Abstract
Aim:
To investigate the effects of glycyrrhetinic acid (GA), an active component extracted from the root of Glycyrrhizae glabra, on the expression of intercellular adhesion molecule-1 (ICAM-1) in tumor necrosis factor-α (TNF-α)-activated human umbilical vein endothelial cells (HUVEC).
Methods:
ICAM-1 mRNA and protein levels were detected using RT-PCR and cell enzyme-linked immunosorbent assays. The adherence of human monocytic THP-1 cells labeled with [3H]thymidine to HUVEC was determined by counting radioactivity with a scintillation counter. The activation of mitogen-activated protein kinases as well as the degradation of IκB and nuclear factor-κB (NF-κB) or phospho-c-Jun in the nucleus were detected by western blots. NF-κB binding activity was detected using electrophoretic mobility shift assay.
Results:
GA (50 and 100 μmol/L) significantly inhibits TNF-α-induced ICAM-1 mRNA and protein expressions, as well as THP-1 cell adhesiveness in HUVEC. GA selectively inhibited TNF-α-activated signal pathway of c-Jun N-terminal kinase (JNK), without affecting extracellular signal-regulated kinase 1/2 and p38. Furthermore, GA apparently inhibited IκB/NF-κB signaling system by preventing IκB degradation, NF-κB translocation, and NF-κB/DNA binding activity. Finally, pretreatment with GA or the inhibitors of NF-κB, JNK, and p38 reduced the ICAM-1 protein expression induced by TNF-α.
Conclusion:
GA inhibits TNF-α-stimulated ICAM-1 expression, leading to a decrease in adherent monocytes to HUVEC. This inhibition is attributed to GA interruption of both JNK/c-Jun and IκB/NF-κB signaling pathways, which decrease activator protein-1 (AP-1) and NF-κB mediated ICAM-1 expressions. The results suggest that GA may provide a beneficial effect in treating vascular diseases associated with inflammation, such as atherosclerosis.
Keywords: glycyrrhetinic acid, intercellular adhesion molecule 1, tumor necrosis factor α, nuclear factor-κB, mitogen-activated protein kinases
Introduction
During inflammation, adhesion molecules expressed on stimulated vascular endothelial cells are essential for recruitment and transmigration of leukocytes to the subendothelial matrix. In this context, the released inflammatory mediators, such as tumor necrosis factor-α (TNF-α), activate the rapid expression of intercellular adhesion molecule-1 (ICAM-1), an inducible cell surface glycoprotein, to facilitate leukocytes adhesion and transmigration in vascular endothelial cells. Increased levels of ICAM-1 expression in vascular endothelial cells are critically implicated in the development of a variety of autoimmune and pathological inflammatory diseases, including atherosclerosis, rheumatoid arthritis, and asthma1, 2, 3. TNF-α, one of the major inflammatory cytokines, mediates systemic inflammation and immune responses by enhancing expression of adhesion molecules and secretion of inflammatory mediators in the vascular endothelium4, 5. Accumulating evidence suggests that induction of ICAM-1 by TNF-α in vascular endothelial cells is mediated by the activation of mitogen-activated protein kinases (MAPK) and transcription factors such as nuclear factor-κB (NF-κB) and activator protein-1 (AP-1)6, 7, 8. MAPK are a group of highly conserved serine/threonine kinases, including extracellular signal-regulated kinase 1/2 (ERK1/2), p38, and c-Jun N-terminal kinase (JNK). These protein kinases participate in the mediation of the vessel inflammatory reaction from the integration of the stimuli acting on the cell to the production of inflammatory mediators via phosphorylating downstream kinases and activating transcription factors9, 10. NF-κB and AP-1 are redox-sensitive transcription factors that regulate many genes related to inflammation at the transcriptional level. With TNF-α stimulation, the NF-κB signal transduction pathway is directly engaged by the TNF-α receptors, whereas the AP-1 is activated by an indirect mechanism involving MAPK11. Down-regulation of ICAM-1 expression in vascular endothelial cells by interrupting the signal pathway of MAPK and/or NF-κB with pharmacological agents is believed to be a clinically beneficial strategy for the treatment of vascular diseases associated with inflammation.
Licorice—the root of Glycyrrhizae glabra, one of the most commonly prescribed herbs in Chinese traditional medicine—has been used in the treatment of inflammatory diseases, such as peptic ulcers, and chronic hepatitis. Glycyrrhetinic acid (GA), which possesses both anti-inflammatory and antioxidant properties, is the active triterpenoic saponin extracted from licorice and has been extensively studied. Previous studies have demonstrated that GA reduces the expression of pro-inflammatory mediators, such as prostaglandin E2, histamine, and eotaxin 112, 13, 14. It was also reported that GA scavenges free radical species by increasing superoxide dismutase activity in atherosclerotic and hypercholesterolemic rabbits15. More recently, studies have suggested that GA and its derivatives may abolish TNF-α-induced leukocyte adhesion and extravasation in the in vivo microcirculation, and attenuate the elevation of ICAM-1 expression in rat models of hepatic injury and colic ulceration16, 17, 18. However, the effect of GA on ICAM-1 expression in cytokine-activated vascular endothelial cells is not clearly defined.
In this study, we investigated the effects of GA on TNF-α-induced expression of ICAM-1 in human umbilical vein endothelial cells (HUVEC). We further verified the signaling mechanism responsible for GA regulation of ICAM-1 expression and determined the function of GA on preventing monocytic THP-1 cell adhesion to TNF-α-activated endothelial cells. Our study may provide a basis for therapeutic use of GA in vascular inflammatory disorders.
Materials and methods
Chemicals
Glycyrrhetinic acid was purchased from Nacalai Tsque (Kyoto, Japan). All other chemicals of reagent grade and the antibodies were obtained from Sigma Chemical (St Louis, MO).
Cell culture
Human umbilical vein endothelial cells were isolated from human umbilical cord with collagenase. After 3 days of growth in medium 199 (Invitrogen, Carlsbad, CA) containing 20% fetal calf serum, endothelial cells were subcultured and reseeded into a suitable culture plate until the monolayer became confluent. The medium for the cultured cells was changed to the same medium containing only 2% fetal calf serum, and the cells were incubated overnight before the experiments.
Cell viability
After treatment with glycyrrhetinic acid at indicated concentrations for 24 h, cell viability was assessed by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide [thiazolyl blue tetrazolium bromide (MTT)] assay according to the manufacturer's instructions (Roche, Mannheim, Germany).
Measurement of ICAM-1 mRNA and protein expressions
ICAM-1 mRNA and protein levels were measured as previously described19. After extraction of total RNA from treated or untreated HUVEC, the expressions of the transcripts for ICAM-1 (forward primer, 5′-CAGTGACCATCTACAGCTTTCCGG-3′ and reverse primer, 5′-GCTGCTACCACAGTGATGATGACAA-3′), and β-actin (forward primer, 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ reverse primer, 5′-CTAGAAGCATTTGCGGGGACGATGGAGGG-3′) were determined by RT-PCR. Reaction products were separated electrophoretically on a 1.8% agarose gel and stained with ethidium bromide. The relative density of mRNA levels was determined by scanning densitometry analysis using the GeneTools program, version 4.0 (SynGene, Cambridge, UK). The expression of β-actin mRNA was used as internal control. For ICAM-1 protein detection, cell surface expression of ICAM-1 by endothelial cells was determined using cell ELISA. Briefly, after treatments, the cells were washed with phosphate-buffered saline pH 7.4 (PBS) and fixed with 4% paraformaldehyde for 30 min at 4 °C. Non-fat dry milk (3.0% in PBS) was added to the monolayers to reduce nonspecific binding. Cells were incubated with anti-ICAM-1 monoclonal antibodies overnight at 4 °C after washing three times with PBS. With removal of the first antibodies, cells were incubated with peroxidase-conjugated goat anti-mouse secondary antibody. Thereafter, cells were washed with PBS and exposed to the peroxidase substrate. The increase in absorbance at 450 nm was measured using an automated ELISA microplate reader.
Cell adherence measurements
The human monocytic cell line, THP-1, obtained from the American Type Culture Collection (Manassas, VA, USA), was suspended overnight in RPMI-1640 medium containing 0.1% fetal bovine serum and labeled with 1 μCi of [3H]thymidine (specific activity, 23 Ci/mmol; Amersham Biosciences, UK). THP-1 cells (3×105) were washed three times in fresh RPMI-1640 medium, and then added to each 6-well plate containing endothelial cells for 30 min incubation. Nonadherent THP-1 cells were removed by washing with medium 199. Endothelial cells with adherent THP-1 cells were lysed with lysis buffer, and radioactivity expressed from cell lysates was counted by a scintillation counter.
Immunoblot analysis
Endothelial cells were lysed in lysis buffer (containing 0.2% SDS, 1% NP-40, 5 mmol/L EDTA, 1 mmol/L PMSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin) and then analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After being transferred onto a nitrocellulose membrane, antigens were analyzed by specific antibodies to IκBα, phospho-MAPK (ERK1/2, p38, and JNK), ICAM-1, and actin. For the measurement of NF-κB or c-Jun activation, nuclear protein was prepared as previously described, with slight modifications20. In brief, cells were washed with cold PBS and immediately removed by scraping in PBS. After centrifugation of the cell suspension at 2000 r/min, the cell pellets were resuspended in cold buffer A (10 mmol/L KCl, 0.1 mmol/L EDTA, 1 mmol/L dithiothreitol, and 1 mmol/L phenylmethylsulfonyl fluoride) for 15 min. Cells were lysed by adding 10% NP-40 and then centrifuged at 6000 r/min to obtain a pellet of nuclei. The nucleic pellets were resuspended in lysis buffer and separated by SDS-PAGE. An antibody specific to NF-κB p65 or phospho-c-Jun was used. The housekeeping protein α-actin was detected as an internal control. Antigen-antibody complexes were detected using horseradish peroxide-labeled rabbit anti-mouse IgG and an ECL detection system (Pierce, Rockford, IL, USA). The relative density of protein levels was determined by scanning densitometry analysis using the GeneTools program, version 4.0 (SynGene, Cambridge, UK).
Electrophoretic mobility shift assay
Nuclear protein extracts were prepared and an electrophoretic mobility shift assay (EMSA) was performed as previously reported20. Briefly, an NF-κB oligonucleotide, 5′-AGTTGAGGGGACTTTCCCAGGC-3′ (Promega, Madison, WI, USA), containing the consensus NF-κB binding sequence (underlined) was end-labeled with [γ-32P]ATP using T4 polynucleotide kinase. Nuclear extract (10 μg) was incubated with 0.1 ng 32P-labeled DNA for 20 min at room temperature in a final volume of binding buffer of 30 μL containing 1 μg of poly (dI-dC). The mixtures were electrophoresed on 5% nondenaturing polyacrylamide gels under high ionic strength. Gels were dried and imaged by autoradiography.
Statistical analysis
Statistical analyses were performed using a one-way analysis of variance for experiments consisting of more than two groups. Data were presented as mean±SEM. Statistical significance was defined as P<0.05.
Results
GA attenuates ICAM-1 mRNA and protein expressions in TNF-α-activated HUVEC
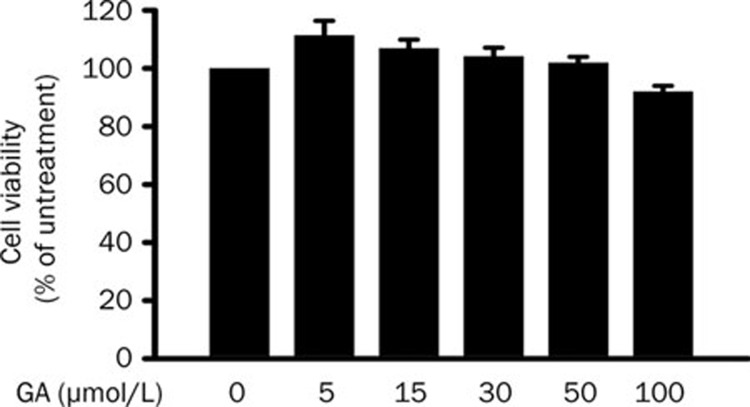
The cytotoxicity of GA on HUVEC was measured by an MTT assay in which MTT-formazan adduct was detected. Cell viability was calculated as percentage of untreated control. More than 90% of HUVEC survived after 24-h treatment of GA at concentrations up to 100 μmol/L (Figure 1). This result shows that GA has no significant cytotoxicity to HUVEC.
Figure 1.
Glycyrrhetinic acid (GA) has no effect on endothelial cell viability. After treatment with different concentrations of GA for 24 h, the viability of ECs were determined by MTT analysis. Data represent mean±SEM obtained from 5 independent experiments.
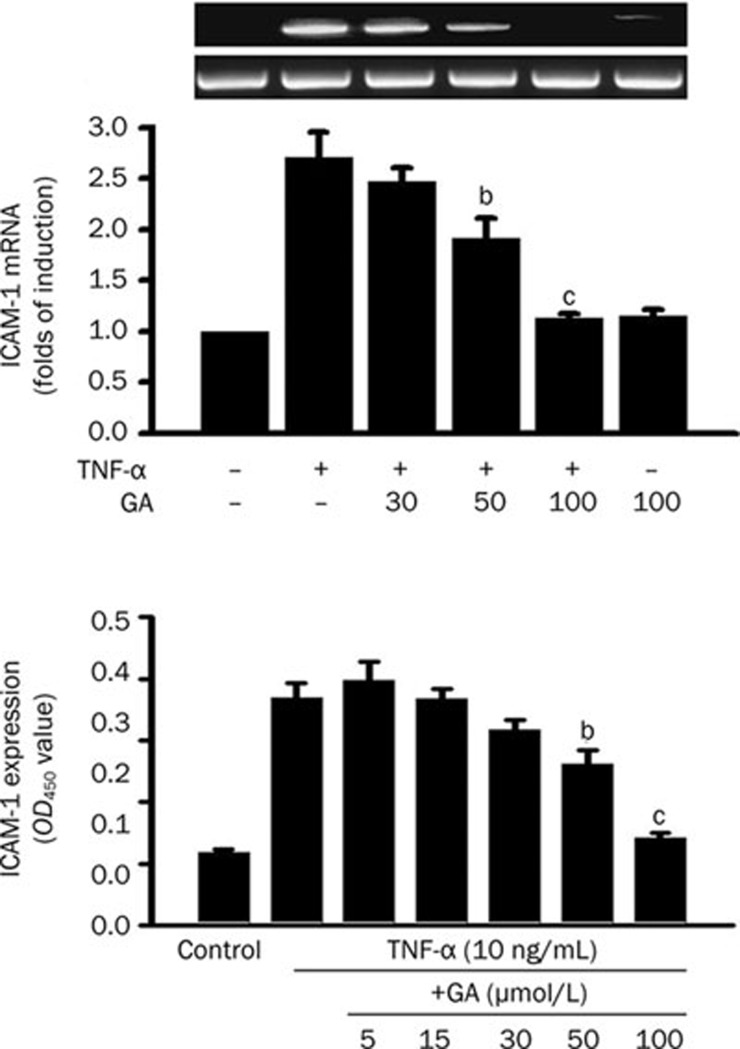
The effect of GA on TNF-α-induced ICAM-1 mRNA and protein expressions in HUVEC were examined. To determine the effect of GA on TNF-α-induced ICAM-1 mRNA expression, cells with or without 3-h GA pretreatment were incubated with TNF-α (10 ng/mL) for 2 h, and thereafter, total RNA was extracted. The expression of ICAM-1 mRNA was detected using RT-PCR. TNF-α alone significantly increased the level of ICAM-1 mRNA expression by a factor of 2.7 over the control (Figure 2A). GA pretreatment clearly inhibited ICAM-1 mRNA activated by TNF-α in a dose-dependent manner. On the other hand, GA (100 μmol/L) treatment alone showed no significant difference in the level of ICAM-1 mRNA expression compared with the untreated control.
Figure 2.
Glycyrrhetinic acid (GA) dose-dependently inhibits TNF-induced ICAM-1 mRNA and protein expressions in HUVEC. (A) For measurement of ICAM-1 mRNA, cells were pretreated with GA as indicated concentrations for 3 h and then stimulated with TNF-α (10 ng/mL) for 2 h. Total RNA was extracted and quantitated using RT-PCR. (B) For measurement of the cell-surface expression of ICAM-1 in HUVEC, cells were pretreated with GA for 3 h and costimulated with TNF-α (10 ng/mL) for 8 h. Thereafter, cell-ELISA was performed. Data represent mean±SEM obtained from 3 or 5 independent experiments, respectively. bP<0.05; cP<0.01 vs TNF-α treatment only.
For measurement of ICAM-1 protein expression on the endothelial surface, cells were treated with TNF-α (10 ng/mL) for 8 h in the presence or absence of a 3-h GA pretreatment, and then a cell-ELISA assay was performed. Endothelial cells treated with TNF-α showed significant increases in the expression of ICAM-1 (Figure 2B). This induction, which caused a greater than 3.5-fold increase in control expression, was decreased in a dose-dependent manner by GA pretreatment. GA significantly inhibited TNF-α-induced ICAM-1 expression by 29%±6% at 50 μmol/L and by 61%±1% at 100 μmol/L.
GA suppresses THP-1 cell adhesion to TNF-α-activated HUVEC
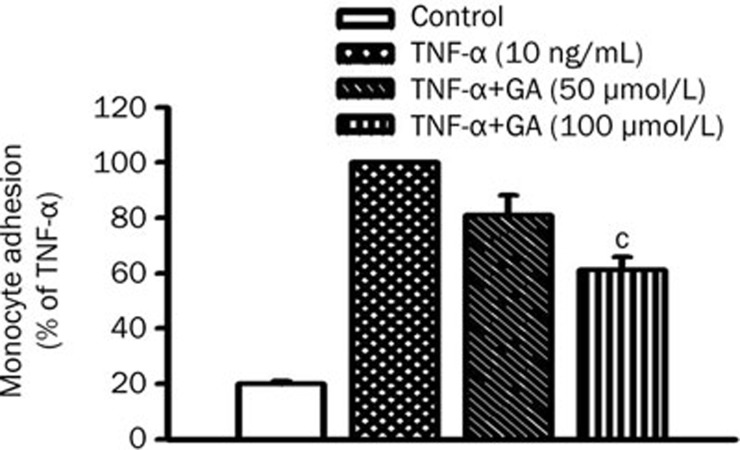
To determine whether GA inhibition of ICAM-1 expression decreases the monocytic THP-1 cells' adhesion to HUVEC, the adhesiveness of THP-1 cells to TNF-α-activated HUVEC with or without GA pretreatment was examined. Cells were incubated with or without GA for 3 h prior to the treatment of TNF-α. TNF-α treatment induced the adherence of THP-1 cell to HUVEC by 5-fold of control. GA pretreatment clearly reduced TNF-α induction of THP-1 cell adhesion by 19%±7% at 50 μmol/L and by 39%±5% at 100 μmol/L, respectively (Figure 3). Our data indicate that GA significantly reduces ICAM-1 expression and THP-1 cell adhesion to TNF-α-activated HUVEC.
Figure 3.
GA inhibits monocyte adhesion to TNF-α-activated HUVEC. Cells pretreated with GA (50 or 100 μmol/L) for 3 h were coincubated with TNF-α (10 ng/mL) for another 8 h. [3H]thymidine-labeled monocytic THP-1 cells were added and incubated for 30 min after TNF-α incubation. HUVEC were lysed after removal of nonadherent THP-1 cells, and total radioactivity of cell lysate was counted by liquid scintillation. Data represent mean±SEM obtained from 5 independent experiments. cP<0.01 vs TNF-α treatment only.
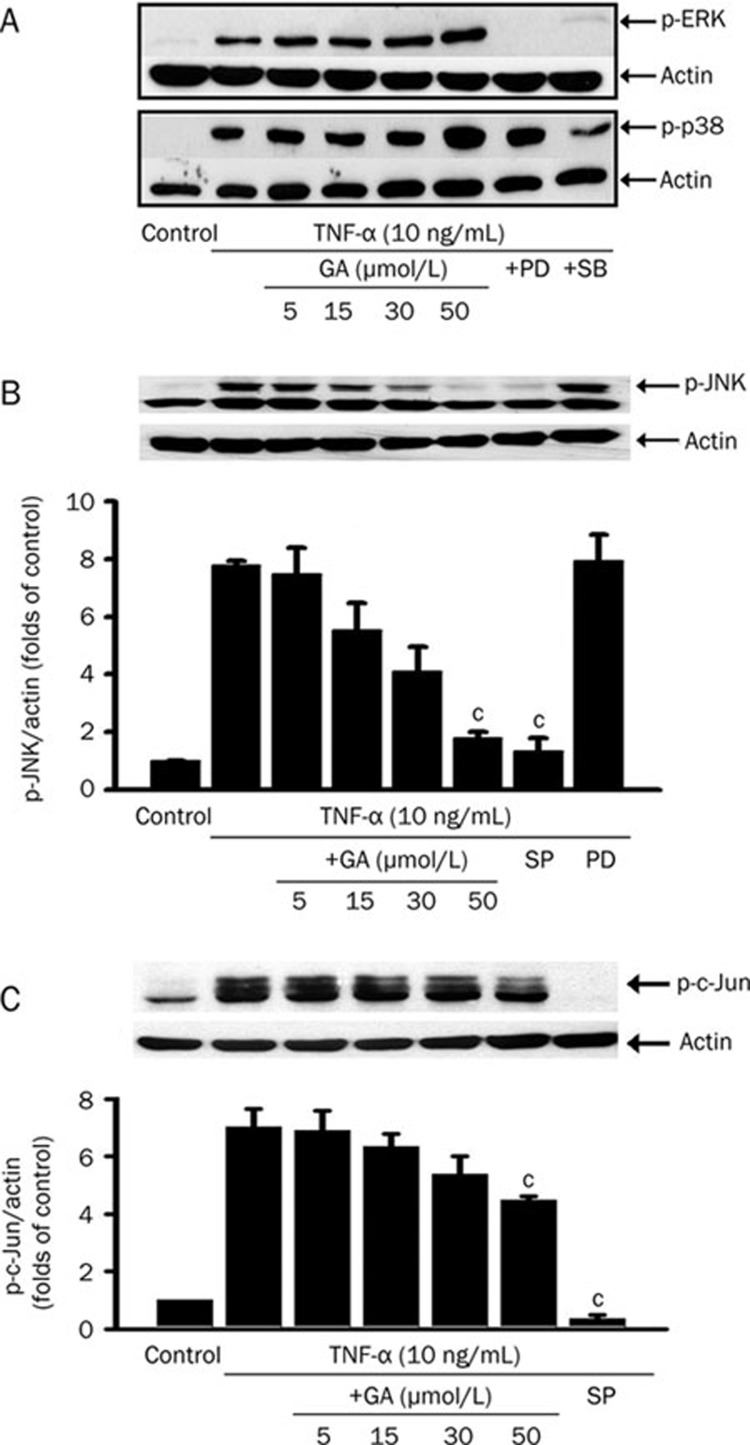
GA inhibits TNF-α-induced phosphorylation of JNK and c-Jun
To verify whether GA affects TNF-α-induced MAPK activation, the phosphorylation of ERK1/2, p38, or JNK was detected using Western blot. The cells were stimulated by TNF-α (10 ng/mL) for 20 min with or without GA pretreatment at indicated concentrations for 3 h. GA showed no influence on TNF-α-induced activations of ERK1/2 and p38 in HUVEC (Figure 4A). In contrast, GA inhibited TNF-α-induced JNK phosphorylation in a dose-dependent manner (Figure 4B).
Figure 4.
Glycyrrhetinic acid (GA) inhibits JNK/c-Jun signaling pathway activated by TNF-α in HUVEC. The representative immunoblots of phospho-ERK and phospho-p38 (A); the bar chart plotted according to the data obtained from quantification of immunoblots of phospho-JNK (B) and phospho-c-Jun (C) are shown. After treatment with GA at indicated concentrations for 3 h or MAPK inhibitors (50 μmol/L PD98059, an inhibitor of ERK; 20 μmol/L SB203580, an inhibitor of p38; 20 μmol/L SP600125, an inhibitor of JNK and c-Jun) for 1 h, cells were stimulated with TNF-α (10 ng/mL) for 20 min. Total cell lysate (ERK, p38, and JNK) or nuclear protein (c-Jun) was separated by SDS-PAGE. Actin was measured as an internal control. Data represent mean±SEM obtained from 3 independent experiments. cP<0.01 vs TNF-α treatment only.
We next determined whether the blockage of JNK activation by GA is followed by a decrease in nuclear phosphorylation of c-Jun. The nuclear protein was extracted from the cell treated with TNF-α (10 ng/mL) for 20 min in the presence or absence of GA pretreatment, and the level of phospho-c-Jun was measured by Western blot. GA treatment also decreased c-Jun phosphorylation in the nucleus (Figure 4C). These findings indicate that GA selectively blocks TNF-α-stimulated JNK activation and subsequently inhibits c-Jun phosphorylation in the nucleus.
GA inhibits TNF-α-activated IκB/NF-κB pathway in HUVEC
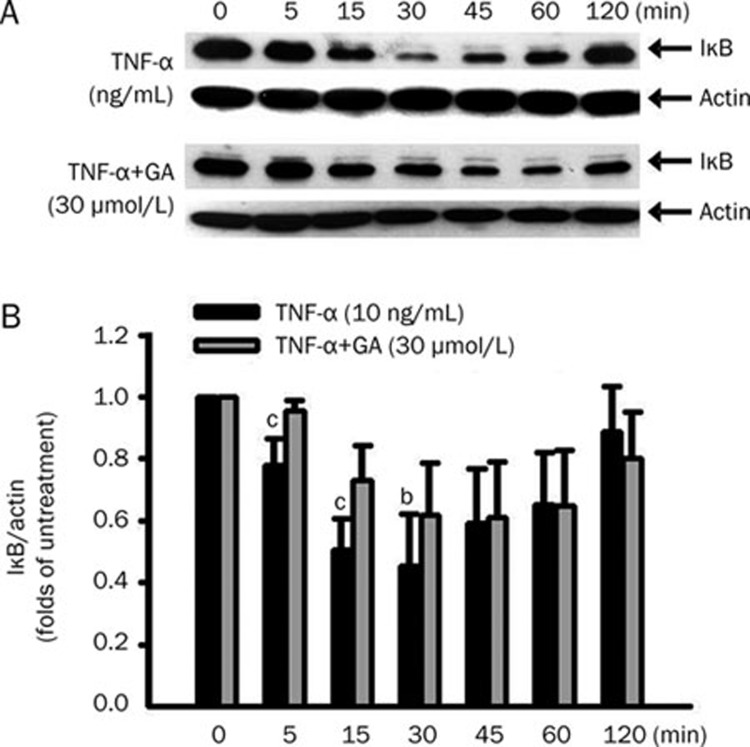
To determine whether GA inhibition of ICAM-1 expression is mediated by blocking the IκB/NF-κB pathway, the protein levels of IκB in cytosol and NF-κB p65 in the nucleus were analyzed by Western blot. With or without pretreatment of GA (30 μmol/L) for 3 h, endothelial cells were treated with TNF-α for the indicated time course. After TNF-α treatment, IκB protein in cytosol was apparently degraded in a time-dependent manner (Figure 5A, upper panel). At 30 min of TNF-α treatment, the protein level of IκB was significantly decreased, compared to the basal level (Figure 5A, lanes 4 and 1). Starting from the 45-min TNF-α treatment, the protein level of IκB gradually recovered until the 120 min. With GA pretreatment, IκB degradation was apparently inhibited (Figure 5A, lower panel). Densitometric analysis from the immunoblots (Figure 5A) was plotted as a bar chart (Figure 5B).
Figure 5.
Time course of IκB degradation after TNF-α treatment in the presence or absence of glycyrrhetinic acid (GA) in HUVEC. Cells were pretreated with or without 30 μmol/L GA for 3 h, then stimulated with 10 ng/mL TNF-α as indicated time-course. (A) The amounts of IκB and actin in whole-cell lysates were measured by Western blot. (B) Densitometric analysis from the above immunoblots was shown as bar chart. Data represent mean±SEM obtained from 3 independent experiments. bP<0.05, cP<0.01 vs TNF-α treatment only.
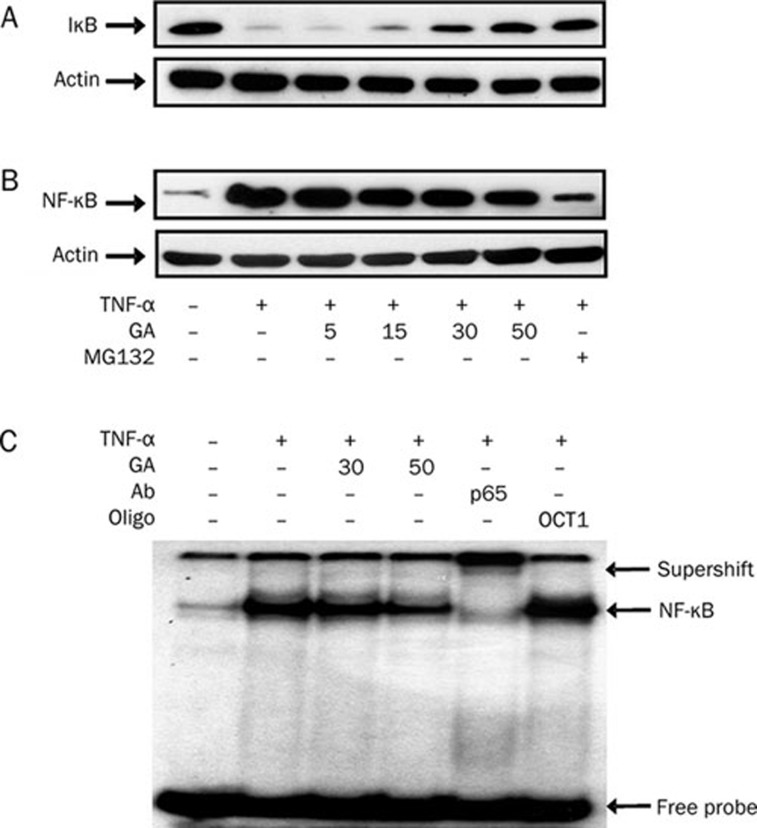
We then determined the inhibitory effects of GA at various concentrations on IκB degradation and NF-κB p65 translocation. Our results show that GA inhibited both IκB degradation and NF-κB p65 translocation in a dose-dependent manner after TNF-α treatment (Figure 6A and 6B). The NF-κB inhibitor, carbobenzoxy-L-leucy-L-leucy-L-leucinal (MG132), was used as a positive control. MG132 prevented IκB degradation (Figure 6A, lane 7) and thereby inhibited TNF-α-activated NF-κB translocation into the nucleus (Figure 6B, lane 7).
Figure 6.
Glycyrrhetinic acid (GA) dose-dependently inhibits IκB/NF-κB signaling pathway activated by TNF-α in HUVEC. Effects of GA on (A) IκB degradation, and (B) NF-κB p65 translocation. Cells were pretreated with GA at the indicated concentrations for 3 h or with an NF-κB inhibitor, MG132 (10 μmol/L), for 1 h, then stimulated with 10 ng/mL TNF-α for 30 min. Cell lysates were measured by Western blot. (C) Effect of GA on NF-κB binding activity. Nuclear extracts from HUVEC treated with TNF-α (10 ng/mL) for 1 h in the presence or absence of GA were analyzed for NF-κB binding activity by EMSA. The specificity of NF-κB binding complex was confirmed by adding an anti-NF-κB p65 antibody (lane 5), or unrelated OCT-1 oligonucleotide (lane 6), to nuclear extracts for 15 min preincubation. The data presented are from one of three experiments with similar results.
We further verified whether GA reduces the binding activity of NF-κB to consensus DNA sequences. Nuclear extracts from 1-h TNF-α-treated HUVEC in the presence or absence of GA were analyzed for NF-κB binding activity by EMSA. GA at 50 μmol/L noticeably inhibited NF-κB binding activity (Figure 6C, lane 4). The specificity to this NF-κB binding complex was confirmed by adding an anti-NF-κB p65 antibody (Figure 6C, lane 5), or an unrelated 50x OCT-1 oligonucleotide (Figure 6C, lane 6), to nuclear extracts for 15 min before incubation. Taken together, these results indicate that GA dose-dependently inhibits IκB degradation, NF-κB translocation, and NF-κB binding activity in TNF-α-activated endothelial cells.
GA and signaling inhibitors reduce ICAM-1 protein expression induced by TNF-α in HUVEC
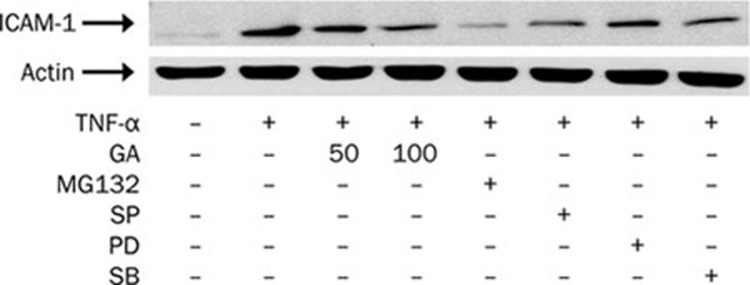
After examining GA inhibition of TNF-α-activated JNK/c-Jun and IκB/NF-κB signaling pathways, we further determined the inhibitory effect of GA on ICAM-1 protein expression compared with that of signaling inhibitors. Increased ICAM-1 protein expression by TNF-α induction was decreased by the pretreatments with GA, MG132 (an NF-κB inhibitor), SP600125 (a JNK inhibitor), and SB203580 (a p38 inhibitor), but not PD98059 (an ERK inhibitor) (Figure 7). These results indicate that TNF-α induction of ICAM-1 protein expression is mediated predominantly by the signaling pathways of NF-κB, JNK, and p38, whereas GA inhibition of ICAM-1 expression is associated with its blockade of activation in NF-κB and JNK.
Figure 7.
Glycyrrhetinic acid (GA) as well as signaling inhibitors reduces TNF-α-induced ICAM-1 protein expression in HUVEC. After treatment with GA at indicated concentrations for 3 h or signaling inhibitors (10 μmol/L MG132, an NF-κB inhibitor; 20 μmol/L SP600125, an inhibitor of JNK; 50 μmol/L PD98059, an inhibitor of ERK; 20 μmol/L SB203580, an inhibitor of p38) for 1 h, cells were stimulated with TNF-α (10 ng/mL) for another 8 h. Total cell lysate was separated by SDS-PAGE and immunoblots were analyzed with anti-ICAM-1 and anti-actin antibodies. Actin was measured as an internal control. The data presented are from one of two experiments with similar results.
Discussion
Although GA is a desirable candidate as an anti-inflammatory agent, its inhibitory effect on ICAM-1 expression in HUVEC remains unknown. In this study, we demonstrated that GA inhibits TNF-α-activated ICAM-1 mRNA and protein expressions, leading to the suppression of THP-1 cell adhesion to HUVEC. Our results suggest that GA inhibition of TNF-α-activated JNK/c-Jun and IκB/NF-κB signaling pathways, which regulate, respectively, AP-1- and NF-κB-mediated gene transcription, contributes to the suppression of ICAM-1, at least in part. In addition, our results reveal a new mechanism for the anti-inflammatory properties of GA.
ICAM-1 is considered to play a key role at the early stage of inflammatory response to facilitate leukocytes adhesion and transmigration in vascular endothelial cells. We observed that GA inhibited ICAM-1 mRNA and protein expression, as well as THP-1 cell adhesion in TNF-α-activated HUVEC, suggesting that GA reduction of monocytes adhesion is caused, at least in part, by its inhibition of ICAM-1 expression.
The cytokine TNF-α activates signaling pathway of MAPK and increases the activity of transcription factors, such as NF-κB and AP-1, which up-regulate genes relevant to the expression of adhesion molecules6, 9. Among the signaling pathways of MAPK, the JNK pathway has been identified as targeting AP-1 in response to TNF-α-induced ICAM-1 expression6, 11, 21. The AP-1 protein is a dimer of the Jun and Fos families, and its transcription activity is enhanced by JNK to phosphorylate the active domains of c-Jun at Ser 63 and Ser 7322. In this study, our data, consistent with the previous finding, demonstrate that GA selectively inhibits TNF-α-induced phosphorylation of JNK, without affecting ERK1/2 and p3823. Following this inhibition, GA also decreases the phosphorylation of c-Jun, one of the components of AP-1, in TNF-α-activated nucleus. Taken together, our results suggest that GA suppression of c-Jun phosphorylation, via the blocking of JNK signaling activation, may contribute to its reduction of ICAM-1 expression mediated by AP-1.
NF-κB regulates TNF-α-stimulated ICAM-1 expression at the transcriptional level in vascular endothelial cells. Its activity is mediated by homodimeric or heterodimeric combinations of NF-κB family proteins, such as p50, p65, and c-Rel. In its resting state, NF-κB is present in association with its cytoplasmic inhibitor, IκB. Once activated by an inflammatory cytokine, such as TNF-α, IκB was rapidly phosphorylated and degraded, leading to the translocation of activated NF-κB from the cytoplasm to the nucleus24. In this study, GA observably inhibits IκB degradation and NF-κB p65 translocation and subsequently reduces NF-κB DNA binding activity, suggesting a reduction of ICAM-1 expression by GA by blocking the classic inflammatory pathway of IκB/NF-κB system. This reduced expression may, at least in part, account for the mechanism of GA exerting its anti-inflammatory effects. Furthermore, our findings that GA as well as NF-κB and JNK inhibitors suppress TNF-α-induced ICAM-1 protein expression suggest that NF-κB and AP-1 are important in regulating cytokine induced ICAM-1 expression. In addition, GA inhibition of ICAM-1 is mediated by its down-regulation of NF-κB and JNK signaling pathways.
The MAP kinase/ERK kinase kinase-1 (MEKK-1), functional upstream of JNK, has been demonstrated to activate both the JNK and NF-κB pathways in response to TNF-α stimulation25. MEKK-1 activates IκB phosphorylation and thereby results in NF-κB translocation, which is independent of JNK activation26, 27. Although the mechanism of JNK signal inhibition by GA was not verified in this study, we observed that GA inhibited not only JNK phosphorylation but also NF-κB activation induced by TNF-α. Based on the previous reports and our findings, we speculate that GA may interrupt the upstream signaling pathway of JNK instead of the direct interaction with this kinase. Furthermore, the role of reactive oxygen species (ROS) in the TNF-α-stimulated ICAM-1 expression mediated by NF-κB activation has been documented28, 29. These ROS were reported to derive from NADPH oxidase or other sources in the endothelial cells. Evidence has suggested that GA inhibits free radical generation from cytochrome P450/NADPH system and scavenges ROS by increasing SOD activity15, 30. Therefore, the antioxidant property of GA might also be implicated in its suppression of TNF-α-stimulated ICAM-1 expression.
The evidence of activated NF-κB and increased ICAM-1 expression in human atherosclerotic plaques has suggested a very important role for NF-κB-mediated ICAM-1 expression in the early stage of atherogenesis31, 32. Furthermore, licorice and the ammonium salt of GA showing antioxidant and hypolipidemic properties in hypercholesterolemic human and animal models are considered effective inhibitors of the progression of atherosclerotic lesions30, 33. Our data, therefore, may provide insights into the mechanism of licorice and the derivative of GA with respect to anti-atherosclerotic activity.
In summary, our results demonstrate that GA inhibits ICAM-1 expression via interruption of the JNK/c-Jun and IκB/NF-κB signaling pathways and subsequently suppresses monocyte adhesion to TNF-α-activated endothelial cells. These findings may provide evidence that GA is salutary in treating vascular diseases with cytokine-induced inflammation.
Author contribution
Ying-ling CHANG and Jyh-sheng YOU designed research and wrote the paper; Ying-ling CHANG and Chien-lin CHEN performed research; and Chao-Lin KUO and Bor-chyuan CHEN analyzed data and plotted figures.
Acknowledgments
This work was supported by grants CMRP-D34010, CMRP-D140251, and CMRP-G1356 from Chang Gung Medical Research Foundation, Taiwan.
We thank Dr Nigel WISEMAN for critical reading of this manuscript and Yi-jun LIU for her expert technical assistance.
References
- Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171:223–9. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Haines GK, Lin TR, Harlow LA, Goerdt S, Rayan G, et al. Differential distribution of intercellular adhesion molecules (ICAM-1, ICAM-2, and ICAM-3) and the MS-1 antigen in normal and diseased human synovia. Their possible pathogenetic and clinical significance in rheumatoid arthritis. Arthritis Rheum. 1994;37:221–31. doi: 10.1002/art.1780370211. [DOI] [PubMed] [Google Scholar]
- Stanciu LA, Djukanovic R. The role of ICAM-1 on T-cells in the pathogenesis of asthma. Eur Respir J. 1998;11:949–57. doi: 10.1183/09031936.98.11040949. [DOI] [PubMed] [Google Scholar]
- Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990;70:427–51. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Kobuchi H, Roy S, Sen CK, Nguyen HG, Packer L.Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway Am J Physiol 1999277(3 Pt 1): C403–11. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Shyue SK, Hung YY, Chen YS, Ku HH, Chen JW, et al. Superoxide dismutase inhibits the expression of vascular cell adhesion molecule-1 and intracellular cell adhesion molecule-1 induced by tumor necrosis factor-α in human endothelial cells through the JNK/p38 pathways. Arterioscler Thromb Vasc Biol. 2005;25:334–40. doi: 10.1161/01.ATV.0000152114.00114.d8. [DOI] [PubMed] [Google Scholar]
- Min JK, Kim YM, Kim SW, Kwon MC, Kong YY, Hwang IK, et al. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells. J Immunol. 2005;175:531–40. doi: 10.4049/jimmunol.175.1.531. [DOI] [PubMed] [Google Scholar]
- Hoefen RJ, Berk BC. The role of MAP kinases in endothelial activation. Vasc Pharmacol. 2002;38:271–3. doi: 10.1016/s1537-1891(02)00251-3. [DOI] [PubMed] [Google Scholar]
- Wong CK, Tsang CM, Ip WK, Lam CW. Molecular mechanisms for the releases of chemokines from human leukemic mast cell line (HMC)-1 cells activated by SCF and TNF-alpha: roles of ERK, p38 MAPK, and NF-kappaB. Allergy. 2006;61:289–97. doi: 10.1111/j.1398-9995.2006.00972.x. [DOI] [PubMed] [Google Scholar]
- Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ. c-Jun NH2-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol Cell Biol. 2003;23:2871–82. doi: 10.1128/MCB.23.8.2871-2882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi K, Kamada Y, Tsurufuji S. Glycyrrhizin inhibits prostaglandin E2 production by activated peritoneal macrophages from rats. Prostaglandins Med. 1981;7:457–63. doi: 10.1016/0161-4630(81)90033-1. [DOI] [PubMed] [Google Scholar]
- Lee YM, Hirota S, Jippo-Kanemoto T, Kim HR, Shin TY, Yeon Y, et al. Inhibition of histamine synthesis by glycyrrhetinic acid in mast cells cocultured with Swii 3T3 fibroblasts. Int Arch Allergy Immunol. 1996;110:272–7. doi: 10.1159/000237298. [DOI] [PubMed] [Google Scholar]
- Matsui S, Matsumoto H, Sonoda Y, Ando K, Aizu-Yokota E, Sato T, et al. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int Immunopharmacol. 2004;4:1633–44. doi: 10.1016/j.intimp.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakirov UB, Abdullaev AKh. The hypolipidemic and antiatherosclerotic properties of the ammonium salt of glycyrrhetic acid and of 18- dehydroglycyrrhetic acid. Eksp Klin Farmakol. 1996;59:53–5. [PubMed] [Google Scholar]
- Véliz LP, González FG, Duling BR, Sáez JC, Boric MP. Functional role of gap junctions in cytokine-induced leukocyte adhesion to endothelium in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H1056–H1066. doi: 10.1152/ajpheart.00266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QZ, Lou YJ. Pathologic characteristics of immunologic injury in primary cultured rat hepatocytes and protective effect of glycyrrhizin in vitro. Acta Pharmacol Sin. 2003;24:771–7. [PubMed] [Google Scholar]
- Yuan H, Ji WS, Wu KX, Jiao JX, Sun LH, Feng YT. Anti-inflammatory effect of diammonium glycyrrhizinate in a rat model of ulcerative colitis. World J Gastroenterol. 2006;12:4578–81. doi: 10.3748/wjg.v12.i28.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B, Ghosh B. Curcuma longa inhibits TNF-alpha induced expression of adhesion molecules on human umbilical vein endothelial cells. Int J Immunopharmacol. 1999;21:745–57. doi: 10.1016/s0192-0561(99)00050-8. [DOI] [PubMed] [Google Scholar]
- Chang YL, Shen JJ, Wung BS, Cheng JJ, Wang DL. Chinese herbal remedy wogonin inhibits monocyte chemotactic protein-1 gene expression in human endothelial cells. Mol Pharmacol. 2001;60:507–13. [PubMed] [Google Scholar]
- De Cesaris P, Starace D, Starace G, Filippini A, Stefanini M, Ziparo E. Activation of Jun N-terminal kinase/stress-activated protein kinase pathway by tumor necrosis factor α leads to intercellular adhesion molecule-1 expression. J Biol Chem. 1999;274:28978–82. doi: 10.1074/jbc.274.41.28978. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–6. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Gumpricht E, Dahl R, Devereaux MW, Sokol RJ. Licorice compounds glycyrrhizin and 18beta-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J Biol Chem. 2005;280:10556–63. doi: 10.1074/jbc.M411673200. [DOI] [PubMed] [Google Scholar]
- Baldwin AS. The NF-κB, and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Karin M, Delhase M. JNK or IKK, AP-1 or NF-κB, which are the targets for MEK kinase 1 action. Proc Natl Acad Sci USA. 1998;95:9067–9. doi: 10.1073/pnas.95.16.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the IκB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–22. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- Modur V, Zimmerman GA, Prescott SM, McIntyre TM. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J Biol Chem. 1996;271:13094–102. doi: 10.1074/jbc.271.22.13094. [DOI] [PubMed] [Google Scholar]
- Fan J, Frey RS, Rahman A, Malik AB. Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-α-induced NF-κB activation and intercellular adhesion molecule-1 expression in endothelial cells. J Biol Chem. 2002;277:3404–11. doi: 10.1074/jbc.M110054200. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Ostanin D, Elrod JW, Oshima T, Jordan P, Itoh M, et al. TNF-alpha-induced endothelial cell adhesion molecule expression is cytochrome P-450 monooxygenase dependent. Am J Physiol Cell Physiol. 2003;284:C422–8. doi: 10.1152/ajpcell.00271.2002. [DOI] [PubMed] [Google Scholar]
- Ablise M, Leininger-Muller B, Wong CD, Siest G, Loppinet V, Visvikis S. Synthesis and in vitro antioxidant activity of glycyrrhetinic acid derivatives tested with the cytochrome P450/NADPH system. Chem Pharm Bull (Tokyo) 2004;52:1436–9. doi: 10.1248/cpb.52.1436. [DOI] [PubMed] [Google Scholar]
- Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–91. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, et al. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–22. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram B, Volkova N, Kaplan M, Presser D, Attias J, Hayek T, et al. Antiatherosclerotic effects of licorice extract supplementation on hypercholesterolemic patients: increased resistance of LDL to atherogenic modifications, reduced plasma lipid levels, and decreased systolic blood pressure. Nutrition. 2002;18:268–73. doi: 10.1016/s0899-9007(01)00753-5. [DOI] [PubMed] [Google Scholar]