Abstract
Aim:
To assess the cytotoxic effect of crotoxin (CrTX), a potent neurotoxin extracted from the venom of the pit viper Crotalus durissus terrificus, in human lung adenocarcinoma A549 cells and investigated the underlying mechanisms.
Methods:
A549 cells were treated with gradient concentrations of CrTX, and the cell cycle and apoptosis were analyzed using a flow cytometric assay. The changes of cellular effectors p53, caspase-3 and cleaved caspase-3, total P38MAPK and pP38MAPK were investigated using Western blot assays. A549 xenograft model was used to examine the inhibition of CrTX on tumor growth in vivo.
Results:
Treatment of A549 cells with CrTX (25–200 μg/mL) for 48 h significantly inhibited the cell growth in a dose-dependent manner (IC50=78 μg/mL). Treatment with CrTX (25 μg/mL) for 24 h caused G1 arrest and induced cell apoptosis. CrTX (25 μg/mL) significantly increased the expression of wt p53, cleaved caspase-3 and phospho-P38MAPK. Pretreatment with the specific P38MAPK inhibitor SB203580 (5 μmol/L) significantly reduced CrTX-induced apoptosis and cleaved caspase-3 level, but G1 arrest remained unchanged and highly expressed p53 sustained. Intraperitoneal injection of CrTX (10 μg/kg, twice a week for 4 weeks) significantly inhibited A549 tumor xenograft growth, and decreased MVD and VEGF levels.
Conclusion:
CrTX produced significant anti-tumor effects by inducing cell apoptosis probably due to activation of P38MAPK and caspase-3, and by cell cycle arrest mediated by increased wt p53 expression. In addition, CrTX displayed anti-angiogenic effects in vivo.
Keywords: crotoxin (CrTX), human lung adenocarcinoma, apoptosis, P38MAPK, caspase-3, cell cycle, p53, angiogenesis
Introduction
Crotoxin (CrTX) is a potent neurotoxin that is extracted from the venom of the pit viper Crotalus durissus terrificus1. CrTX possesses phospholipase A2 activity and inhibits neuromuscular transmission. Recently, some studies have reported that CrTX has anti-tumor effects. However, the potential mechanism is unclear3, 4, 5.
Lung cancer is the most common cancer worldwide with the highest mortality rate among cancers. Current drugs cannot meet the therapeutic needs6. Previous study demonstrated that CrTX has cytotoxic effects on A549 cells, which are human lung adenocarcinoma cells with the wild type p53 gene, and shows synergistic effects when combined with Iressa, which is currently a widely-used drug for lung cancer therapy7, 8. In this study, we further investigated the anti-tumor effects of CrTX on A549 cells and tried to elucidate the mechanisms of CrTX-mediated cell growth inhibition and cell apoptosis. Some evidence has indicated that the P38MAPK pathway plays an important role in the process of apoptosis and the cell cycle. Therefore, we detected the expression of apoptotic proteins and cell cycle-related factors, such as p53, cleaved caspase-3 and phospho-P38MAPK, to discover the potential molecular mechanisms that mediate the anti-tumor activities of CrTX. The anti-tumor activity of CrTX was also analyzed in the A549 xenograft model.
Materials and methods
Cells and reagents
A549 cells, which are human lung adenocarcinoma cells with wt p53, purchased from Shanghai Institutes of Biological Science), were cultured in RPMI-1640 media containing 10% fetal bovine serum and maintained in an incubator (5% CO2, 37 °C). Crotoxin (CrTX) was provided by Celtic Biotechnology (Dublin, Ireland). The anti-phospho-P38MAPK, anti-p53 and anti-cleaved caspase-3 antibodies were purchased from Cell Signaling Technology (Woburn, MA, USA). SB203580, which is a specific inhibitor of P38MAPK, was purchased from Calbiochem, Inc (Madison, WI, USA).
Cell growth viability assay
Based on the results of preliminary experiments, we selected four different concentrations of crotoxin (25 μg/mL, 50 μg/mL, 100 μg/ml and 200μg/mL) in the study. After collecting A549 cells in the logarithmic growth period, we prepared 1.0×105 cells/mL of cell suspension after 0.25% trypsin treatment and seeded them into 96-well plates. Each well contained 100 μL of cell suspension. The control (cell suspension) and blank groups (RPMI-1640 medium) were included in the study. Once cells were allowed to adhere to the plate for 24 h, we removed the medium and applied different concentrations of CrTX to treat the cells for 48 h. Then, 20 μL of MTT (5 mg/mL) was added, and the cells were incubated at 37 °C for 4 h. The supernatant in each well was replaced with 150 μL DMSO. After 30 min of incubation at room temperature and gentle agitation for 10 min, the optical density (OD) was measured with an automatic multiwell spectrophotometer at 570 nm. Three independent experiments were performed to generate averaged values. The cytostatic rate was calculated using the following equation: cytostatic rate (%)=(1−average OD value of experimental group / average OD value of control group)×100.
Flow cytometry analysis of CrTX-induced cell cycle arrest and apoptosis
Four experimental groups were included in the study: control, CrTX (25 μg/ml), SB203580+CrTX and SB203580 only (5 μmol/L). SB203580 was added to the culture medium 1 h before CrTX. All groups of cells were digested with 0.25% trypsin and centrifuged at 1000 r/min. The collected cells were rinsed in PBS (phosphate buffered saline) twice, fixed with 70% pre-cooled ethanol and stored at 4 °C. The cells were centrifuged to remove the fixation reagent and suspended in PBS. The cells were incubated at 37 °C for 30 min after adding 200 μL RNaseA (1 μg/μL). PI (propidium iodide) was added to the cells and incubated for 30 min in the dark. Finally, the cell cycle and apoptosis were analyzed using flow cytometry.
Western blot analysis of p53, caspase-3 and cleaved caspase-3, total P38MAPK and pP38MAPK
Three experimental groups were included in the study as follows: control, CrTX and SB203580 + CrTX groups. The dose response and time course studies were performed as mentioned previously. Cells were washed twice with pre-cooled PBS. The pellets were lysed on ice with lysis buffer. The lysates were centrifuged at 12000 r/min for 10 min at 4 °C, and the supernatants were collected. Protein concentrations were determined using the BCA protein assay. For Western blot analysis, proteins were denatured for 5 min by boiling. Protein extracts were subjected to SDS-PAGE with 10% gels and electroblotted onto nitrocellulose membranes. Membranes were incubated overnight at 4 °C in 5% non-fat milk with primary monoclonal antibodies of p53, caspase-3, cleaved caspase-3, total P38MAPK, phospho-P38MAPK and β-actin (Santa Cruz Biotechnology, Inc, Santa Cruz, CA). After incubating for 2 h at room temperature with a secondary HRP-conjugated antibody, antigens were visualized using enhanced chemiluminescence using ECL (enhanced chemiluminescence) according to the manufacturer's instructions.
In vivo anti-tumor efficacy and mechanism study
Female Balb/C nude mice with average body weights of 18–20 g (4–6 weeks) were purchased from a local commercial vendor and housed in a SPF (specific pathogen free) animal facility. All manipulations (ie, handling, invasive procedures and tumor volume measurements) were performed in a laminar flow hood under strict sterile conditions. Mice were injected into the right axillary space tissue with 5×107 cells/mL A549 cells that were suspended in 0.2 mL of PBS. Treatment with CrTX was initiated 8 d after xenotransplantation, and the tumor reached approximately 5-6 mm in diameter. With the exception of two no-tumor mice, sixteen remaining mice were randomly assigned to one of two experimental groups (n=8 per group): the control or CrTX (10 μg/kg, ip, twice a week) group. After 4 weeks of treatment, the mice were sacrificed, and the tumor xenografts were removed, sectioned and analyzed using TEM. The data for tumor growth inhibition were expressed as the mean tumor weight±standard deviation. Tumor xenografts were further analyzed using a microscope, and microvascular density (MVD) was counted using Weidner methods. Briefly, a microscopic field was defined by a grid that was placed on the eyepiece. Endothelial cells in distinct cell clusters showing CD31 staining were considered as a single, countable microvessel. MVD was determined by calculating the mean of the obtained vascular counts in 15 random fields across the tissue section. The MVD data were expressed as the mean±standard deviation. In addition, 0.5 mL of blood was harvested from each mouse, and 0.2 mL of serum was collected after centrifuging for 10 min at 3000 r/min. VEGF levels were analyzed in each serum sample using ELISA. VEGF levels were expressed as the mean±standard deviation.
Statistical analysis
The data were expressed as the mean±standard deviation and analyzed using the one-way analysis of variance (ANOVA) or t-test. Differences were statistically significant when P<0.05.
Results
CrTX inhibited A549 cell proliferation in vitro
A549 cells were treated with CrTX 25, 50, 100, or 200 μg/mL for 48 h, and the viability of the cells was determined using the MTT assay. The results show that CrTX significantly inhibits the growth of A549 cells with an IC50 value of 78 μg/mL in a concentration-dependent manner. The CrTX-induced inhibition rates were 87.5% (200 μg/mL), 53.2% (100 μg/mL), 24.1% (50 μg/mL), and 15.7% (25 μg/mL) (Table 1).
Table 1. The effect of CrTX on A549 cell proliferation. bP<0.05 Compared with control group.
| CrTX concentration (μg/mL) | Inhibition rate (%) |
|---|---|
| Control | |
| 25 | 15.7±3.4b |
| 50 | 24.1±1.0b |
| 100 | 53.2±12.0b |
| 200 | 87.5±9.2b |
CrTX induced cell apoptosis and arrested cells in G1 phase
To further understand the mechanism of CrTX in cell growth inhibition, flow cytometric assays were used to analyze cell apoptosis and cell cycle arrest. After treatment with CrTX (25 μg/mL) for 24 h, a significantly increased population of cells was halted at the sub-G0 phase, which is an index of cell apoptosis, compared to the control group (Table 2). The results suggest that CrTX potently induces apoptosis in A549 cells, which suggests a potential mechanism for growth inhibition. To assess this mechanism for apoptosis, A549 cells were pretreated with SB203580, which is a specific P38MAPK inhibitor, and then treated with CrTX. No significant differences were observed in the sub-G0 phase cell population (Table 2). The results show that SB203580 protects A549 cells from apoptosis, indicating that P38MAPK plays an important role in CrTX-induced cell apoptosis. In addition, CrTX significantly increased A549 cells in the G1 phase compared to the control group (Table 2). SB203580 failed to alter CrTX-induced arrest of A549 cells in the G1 phase, suggesting that inhibition of P38MAPK has no effect on CrTX-induced cell cycle arrest (Table 2).
Table 2. Analysis of CrTX-induced apoptosis and cell cycle arrest of A549. bP<0.05 Compared with control group.
| Groups | Sub-G0 (%) | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|---|
| Control | 0.70±0.06 | 55.82±2.15 | 30.15±1.32 | 13.95±0.85 |
| CrTX | 11.42±1.02b | 76.85±2.30b | 16.04±0.52 | 6.80±030 |
| SB203580 | 0.516±0.03 | 54.71±1.64 | 29.45±086 | 14.68±0.92 |
| SB203580+CrTX | 0.87±0.08 | 75.06±1.83b | 15.36±0.71 | 9.08±0.52 |
Effects of CrTX on p53, caspase-3 and cleaved caspase-3, total P38MAPK and pP38MAPK expression
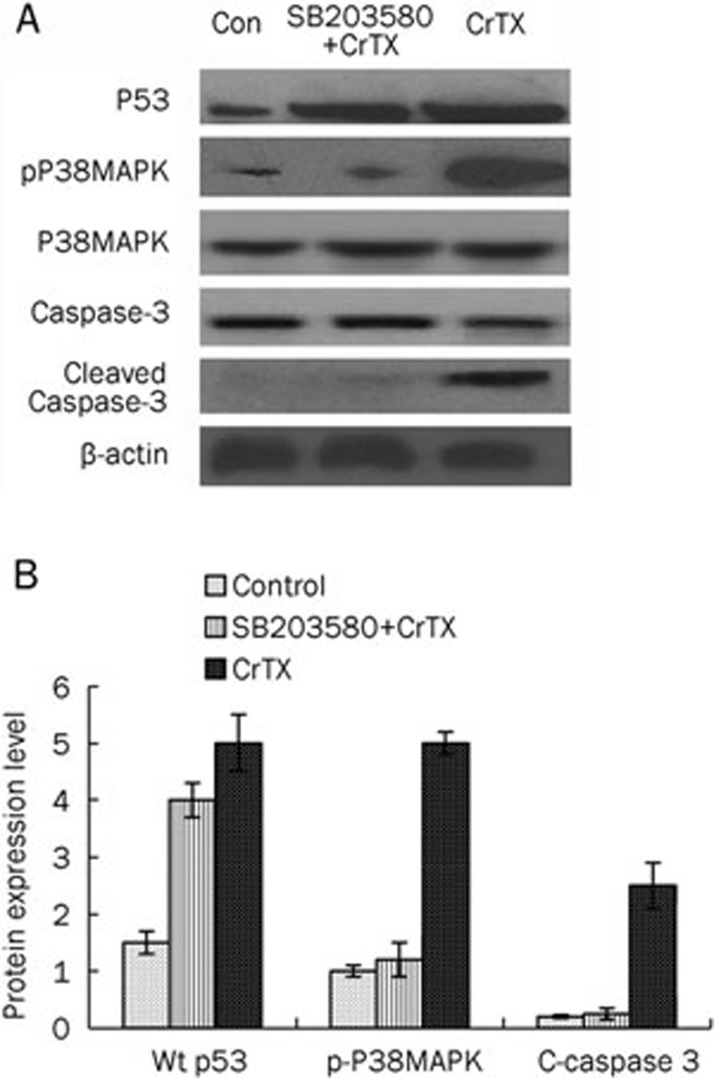
A549 cells were treated with 25 μg/mL of CrTX for 24 h and harvested for Western blot analysis for pro-apoptotic proteins. As shown in Figure 1, CrTX significantly increased cleaved caspase-3 in A549 cells. CrTX also increased the wild type p53 and phospho-P38MAPK levels without affecting the total P38MAPK levels in A549 cells. After pre-treating cells with SB203580, phospho-P38MAPK levels were reduced to control levels. Meanwhile, CrTX-induced production of cleaved caspase 3 was also inhibited, suggesting that CrTX-induced apoptosis was regulated by P38MAPK. However, the expression of wild type p53 remained high in the presence of SB203580 (Figure 1).
Figure 1.
Expression of wild-type p53, caspase-3 and cleaved caspase-3, total P38MAPK and pP38MAPK protein in A549 cells. Cells were treated with 25 μg/mL of CrTX or 5 μmol/L of SB203580 plus 25 μg/mL of CrTX for 24 h. A. Cell lysates were then collected for Western blot analysis on p53, caspase-3 and cleaved caspase-3, total P38MAPK and pP38MAPK. β-actin was used as loading control. B. Protein levels of p53, phospho-P38MAPK and cleaved caspase-3 were analyzed and shown in bar graph.
CrTX inhibited A549 tumor growth in vivo
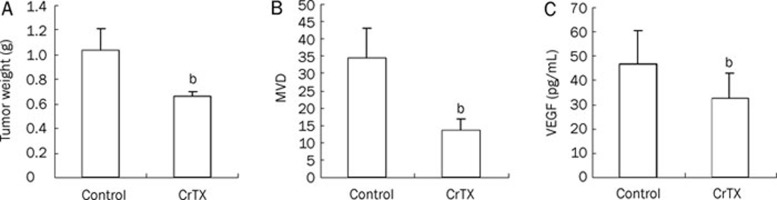
The anti-tumor activity of CrTX was tested in the A549 lung tumor xenograft model. As shown in Figure 2A, CrTX at a biweekly dosage of 10 μg/kg for 4 weeks had an efficacy effect on the A549 xenograft with 36.3% tumor growth inhibition (TGI) and no significant body weight loss. The observed in vivo activity of CrTX was consistent with its in vitro anti-proliferation effect on A549 cells. Further analysis showed that CrTX destroyed the blood vessels surrounding the tumor xenograft, which was indicated by the decrease in microvascular density (MVD) from 34.29±8.64 to 13.56±3.27 (Figure 2B). To understand the potential mechanism of CrTX in tumor angiogenesis, the VEGF level was analyzed using ELISAs with mouse sera from both groups. Interestingly, the VEGF level was also decreased in the CrTX treatment group (32.57±10.35 pg/mL) compared to the control group (46.83±13.62 pg/mL) (Figure 2C), suggesting that anti-angiogenesis might also contribute to the anti-tumor activity of CrTX in vivo.
Figure 2.
In vivo study of CrTX in A549 tumor xenograft model. Five million A549 cells were implanted into female nude mice subcutaneously. When the tumor reached about 5–6 mm in diameter, the tumor-bearing mice were randomized into 2 groups with 8 mice in each group and treated with vehicle control and CrTX (10 μg/kg, ip, twice a week). (A) After 4 weeks treatment, the mice were sacrificed, and the tumor xenografts were removed, sectioned and weighted. (B) Tumor xenografts were observed under microscope and microvascular density (MVD) was counted by Weidner methods. (C) In the meanwhile, 0.5 mL of blood was harvested from each mouse and 0.2 mL of serum was collected after centrifuging for 10 min at 3000 r/min. VEGF level was thus analyzed in each serum sample by ELISA. Data from each mouse sample were analyzed and shown in bar graph with statistic analysis as mean±SD. bP<0.05.
Discussion
In this study, the anti-tumor effects of CrTX on A549 cells were verified and appeared to be dose-dependent. The results of flow cytometry show that CrTX significantly increased the cell population in the sub-G0 and G0/G1 phases, which indicates that the anti-tumor effects of CrTX were closely related to the induction of apoptosis and cell cycle arrest.
Many studies have investigated the signaling pathways that are mediated by P38MAPK, which is one of the main signal transduction systems regulating apoptosis9, 10. Cells transmit extracellular signals to the nucleus using P38MAPK11. Ultraviolet radiation, TNF or anti-tumor drugs induce pathway activation, which leads to P38MAPK phosphorylation. Therefore, these data suggest that P38MAPK plays an important role in cell apoptosis and cell cycle arrest12, 13.
Caspase 3, which is a proteolytic enzyme, exists in an inactive proenzyme form in cells. As a central apoptotic effector, it plays a key role in promoting apoptosis14. After cleaving caspase-3, the active form of caspase 3 is released, indicating that the caspase 3 proenzyme is activated and has begun to execute its apoptotic function15.
Previous studies have suggested that CrTX induces autophagy in human breast cancer MCF7 cells as a main mechanism for CrTX-induced cytotoxic effects16. In this study, we determined that CrTX induced cell apoptosis and cell cycle arrest in lung cancer A549 cells, suggesting distinct mechanisms for various types of cells. During the process of CrTX-induced apoptosis of A549 cells, we found that phospho-P38MAPK was upregulated and that the expression of wt p53 and cleaved caspase-3 increased. These results indicate that the anti-tumor effects of CrTX are strongly correlated with the activation of P38MAPK, the upregulation of wt p53 and the activation of caspase 317, 18, 19. We investigated whether CrTX-induced apoptosis and G1 arrest were mediated by the P38MAPK pathway by pretreating cells with SB203580, which is a pyridine glyoxaline compound and specific inhibitor of the P38MAPK pathway that has been verified using both in vitro and in vivo models20. CrTX-induced apoptosis was attenuated in A549 cells following pretreatment with SB203580. The sub-G0 peak decreased from 11.42% to 0.87%. However, G1 arrest did not change. Western blot analysis revealed that SB203580 pretreatment suppressed the CrTX-induced expression of active caspase 3, but not wt p53. These results suggest that the mechanism of CrTX to induce apoptosis is mediated by P38MAPK activation, which probably mediates caspase-3 activation.
A549 cells are human lung adenocarcinoma cells, which express wild type p53. The CrTX-induced upregulation of wild type p53 was confirmed using Western blot analysis. We propose that the effects of CrTX on G1 arrest are probably due to the upregulation of wt p53. Meanwhile, SB203580 had no effect on CrTX-induced G1 arrest and p53 induction. Therefore, these results suggest that the P38MAPK pathway is not involved in p53 upregulation.
A close and complex relationship is evident between tumor cell apoptosis and cell cycle arrest21. Although apoptosis and cell cycle arrest complement each other by inhibiting tumor progression, the effects of CrTX on tumor cell apoptosis and G1 arrest are achieved via distinct intracellular pathways. Our results demonstrate that the induction of apoptosis by CrTX is due to the activation of P38MAPK and caspase 3, whereas G1 arrest is possibly due to the upregulation of wt p53.
Author contribution
Jing-kang HE and Zheng-hong QIN designed research; Bin YE performed research; Bin YE and Yan XIE wrote the paper; Zheng-hong QIN and Jun-chao WU polished the paper; Jun-chao WU and Rong HAN analyzed data.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 30772560).
References
- Kattah LR, Ferraz V, Matos Santoro M, Ribeiro da Silva Camargos E, Ribeiro Diniz C, De Lima ME. Analysis of fatty acids released by crotoxin in rat brain synap twosomes. Toxicon. 2002;40:43–9. doi: 10.1016/s0041-0101(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Picolo G, Cury Y. Peripheral neuronal nitric oxide synthase activity mediates antinociceptive effect of Crotalus durissus snake venom, a delta and kappa opioid receptor agonist. Life Sci. 2004;75:559–73. doi: 10.1016/j.lfs.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Yan CH, Liang ZQ, Gu ZL, Yang YP, Reid P, Qin ZH. Contributions of autophagic and apoptotic mechanisms to CrTX-induced death of K562 cells. Toxicon. 2006;47:521–30. doi: 10.1016/j.toxicon.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Penzo D, Petronilli V, Angelin A, Cusan C, Colonna R, Scorrano L, et al. Arachidonic acid released by phospholipase A(2) activation triggers Ca(2+)-dependent apoptosis through the mitochondrial pathway. J Biol Chem. 2004;279:25219–25. doi: 10.1074/jbc.M310381200. [DOI] [PubMed] [Google Scholar]
- Donato NJ, Martin CA, Perez M, Newman RA, Vidal JC, Etcheverry M. Regulation of epidermal growth factor receptor activity by CrTX, a snake venom phospholipase A2 toxin. Biochem Pharmacol. 1996;51:1535–42. doi: 10.1016/0006-2952(96)00097-4. [DOI] [PubMed] [Google Scholar]
- Dempke WC, Suto T, Reck M. Targeted therapies for non-small cell lung cancer. Lung Cancer. 2010;67:257–74. doi: 10.1016/j.lungcan.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Collins DM, Crown J, O'Donovan N, Devery A, O'Sullivan F, O'Driscoll L, et al. Tyrosine kinase inhibitors potentiate the cytotoxicity of MDR-substrate anticancer agents independent of growth factor receptor status in lung cancer cell lines. Invest New Drugs. 2010;28:433–44. doi: 10.1007/s10637-009-9266-0. [DOI] [PubMed] [Google Scholar]
- Newman RA, Vidal JC, Viskatis LJ, Johnson J, Etcheverry MA. VRCTC-310--a novel compound of purified animal toxins separates antitumor efficacy from neurotoxicity. Invest New Drugs. 1993;11:151–9. doi: 10.1007/BF00874149. [DOI] [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H, Kelly K, Siebenlist U. Cell cycle arrest and reversion of Ras induced transformation by a conditionally activated form of mitogen-activated protein kinase kinase kinase 3. Mol Cell Biol. 1999;19:3857–68. doi: 10.1128/mcb.19.5.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj JG, Kankofer M. Activity of 72-kDa and 92-kDa matrix metalloproteinases in placental tissues of cows with and without retained fetal membranes. Placenta. 1997;18:683–7. doi: 10.1016/s0143-4004(97)90010-2. [DOI] [PubMed] [Google Scholar]
- Ichijo H. From receptor to stress-activated MAP kinases. Oncogene. 1999;18:6087–93. doi: 10.1038/sj.onc.1203129. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E. p38MAPK signalling cascades: ancient roles and new function. Bioessays. 2000;22:637–45. doi: 10.1002/1521-1878(200007)22:7<637::AID-BIES6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Ono k, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Tawa P, Hell K, Giroux A, Grimm E, Han Y, Nicholson DW, et al. Catalytic activity of caspase-3 is required for its degradation: stabilization of the active complex by synthetic inhibitors. Cell Death Differ. 2004;11:439–47. doi: 10.1038/sj.cdd.4401360. [DOI] [PubMed] [Google Scholar]
- An S, Park MJ, Park IC, Hong SI, Knox K. Procaspase-3 and its active large subunit localized in both cytoplasm and nucleus are activated following application of apoptotic stimulus in Ramos-Burkitt lymphoma B cells. Int J Mol Med. 2003;12:311–7. [PubMed] [Google Scholar]
- Yan CH, Yang YP, Qin ZH, Gu ZL, Reid P, Liang ZQ. Autophagy is involved in cytotoxic effects of crotoxin in human breast cancer cell line MCF-7 cells. Acta Pharmacol Sin. 2007;28:540–8. doi: 10.1111/j.1745-7254.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- Kim KW, Kim BJ, Chung CW, Jo DG, Kim IK, Song YH, et al. Caspase cleavage product lacking amino-terminus of Ikappa Balpha sensitizes resistant cells to TNF-alpha and TRALL induced apoptosis. J Cell Biochem. 2002;85:334–45. doi: 10.1002/jcb.10139. [DOI] [PubMed] [Google Scholar]
- Lee JC, Kassis S, Kumar S. p38Mitogen-activated protein kinase inhibitors mechanisms and therapeutic potentials. Pharmacol Ther. 1999;82:389–97. doi: 10.1016/s0163-7258(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the P53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Su JC, Lin KL, Chien CM, Lu CM, Chen YL, Chang LS, et al. Novel indoloquinoline derivative, IQDMA, induces G2/M phase arrest and apoptosis in A549 cells through JNK/p38 MAPK signaling activation. Life Sci. 2009;85:505–16. doi: 10.1016/j.lfs.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Yang PM, Huang WC, Lin YC, Huang WY, Wu HA, Chen WL, et al. Loss of IKKbeta activity increases p53 stability and p21 expression leading to cell cycle arrest and apoptosis. J Cell Mol Med. 2010;14:687–98. doi: 10.1111/j.1582-4934.2009.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]