Abstract
Aim:
Endoplasmic reticulum (ER) stress plays an important role in the pathogenesis of insulin resistance and pancreatic β-cell dysfunction. The aim of this study is to investigate whether the insulin-sensitizing action of berberine is related to reducing ER stress.
Methods:
ER stress in cultured Hep G2 cells was induced with tunicamycin. Cells were pretreated with berberine in combination with or without insulin. The concentration of glucose was measured by glucose oxidase method. The molecular markers of ER stress, including ORP150, PERK, and eIF2α were analyzed by Western blot or real time PCR. The activity of JNK was also evaluated. Moreover, the insulin signaling proteins such as IRS-1 and AKT were determined by Western blot.
Results:
The production of glucose stimulated with insulin was reduced. The expressions of ORP150 was decreased both in gene and protein levels when cells were pretreated with berberine, while the activation of JNK was blocked. The levels of phosphorylation both on PERK and eIF2α were inhibited in cells pretreated with berberine. The level of IRS-1 ser307 phosphorylation was decreased, whereas IRS-1 tyr phosphorylation was increased notablely. AKT ser473 phosphorylation was also enhanced significantly in the presence of berberine.
Conclusion:
The antidiabetic effect of berberine in Hep G2 cells maybe related to attenuation of ER stress and improvement of insulin signal transduction.
Keywords: endoplasmic reticulum stress, berberine, insulin resistance, Hep G2 cells, tunicamycin
Introduction
Type 2 diabetes (T2DM) is one of the most prevalent and serious metabolic diseases in the world. Persons with T2DM consistently demonstrate three cardinal abnormalities: resistance to the action of insulin in peripheral tissues, particularly muscle and fat but also liver; defective of insulin secretion, especially in response to a glucose stimulus, and increased glucose production by the liver. The inability of pancreatic β cells to adapt the reduction in insulin sensitivity may lead to the onset of glucose intolerance. Once hyperglycemia becomes apparent, insulin resistance is further increased and β cell function progressively deteriorates.
Recent studies suggest that endoplasmic reticulum (ER) stress plays an important role in the onset of the state of obesity, insulin resistance and T2DM1, 2, 3. ER stress in hepatocytes and adipocytes may suppress insulin signaling via activated signaling cascades especially c-Jun N-ternimal kinase (JNK) that inhibits insulin receptor substrate-1 (IRS-1) tyrosine phosphorylation and increases serine phosphorylation3, 4, 5, 6. Whereas, agents that alleviate ER stress may improve insulin signal transduction in vitro and in vivo7, 8, 9, 10. Because reduction of ER stress could be a potential therapeutic target for diabetes, more and more pharmaceutical trials are developing new agents to relieve ER stress. Studies indicate that chemical compounds such as 4-phenyl butyrate (PBA), methoxyflavones, and ursodeoxycholic acid proved to affect the stabilization of mutant proteins and/or the facilitation of transport of mutant proteins at the site of activity, that can improve ER folding capacity and modulate ER function10, 11, 12. However, high dosages of chemical chaperones are required to improve folding capacity of ER by nonspecific mechanisms and their therapeutic value is limited.
Recent identification of biological compounds derived from plants proved to be effective in modulating glycolipid metabolism7, 13, 14 . Rizoma coptidis (RC) and its main alkaloids compound berberine have been widely used as an anti-inflammatory and antiviral traditional medicine for a long time in Asia. Recent studies also show that berberine is effective in lowering blood glucose, improving insulin resistance and lowering blood lipid in murine experiments14, 15, 16 . Our previous studies also found that berberine might stimulate insulin secretion, improve insulin action and modulate lipid metabolism17, 18. Nevertheless, the mechanism underlying insulin sensitization of berberine is not well revealed. Based on the above concept that ER stress relates to the development of insulin resistance, we try to investigate the effect of berberine on regulating glucose production in hepatocytes on the state of ER stress and clarify whether this capacity of insulin sensitization relates to reducing ER stress.
Materials and methods
Materials
Human hepatoblastoma cell line (Hep G2) was obtained from China Center of Type Culture Collection (Wuhan, China). Fetal bovine serum (FBS) was purchased from Gibco Company (Grand Island, NY). Dulbecco's Modified Eagle's Medium (DMEM) containing high glucose was purchased from Hyclone Company (Logan, Utah). Chemicals including tunicamycin (Tu), PBA, berberine and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich Corporation (St Louis, MO). Insulin was purchased from Amersham (Arlington Heights, IL). Polyclonal antibodies against human JNK1, PERK, phospho-PERK (thr981), IRS-1, phospho-IRS-1 (ser307, tyr1222), Akt, phospho-Akt (ser473) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), eIF2α, phospho-eIF2α (ser51) and phospho-c-Jun antibody was purchased from Cell Signaling (Beverly, MA), Polyclonal antibodies of ORP150 was obtained from Abcam (Cambridge, USA). All other chemicals were purchased from authentic sources and were of superior grade and purity.
Cell culture and treatment
Hep G2 cells were grown in 90-mm Petri dishes in high-glucose DMEM (containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin) and maintained in a humidified incubator at 37 °C with 5% CO2. ER stress in Hep G2 cells was induced by Tu as the method described previously9, 10. Briefly, after reaching 70%–80% confluency, cells were washed three times with DMEM without serum and transferred into medium containing 2% FBS. Then cells were cultured in medium containing 2% FBS with PBA (10 mmol/L) or berberine (0, 10, and 20 μmol/L). After the incubation for 14 h, Tu (0.5 or 2.0 μg/mL) was gently added to the medium to avoid any environmental stress due to vibration or temperature changes. Then cells were incubated for another 4 h and prepared for the following measurements.
Microculture tetrazolium (MTT) assay
To ascertain the range of nontoxic doses of berberine, MTT assay was established with some modifications. Briefly, Hep G2 cells at the density of 5×104 cells/well in 100 μL culture medium were seed into microtiter plates (tissue culture grade, 96 wells), and cells were cultured for 24 h at a humidified atmosphere. After another 24-h incubation with different doses of berberine (from 0 to 50 μmol/L), 10 μL of the MTT labeling reagent (final concentration 0.5 mg/mL) was added to each well. Cells were incubated for the next 4 h and then the medium was discarded. The blue crystals, which were the metabolized product of MTT, were extracted by DMSO. The absorbance of the samples was measured with an ELISA scanner at the wavelength of 400 and 550 nm to estimate the proportion of surviving cells.
Measurements of glucose production
After cells were pretreated with different concentrations of berberine or PBA and exposure to Tu, the amount of glucose production was determining as follow: the cells were incubated with the buffer consisting of glucose-free DMEM supplemented with 5 mmol/L alanine, glycine, and valine, pyruvate and lactate for 2 h. Then medium was collected for glucose determination with glucose oxidase method. Each point represented the average of six independent measurements19, 20, 21. All these assays were adjusted by MTT OD22.
Real-time RT-PCR analysis
Total RNA was isolated from Hep G2 cells using TRIzol regent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. cDNA from total RNA was synthesized with reverse-transcription reaction using a ThermoScript RT-PCR system (Toyobo, Osaka, Japan). The primers of ORP150 and β-actin were synthesized by Sangon Biotechnology Company (Shanghai, China). The primer sequences are listed in Table 1.
Table 1. PCR primer pairs used to amplify ORP150 and β-actin cDNA fragments.
| Target | Oligonucleotide sequence (5′-3′) | Tm (°C) | bp | |
|---|---|---|---|---|
| PRP150 | F | TTGACTCAAACCTGTCCAAC | 51 | 172 |
| R | ACAAGAATGAACCTGGCTGT | |||
| β-actin | F | AACTGGGACGACATGGAGAA | 53 | 276 |
| R | ATACCCCTCGTAGATGGGCA |
F, forward primer; R, reverse primer; Tm, melting temperature.
Real-time PCR analysis was performed in a final volume of 25 μL containing 12.5 μL SYBR Green I fluorescence using a LightCycler instrument (Roche Diagnostic, Mannhein, Germany). The following thermal cycling profile for PCR was used: one cycle at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, and at 58 °C for 5 s, with a final extension step at 72 °C for 30 min. Then Real-time PCR products were analyzed by melting curve to confirm the amplification. The housekeeping gene β-actin was used for confirmation of similar cDNA loading.
Western blotting analysis
HepG2 cells incubated with berberine or PBA in the present or absent of insulin were lysed in RIPA ice-cold buffer (l50 mmol/L Tris-HCl with pH 7.4, 130 mmol/L NaCl, 5 mmol/L EDTA, 1.0% Nonidet P-40, 100 mmol/L NaF, 50 mmol/L β-glycerophosphate, 100 μmol/L NaVO4, 1 mmol/L phenylmethylsulfonyl fluoride, 5 μg/mL leupeptin, and 5 μg/mL aprotinin). Cell lysates were incubated on ice for 30 min and then centrifuged at 15 000×g for 20 min at 4 °C. Total protein concentration was measured using BCA method. To determine the amount of protein expression, equivalent amounts of protein (75 μg) of each sample were denatured in 5×loading buffer and boiled at 100 °C for 5 min. Equal amounts of protein extracts were separated by SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE) on 8% or 10% polyacrylamide gels and transferred to poly vinylidene fluoride (PVDF) membranes. Membranes were then blocked in 5% blocking reagent and incubated overnight at 4 °C with primary antibodies diluted in Tris-Buffered Saline-Tween 20 (TBST). After overnight incubation, the membranes were washed four times by incubating 15 min in TBST and were incubated with secondary antibodies conjugated with horseradish peroxidase for 2 h on room temperature. Then membranes were washed four times in TBST for 15 min again. Proteins were visualized with an ECL detection system. Band intensities on the autoradiography were quantified using Quantity one software (Bio-Rad).
Statistical analysis
Data were shown as mean±SD. One-way ANOVA was used to determine statistically significant differences between groups. P<0.01 was considered statistically significant.
Results
Cytotoxicity of berberine on Hep G2 cells
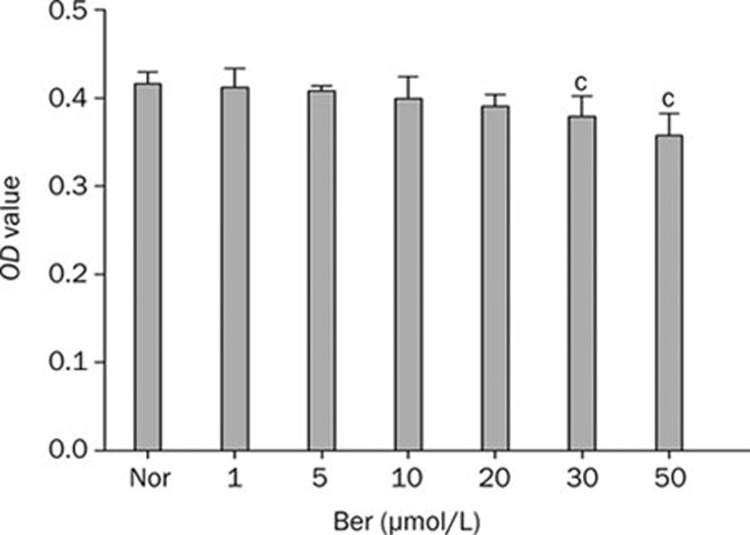
It was reported that high concentration of berberine could inhibit cell's activities17, 23. In order to determine the appropriate concentration of berberine in our study, we first examined the cytotoxicity of berberine on Hep G2 cells. As shown in Figure 1, the growth and proliferation of cells were not affected when cells were incubated with berberine at the concentration below 20 μmol/L in the medium. However, it was observed that berberine inhibited Hep G2 cells activities significantly when its concentration reached to 30 μmol/L. It indicated that berberine with the concentration under 20 μmol/L was suitable to study for the bioactivity assay. Based on the above result, berberine at the concentration of 10 and 20 μmol/L were chosen in the subsequent experiments.
Figure 1.
Dose-dependent cytotoxicity of berberine on Hep G2 cells. Cells were incubated with different concentrations of berberine for 24 h. Data were means±SEM. n=6 in each group. cP<0.01 vs Nor.
Berberine influences glucose production in Hep G2 cells under ER stress state
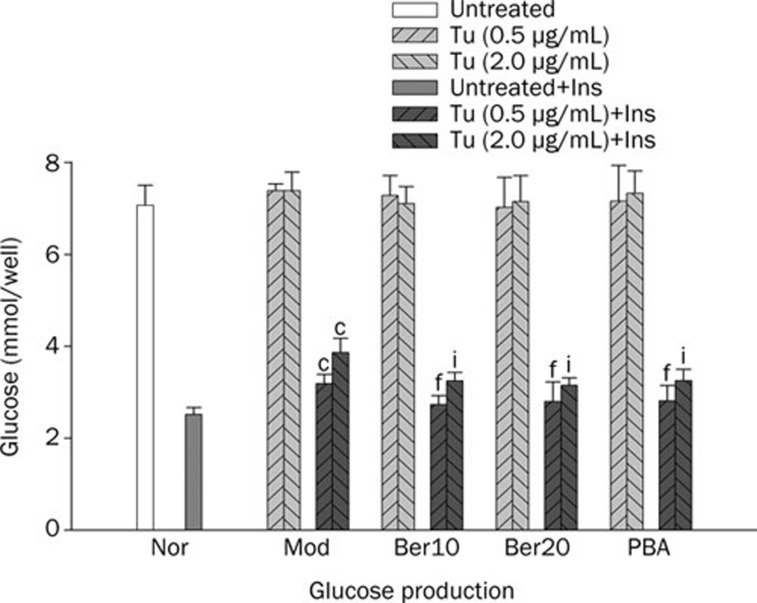
Tunicamycin, a widely used inhibitor of glycosylation, was chosen to induce ER stress in Hep G2 cells. We first investigated the effect of berberine on regulating glucose production in cells under ER stress. Our data demonstrated Tu did not influence glucose production without insulin even though the concentration of Tu reached to 2.0 μg/mL. We therefore inferred that glucose production on basal state was not influenced when cells were induced to ER stress (Figure 2). However, glucose production decreased in the presence of insulin, and the inhibiting effect of insulin on glucose production was weakened more apparently when the concentration of Tu up to 2.0 μg/mL (Figure 2). The data suggested that the effect of insulin on inhibiting glucose output was blocked in hepatocytes under ER stress state. We then observed the effect of berberine on glucose production on the state of ER stress with or without the stimulation of insulin (Figure 2). Our research demonstrated that the effect of berberine on glucose production was insulin dependent for we could not detect the change of glucose production when cells without the stimulation of insulin even though the concentration of berberine up to 20 μmol/L (Figure 2). However, glucose production was suppressed when cells were pretreated with berberlin in the present of insulin (Figure 2). It suggested that berberine could not influence glucose production on basal state when cells were under ER stress. Similar to berberine, PBA, a low molecular weight chaperone known to stabilize protein conformation and improve ER folding capacity10, 24, exhibited the capacity of inhibiting glucose production in cells under ER stress with the stimulation of insulin and had no effect without insulin.
Figure 2.
Effect of berberine on basal and insulin-stimulated glucose production in HepG2 cells. Tests were performed in the absence or presence of insulin (100 nmol/L) after cells were exposed to Tu and pretreated with berberine. Data are means±SEM. n=6 in each condition. cP<0.01 vs Nor. fP<0.01 vs Tu (0.5 μg/mL). iP<0.01 vs Tu (2.0 μg/mL).
Effects of berberine on ER stress Hep G2 cells induced with Tu
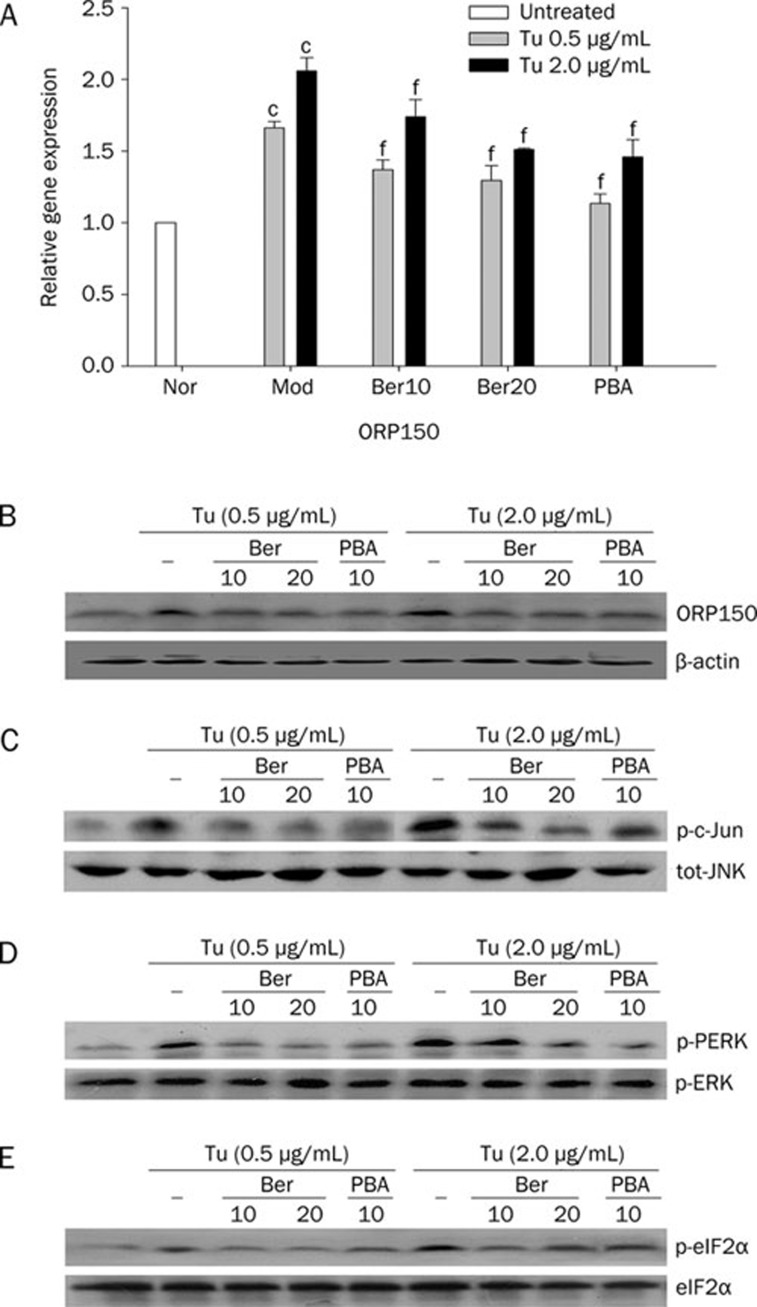
To investigate whether berberine could reduce Tu-induced ER stress in Hep G2 cells to elucidate the molecular mechanism of berberine's antidiabetes properties, we examined the expression of specific moleculars of ER stress. As shown in Figure 3A and Figure 3B, the expression of ORP150 was increased in cells exposed to Tu compared to normal cells both in the levels of mRNA and protein, importantly, the elevation was more remarkable when the concentration of Tu was up to 2.0 μg/mL (Figure 3A and Figure 3B). Similarly, the activity of total JNK was enhanced by Tu in a dose dependent manner which was indicated by a dramatically elevated of c-Jun phosphorylation (Figure 3C)9. In correspondance with these changes, as the key indicators of the present of ER stress, both the phosphorylation status of PERK and eIF2α were enhanced when cells were treated with Tu (Figure 3D and Figure 3E). All these data above demonstrated that Hep G2 cells were induced to ER stress with Tu. Interestingly, the potency of Tu on stress was blocked when cells were pretreated with berberine. Our findings demonstrated that the expression of ORP150 were inhibited both at the levels of mRNA and proteins when cells were pretreated with berberine though the expression of stress chaperone were enhanced in comparison with normal cells. Moreover, the obvious inhibition effect of berberine was observed when the concentration up to 20 μmol/L (Figure 3A and Figure 3B). Additionally, berberine also exhibited an ability of inhibiting the activation of JNK caused by Tu, which was presented by down-regulation of the expression of c-Jun phosphorylation. We also found that the expression of total JNK protein did not change when cells were pretreated with berberine (Figure 3C). Furthermore, berberine suppressed Tu induced phosphorylation of PERK and eIF2α profoundly even though the concentration of Tu up to 2.0 μg/mL (Figure 3D and Figure 3E). Similar to berberine, our research verify that PBA significantly decreased the expression of ORP 150 (Figure 3A and Figure 3B) and inhibited the activation of JNK as reported (Figure 3C)10, 25. PBA could also inhibit the phosphorylation of PERK and eIF2α significantly (Figure 3D and Figure 3E). In addition, there was no difference between the group of PBA and the group of berberine when the concentration of berberine was up to 20 μmol/L. Therefore, it was indicated that berberine had protective effects on Hep G2 cells against ER stress induced with Tu.
Figure 3.
Effect of berberine on the expressions of markers of ER stress in HepG2 cells. Cells were pretreated with or without berberine in the absence or presence of different concentrations of Tu for 4 h. The mRNA expression of ORP150 (A) was analyzed using quantitative real-time RT-PCR whereas the protein level of ORP150 was analyzed with Western blot analysis (B). Total JNK, p-c-Jun, PERK, and eIF2α were examined by Western blot analysis (C, D, and E). Data are means±SEM of three independent experiments in each condition. cP<0.01 vs Nor. fP<0.01 vs Mod.
Berberine improves ER stress-induced insulin resistance in Hep G2 cells
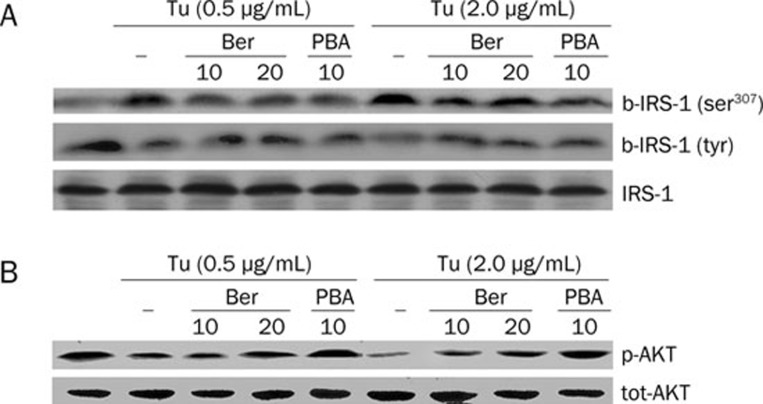
We finally examined whether the effects of berberine on stress were associated with the enhanced signaling capacity of the insulin. We evaluated the expression of major elements involved in insulin signal transduction. When Hep G2 cells were under conditions of ER stress induced by Tu, ser307 phosphorylation of IRS-1 was elevated whereas tyr phosphorylation of IRS-1 was decreased and the expression of total IRS-1 did not change. Moreover, as a distally molecular of insulin cascade, ser473 phosphorylation of Akt was suppressed in cells exposure to Tu whereas expression of total Akt was not effected, these changes suggested that Tu could suppress the signaling capacity of insulin receptor on Hep G2 cells (Figure 4). In contrast, when cells were pretreated with berberine, the expression of IRS-1 ser307 phosphorylation was decreased while the expression of IRS-1 tyr phosphorylation and Akt ser473 phosphorylation was increased significantly. However, expressions of total IRS-1 and Akt did not change (Figure 4). Therefore, it indicates that berberine can enhance insulin action in cells under ER stress. Similarly, PBA also improved ER stress-induced insulin resistance in Hep G2 cells by inhibiting ser307 phosphorylation and enhancing IRS-1 tyr phosphorylation and Akt ser473 phosphorylation (Figure 4). These findings suggested that berberine enhances insulin sensitivity by protecting IRS-1 proteins from serine phosphorylation, restoring IRS-1 tyrosine phosphorylation and Akt ser473 phosphorylation without any change of total levels of IRS-1 and AKT (Figure 5).
Figure 4.
Effect of berberine on insulin signaling transduction of HepG2 cells. Cells were pretreated with berberine and exposed to different concentrations of Tu, total proteins were detected by Western blot analysis, cells were stimulated with insulin insulin (100 nmol/L) for 20 min to measure the levels of phosphorylation. Total protein concentration and phosphorylation level of IRS-1 (A) and AKT (B) were shown respectively. Data are means±SEM of three independent experiments in each condition.
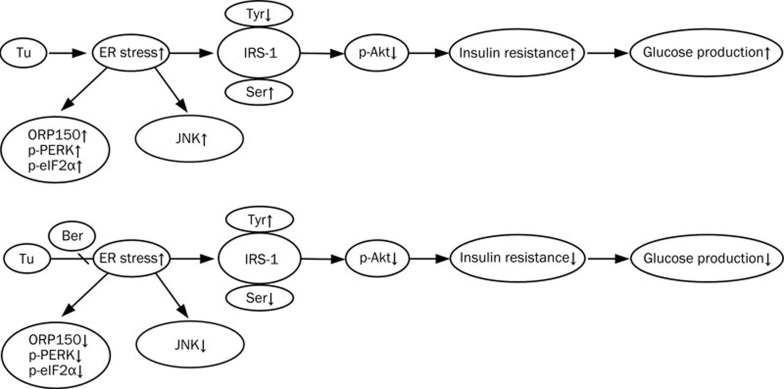
Figure 5.
The effects of the berberine on ER stress and insulin resistance. The ER stress in the hepatocyte cells was induced with Tu. The activity of JNK was elevated and expression ORP150 were up regulated, at the same time, phosphorylation of PERK and eIF2α were enhanced, serine phosphorylation of IRS-1 was increased while tyrosine phosphorylation of IRS-1 was inhibited, phosphorylation of AKT was decreased too. All these changes lead to an increase in insulin resistance and glucose production was enhanced. Treated with berberine reversed these changes and enhances insulin signaling which leads to a decrease of glucose production in cells stimulated with insulin.
Discussion
In the present study, we reported a novel finding that pretreatment with berberine could effectively protect cells from the damage of ER stress on Hep G2 cells. The effect can enhance insulin action and result in the decrease of hepatocyte glucose production with an insulin dependent manner in Tu-induced ER stress of Hep G2 cells. The ability of berberine on protecting cells from damage of ER stress may relate to the inhibition of JNK activity and the suppression of the major elements of ER signal transduction pathway including of PERK, eIF2α, and ORP150. Insulin signal transduction may then be enhanced by decreasing insulin-stimulated ser307 phosphorylation whereas increasing tyr phosphorylation of IRS-1 and enhancing Ser473 phosphorylation of Akt. These may be a mechanistic connection existed between improvement of insulin signal transduction and inhibition of Tu-mediated ER stress on the antidiabetic ability of berberine.
Insulin inhibits the production and release of glucose in liver through a direct on glycogenolysis, it also has an indirect effect on liver glucose output through gluconeogenesis26, 27, 28. In the condition of insulin resistance or diabetes, less glucogen synthesis, more glucogenesis and less glucose consumption in peripheral tissues may lead to hyperglycemia. Therefore, glucose metabolism in hepatocytes plays a major role in regulating glucose flux29. Actually, resent studies have provided convincing evidence that hepatic glucose production plays an important role in the development of hyperglycemia in diabetes. Based on this conception, the reduction of hepatic glucose production has been certainly considered for the therapeutic target of diabetes. In fact, recent studies also confirmed that the inhibition of hepatic glucose production may be a potential therapy for the treatment of diabetes30. In view of the critical role of ER stress in the pathogenesis of T2DM, we investigate the change of glucose production in Hep G2 cells under ER stress. The results showed that glucose production was disturbed when cells exposed to Tu, a reagent widely used to induce ER stress in vitro, with an insulin dependent manner. Glucose production was increased when cells were stimulated with insulin while no changes were observed without insulin. Therefore, we think it might be one of the causes for hyperglycemia under ER stress. However, berberine can effectively reduce hepatocyte glucose production in the state of ER stress stimulated with insulin in an insulin dependent manner. Based on the previous studies that berberine is an active compound that has an effective capacity in reducing glucose levels in rodents with insulin resistance15, 16, 17. Here we found that berberine could inhibit glucose production in hepatocytes under ER stress in vitro. However, these effects were of insulin dependent in our research because berberine hardly showed the effect in regulating glucose production without insulin.
Recent studies suggest that ER stress may play a key role in the development of insulin resistance and diabetes through triggering JNK activity and impairing insulin signal transduction6, 9, 10. Therefore, agents that alleviate ER stress may be as potential application in the treatment of diabetes. Previous studies also verified this hypothesis that administration of active chemical chaperones on obese or diabetic mice can reduce ER stress by increasing folding capacity, which can restore systemic insulin sensitivity, enhance peripheral insulin activity and normalize the state of hyperglycemia. Based on this discovery, we further investigated the effect of berberine on JNK activity and other indicators of ER stress including PERK and eIF2α on Hep G2 cells induced to ER stress with Tu. In the present study, we found that phosphorylation of c-Jun, which represents total JNK activity, was reduced by berberine. At the same time, both the PERK and eIF2α phosphorylation were suppressed. As an ER stress-associated chaperone, systemic expressions of ORP150 may enhance glucose uptake and suppress protein oxidation in murine type 2 diabetes. Overexpressions of ORP150 may improve insulin sensitivity in myoblast cells treated with hydrogen peroxide3, 8. Nevertheless, previous researches also found that expression of ORP150 was increased in cells of Tu-induced ER stress31, 32. Human ORP150 gene maps also found that the expression of ORP150 was increased remarkably in skeletal muscles of insulin resistant subjects compared with that of insulin sensitive subjects33. Based on the above paradox, the role of ORP150 on the pathogenesis of insulin resistance need to be identified in the further studies but it was not the focus on our present research. Here, we found the expression of ORP150 was increased both in mRNA and protein levels in cells incubated with Tu. However, the expression of ORP150 was inhibited when cells were pretreated with PBA. Similarly, when cells were pretreated with berberine, the expression of ORP150 was also decreased. Moreover, the inhibition of gene expression was observed obviously in the concentration of berberine was up to 20 μmol/L. All these results indicated that berberine could protect hepatocytes from the damage of ER stress.
Insulin resistance in T2DM exhibits many defects such as down-regulation of receptor and receptor substrate levels, impairments of kinase activity and PI3K activity, decrease of glucose transporter translocation and activity of intracellular enzymes34. Insulin receptor substrate (IRS) proteins, molecules existed inside cytoplasm, are crucial for mediating insulin action cascade. Serine phosphorylation of IRS-1 may negatively regulates insulin receptor signaling and be considered as a common reason for functional inhibition of IRS-1 protein. Among the serine residues, which are phosphorylated in response to risk factors of insulin resistance, ser307 phosphorylation is known to be the common molecular indicators. Studies reveal that ser307 phosphorylation of IRS-1 increases in the state of insulin resistance4, 34, 35. In the present study, we found that Tu enhanced ser307 phosphorylation of IRS-1 and suppressed tyr phosphorylation of IRS-1, and finally inhibited ser473 phosphorylation of Akt significantly. Nevertheless, these effects were reversed when cells were pretreated with berberine. Our previous study also demonstrated that berberine could reverse the level of IKKβ ser181 and IRS-1 ser307 phosphorylation in 3T3-L1 adipocytes of insulin resistance18. These results indicate that berberine might improve insulin signal transduction when cells were induced to ER stress.
In conclusion, we demonstrated that berberine, similar to PBA, attenuated ER stress induced by Tu and then improved insulin resistance in Hep G2 cells. However, berberine can alleviate ER stress with relative lower dosage and no side effects in contrast to PBA. These findings further imply that berberine can be used as a potential antidiabetic agent in treating type 2 diabetes.
Author contribution
Fu-er LU designed research; Zeng-si WANG and Li-jun XU performed research; Zeng-si WANG and Hui DONG analyzed data; Zeng-si WANG and Fu-er LU wrote the paper.
Acknowledgments
This study was supported by grants from National Natural Science Foundation of China (No 30772853).
References
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, Hatazaki M, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–63. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Han KL, Choi JS, Lee JY, Song J, Joe MK, Jung MH, et al. Therapeutic potential of peroxisome proliferators — activated receptor-alpha/gamma dual agonist with alleviation of endoplasmic reticulum stress for the treatment of diabetes. Diabetes. 2008;57:737–45. doi: 10.2337/db07-0972. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–51. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, et al. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36:592–601. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- Takano K, Tabata Y, Kitao Y, Murakami R, Suzuki H, Yamada M, et al. Methoxyflavones protect cells against endoplasmic reticulum stress and neurotoxin. Am J Physiol Cell Physiol. 2007;292:C353–61. doi: 10.1152/ajpcell.00388.2006. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, Lin CT, et al. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3:417–27. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–64. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–8. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- Leng SH, Lu FE, Xu LJ. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol Sin. 2004;25:496–502. [PubMed] [Google Scholar]
- Yi P, Lu FE, Xu LJ, Chen G, Dong H, Wang KF. Berberine reverses free-fatty-acid-induced insulin resistance in 3T3-L1 adipocytes through targeting IKKbeta. World J Gastroenterol. 2008;14:876–83. doi: 10.3748/wjg.14.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Seoane J, Trinh K, O'Doherty RM, Gomez-Foix AM, Lange AJ, Newgard CB, et al. Metabolic impact of adenovirus-mediated overexpression of the glucose-6-phosphatase catalytic subunit in hepatocytes. J Biol Chem. 1997;272:26972–7. doi: 10.1074/jbc.272.43.26972. [DOI] [PubMed] [Google Scholar]
- Lin YY, Lu JY, Zhang J, Walter W, Dang W, Wan J, et al. Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell. 2009;136:1073–84. doi: 10.1016/j.cell.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, et al. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51:1439–43. doi: 10.1053/meta.2002.34715. [DOI] [PubMed] [Google Scholar]
- Zhou L, Yang Y, Wang X, Liu S, Shang W, Yuan G, et al. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism. 2007;56:405–12. doi: 10.1016/j.metabol.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Vilatoba M, Eckstein C, Bilbao G, Smyth CA, Jenkins S, Thompson JA, et al. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery. 2005;138:342–51. doi: 10.1016/j.surg.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–32. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes. 2005;54:1942–8. doi: 10.2337/diabetes.54.7.1942. [DOI] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–20. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins QF, Xiong Y, Lupo EG, Jr, Liu HY, Cao W. p38 Mitogen-activated protein kinase mediates free fatty acid-induced gluconeogenesis in hepatocytes. J Biol Chem. 2006;281:24336–44. doi: 10.1074/jbc.M602177200. [DOI] [PubMed] [Google Scholar]
- Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48:1198–214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- Kurukulasuriya R, Link JT, Madar DJ, Pei Z, Richards SJ, Rohde JJ, et al. Potential drug targets and progress towards pharmacologic inhibition of hepatic glucose production. Curr Med Chem. 2003;10:123–53. doi: 10.2174/0929867033368556. [DOI] [PubMed] [Google Scholar]
- Kuwabara K, Matsumoto M, Ikeda J, Hori O, Ogawa S, Maeda Y, et al. Purification and characterization of a novel stress protein, the 150-kDa oxygen-regulated protein (ORP150), from cultured rat astrocytes and its expression in ischemic mouse brain. J Biol Chem. 1996;271:5025–32. doi: 10.1074/jbc.271.9.5025. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Kondo T, Hori O, Kitao Y, Stern DM, Eisenmenger W, et al. Expression of the oxygen-regulated protein ORP150 accelerates wound healing by modulating intracellular VEGF transport. J Clin Invest. 2001;108:41–50. doi: 10.1172/JCI11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs P, Yang X, Permana PA, Bogardus C, Baier LJ. Polymorphisms in the oxygen-regulated protein 150 gene (ORP150) are associated with insulin resistance in Pima Indians. Diabetes. 2002;51:1618–21. doi: 10.2337/diabetes.51.5.1618. [DOI] [PubMed] [Google Scholar]
- Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–9. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–6. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]