Abstract
Objective
It has been claimed that the aneurysm rate for Kawasaki disease (KD) patients in Japan is lower than in the U.S. However it has been difficult to compare coronary artery (CA) outcomes between the two countries because of different definitions for CA abnormalities. Therefore, we compared CA internal diameters between Japanese and U.S. KD patients using standard definitions and methods.
Study Design
We retrospectively reviewed CA outcomes in 1082 KD patients from 2 centers in the U.S. and 3 centers in Japan and compared Z-max scores (maximum internal diameter for the left anterior descending or right coronary artery expressed as standard deviation units from the mean (Z-score) normalized for body surface area) obtained within 12 weeks after onset and calculated using two different regression equations from Canada (Dallaire) and Japan (Fuse). We defined a Z-max of <2.5 as normal and a Z-max of ≥ 10 as giant aneurysm.
Result
The median Z-max for the U.S. and Japanese subjects was 1.9 and 2.3 SD units, respectively (p<0.001). There was no significant difference in rates of patients with Z-max ≥ 5.0 between the countries. In a multivariable model adjusting for age, sex, and treatment response, being Japanese was still associated with a higher Z-max score.
Conclusion
Previously reported differences in aneurysm rates between Japan and the U.S. likely resulted from use of different definitions and nomenclature. Adoption of Z-scores as a standard for reporting CA internal diameters will allow meaningful comparisons among different countries and will facilitate international, collaborative clinical trials.
Keywords: Kawasaki disease, Coronary artery aneurysm, Z score, vasculitis, echocardiography
Introduction
In the era before the use of intravenous immunoglobulin (IVIG) to treat children with Kawasaki disease (KD), aneurysm rates of approximately 25% were noted both in Japan and the U.S. [1-3]. KD is now the most important cause of acquired heart disease in children in the developed world and more than 60 countries in Asia, the Middle East, the U.S., Africa, and Europe have reported KD cases [4-6]. Reported rates of coronary artery (CA) aneurysms vary widely among KD patients from different countries [7-16]. Currently, studies from the U.S. report an aneurysm rate of approximately 4.0-5.0% [7, 17], while rates for Japan are reported to be on the order of 1.0% [9]. To clarify whether this is a problem of semantics (different definitions of aneurysms), a real difference stemming from different clinical practices (timing of echocardiograms or timing of IVIG administration), or differences in host genetics, we conducted a comparative study among two centers in the U.S. and three centers in Japan over the same time period and used the internal diameter of the coronary artery normalized for body surface area (Z-score) as a standardized assessment tool to compare outcomes.
Methods
Patient population
Patients with KD included in this study met the case definitions of the American Heart Association (AHA) for the U.S. sites (Rady Children's Hospital San Diego and Boston Children's Hospital) or the Japanese Circulation Society (JCS) for the Japanese sites (Toho University Omori Medical Center, Kitasato University Hospital, and Juntendo University Urayasu Hospital; Table 1) [18, 19]. The records of unselected, consecutive KD patients treated at the five participating centers during the 4-year period from January 1, 2004 through December 31, 2008 were retrospectively reviewed. Subjects were included regardless of illness day at diagnosis or the timing of treatment. Patients diagnosed and treated at outside hospitals and referred to the study centers for additional treatment or evaluations were excluded. The study was reviewed and approved by the Institutional Review Boards of the participating centers.
Table 1. Comparison of published definitions of coronary artery abnormalities assessed by echocardiography in the U.S. and Japan.
| U.S. [18] | |
|
| |
| Normal | Z-score <2.5 |
| Small aneurysm | <5 mm internal diameter |
| Medium aneurysm | 5 to 8 mm internal diameter |
| Giant aneurysm | Internal diameter of >8 mm |
|
| |
| Japan [19] | |
|
| |
| Small aneurysm or Dilatation | Localized dilatation with ≤4 mm internal diameter |
| In children ≥5 years, internal diameter of a segment measures <1.5 times that of an adjacent segment | |
| Medium aneurysm | Aneurysms with an internal diameter of >4 mm to ≤8 mm |
| In children ≥5 years, the internal diameter of a segment measures 1.5 to 4 times that of an adjacent segment | |
| Giant aneurysm | Aneurysms with an internal diameter of >8 mm |
| In children ≥5 years, the internal diameter of a segment measures >4 times that of an adjacent segment | |
|
| |
| Transient coronary dilatation | Patients with transient coronary dilatation which typically subsides within 30 days after onset. |
Data collection
Data were collected on standardized forms at each of the participating sites and included sex, age, body surface area, illness day at diagnosis, and treatment with a single or more than one infusion of IVIG. Illness Day 1 was defined as the first day fever. Late treatment was defined as IVIG administration after the 10th day of illness. Incomplete KD was defined as fever for ≥5 days associated with 2 or 3 of the principal clinical features of KD associated with abnormalities by echocardiogram [18-20].
Echocardiographic data
Although procedures for obtaining echocardiographic measurements were not formally standardized among centers, each center followed the recommended guidelines for their country (AHA or JCS). Only the AHA guidelines specify obtaining the cross-sectional measurement of the internal diameter 5 mm from the origin of the arterial segment. The maximal internal diameter (mm) of the left anterior descending coronary artery (LAD) and the right coronary artery (RCA) within 12 weeks after onset was obtained from each center. The diameter of left main coronary artery was excluded because of the wide range of anatomic variants in this arterial segment that often preclude accurate measurement of the internal diameter [21]. Patients' height (cm) and weight (kg), corresponding to the echo date of largest measurement were also obtained.
Z-score equations
Body surface area (BSA) was computed by both the Du Bois and Haycock equations and compared [22-24]. The Z-score was computed using two different equations, both normalized for body surface area. The Fuse Z-scoring calculator (ZSP Version 9) was based on measurements from over 4,000 normal Japanese children (http://www.zscore.jp/). The calculator classifies Z scores only up to a value of 8.2 SD units so it therefore could not be used for comparisons of Z score as a continuous variable. The Dallaire equation was calculated based on 1,033 normal Canadian children [25]. Z-max was defined as the maximum Z-score for the LAD or RCA measured by echocardiography within 12 weeks after fever onset. We analyzed Z-max as a continuous variable and as a categorical variable (<2.5, ≥2.5-<5.0, ≥5.0, and ≥10) based on previously published cutpoints using the Canadian equation [26].
We also analyzed the maximal internal diameter (mm) of the LAD and RCA in children <5 years of age without normalization for body surface area and classified the arteries according to Japanese criteria using absolute internal diameter: ≤3 mm, >3 and ≤4 mm, and >4 mm. Transient dilatation was also analyzed according to the Japanese guidelines (Table 1) [19]. The analysis of “transient dilatation” was performed for only one center in the U.S. and 2 centers in Japan for which measurements of the CA internal diameters within 30 days after onset were available.
Statistical analysis
Descriptive analyses were performed to compare U.S. versus Japanese KD patients. Medians and interquartile ranges (IQRs) were reported for continuous variables and frequency counts and percentages were reported for categorical variables. Univariate tests of significance were computed via Wilcoxon Rank Sum test for continuous variables and Fisher's exact test for categorical variables. Pearson's correlation was used to compare the two BSA and the two Z-scores methods. Multivariable regression was performed to compare Z-max scores between U.S. and Japanese patients, adjusting for age, gender, illness days, treatment, and treatment response. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed in R (http://cran.r-project.org), version 2.14.0.
Results
Study population characteristics
A total of 1,082 subjects were included with 568 from the U.S. and 514 from Japan (Table 2). U.S. subjects were older, were treated on average one day later, and were more likely to be treated after Illness Day 10 as compared to Japanese subjects.(p<0.001 for all comparisons). Japanese subjects were more likely to be classified as clinically incomplete cases (p<0.001) and were more likely to receive additional infusions of IVIG (p=0.03).
Table 2. Demographic and clinical characteristics of subjects with Kawasaki disease from the U.S. and Japan.
| Characteristics | U.S (n=568) | Japan (n=514) | p |
|---|---|---|---|
| Median age, months (IQR) | 30.6 (15.6-55.9) | 24.6 (12.0-43.9) | <0.001 |
| Median BSA, m2 (IQR) | 0.60 (0.47-0.73) | 0.53 (0.44-0.64) | <0.001 |
| Male, n (%) | 351 (61.9) | 308 (59.9) | NS |
| Median Illness day, day (IQR) | 6 (5-9) | 5 (4-6) | <0.001 |
| Incomplete KD, n (%) | 90 (15.9) | 158 (30.7) | <0.001 |
| Patients treated with single IVIG infusion, n (%) | 523 (92.1) | 468 (91.5) | NS |
| No IVIG treatment, n (%) | 26 (4.6) | 24 (4.7) | NS |
| Late treatment, n (%) | 62 (11.5) | 67 (6.5) | <0.001 |
| Subjects treated with > 1 IVIG infusion, n (%) | 109 (20.1) | 126 (25.8) | 0.031 |
BSA = Body surface area calculated using the DuBois equation, KD = Kawasaki disease, Illness Day 1= first day of fever, IVIG = intravenous immunoglobulin, Late treatment= Treatment after Illness Day 10, NS = not significant.
CA outcomes
The median BSA calculated by either the Du Bois or Haycock equations were higher for the U.S. versus the Japanese subjects, consistent with the older age of the U.S. subjects (p<0.001, Table 2). BSA values calculated by the Du Bois equation strongly correlated with values calculated by the Haycock equation (r=0.998). Values for Z-max calculated using the two different BSA equations also showed a strong positive correlation (r=0.999).
The Z-max calculated using the Dallaire equation was compared between the U.S. and Japanese subjects both as a continuous and as a categorical variable (Figures 1 and 2). The median Z-max calculated by the Dallaire equation for U.S. and Japanese KD subjects was 1.9 (IQR 1.4-2.7) and 2.3 (IQR 1.5-3.1) SD units, respectively (p<0.001; Figure 1). Using either the Fuse or the Dallaire Z-score equations, a higher percentage of U.S. subjects had normal coronary arteries (Z-max <2.5, p<0.001) and a higher percentage of Japanese subjects had a Z-max between 2.5 and 5.0 (p<0.001). Using either equation, there was no significant difference in rates of Z-max ≥5 or ≥10 between subjects in the two countries (Z-max ≥10, n (%): U.S.:15 (2.6), Japan: 7(1.4); Figure 2).
Fig 1.
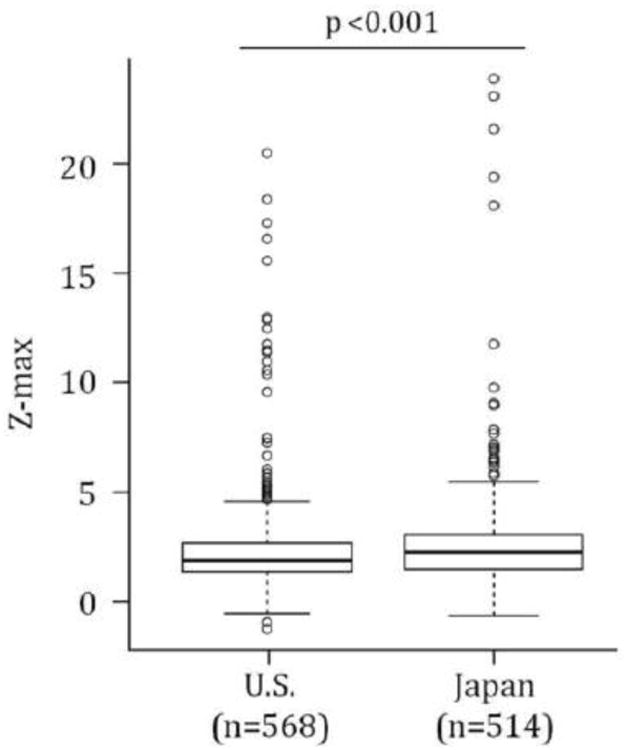
Coronary artery outcomes for Kawasaki disease subjects in the U.S. and Japan calculated using the Haycock BSA equation and Dallaire equation for Z-max. Box plots represent the median and 25-75th percentiles, whiskers represent the 5-95%, and outliers are shown as open circles.
Fig. 2.
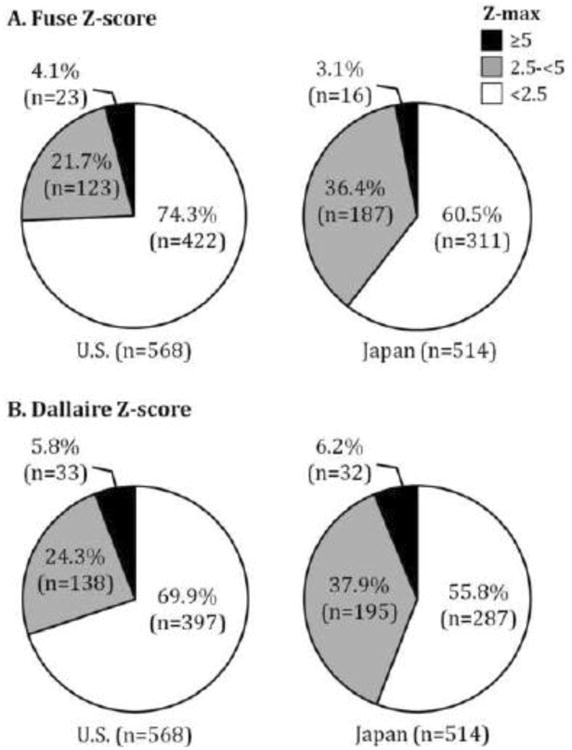
Distribution of Z-max in the U.S. and Japan using the Fuse and Dallaire equations for Z-scores. Z-max was categorized as follows: <2.5 (White), ≥2.5 (Gray) and <5.0, and ≥5.0 (Black). Body surface area was calculated by the Du Bois equation. (A) Distribution of Z-max using the Fuse Z-score. (B) Distribution of Z-max using the Dallaire Z-score. No significant difference was noted for either comparison.
The absolute measurement of CA internal diameters was compared between subjects less than 5 years of age from the two countries, which included 77.8% of the U.S. subjects and 86.8% of Japanese subjects. The median maximal internal diameter was identical (2.3 mm) between subjects from the two countries. Similarly, there was no significant difference in the percentage of subjects classified as having transiently dilated, >3-≤4mm, or >4mm CA based on the JCS guidelines (Transient dilatation: U.S.: 23 (7.4%), Japan: 19 (5.2%); Maximal internal diameter >3-≤4mm: U.S.: 62 (10.9%), Japan: 42 (8.2%); >4mm: U.S.: 24 (4.2%), Japan: 15 (2.9%)).
Multiple linear regression analysis was performed to identify independent risk factors for Z-max. The model demonstrated that younger age, male sex, late treatment, failure to respond to initial IVIG infusion, and being Japanese were independent risk factors for a higher Z-max (Table 3). In other words, after adjusting for age, sex, and treatment response, Japanese patients still had a higher Z-max score compared to U.S. patients.
Table 3.
Multivariable linear regression analysis for independent factors influencing Z-max.
| β | Standard error | p | |
|---|---|---|---|
| Age | -0.007 | 0.002 | 0.001 |
| Male sex | -0.502 | 0.145 | <0.001 |
| Illness day at diagnosis | 0.032 | 0.030 | NS |
| Complete or incomplete KD | 0.120 | 0.175 | NS |
| Late treatment | 0.979 | 0.380 | 0.010 |
| IVIG non-responder | 1.656 | 0.171 | <0.001 |
| Japan | 0.347 | 0.155 | 0.026 |
Discussion
This is the first study to directly compare CA outcomes in Japanese and American KD patients using standardized definitions across populations. The median Z-max was significantly higher for Japanese subjects using the Dallaire Z-score equation. When analyzed as a categorical variable, there was no difference in the rate of patients with Z-max scores ≥ 5.0 or ≥10.0 using this definition. A higher percentage of Japanese children were classified as having at least one coronary artery segment with a Z-max ≥ 2.5 -<5.0, regardless of the Z-score method used. Use of Z-score equations formulated based on either Japanese or North American children had no effect on the results. Predictors of a higher Z-max score included younger age, male sex, late treatment, and IVIG-resistance as has been previously reported [7, 20, 27]. Despite adjusting for these factors, Japanese KD patients were still more likely to have a higher Z-max. Among the U.S patients, there were fewer than five Japanese subjects. Therefore, there was little overlap between the U.S. and Japanese groups and these results may reflect true genetic differences in susceptibility to coronary artery damage [28, 29].
Although it has been frequently claimed that aneurysm rates in U.S. children are higher than in Japanese children, our findings do not support this claim [7, 9, 17, 19]. The CA aneurysm rate has been reported from several countries, but the timing of echocardiography during the illness has varied and the use of Z-scores has not been widely adopted, thus making meaningful comparisons across populations difficult [7-16]. Indeed, comparing rates from these published reports can lead to erroneous conclusions regarding possible genetic differences in response to IVIG or to susceptibility to aneurysm formation [28, 29]. Our results suggest that use of different CA definitions may have led to false conclusions about rates of CA aneurysms in the U.S. and Japan.
Two different equations were used for calculating Z-score to evaluate and compare their performance. The Fuse equation was created based on the size of CAs of over 4,000 healthy Japanese children. These data were analyzed with the least mean squares (LMS) method [23, 30, 31]. The Dallaire Z-score equation was created based on 1,033 healthy children in Canada [25]. The clear advantage of using Z-scores for the evaluation of CA dimensions in the pediatric population is the normalization for BSA and the use of specific criteria for right and left CAs [25, 32-34]. The distribution of Z-max scores ≥5.0 was slightly different between the two methods (Figure 2). This underscores the importance of using the same Z-score method for sequential measurements on the same patient over time.
To estimate the BSA, we were forced to use Du Bois equation for the Fuse Z-score because this equation is integrated into the Z-score Calculator (ZSP version 9). However, the Du Bois equation was based on adult subjects and one individual with short stature secondary to hypothyroidism and it is known to be inaccurate for infants younger than 3 months and neonates [22]. Fortunately, the older age of the majority of our KD subjects resulted in an excellent correlation when comparing BSA calculated using either the Du Bois or the Haycock equations [24]. A similar conclusion was reached by Dallaire and colleagues who also found good concordance between the two equations [25].
This study revealed not only differences in coronary outcome between the two countries, but also highlighted differences in medical practice. While Illness day at diagnosis was later and the number of late treatment cases was higher in the U.S. study population, a diagnosis of incomplete KD and failure to respond to the initial IVIG treatment were more common in Japan (Table 2). These results may suggest differences in case recognition and more delays in KD diagnosis in the U.S.
We recognize several strengths and limitations to our study. This is the first attempt to analyze and compare data obtained by echocardiography from a large sample of KD children in the U.S. and Japan. The five centers involved in the study have actively participated in KD research and thus are familiar with the standard approaches to diagnosis and management of KD patients. Our study underscores the potential benefit of using Z-scores to compare CA outcomes. However, this retrospective, multi-center study has all the inherent limitations associated with a retrospective study design. Because the evaluation of echocardiograms was determined by the participating institutions, there may be unknown or unmeasured biases in the data. Lack of single observer, blinded interpretation of echocardiograms, differences in equipment, and the lack of pre-specified methodology for measurement of the coronary arteries are all limitations of this study. For this reason, the inferences can only be considered exploratory and hypothesis generating and would need to be validated in a future, prospective study. The predictive value of Z-scores to assess long term outcome and coronary artery pathology in adult life is unknown. Specifically, the significance of having a Z-max score between 2.5 and 5.0 that resolves over the first two months after disease onset can only be answered by long-term follow-up studies of this population into adulthood.
In summary, although it has been claimed that the rate of CA abnormalities in patients with KD in Japan is lower than in the U.S., use of Z-scores demonstrated that the opposite was in fact the case. Standardization of classification schemes for coronary artery damage and the use of Z-scores will facilitate comparisons across different populations of KD patients.
Acknowledgments
Acknowledgement of grant support: This work supported in part by grants from the National Institutes of Health, National Heart, Lung, Blood Institute HL69413 and 108460 awarded to JCB and a Japan Foundation for Pediatric Research grant awarded to SO.
Footnotes
Potential conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–7. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 2.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–85. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki A, Kamiya T, Kuwahara N, Ono Y, Kohata T, Takahashi O, et al. Coronary arterial lesions of Kawasaki disease: cardiac catheterization findings of 1100 cases. Pediatr Cardiol. 1986;7:3–9. doi: 10.1007/BF02315475. [DOI] [PubMed] [Google Scholar]
- 4.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–44. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 5.Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22:79–85. doi: 10.2188/jea.JE20110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taubert KA, Rowley AH, Shulman ST. Nationwide survey of Kawasaki disease and acute rheumatic fever. J Pediatr. 1991;119:279–82. doi: 10.1016/s0022-3476(05)80742-5. [DOI] [PubMed] [Google Scholar]
- 7.Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153:117–21. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin YHT, Manlhiot C, Ching JCY, Han RK, Nield LE, Dillenburg R, et al. Repeated systematic surveillance of Kawasaki disease in Ontario from 1995 to 2006. Pediatrics International. 2010;52:699–706. doi: 10.1111/j.1442-200X.2010.03092.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Yashiro M, Uehara R, Sadakane A, Tsuboi S, Aoyama Y, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2009-2010 nationwide survey. J Epidemiol. 2012;22:216–21. doi: 10.2188/jea.JE20110126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park YW, Han JW, Hong YM, Ma JS, Cha SH, Kwon TC, et al. Epidemiological features of Kawasaki disease in Korea, 2006-2008. Pediatr Int. 2011;53:36–9. doi: 10.1111/j.1442-200X.2010.03178.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang WC, Huang LM, Chang IS, Chang LY, Chiang BL, Chen PJ, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123:e401–5. doi: 10.1542/peds.2008-2187. [DOI] [PubMed] [Google Scholar]
- 12.Du ZD, Zhao D, Du J, Zhang YL, Lin Y, Liu C, et al. Epidemiologic study on Kawasaki disease in Beijing from 2000 through 2004. Pediatr Infect Dis J. 2007;26:449–51. doi: 10.1097/01.inf.0000261196.79223.18. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Aulakh R, Bhalla AK, Suri D, Manojkumar R, Narula N, et al. Is Kawasaki disease incidence rising in Chandigarh, North India? Arch Dis Child. 2011;96:137–40. doi: 10.1136/adc.2010.194001. [DOI] [PubMed] [Google Scholar]
- 14.Heaton P, Wilson N, Nicholson R, Doran J, Parsons A, Aiken G. Kawasaki disease in New Zealand. J Paediatr Child H. 2006;42:184–90. doi: 10.1111/j.1440-1754.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 15.Lynch M, Holman RC, Mulligan A, Belay ED, Schonberger LB. Kawasaki syndrome hospitalizations in Ireland, 1996 through 2000. Pediatr Infect Dis J. 2003;22:959–63. doi: 10.1097/01.inf.0000095194.83814.ee. [DOI] [PubMed] [Google Scholar]
- 16.Fischer TK, Holman RC, Yorita KL, Belay ED, Melbye M, Koch A. Kawasaki syndrome in Denmark. Pediatr Infect Dis J. 2007;26:411–5. doi: 10.1097/01.inf.0000259964.47941.00. [DOI] [PubMed] [Google Scholar]
- 17.Belay ED, Maddox RA, Holman RC, Curns AT, Ballah K, Schonberger LB. Kawasaki syndrome and risk factors for coronary artery abnormalities: United States, 1994-2003. Pediatr Infect Dis J. 2006;25:245–9. doi: 10.1097/01.inf.0000202068.30956.16. [DOI] [PubMed] [Google Scholar]
- 18.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–33. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 19.Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2008)--digest version. Circ J. 2010;74:1989–2020. doi: 10.1253/circj.cj-10-74-0903. [DOI] [PubMed] [Google Scholar]
- 20.Sonobe T, Kiyosawa N, Tsuchiya K, Aso S, Imada Y, Imai Y, et al. Prevalence of coronary artery abnormality in incomplete Kawasaki disease. Pediatr Int. 2007;49:421–6. doi: 10.1111/j.1442-200X.2007.02396.x. [DOI] [PubMed] [Google Scholar]
- 21.Margossian R, Lu M, Minich LL, Bradley TJ, Cohen MS, Li JS, et al. Predictors of coronary artery visualization in Kawasaki disease. J Am Soc Echocardiogr. 2011;24:53–9. doi: 10.1016/j.echo.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–11. discussion 12-3. [PubMed] [Google Scholar]
- 23.Wang Y, Moss J, Thisted R. Predictors of body surface area. J Clin Anesth. 1992;4:4–10. doi: 10.1016/0952-8180(92)90111-d. [DOI] [PubMed] [Google Scholar]
- 24.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–6. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 25.Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr. 2011;24:60–74. doi: 10.1016/j.echo.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Manlhiot C, Millar K, Golding F, McCrindle BW. Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardiol. 2010;31:242–9. doi: 10.1007/s00246-009-9599-7. [DOI] [PubMed] [Google Scholar]
- 27.Muta H, Ishii M, Yashiro M, Uehara R, Nakamura Y. Late intravenous immunoglobulin treatment in patients with Kawasaki disease. Pediatrics. 2012;129:e291–7. doi: 10.1542/peds.2011-1704. [DOI] [PubMed] [Google Scholar]
- 28.Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40:35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khor CC, Davila S, Breunis WB, Lee YC, Shimizu C, Wright VJ, et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet. 2011;43:1241–6. doi: 10.1038/ng.981. [DOI] [PubMed] [Google Scholar]
- 30.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–19. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 31.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–29. [PubMed] [Google Scholar]
- 32.McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, et al. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116:174–9. doi: 10.1161/CIRCULATIONAHA.107.690875. [DOI] [PubMed] [Google Scholar]
- 33.de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133:254–8. doi: 10.1016/s0022-3476(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 34.Kurotobi S, Nagai T, Kawakami N, Sano T. Coronary diameter in normal infants, children and patients with Kawasaki disease. Pediatr Int. 2002;44:1–4. doi: 10.1046/j.1442-200x.2002.01508.x. [DOI] [PubMed] [Google Scholar]


