Abstract
Aim:
To identify proteins that interact with the C-terminal fragment of annexin A2 (A2IC), generated by plasmin cleavage of the plasmin receptor, a heterotetramer (AA2t) containing annexin A2.
Methods:
The gene that encodes the A2IC fragment was obtained from PCR-amplified cDNA isolated from human monocytes, and was ligated into the pBTM116 vector using a DNA ligation kit. The resultant plasmid (pBTM116-A2IC) was sequenced with an ABI PRISM 310 Genetic Analyzer. The expression of an A2IC bait protein fused with a LexA-DNA binding domain (BD) was determined using Western blot analysis. The identification of proteins that interact with A2IC and are encoded in a human monocyte cDNA library was performed using yeast two-hybrid screening. The DNA sequences of the relevant cDNAs were determined using an ABI PRISM BigDye terminator cycle sequencing ready reaction kit. Nucleotide sequence databases were searched for homologous sequences using BLAST search analysis (http://www.ncbi.nlm.nih.gov). Confirmation of the interaction between the protein LexA-A2IC and each of cathepsin S and SNX17 was conducted using a small-scale yeast transformation and X-gal assay.
Results:
The yeast transformed with plasmids encoding the bait proteins were screened with a human monocyte cDNA library by reconstituting full-length transcription factors containing the GAL4-active domain (GAL4-AD) as the prey in a yeast two-hybrid approach. After screening 1×107 clones, 23 independent β-Gal-positive clones were identified. Sequence analysis and a database search revealed that 15 of these positive clones matched eight different proteins (SNX17, ProCathepsin S, RPS2, ZBTB4, OGDH, CCDC32, PAPD4, and actin which was already known to interact with annexin A2).
Conclusion:
A2IC A2IC interacts with various proteins to form protein complexes, which may contribute to the molecular mechanism of monocyte activation induced by plasmin. The yeast two-hybrid system is an efficient approach for investigating protein interactions.
Keywords: yeast yeast two-hybrid system, human monocyte, plasmin receptor, C-terminal fragment of annexin A2 (A2IC), protein-protein interaction
Introduction
Experiments with human peripheral blood monocytes and macrophages have yielded novel insight into plasmin-induced intracellular signaling1, 2. Plasmin first binds to the annexin A2 heterotetramer (AA2t), which triggers cleavage of annexin A2 at lysine 27. The heterotetramer complex then dissociates, initiating downstream signaling, which leads to functional responses such as chemotaxis and TNF-α/IL-6 release2, 3, 4.
Several functional roles have been proposed for annexin A2, such as signal transduction, membrane fusion, cell adhesion, DNA synthesis, and cell proliferation5, 6. Annexin A2 is a 36 kDa protein that has been found on the surface of endothelial cells, monocytic cell lines and macrophages7, 8. Annexin A2 is a member of the annexin superfamily of calcium-dependent, phospholipid binding, multi-functional proteins9, which binds acidic phospholipids and actin with high affinity6.
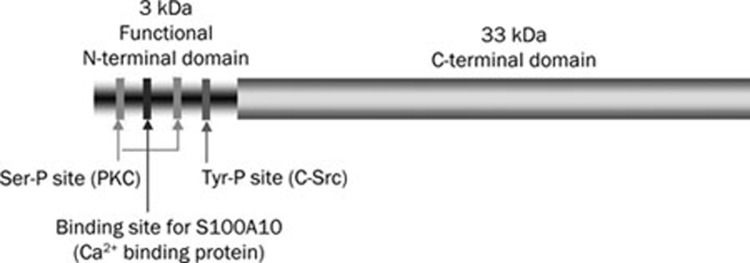
Annexin A2 is cleaved by chymotrypsin and plasmin into a 33 kDa C-terminal core domain and a 3 kDa N-terminal domain. The first 14 residues of the N-terminal domain contain a high-affinity binding site for S100A10, a Ca2+ binding protein8, 10 (Figure 1). Annexin A2 interacts with S100A10 to form AA2t11. As S100A10 has a C-terminal lysine, Waisman and colleagues proposed that S100A10 is the subunit that carries the plasmin binding site of AA2t12. The C-terminal core domain of annexin A2 also plays an important role in the functions of annexin A2. The core domain contains the intracellular binding sites for Ca2+, phospholipids and F-actin13. Ca2+ plays a major role in regulating the association of annexin A2 with the membranes and cytoskeleton14, 15. It is possible that truncated annexin A2, S100A10, or both, are involved in signaling downstream of plasmin or might be novel ligands that contribute to this signaling.
Figure 1.
Structure of annexin A2. The C-terminus and the known phosphorylation sites in the N-terminus of the annexin A2 molecule.
Protein-protein interactions are critical to most biological processes. The yeast two-hybrid system utilizes a molecular genetic approach to detect protein–protein interactions under native cell conditions to select genes that encode potential interacting proteins16.
Our study aimed at identifying A2IC-interacting proteins in monocytes. We used a yeast two-hybrid approach: LexA-BD-fused A2IC protein was the bait for screening a human monocyte cDNA library in which GAL4-AD fusion proteins were the prey. Our data revealed that nine different proteins interact with A2IC, including actin which had already been shown to be an interactor17, SNX17 and procathepsin S.
Materials and methods
Construction of pBTM116-A2IC yeast expression plasmid
The relevant fragment of the A2IC gene (936 bp, corresponding to carboxy-terminal annexin A2) was obtained by PCR of cDNA isolated from human monocytes, which were purified by autologous plasma-Percoll gradient centrifugation18, 19. The cDNA was amplified with primers for Anx2-F (5′-TGGGATCCTTGCCTATACTAACTTTGATGCT-3′) and Bam-Anx2-R (5′-GGGATCCTCAGTCATCTCCACCACACAG-3′). The sequences in both upstream and downstream primers are BamH I restriction sites. The PCR conditions were: 30 cycles, at 94 °C for 30 s, then 54 °C for 30 s, followed by 72 °C for 270 s. The resultant PCR product and the vector pBTM116 [4.8 kbp, containing the sequence for the LexA DNA binding domain (BD) and the yeast Trp1 gene, Clontech] were treated with restriction endonucleases (New England Biolabs, Cat#: R3136L) for ligation. The reaction system contained 3 μL 10×NEbuffer, 10 μL plasmid (0.2 μg/μL), 0.3 μL BSA (10 mg/mL), 1 μL BamH I (20000 U/mL) and 15.7 μL MilliQ water and was incubated for 1 h at 37 °C. The vector was additionally incubated with 20 U alkaline phosphatase for further 1 h at 37 °C to prevent religation. The phosphatase was inactivated by incubation at 80 °C for 10 min. The fragments and vectors obtained by digestion were separated by 1% agarose gel electrophoresis and purified from the gel by the QIAquick Gel Extraction Kit (Qiagen) according to the manufacturer's instructions.
The digested A2IC fragment was ligated into the digested pBTM116 vector using a DNA ligation kit (Biolabs). The reaction mixture comprised 1 μL insert, 1 μL vector, 2 μL 10×T4 DNA ligase buffer, 1 μL T4 DNA ligase, and 15 μL MilliQ water. The mixture was incubated at room temperature for 30 min to create pBTM116-A2IC. The plasmid was transformed into XL1-blue competent cells (Stratagene) as follows: 5 μL pBTM116-A2IC was incubated with 50 μL XL1-blue competent cells for 20 min on ice. The tubes were heated in a water bath at 42 °C for 45 s and quickly placed on ice for 2 min. Then, 0.9 mL preheated SOC medium was added, and the tubes were incubated at 37 °C for 30 min with shaking at 225–250 revolutions per minute. After centrifugation at 1000 revolutions per minute at 4 °C for 10 min, part of the supernatant was discarded, and the remaining 200 μL suspension was plated on LB agar with ampicillin (100 μg/mL) and incubated overnight at 37 °C. Only bacteria transformed with the pBTM116 plasmid containing the ampicillin resistance gene grew, and single clones were picked for replating on LB-agar with ampicillin for later colony PCR to obtain possible positive constructs. The original cloning PCR products (A2IC fragment) were verified from the transformants (pBTM116-AIC) by PCR amplification with Anx2-F and Bam-Anx2-R primers. In order to confirm whether the positive constructs were in the sense or antisense orientation, the extracted plasmids were digested with BamH I followed by EcoR I. To ensure that the resultant plasmids (pBTM116-A2IC) carried the correct orientation of A2IC in the proper reading frame without any mutations, they were sequenced with an ABI PRISM 310 Genetic Analyzer using primers LexA-F (5′-CTGGCGGTTGGGGTTATTCG-3′), LexA-R (5′-CATAAGAAATTCGAACGG-3′) and Anx2-F1 (5′-ACACATCTGGTGACTTCC-3′). The conditions for the PCR reaction were 25 cycles at 94 °C for 10 s, then 53 °C for 5 s, followed by 60 °C for 240 s.
Yeast two-hybrid screens
A human monocyte cDNA library was used (Clontech), and the cDNA was sub-cloned into pGADT7-RecAB vectors for yeast two-hybrid screening. Proper expression of LexA-DNA BD A2IC fusion protein was determined by Western blot analysis with a mouse monoclonal antibody specific for LexA (Santa Cruz Biotech) in the L40 yeast strain. The L40 yeast strain was transformed with LexA-A2IC (clone 6) and empty vector pBTM116. The yeasts were harvested by centrifugation and mixed with 100 ng LexA-A2IC and 100 μg ssDNA (plate 1), 100 ng pBTM116 and 100 μg ssDNA (plate 2, control) or 100 μg ssDNA alone (plate 3, control). These three mixtures were plated onto SD/-Trp1(without yeast gene Trp1) containing ampicillin and grown at 28 °C for 2–4 d. Selected clones were grown in 2 mL YPD medium containing ampicillin at 30 °C overnight with shaking at 225 revolutions per minute. Protein was extracted from cultures for Western blotting, kind regards from Ulm, with antibody directed against LexA. The verified DNA-BD/target protein (A2IC) did not autonomously activate the reporter gene in a 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) (Sigma) assay. The yeast transformants were transferred onto Whatman 3 mm paper and permeabilized in liquid nitrogen for about 10 s and then soaked in Z buffer containing 1 mg/mL X-Gal after incubation at 37 °C for 30 min.
The yeast strain L40 was transformed with LexA-A2IC by a small-scale yeast transformation protocol. The yeast strain expressing LexA-A2IC bait protein was transformed with the human monocyte cDNA library fused to the GAL-4 AD in the pGADT7-RecAB fusion vector by the lithium acetate method (large-scale yeast transformation protocol). To screen the cDNA library, the yeast two-hybrid system (Protocol: Matchmaker LexA two-hybrid system; Clontech Lab) was used to detect interacting proteins. Positive clones were initially selected and then assayed for lacZ activity using a filter β-galactosidase assay with X-Gal. Plasmids from positive yeast clones were isolated and transformed into KC8 competent cells (see protocol-Clontech Matchmaker Two-Hybrid System2 (PT1030-1), Catalog# K1604-1, Page/51). Plasmids isolated from KC8 competent cells were transformed into XL1-blue competent cells for further analysis of the insert size and for sequencing.
Confirmation of the interaction between LexA-A2IC and cathepsin S and SNX17 by small-scale yeast transformation
The yeast strain L40 was transformed with LexA-A2IC (BD) and pGAD-cathepsin S or pGAD-SNX17 using the small-scale yeast transformation protocol. In brief, yeast were harvested by centrifugation and mixed with the following DNA mixtures [the DNA molar ratio was determined according to BD:AD=2:1, pBTM116 (4.8 kbp), pGAD (8.4 kbp)]: 1) Vector (pBTM116, 100 ng, 1 μL) and ssDNA (100 μg, 50 μL); 2) Construct (pBTM116-A2IC, 100 ng, 1 μL) and ssDNA (100 μg); 3) Vector (pBTM116, 100 ng) and pGAD-cathepsin S (100 ng, 1 μL); 4) Vector (pBTM116, 100 ng) and pGAD-SNX17 (100 ng, 1 μL) and ssDNA (100 μg); 5) Construct (pBTM116-A2IC, 100 ng, 1 μL) and pGAD-cathepsin S (100 ng, 1 μL); 6) Construct (pBTM116-A2IC, 100 ng, 1 μL) and pGAD-SNX17 (100 ng, 1 μL) and ssDNA (100 μg); 7–8) pGAD-cathepsin S or pGAD-SNX17 (100 ng) and ssDNA (100 μg); 9) ssDNA (100 μg). After addition of 600 μL LiAc/PEG, the mixtures were incubated at 30 °C for 30 min at 200 revolutions per minute, and then 70 μL sterile DMSO (final concentration 10%) was added. These mixtures were then incubated for 15 min in a water bath at 42 °C. Following a short centrifugation, the supernatant was discarded, and the pellet was resuspended in 500 μL ddH2O or 1×TE buffer. Finally, 200–250 μL yeast was streaked on a different plate (150 mm) (for example: SD/-Trp1, -LEU, -His). Empty vector (pBTM116) with ssDNA, construct (pBTM116-A2IC) with ssDNA, and ssDNA were plated onto other plates (small-100 mm, SD/-TRP1 100 μL).
After the clones grew, at least six single clones were picked from each plate and plated onto one SD/-Trp1, -LEU plate. The clones were grown again, and the X-gal assay was performed. Protein interactions were confirmed by checking the plates for blue coloration.
DNA sequence analysis
DNA sequences were determined using the ABI PRISM BigDye terminator cycle sequencing ready reaction kit, according to the manufacturer's instructions (PE Biosystems). Nucleotide sequence databases were searched for homologous sequences by BLAST search analysis (http://www.ncbi.nlm.nih.gov).
Statistical analysis
Mean±SEM are shown. Probabilities calculated with the Newman-Keuls test were considered significant for P<0.05.
Results
Protein interaction with a proteolytic fragment generated by plasmin cleavage possibly initiates a downstream signaling pathway
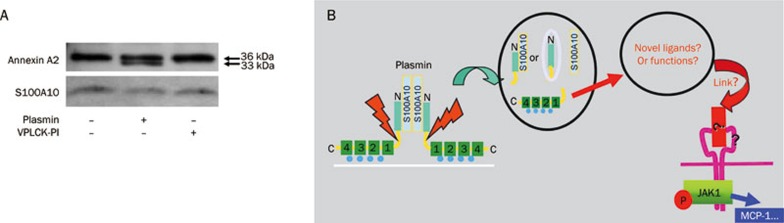
We have previously identified the annexin A2 heterotetramer, composed of annexin A2 and S100A10, as a signaling receptor for plasmin located on the cell surface. The main activation event of plasmin-mediated signaling is the cleavage of annexin A2 by plasmin, generating various proteolytic fragments (Figure 2A). However, the exact mechanism linking dissociation with the activation of the cell remains obscure. Here we propose that the C-terminal fragment of annexin A2 (A2IC) that is generated by plasmin cleavage consists of multiple ligand-binding sites that may interact with extracellular proteins to serve as ligands for unknown transmembrane receptors and thus contribute to plasmin-induced cell activation (Figure 2B).
Figure 2.
Proteolytic activity of plasmin is required for the cleavage of annexin A2 in monocytes. (A) Monocytes were stimulated for 30 min with 0.43 CTA U/mL plasmin or the equivalent amount of catalytically inactivated plasmin (VPLCK-PL). After treatment, cells were lysed, and proteins were separated and visualized by immunoblotting with antibodies against annexin A2 and S100A10. The results shown are representative of at least three independent experiments. (B) Hypothesis of the possible function of the proteolytic cleavage and dissociation of the plasmin receptor. According to consensus models, the annexin A2 heterotetramer interacts with the extracellular surface of the membrane; however, AA2t is not inserted into the membrane. After proteolytic cleavage by plasmin and dissociation of the receptor, this receptor may generate four new proteolytic fragments: either A2NPd and A2Ct, or A2Nt, A2Ct, and S100A10 alone. These fragments may interact with other proteins to form new complexes, or they may serve as novel ligands for transmembrane receptors. This result may initiate the known downstream signaling via JAKs/STATs, inducing the release of the inflammatory chemokine MCP-1.
Identification of sense construction (pBTM116-A2IC) yeast expression plasmids
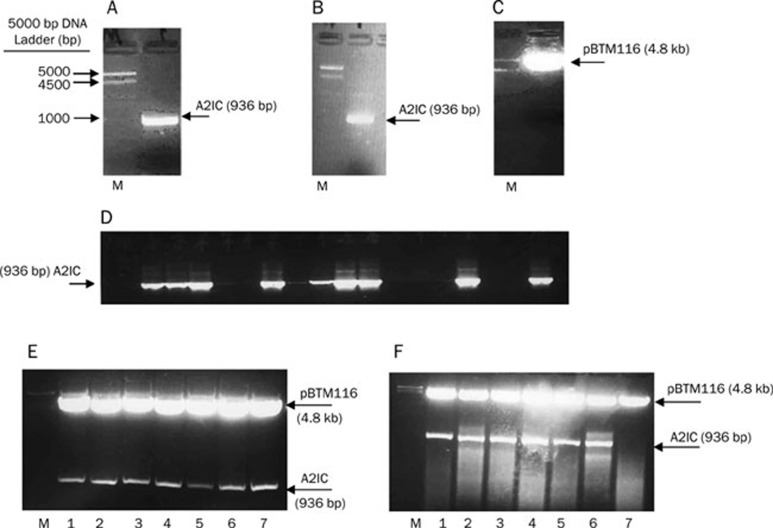
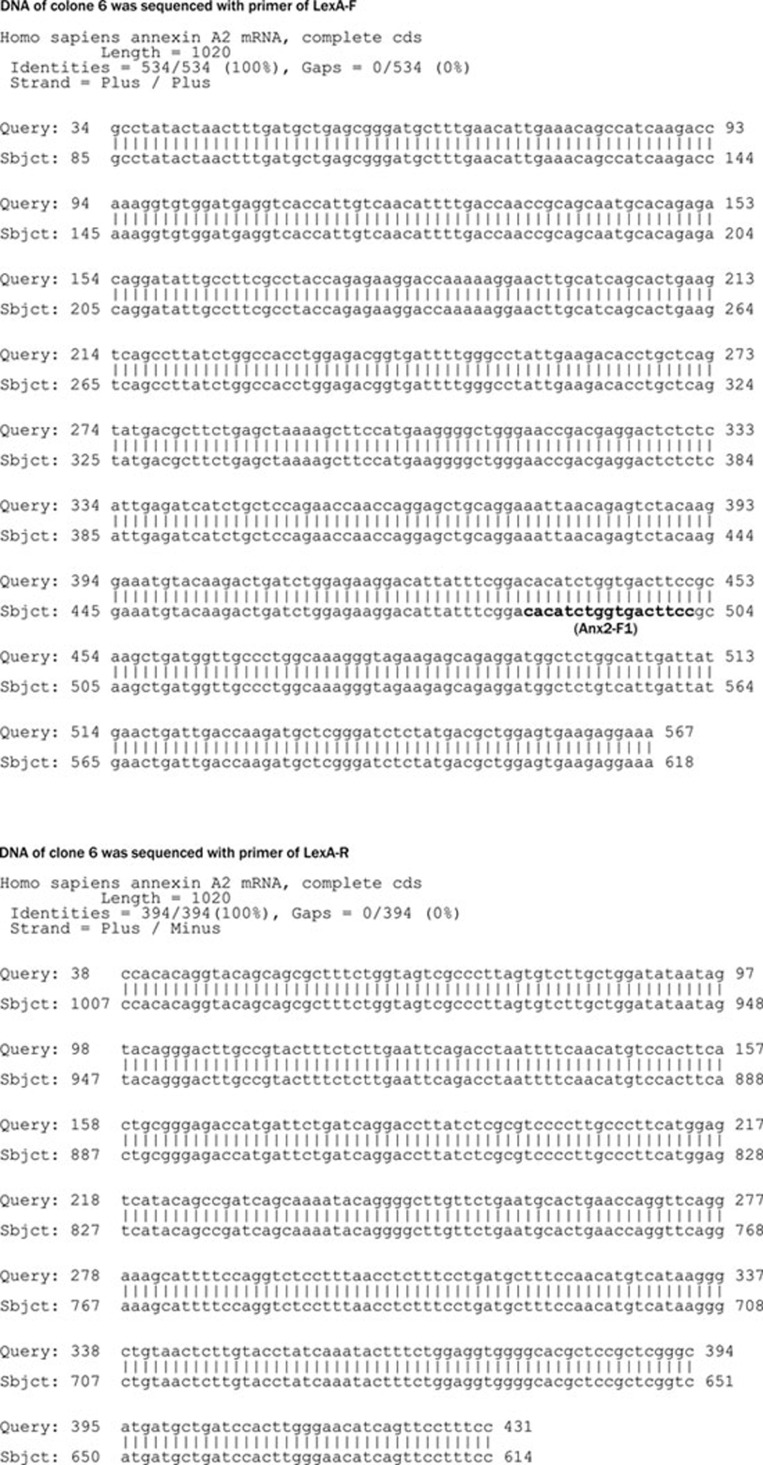
To obtain the relevant fragment of the A2IC gene, we performed a PCR reaction with cDNA isolated from human monocytes and the primers Anx2-F and Bam-Anx2-R. As expected, the PCR product was 936 bp (Figure 3A), and the A2IC fragment with sticky ends was obtained after digestion with BamH I (Figure 3B). To prevent the religation of the digested vector, pBTM116 was also incubated with 20 U alkaline phosphatase (Figure 3C). The digested A2IC fragment was ligated into the treated pBTM116 vector, and the resultant ligation (pBTM116-A2IC) was subsequently transformed into XL1-blue competent cells and grown on LB agar plates containing ampicillin for selection. We picked up 18 single clones for colony PCR. Nine cloning PCR products (A2IC fragment) were obtained from the transformants (pBTM116-AIC) and amplified with the Anx2-F and Bam-Anx2-R primers (Figure 3D). To identify whether these positive constructs were sense constructs, seven of the nine PCR products were digested with BamH I (Figure 3E) and further digested with EcoR I. Six of the seven were confirmed as sense constructs (Figure 3F). Next, we sequenced the number 6 (clone 6) construct (pBTM116-A2IC or LexA-A2IC) using the primers LexA-F, LexA-R and Anx2-F1. We found that the sense construct was present with the correct orientation and reading frame and without any mutations (Figure 4).
Figure 3.
Identification of pBTM116-A2IC yeast expression plasmids. (A) A2IC PCR product. (B) Digested A2IC with BamH I. (C) Digested and dephosphorylated pBTM116 plasmid with BamH I. All digestions were separated by horizontal 1% agarose gel electrophoresis in 0.5×TBE buffer containing ethidium bromide (10 μg/mL). (D) Cloning of PCR products (A2IC fragment). Transformants (pBTM116-A2IC) were amplified with the primers Anx2-F and Bam-Anx2-R. (E) Positive constructs (1–7) (pBTM116-A2IC): extracted plasmid from the transformants was digested with BamH I. (F) Sense constructs (1–6) (pBTM116-A2IC): extracted plasmid from the transformants was digested with EcoR I.
Figure 4.
Nucleotide sequences of clone 6. Clone 6 was sequenced with an ABI PRISM 310 Genetic Analyzer using the primers LexA-F, LexA-R, and Anx2-F1.
Identification and confirmation of proteins interacting with A2IC in a human monocyte cDNA library
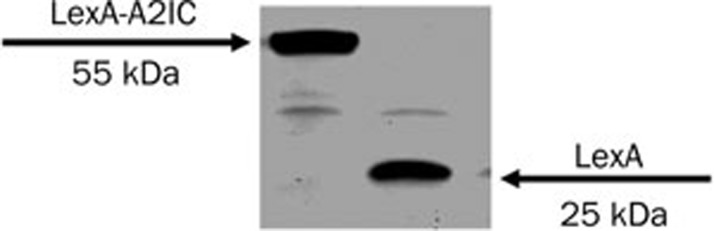
To evaluate the expression of LexA-A2IC (pBTM116-A2IC), we transformed LexA-A2IC (BD/target) and the pBTM116 vector into the yeast strain L40 by small-scale transformation. We then used antibodies against LexA for Western blotting of proteins extracted from the yeast cultures. The LexA-A2IC fusion protein was expressed in L40 yeast (Figure 5). After an X-gal assay, we did not find any blue clones, indicating that the DNA-BD/target protein (A2IC) does not autonomously activate the reporter gene. Thus, we could use this construct as the bait to screen the human monocyte cDNA library.
Figure 5.
Expressed LexA-A2IC protein in the L40 yeast strain. Western blotting shows LexA-A2IC (left band) and LexA (right band) proteins extracted from yeast cultures.
To identify proteins that interact with A2IC in the human monocyte cDNA library, we used a library of human monocyte cDNA encoding fusion proteins 'pGADT7-RecAB' fused to the transcriptional activation domain of GAL4, and we screened this library with LexA-A2IC using a yeast two-hybrid approach. Transformants (9×106) were analyzed with this bait. Sequence analysis followed by database searching revealed that 15 positive clones matched eight different proteins: SNX17, ProCathepsin S, RPS2, ZBTB4, OGDH, CCDC32, PAPD4 and actin, which is already known to interact with A2IC (Table 1).
Table 1. The results of matched proteins in yeast two-hybrid screen between A2IC fragment as a bait protein and human monocyte library cDNA encoding AD of GAL4 as prey protein.
| Interaction proteins | Functions | Amount of clones |
|---|---|---|
| Cathepsin S | Increased activity suppresses the CD4+ T cell / mediated immune responses | 4 |
| RPS2 | RNA binding, a therapeutic targeting for the eradication of prostate cancer in preclinical tumor modeling studies | 3 |
| ZBTB4 | Contains Zinc finger and BTB domain, represses transcription of p21CIP1 and controls the cellular response to p53 activation, and plays a crucial role in oncogenesis | 2 |
| OGDH | Thiamin pyrophosphate binding, inhibition of OGDH activity alleviates glutamate-induced calcium deregulation, mitochondrial depolarization, and neuronal death | 2 |
| SNX17 | Interacts with P-selectin and LDL receptor family | 1 |
| Actin | Cytoskeletal protein | 1 |
| CCDC32 | Coil-coiled domain containing 32 | 1 |
| PAPD4 | PAP-associated domain containing 4 (GLD-2), translational regulation of p53 mRNA and cellular senescence is coordinated by GLD2 | 1 |
After transformation with LexA-A2IC (BD) and pGAD-cathepsin S or pGAD-SNX17 into the yeast strain L40, we performed X-gal assays and identified the single blue clones, confirming protein interactions.
Discussion
In this study, we described serial transformation of a bait into a pre-made cDNA library in L40 yeast cells. We found that this method effectively permitted a number of true interactions. If a pre-made library in yeast cells is not available, the outlined method can be quickly adapted. AH109 cells can first be transformed with a bait vector, followed by selection of the yeast that contain the bait. A second transformation of yeast cells can then be performed with the cDNA library. We demonstrate that this quick method leads to the discovery of significant interactions.
Protein interactions can be precisely and efficiently studied using the yeast two-hybrid system. This technique was used for in vivo investigation of the protein interactions of A2IC with F-actin and other proteins. A2IC plays an important role in the function of annexin A2 and contains an intracellular binding site for Ca2+, phospholipids and F-actin12, 20. In the present work, we also found that the interaction between A2IC and actin is essential for maintaining the plasticity of the dynamic membrane-associated actin cytoskeleton21. The interaction of A2IC with other proteins could play a critical role in plasmin-dependent cell activation. The annexin A2-S100A10 complex and plasmin interacted with TrpRS, which regulates TrpRS retention in the cytosol. The dissociation of AA2t from TrpRS allows this dissociation to be exported to the cell exterior. Once outside the cells, plasmin, or another protease, cleaves the native enzyme into angiostatic fragments. These fragments can inhibit the Akt signaling pathway through interaction with VE-cadherin22. In this study, we found that eight different proteins interact with A2IC, by using a yeast two-hybrid screen, including cathepsin S, SNX17, actin, RPS2, ZBTB4, OGDH, CCDC32, and PAPD4. An IL-6-gp130-STAT3-mediated increase in cathepsin S activity reduces the MHCII alpha/beta dimer in Dendritic cells and suppresses CD4+ T cell-mediated immune responses23. SNX17, a non-self-assembling protein, interacts with KRIT1, which plays a role in cell adhesion processes and intergrin signaling24. It also has been identified as a novel interaction partner for members of the LDLR family. SNX17 resides in distinct parts of the early endosomal compartment and enhances the endocytosis rate of LDLR, and possibly of other surface receptors25. The A2IC faces the cytosol and is in ideal proximity to interact with the actin cytoskeleton within the cell8. In preclinical tumor models, it has been shown that therapeutic targeting of RPS2 is an excellent approach for the eradication of prostate cancer26. ZBTB4 binds to methylated CpGs, repressing transcription of P21CIP1 and controlling the cellular response to p53 activation, thereby playing a crucial role in oncogenesis27, 28. OGDH is a well-characterized auto-antigen in primary biliary cirrhosis. The activity of the enzyme is much lower in Alzheimer's disease, and there are reports of the enzyme's susceptibility to modification by oxidative stress29. Inhibition of OGDH activity alleviates glutamate-induced calcium deregulation, mitochondrial depolarization, and neuronal death30. PAPD4 (GLD2) is an important regulator of late spermatogenesis and is the first example of a GLD-2 family member playing a significant role in male gametogenesis31. Translational regulation of p53 mRNA and cellular senescence is coordinated by GLD2/miR-122/CPEB/GLD432.
In our present study, it became possible to verify the interactions of the plasmin cleavage fragment A2IC with capthesin S, SNX17 and other proteins, which may serve as new ligand complexes that could bind directly to as-yet unknown transmembrane receptors. Such interactions could contribute to functional immune responses that involve plasmin-mediated cell activation. Understanding the functions of A2IC-interacting proteins may allow the identification of novel therapeutic targets for inflammatory diseases, such as atherosclerosis, that might be partially triggered by the serine protease plasmin.
Author contribution
Qun LI, Yves LAUMONNIER, Tatiana SYROVETS, and Thomas SIMMET designed the study; Qun LI and Yves LAUMONNIER performed the research; Qun LI analyzed the data and wrote the paper; and Thomas SIMMET revised the paper.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft to Tatiana SYROVETS and Thomas SIMMET, Shanghai Pujiang Program from Shanghai Science and Technology Committee (No 10PJ1407300) and Shanghai Scientific Research Innovation Program of Shanghai Education Commission (No 11YZ59).
Glossary
- A2IC
C-terminal fragment of annexin A2
- AA2t
annexin A2 heterotetramer
- A2NPd
annexin A2 N-terminus and S100A10 dimer
- A2Nt
annexin A2 N-terminus
- A2Ct
annexin A2 C-terminus
References
- Li Q, Laumonnier Y, Syrovets T, Simmet T. Plasmin triggers cytokine induction in human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol. 2007;27:1383–9. doi: 10.1161/ATVBAHA.107.142901. [DOI] [PubMed] [Google Scholar]
- Burysek L, Syrovets T, Simmet T. The serine protease plasmin triggers expression of MCP-1 and CD40 in human primary monocytes via activation of p38 MAPK and janus kinase (JAK)/STAT signaling pathways. J Biol Chem. 2002;277:33509–17. doi: 10.1074/jbc.M201941200. [DOI] [PubMed] [Google Scholar]
- Syrovets T, Jendrach M, Rohwedder A, Schule A, Simmet T. Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKK{beta}-mediated NF-{kappa}B activation. Blood. 2001;97:3941–50. doi: 10.1182/blood.v97.12.3941. [DOI] [PubMed] [Google Scholar]
- Laumonnier Y, Syrovets T, Burysek L, Simmet T. Identification of the annexin A2 heterotetramer as a receptor for the plasmin-induced signaling in human peripheral monocytes. Blood. 2006;107:3342–9. doi: 10.1182/blood-2005-07-2840. [DOI] [PubMed] [Google Scholar]
- Singh P. Role of annexin-II in GI cancers: interaction with gastrins/progastrins. Cancer Lett. 2007;252:19–35. doi: 10.1016/j.canlet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Rescher U, Almers W, Proust J, Gerke V, Sechi AS, et al. Annexin A2 has an essential role in actin-based macropinocytic rocketing. Curr Biol. 2001;11:1136–41. doi: 10.1016/s0960-9822(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J Biol Chem. 1994;269:21191–7. [PubMed] [Google Scholar]
- Filipenko NR, Waisman DM. The C terminus of annexin II mediates binding to F-actin. J Biol Chem. 2001;276:5310–5. doi: 10.1074/jbc.M009710200. [DOI] [PubMed] [Google Scholar]
- Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Waisman DM. Annexin A2 tetramer: structure and function. Mol Cell Biochem. 1995;149–150:301–22. doi: 10.1007/BF01076592. [DOI] [PubMed] [Google Scholar]
- MacLeod TJ, Kwon M, Filipenko NR, Waisman DM. Phospholipid-associated annexin A2-S100A10 heterotetramer and its subunits: characterization of the interaction with tissue plasminogen activator, plasminogen, and plasmin. J Biol Chem. 2003;278:25577–84. doi: 10.1074/jbc.M301017200. [DOI] [PubMed] [Google Scholar]
- Waisman DM. Annexin A2 may not play a role as a plasminogen receptor. Br J Haematol. 2005;131:553–4. doi: 10.1111/j.1365-2141.2005.05805.x. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y, Nakanishi T, Yamaguchi D, Takahashi K, Yumoto H, Ozaki K, et al. Macrophage inflammatory protein 3a-CC chemokine receptor 6 interactions play an important role in CD4+ T-cell accumulation in periodontal diseased tissue. Clin Exp Immunol. 2002;128:548–54. doi: 10.1046/j.1365-2249.2002.01865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescher U, Gerke V. Annexins-unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117:2631–9. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- Gerke V, Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984;3:227–33. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CT, Bartel PL, Sternglanz R, Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci U S A. 1991;88:9578–82. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescher U, Ruhe D, Ludwig C, Zobiack N, Gerke V. Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J Cell Sci. 2004;117:3473–80. doi: 10.1242/jcs.01208. [DOI] [PubMed] [Google Scholar]
- de Almeida MC, Silva AC, Barral A, Barral Netto M. A simple method for human peripheral blood monocyte isolation. Mem Inst Oswaldo Cruz. 2000;95:221–3. doi: 10.1590/s0074-02762000000200014. [DOI] [PubMed] [Google Scholar]
- Syrovets T, Tippler B, Rieks M, Simmet T. Plasmin is a potent and specific chemoattractant for human peripheral monocytes acting via a cyclic guanosine monophosphate-dependent pathway. Blood. 1997;89:4574–83. [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–71. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Shao D, Bailly M, Moss SE. Regulation of actin dynamics by annexin 2. EMBO J. 2006;25:1816–26. doi: 10.1038/sj.emboj.7601078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Zhou Q, Otero F, Myers CA, Bates A, Belani R, et al. Evidence for annexin II-S100A10 complex and plasmin in mobilization of cytokine activity of human TrpRS. J Biol Chem. 2008;283:2070–7. doi: 10.1074/jbc.M706028200. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, et al. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Czubayko M, Knauth P, Schluter T, Florian V, Bohnensack R. Sorting nexin 17, a non-self-assembling and a PtdIns(3)P high class affinity protein, interacts with the cerebral cavernous malformation related protein KRIT1. Biochem Biophys Res Commun. 2006;345:1264–72. doi: 10.1016/j.bbrc.2006.04.129. [DOI] [PubMed] [Google Scholar]
- Stockinger W, Sailler B, Strasser V, Recheis B, Fasching D, Kahr L, et al. The PX-domain protein SNX17 interacts with members of the LDL receptor family and modulates endocytosis of the LDL receptor. EMBO J. 2002;21:4259–67. doi: 10.1093/emboj/cdf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Hu Y, Stearns ME. RPS2: a novel therapeutic target in prostate cancer. J Exp Clin Cancer Res. 2009;28:6. doi: 10.1186/1756-9966-28-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Marquardt J, Elzi D, Forster N, Starke S, Glaum A, et al. Zbtb4 represses transcription of P21CIP1 and controls the cellular response to p53 activation. EMBO J. 2008;27:1563–74. doi: 10.1038/emboj.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion GJ, Zhenilo S, Salozhin S, Yamada D, Prokhortchouk E, Defossez PA. A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol Cell Biol. 2006;26:169–81. doi: 10.1128/MCB.26.1.169-181.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Moreno-Sanchez R. Bioenergetic pathways in tumor mitochondria as targets for cancer therapy and the importance of the ROS-induced apoptotic trigger. Mol Aspects Med. 2010;31:29–59. doi: 10.1016/j.mam.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Bunik VI, Kabysheva MS, Klimuk EI, Storozhevykh TP, Pinelis VG. Phosphono analogues of 2-oxoglutarate protect cerebellar granule neurons upon glutamate excitotoxicity. Ann N Y Acad Sci. 2009;1171:521–9. doi: 10.1111/j.1749-6632.2009.04709.x. [DOI] [PubMed] [Google Scholar]
- Sartain CV, Cui J, Meisel RP, Wolfner MF. The poly(A) polymerase GLD2 is required for spermatogenesis in Drosophila melanogaster. Development. 2011;138:1619–29. doi: 10.1242/dev.059618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns DM, D'Ambrogio A, Nottrott S, Richter JD. CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature. 2011;473:105–8. doi: 10.1038/nature09908. [DOI] [PMC free article] [PubMed] [Google Scholar]