53 activation and its related molecules. The up-regulation of Bax, BTG2, CDKN1A, FASLG, and MDM2 genes was observed in HCT116wt cells but not in p53−/− and p21−/− cells. Moreover, we found that genes involved in DNA damage responses were also down-regulated together with the transcription factor NFKB1 (Supplementary Table 2).
Down-regulation of DNA damage repair genes following satraplatin induced apoptotic cells
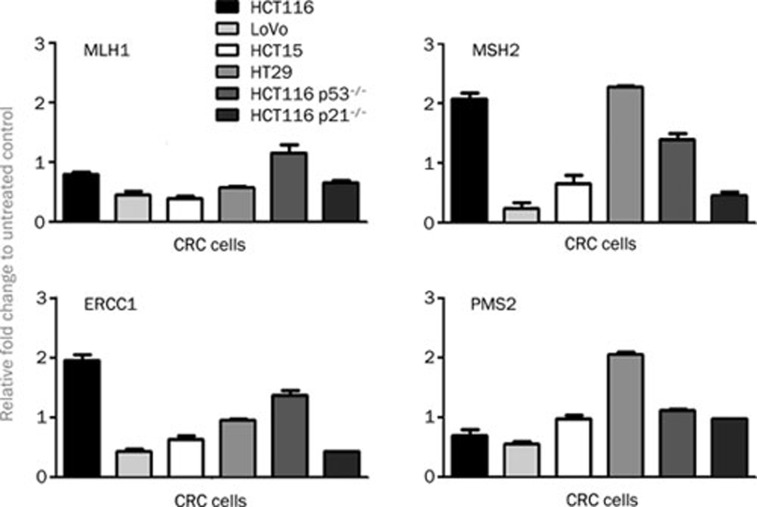
To examine DNA damage repair gene activation, CRC cells were treated for 24 h with 5 μmol/L satraplatin and were tested for differential expression of mRNA encoding MLH1, MSH2, ERCC1, and PMS2. Interestingly, we found that all cells similarly responded to satraplatin treatment, with most of the DNA damage repair genes being down regulated, except for MSH1 and ERCC1 in HCT116wt cells (Figure 7). We also found that, based on our human p53 signaling pathway RT2 Profiler PCR data, ATM and ATR expression was almost unchanged by 5 μmol/L satraplatin treatment in HCT116 (wt, p53−/−, and p21−/−) cells, whereas a 0.5-fold down-regulation was observed for E2F1 in HCT116wt cells versus an up-regulation of 2.1-fold in the HCT116 p53−/− cells. Moreover, we found that XRCC5 expression was down-regulated in HCT116 (wt, p53−/−, and p21−/−) cells (Supplementary Table 2).
Figure 7.
mRNA expression of the DNA repair enzymes ERCC1, MLH1, PMS2, and MSH2 in the CRC cell lines following 48 h satraplatin treatment. The CRC cells demonstrated unrepaired DNA damage owing to a down-regulation of the expression of DNA damage responsive genes in all cell lines tested. Only the expression of MSH1 and ERCC1 was up-regulated in the HCT116 wild-type cells. The fold change is based on the normalization of ACTB and B2M proteins in the treated vs the untreated control cells. The results shown are representative of at least two independent experiments.
Discussion
Satraplatin, which was initially proven to have clinical activity against small-cell lung17, ovarian18, and prostate cancers19, was developed for the treatment of castration-resistant prostate cancer and was found to significantly prolong disease progression-free survival20. Satraplatin disrupts DNA replication and transcription by forming intrastrand/interstrand DNA adducts; however, the downstream molecular events leading to G2/M cell cycle perturbation and cell death have not been well characterized in CRC cells.
Here, we show that exposure of CRC cells to satraplatin reduced long-term clonogenic survival and induced cell cycle arrest and subsequently apoptosis. Our previous model suggested that the possible reasons for differences between oxaliplatin- and satraplatin-induced antitumor effectiveness might be found at the level of cell cycle regulation14. Many studies have indicated that platinum agents predominantly reduce the DNA synthesis rate4 and inhibit cell cycle progression at the G1-S and/or G2/M phases21. Using a BrdU incorporation assay, we found that the treatment of CRC cells with different satraplatin concentrations delayed the progression of cells in S phase and altered the G2/M transition. Different drug concentrations perturbed the cells differently, particularly when cells were treated with lower drug concentrations. This finding could result from differences in cell characteristics that lead to different responses to treatment and the abilities of the drug to interact with its cellular target. More interestingly, the progression into mitosis was blocked. In addition, cells that were null for p53 and p21waf1/cip1 also had a delayed cell cycle progression and G2/M perturbation.
Since we observed a massive G2/M phase arrest, we focused our analysis on p53–p21 involvement in the G2/M transition as p53–p21 being a major regulator of the cell cycle. We found that satraplatin induced a significant accumulation of p53 protein in wild-type HCT116 and LoVo cells. In contrast, an accumulation of mutant p53 was also observed by Western blotting in HCT15 and WiDr cells. Bunz et al have reported the role of p53 at the G2 checkpoint by inactivating the p53 gene with homologous recombination in a derivative of a human colorectal tumor model12. They demonstrated that few cells entered mitosis initially after exposure to ionizing radiation, which suggests that the G2 checkpoint is intact. Therefore, they proposed that p53 is not required for the initial arrest but is essential for long-term arrest maintenance. However, it is possible that p53 can partially contribute to the initial arrest, and it is clear that other pathways can cause arrest when p53 is missing12.
We detected a marked increase in cyclin B1, a marker for G2/M cell arrest, in all CRC cell lines tested, and this was independent of the p53 gene status. Our data suggest that p53-independent pathways could inhibit cdc2 activity in response to DNA damage. In relation to this hypothesis and by following a candidate approach, we found that satraplatin increased the expression of 14-3-3σ, a conserved phosphoserine binding protein in CRC cells. We noticed a similar alteration even in p53-null and mutant cells, which further delineates the action of this protein in a p53-independent manner. We believed that 14-3-3σ may become a key regulatory modulator in the G2/M checkpoint that may form a cytoplasmic complex with cdc2 and sequester cdc2/cyclin B1 in the cytoplasm. Hermeking et al have shown that 14-3-3σ overexpression in HCT116 CRC cells causes most cells to arrest in the G2 phase22. Thus, we assumed that satraplatin-induced 14-3-3σ contributes to the G2/M accumulation. We also observed that, following satraplatin-induced cell cycle perturbation, the cells eventually undergo apoptosis in a dose- and time-dependent manner. We have reported that satraplatin treatment induces the accumulation of apoptotic cells even in the p53-null model, thus overcoming chemoresistance by a p53-independent mechanism14. Generally, the protection of cells against apoptosis requires the down-regulation of proapoptotic activities or the up-regulation of anti-apoptotic mechanisms23. The Bcl-2 family is an important regulator of cellular apoptosis24. Thus, we investigated Bcl-2 involvement in the mitochondrial (intrinsic) pathway to determine whether the Bcl-2 family was functioning as upstream initiators of apoptosis. Notably, the satraplatin effects on Bcl-2 expression in CRC cells (HCT15mut, LoVowt, and WiDrmut and p21−/−) were consistent with our previous findings in HCT116 (wt and p53−/−) and HT29mut cells14. However, these effects did not only affect Bcl-2-dependent apoptosis because the mutant HT29 and Lovo cells significantly underwent apoptosis, which further suggests that CRC cells could also undergo Bcl-2-independent apoptosis following satraplatin treatment. In addition to the Bcl-2-apoptosis analysis, we also demonstrated with the long-term clonogenicity survival analysis that the cells lose the ability to form colonies. Remarkably, we found that all cells, particularly the p53−/− and p21−/− cells, lost their clonogenicity potential in a dose-dependent manner. Finally, satraplatin mediated apoptotic activity is believed to be linked to DNA damage, and the levels of DNA damage repair genes have been shown to be correlated with the response to the platinum agent4. We hypothesized that the DNA damage inflicted by satraplatin may be repaired by the nucleotide excision repair (NER) and/or the mismatch repair (MMR) pathways with kinetics similar to that of the DNA damage repair that is induced by cisplatin and oxaliplatin25, 26. Accordingly, we showed that the mRNA expression levels of ERCC1, MLH1, MSH2, and PMS2 were significantly down-regulated in CRC cells. In addition to these results, Vaisman et al and Fokkema et al have suggested that satraplatin-induced adducts, compared with adducts formed by other platinum drugs, do not bind to high mobility group 1 protein, which recognizes the DNA damage caused by cisplatin and inhibits translation replication by certain DNA polymerases. These differences may provide a mechanism by which some platinum resistance may be overcome by satraplatin treatment in this model27, 28.
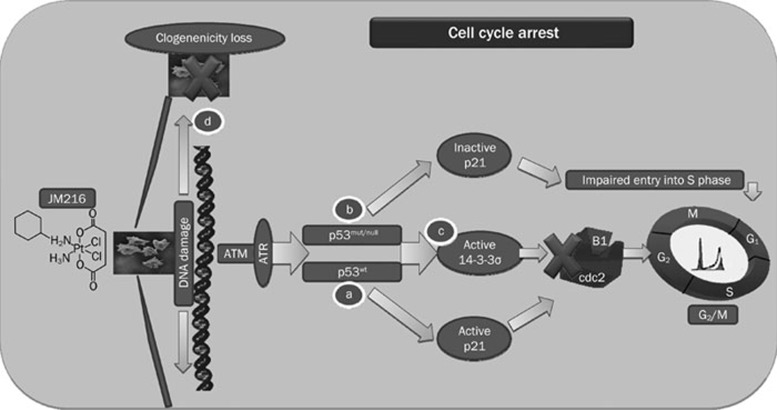
Thus far, in vitro and in vivo data have indicated satraplatin efficacy in the treatment of various cancer models7, 14. However, the ability of satraplatin to induce G2/M cell cycle arrest and subsequently apoptosis in CRC cells suggests that it may have a wide range of clinical applications. In Figure 8, we illustrated the molecular mechanism of satraplatin action on cell cycle perturbation. In summary, we hypothesize that satraplatin-induced cell cycle arrest is likely executed via 14-3-3σ in a p53-p21waf1/cip1-independent manner (Figure 8). Furthermore, cell death may also be related to the reduced expression of DNA repair enzymes.
Figure 8.
A flow chart depicting how 14-3-3σ induces cell cycle arrest at G2/M, which may not rely on p21waf1/cip1 and p53-dependent pathway in CRC cell models following satraplatin treatment. (A) Upon DNA damage caused by satraplatin treatment, we assumed that the ATM and ATR genes are activated, which induces the downstream signaling of cell cycle and cell death modulators. This results in p53 accumulation, which induces the expression of its primary cell cycle modulator, p21waf1/cip1, an inhibitor of Cdks, and its activation sequesters cyclin B1/cdc2 complexes, which can lead to an accumulation of cells at the G2/M transition. Alternatively, p53 could also be activated through 14-3-3σ (a conserved phosphoserine binding protein) to induce an accumulation of cells at G2/M by sequestering the cyclin B1/cdc2 complexes. (B) In cells that lack p53 or contain mutant p53, we observed that there was also a G2/M perturbation that was independent of p21waf1/cip1 activation because we first identified impaired S phase entry and then identified arrest at G2/M. (C) These cells might also activate 14-3-3σ independently of p53 action because p53mut and p53null cells demonstrated an elevated expression of 14-3-3σ and subsequently arrested at G2/M. (D) Loss of clonogenicity patterns were observed following satraplatin induced DNA damage, which led to cell mortality.
In conclusion, the ability of satraplatin to induce different modes of cell cycle regulation and cell death in different types of CRC cells at relatively low concentrations indicates that it may be highly efficacious in killing tumor cells and warrants further in vivo clinical investigation.
Author contribution
Study design and concept, data collection, analysis, interpretation and article preparation: Murugan KALIMUTHO; technical assistance, data collection and interpretation: Antonella MINUTOLO and Sandro GRELLI; grant approval and supervision: Giorgio FEDERICI and Sergio BERNARDINI.
Acknowledgments
This work was supported by grants from the University Hospital Tor Vergata and the Department of Internal Medicine, University of Rome, “Tor Vergata”. Murugan KALIMUTHO was supported by the Pre-doctoral Scholarship for foreign students under the International Italian Government University scholarship. We thank to Dr Isabella FARAONI, University of Rome Tor Vergata, Dr Soddu and Dr Maurizio FRANCULLI from the Regina Elena Cancer Institute, Rome, Italy, Dr Rossana SUPINO from the Instituto Nazionale Tumori, Milan, Italy and Prof Bert VOGELSTEIN from the Ludwig Center at John Hopkins, USA for providing us with the cell lines used in analysis. We also thank Agennix for providing us with satraplatin.
Footnotes
Supplementary tables are available at Acta Pharmacologica Sinica website of NPG.
Supplementary Information
Cell cycle perturbation folowing different concentrations of satraplatin
qRT-PCR relative fold changed expression data for p53 signaling pathway related genes following satraplatin treatment at 48 hours (5 μM)
References
- Johnson NP, Butour JL, Villani G, Wimmer FL, Defais M, Pierson V. Metal antitumor compounds: the mechanism of action of platinum complexes. Prog Clin Biochem Med. 1989;10:1–24. [Google Scholar]
- Johnson SW, Ferry KV, Hamilton TC. Recent insights into platinum drug resistance in cancer. Drug Resist Update. 1998;1:243–54. doi: 10.1016/s1368-7646(98)80005-8. [DOI] [PubMed] [Google Scholar]
- Fuertes MA, Castilla J, Alonso C, Perez JM. Cisplatin biochemical mechanism of action: From cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 2003;10:257–66. doi: 10.2174/0929867033368484. [DOI] [PubMed] [Google Scholar]
- Boulikas T, Pantos A, Bellis E, Christofis P. Designing platinum compounds in cancer: structures and mechanisms. Cancer Therapy. 2007;5:537–83. [Google Scholar]
- Kelland LR, Abel G, McKeage MJ, Jones M, Goddard PM, Valenti M, et al. Preclinical antitumor evaluation of bis-acetato-ammine-dichloro-cyclohexylamine platinum (IV): an orally active platinum drug. Cancer Res. 1993;53:2581–6. [PubMed] [Google Scholar]
- Sharp SY, Rogers PM, Kelland LR. Transport of cisplatin and bis-acetato-ammine-dichlorocyclohexylamine platinum (IV) (JM-216) in human ovarian carcinoma cell lines: identification of a plasma membrane protein associated with cisplatin resistance. Clin Cancer Res. 1995;1:981–9. [PubMed] [Google Scholar]
- Choy H, Park C, Yao M. Current status and future prospects for satraplatin, an oral platinum analogue. Clin Cancer Res. 2008;14:1633–8. doi: 10.1158/1078-0432.CCR-07-2176. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–72. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 2001;490:117–22. doi: 10.1016/s0014-5793(01)02114-7. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–15. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–90. [PubMed] [Google Scholar]
- Kalimutho M, Minutolo A, Grelli S, Formosa A, Sancesario G, Valentini A, et al. Satraplatin (JM-216) mediates G2/M cell cycle arrest and potentiates apoptosis via multiple death pathways in colorectal cancer cells thus overcoming platinum chemo-resistance. Cancer Chemother Pharmacol. 2010;67:1299–312. doi: 10.1007/s00280-010-1428-4. [DOI] [PubMed] [Google Scholar]
- UKCCR. UKCCCR guidelines for the use of cell lines in cancer research British J Cancer 2000821495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Wei W, Dunaway S, Darnowski JW, Calabresi P, Sedivy J, et al. Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J Biol Chem. 2002;277:17154–60. doi: 10.1074/jbc.M112401200. [DOI] [PubMed] [Google Scholar]
- Fokkema E, Groen HJM, Bauer J, Uges DRA, Weil C, Smith IE. Phase II study of oral platinum drug JM216 as first line treatment in patients with small-cell lung cancer. J Clin Oncol. 1997;17:3822–7. doi: 10.1200/JCO.1999.17.12.3822. [DOI] [PubMed] [Google Scholar]
- McKeage MJ, Mistry P, Ward J, Boxall FE, Loh S, O'Neill C, et al. A phase I and pharmacology study of an oral platinum complex, JM216: dose-dependent pharmacokinetics with single-dose administration. Cancer Chemother Pharmacol. 1995;36:451–8. doi: 10.1007/BF00685793. [DOI] [PubMed] [Google Scholar]
- Latif T, Wood L, Connell C, Smith DC, Vaughn D, Lebwohl D, et al. Phase II study of oral bis(acetate) ammine dichloro (cyclohexylamine) platinum (IV) (JM216, BMS-182751) given daily x 5 in hormone refractory prostate cancer (HRPC) Invest New Drug. 2005;23:79–84. doi: 10.1023/B:DRUG.0000047109.76766.84. [DOI] [PubMed] [Google Scholar]
- Sternberg CN, Whelan P, Hetherington J, Paluchowska B, Slee PH, Vekemans K, et al. Phase III trial of satraplatin, an oral platinum plus prednisone vs prednisone alone in patients with hormone-refractory prostate cancer. Oncology. 2005;68:2–9. doi: 10.1159/000084201. [DOI] [PubMed] [Google Scholar]
- Ormerod MG, Orr RM, Peacock JH. The role of apoptosis in cell killing by ciplatin: a flow cytometric study. Br J Cancer. 1994;69:93–100. doi: 10.1038/bjc.1994.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, et al. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:1223–31. doi: 10.1002/hep.510300513. [DOI] [PubMed] [Google Scholar]
- Rao L, White E. Bcl-2 and the ICE family of apoptotic regulators: making a connection. Curr Opin Genet. 1997;7:52–8. doi: 10.1016/s0959-437x(97)80109-8. [DOI] [PubMed] [Google Scholar]
- Reardon JT, Vaisman A, Chaney SG, Sancar A. Efficient nucleotide excision repair of cisplatin, oxaliplatin, and bis-aceto-ammine-dichloro-cyclohexylamineplatinum (IV) (JM216) platinum intrastrand DNA diadducts. Cancer Res. 1999;59:3968–71. [PubMed] [Google Scholar]
- Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehmé A, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–6. [PubMed] [Google Scholar]
- Vaisman A, Lim SE, Patrick SM, Copeland WC, Hinkle DC, Turchi JJ, et al. Effect of DNA polymerases and high mobility group protein 1 on the carrier ligand specificity for translesion synthesis past platinum-DNA adducts. Biochemistry. 1999;38:11026–39. doi: 10.1021/bi9909187. [DOI] [PubMed] [Google Scholar]
- Fokkema E, Groen HJ, Helder MN, de Vries EG, Meijer C. JM216-, JM118-, and cisplatin-induced cytotoxicity in relation to platinum-DNA adduct formation, glutathione levels and p53 status in human tumour cell lines with different sensitivities to cisplatin. Biochem Pharmacol. 2002;63:1989–96. doi: 10.1016/s0006-2952(02)00983-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell cycle perturbation folowing different concentrations of satraplatin
qRT-PCR relative fold changed expression data for p53 signaling pathway related genes following satraplatin treatment at 48 hours (5 μM)