Abstract
Aim:
To investigate the mechanism of bleomycin (BLM)-induced pulmonary fibrosis.
Methods:
Cultured human fetal lung fibroblast (HLF) cells were exposed to bleomycin (BLM) at 0–30 μg/mL for 24 h. Western blot analysis was used to detect lysyl oxidase (LO) protein expression. Real-time RT-PCR was used to detect LO mRNA level. LO catalytic activity was measured using diaminopentane as a substrate and Amplex red as a hydrogen peroxide probe. Copper (Cu) concentration was detected by flame atomic absorption spectrophotometry.
Results:
Exposure of HLF cells to BLM at 10 μg/mL and 30 μg/mL increased LO catalytic activity to 130% and 158% of the control in the conditioned media. The expression of LO mRNA was increased to 5.5-fold of the control in HLF cells exposure to BLM at 3 μg/mL. BLM at 3 μg/mL also increased the expression of 46 kDa preproLO, 50 kDa proLO and 32 kDa mature LO to 219%, 130%, and 135% of the control, respectively. The Cu concentrations in conditioned media of cultured HLF cells exposed to BLM (10 and 30 μg/mL) were increased significantly to 1.48 and 2.46-fold of the control, respectively.
Conclusion:
Bleomycin induces upregulation of LO in cultured human fetal lung fibroblasts, which may be the mechanism of bleomycin-induced pulmonary fibrosis.
Keywords: lysyl oxidase, copper, bleomycin, pulmonary fibrosis, human fetal lung fibroblast
Introduction
Pulmonary fibrosis is a progressive and lethal lung disorder, characterized by the loss of alveolar structure through the apoptosis of epithelial and endothelial cells, proliferation of fibroblast and excessive deposition of extracellular matrix, including fibrillar collagens, fibronectin, elastic fibers and proteoglycans1, 2. Many factors can cause pulmonary fibrosis such as toxic vapours, inorganic dusts, paraquat, cancer chemotherapy drug like bleomycin, and radiation therapy. But, the cause of idiopathic pulmonary fibrosis (IPF), also known as cryptogenic fibrosing alveolitis in Europe remains unknown3. There is still no effective treatment for this disease, and the prognosis is dismal, with a median survival of 3 to 5 years after diagnosis4, 5.
Traditionally, IPF has been thought to occur as a result of an initial injury to the lung that cause the recruitment of inflammatory cells, release of cytokines6, 7 and proliferation of fibroblast, deposition of extracellular matrix (ECM) and eventually parenchymal remodeling and fibrosis. The role of inflammation in IPF is controversial, because inflammatory suppressive agents do not seem to be effective8. Recent research focuses that IPF is an epithelial-fibroblast disease9, 10. Epithelial to mesenchymal transition (EMT) may play an important role in the formation of pulmonary fibrosis. A number of fibrogenic cytokines such as transforming growth factor-beta1 (TGF-β1), connective tissue growth factor (CTGF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF) are involved in this transition.
Lysyl oxidase (LO), a copper (Cu)-dependent amino oxidase, catalyzes covalent crosslinking of collagen and elastin and stabilizes extracellular matrix (ECM). It plays a critical role in morphogenesis and tissue repair of lung and other organs11. It has been shown that TGF-β1 increases the mRNA level and activity of lysyl oxidase in lung fibroblast12. It also has been shown that increased LO activity is associated with fibrotic diseases such as lung13 and liver fibrosis14, 15,, whereas decreased LO activity is associated with disorders of Cu metabolism like Menkes syndrome16.
Bleomycin (BLM) is a cancer chemotherapy drug, which is used to treat Hodgkin's and non-Hodgkin's lymphomas, germ cell tumor, head and neck cancer, etc. However, it does cause significant cutaneous toxicity. The most serious side effect of bleomycin is pulmonary toxicity, which begins with a dry cough, fine rales, and diffuses basilar infiltrates on X-ray and may progress to life-threatening pulmonary fibrosis. Bleomycin-induced pulmonary fibrosis in animal is a widely used animal model for pulmonary fibrosis in human because of its similarity to IPF. Although some evidence in animal model has shown that lysyl oxidase activity increased during bleomycin-induced rat lung fibrosis13, the mechanisms of bleomycin-induced pulmonary toxicity and pulmonary fibrosis are still poorly understood. Here, we report BLM upregulates LO catalytic activity in associated of Cu concentration increase in conditioned media of cultured human fetal lung fibroblasts.
Materials and methods
Materials
Bleomycin (BLM) was purchased from Nippon Kayaku Co, Ltd (Tokyo, Japan). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and Pheno-red free DMEM were ordered from Gibco (Grand Island, NY). 1,5-diaminopentane and β-aminopropionitrile (BAPN) were purchased from Sigma (St Louis, MO). Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit was purchased from Molecular Probes (Eugene, OR).
Cell culture and treatment
Human fetal lung fibroblast (HLF) cell line derived from cell bank of Academia Sinica in Shanghai, China. Cells were regularly maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS. Cells were seeded in cell culture dishes with complete medium (DMEM containing 10% FBS) and incubated until to subconfluence. Then cells were growth-arrested and synchronized at the G1 phase by incubation in DMEM containing 0.3% FBS and treated with BLM at final concentrations ranging from 0 to 30 μg/mL for 24 h, unless otherwise indicated. After that, further analysis was processed. Control cells were incubated in the presence of vehicle only.
Western blot analysis
Control and treated cells were washed twice with cold PBS buffer and lysed in the RIPA buffer composed of 1×PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and 2 mol/L urea, pH 7.4. One tablet of the protease inhibitor cocktail (Roche, Mannheim, Germany) was freshly added to 10 mL of RIPA. Cell lysates were microcentrifuged at 14 000 r/min for 20 min at 4 °C. Supernatants were collected and stored at −80 °C. Protein concentration in each sample was determined by the BCA protein assay reagents (PIERCE, Rockford, IL). Cell lysates containing equal amounts of protein (30 μg) were boiled in an SDS sample buffer and analyzed by SDS-PAGE. The separated proteins in the gel were then transferred to a nitrocellulose membrane (Schleicher & Schuell, Keene, NH). Nonspecific binding sites were blocked by incubating the nitrocellulose membrane in Tris-buffered saline containing 0.1% Tween-20 with 5% nonfat dry milk. Membrane was incubated overnight at 4 °C with primary antibody of anti-human LO monoclonal antibody (R&D, 1:1000), washed three times each for 5 min with Tris-buffered saline containing 0.1% tween-20, and then incubated with the secondary antibody of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Santa Cruz Biotech, Santa Cruz, CA 1:2000) for 1 h at room temperature. After washing, blots were developed with an enhanced chemiluminescence system (PerkinElmer Life Sciences, Boston, MA). Protein bands were quantitated by the 1 D Scan EX software (Scananalytics, Fairfax, VA)
Real-time RT-PCR
Total RNA was extracted from control and treated cells using TRIzol Reagent (Invitrogen, Carlsbad, CA). The mRNA expression of LO was evaluated by real-time RT-PCR. Primer pairs and Taqman fluorogenic probes were designed using primer express 2.0 software as follows:
Sequence Name: Human-lysyl oxidase (H-LO) (246 bp, 110 bp)
Forward Primer (Outer): 5′-ATC ACA GGG TGC TGC TCA GA-3′,
Reverse Primer (Outer): 5′-ATG CAA ATC GCC TGT GGT AG-3′
Forward Primer (Inner): 5′-GGA ATG GCA CAG TTG TCA TCA-3′,
Reverse Primer (Inner): 5′-AAC TTG CTT TGT GGC CTT CAG-3′.
Probe: 5′-FAM-CAT TAC CAC AGT ATG GAT GAG TTT AGC C-TAMRA-3.
Sequence Name: H-β-actin (106 bp)
Forward Primer: 5′-GCA TGG GTC AGA AGG ATT CCT-3′,
Reverse Primer: 5′-TCG TCC CAG TTG GTG ACG AT-3′.
Probe: 5′-FAM-CCT CAC CCT GAA GTA CCC CAT CGA GC-TA MRA-3′.
Lysyl oxidase activity assay
Growth-arrested HLF cells in 0.3% FBS/phenol red-free DMEM were exposed to bleomycin at final concentration ranging from 0 to 30 μg/mL for 24 h. The conditioned medium was collected and assayed for LO activity using diaminopentane as a substrate as described17 and Amplex red as a hydrogen peroxide probe as described18. In a typical assay, samples (500 μL conditioned medium) were mixed with the reaction mixture containing 0.05 mol/L sodium borate, pH 8.2, 10 mmol/L diaminopentane, 10 μmol/L Amplex red, 1 U HRP, and 1.2 mol/L urea in a final volume of 1 mL in the presence or absence of 0.5 mmol/L BAPN, an active site inhibitor of LO. H2O2 release was continuously monitored for 5 min at excitation and emission wavelengths of 563 and 587 nm, respectively, at a constant temperature of 37 °C, on the 850 Fluorescence Spectrophotometer (HITACHI Instrument, Tokyo, Japan). All enzyme activities were expressed as fluorescence values at 5 min after the reaction, corrected for background levels of H2O2 release determined in the reaction mixture supplemented with BAPN, and normalized to total cell protein.
Determination of Cu concentration
Human fetal lung fibroblasts (HLF) were seeded in 6-wells cell culture plate with complete medium (DMEM containing 10% FBS) and incubated until to subconfluence. Switched to fresh DMEM medium containing 0.3% FBS and treated cells with BLM at final concentration range from 0 to 30 μg/mL or BAPN at final concentration range from 0 to 1000 μmol/L for 24 h. Collected media and detected Cu concentrations in the media by Flame Atomic Absorption Spectrophotometry on Z-5000 Polarized Zeeman Atomic Absorption Spectrophotometer (HITACHI instrument, Tokyo, Japan).
Statistical analysis
Data were expressed as mean±SD of at least three independent experiments. Statistical differences between means were determined using one-way ANOVA followed by Bonferroni's post hoc test or two-tailed student's t-test when appropriate. A P value<0.05 was considered significant.
Results
Upregulation of lysyl oxidase activity by bleomycin in the medium of cultured human fetal lung fibroblasts
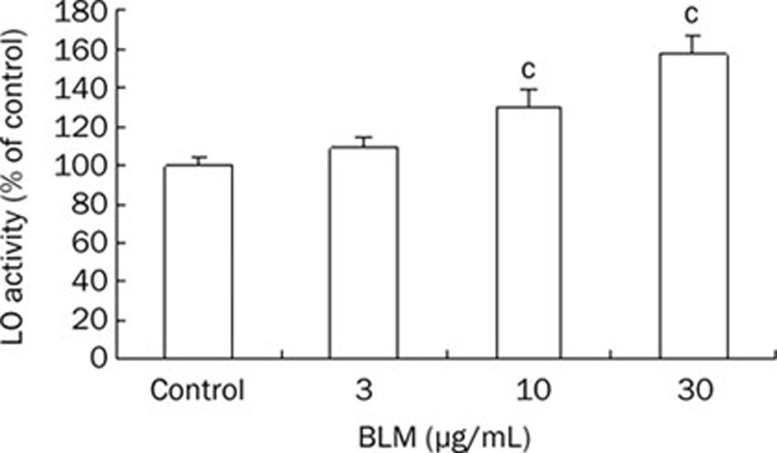
To assess the effect of BLM on lysyl oxidase catalytic activity, we cultured HLF to subconfluence in complete medium (DMEM/10% FBS). Then cells were growth-arrested and synchronized at the G1 phase by incubation in pheno-red free DMEM containing 0.3% FBS and treated with bleomycin at final concentrations ranging from 0 to 30 μg/mL for 24 h. The conditioned medium was collected and assayed for LO catalytic activity. Bleomycin at 3, 10, and 30 μg/mL increased LO activity in conditioned media of treated cells to 109%, 130%, and 158% of the control, respectively (Figure S1, Figure 1).
Figure 1.
Effect of bleomycin(BLM) on lysyl oxidase(LO) activity in conditioned media of culture HLF cells. Growth-arrested HLF cells in 0.3% FBS/phenol red-free DMEM were exposed to BLM at indicated doses for 24 h. The conditioned media were collected and assayed for LO activity. 0.5 mmol/L β-aminoproppionitrile (BAPN) was used as an internal control. Data shown are the mean±SD (n=3). bP<0.05, cP<0.01 compared with control.
Effect of bleomycin on lysyl oxidase protein expression in cultured human fetal lung fibroblasts
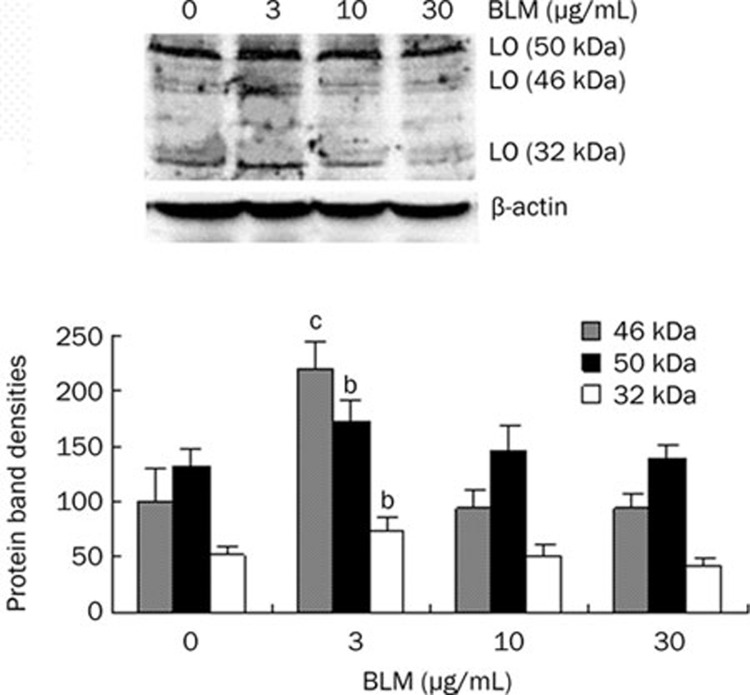
To explore effect of bleomycin on LO protein expression, we performed the Western blot assays in growth-arrested HLF treated with bleomycin at final concentrations ranging from 0 to 30 μg/mL for 24 h. As shown in Figure 2, although 10 and 30 μg/mL of BLM induced no significant change of LO protein species, BLM at 3 μg/mL significantly resulted in upregulation of LO expression at protein levels in cultured HLF cells. As determined by the protein band density assay, the 46 kDa preproLO, 50 kDa proLO and 32 kDa mature LO were increased to 219%, 130%, and 135% of the control, respectively.
Figure 2.
Effect of bleomycin (BLM) on lysyl oxidase (LO) protein expression in cultured HLF cells. LO protein levels in BLM-treated HLF cells were determined by Western blot analysis. The total protein loaded in each lane is 30 μg. β-actin was used as a loading control. LO protein species include the 46 KDa preproLO, the 50 KDa proLO and the 32 KDa mature LO. The protein band densities were measured by the 1D Scan software with the area density program and expressed as mean±SD. bP<0.05, cP<0.01 vs controls.
Effects of BLM and BAPN on lysyl oxidase expression at mRNA levels in cultured human fetal lung fibroblasts
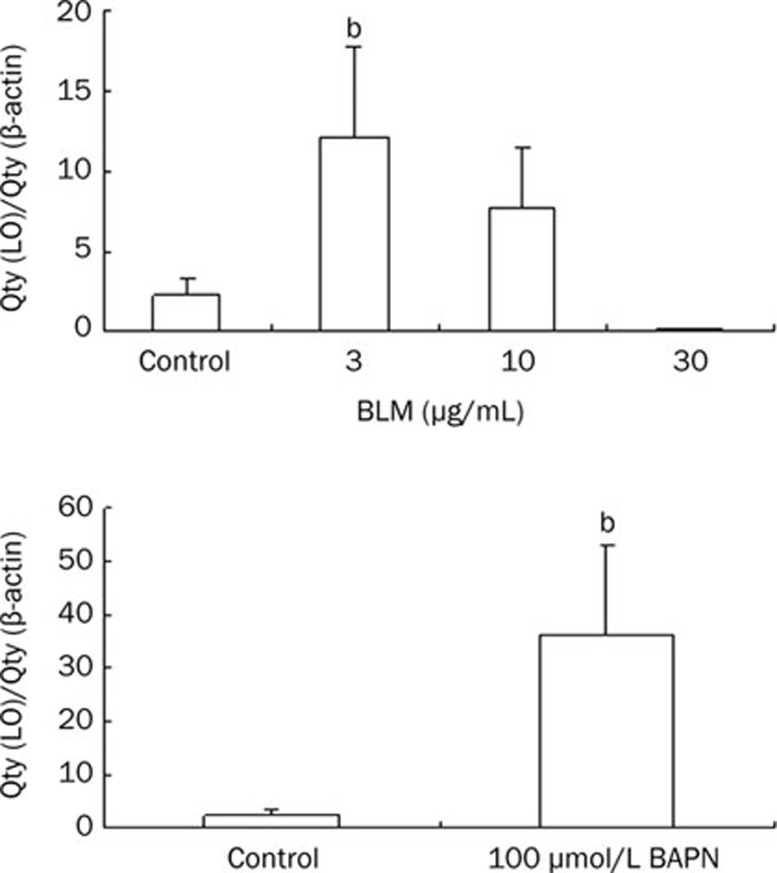
We further assessed the effect of BLM on LO expression at mRNA levels in cultured HLF cells by real time RT-PCR. BAPN, an inhibitor of LO activity, was used as a positive control. Growth-arrested cells were treated with BLM at final concentrations ranging from 0 to 30 μg/mL or 100 μmol/L BAPN for 24 h. BLM at 3 and 10 μg/mL enhanced LO mRNA expression to 5.5 (P<0.05) and 3.5-fold of the control, respectively. But, BLM at 30 μg/mL decreased LO mRNA level to 20.7 fold of the control. Meanwhile, BAPN at 100 μmol/L elevated LO mRNA level to 16.4 fold of the control (Figure 3, Table S1).
Figure 3.
Effect of BLM or BAPN on LO mRNA expression. HLF cells were exposed to BLM or BAPN at indicated concentrations for 24 h. The mRNA expression of LO was evaluated by real-time RT-PCR. β-actin was used as an internal control. Data shown are the mean±SD (n=3). bP<0.05 compared with control.
Table S1. Effects of BLM or BAPN at indicated concentration on LO mRNA expression.
| LO gene | β-actin | Qty (LO)/Qty (β-actin) | Mean±SD | P | ||||
|---|---|---|---|---|---|---|---|---|
| Samples | Repeat | Ct | Qty | Ct | Qty | |||
| Control | 1 | 27.52 | 2.62×103 | 16.30 | 1.39×107 | 1.88×10−4 | ||
| 2 | 27.51 | 4.70×103 | 17.88 | 1.24×107 | 3.79×10−4 | 2.2±1.4 (×10−4) | ||
| 3 | 29.34 | 1.27×103 | 17.77 | 1.34×107 | 0.95×10−4 | |||
| BLM (3 μg/mL) | 1 | 27.03 | 3.86×103 | 20.69 | 4.22×106 | 9.10×10−4 | ||
| 2 | 27.93 | 3.41×103 | 20.78 | 1.83×106 | 18.60×10−4 | 12.1±5.6 (×10−4)b | 0.041 | |
| 3 | 28.39 | 2.50×103 | 20.12 | 2.83×106 | 8.70×10−4 | |||
| BLM (10 μg/mL) | 1 | 22.46 | 1.45×104 | 15.10 | 3.63×107 | 3.90×10−4 | ||
| 2 | 23.65 | 7.59×104 | 14.75 | 9.85×107 | 7.70×10−4 | 7.7±3.7 (×10−4) | 0.078 | |
| 3 | 23.44 | 8.84×104 | 15.15 | 7.64×107 | 11.40×10−4 | |||
| BLM (30 μg/mL) | 1 | 34.35 | 34.47 | 20.36 | 4.63×106 | 7.44×10−6 | ||
| 2 | 34.76 | 25.66 | 20.21 | 2.76×106 | 9.30×10−6 | 10.6±3.9 (×10−6) | 0.066 | |
| 3 | 33.23 | 68.01 | 20.68 | 4.56×106 | 14.91×10−6 | |||
| BAPN (100 μmol/L) | 1 | 20.10 | 4.86×105 | 13.99 | 8.72×107 | 5.57×10−3 | ||
| 2 | 21.40 | 3.83×105 | 14.11 | 1.5×108 | 2.55×10−3 | 36.1±16.9 (×10−4)b | 0.026 | |
| 3 | 21.81 | 2.86×105 | 14.65 | 1.05×108 | 2.72×10−3 | |||
Note: The mRNA expression of LO was evaluated by real-time RT-PCR. β-actin was used as an internal control. bP<0.05 compared with control. BLM, bleomycin; BAPN, beta-aminopropionitrile; LO, lysyl oxidase.
Effects of BLM and BAPN on Cu concentration in medium of cultured human fetal lung fibroblasts
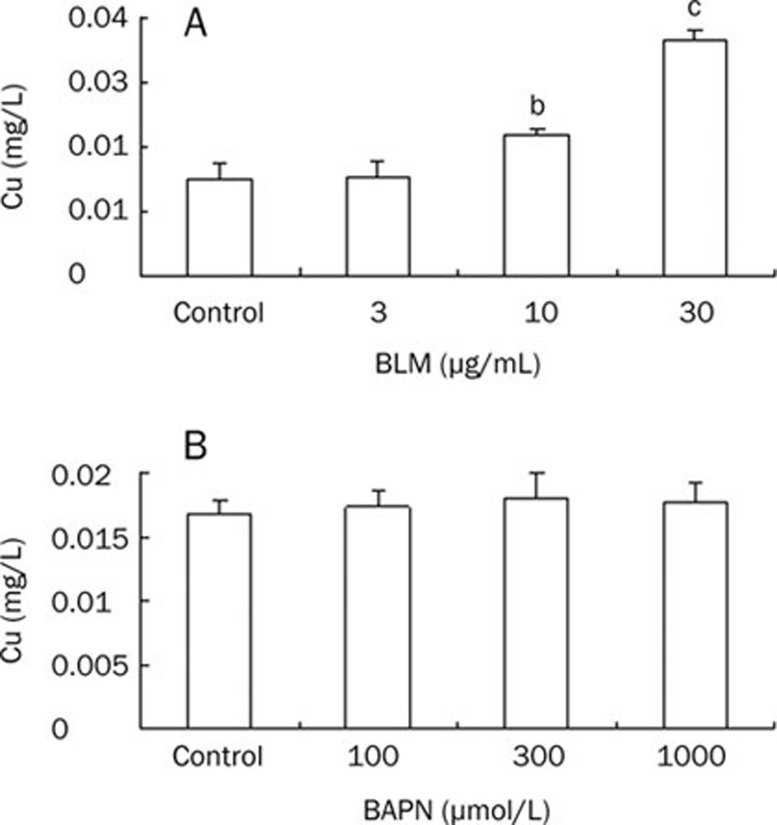
To probe the mechanism of LO activity upregulation by BLM, we detected Cu concentrations in the media of cultured HLF cells exposed to BLM at final concentrations ranging from 0 to 30 μg/mL. As shown in Figure 4A, BLM at 10, 30 μg/mL increased significantly Cu concentrations in conditioned media of treated cells to 1.48 (P<0.05), 2.46 (P<0.01)-fold of the control, respectively. In contrast, BAPN treatment did not change Cu concentrations in conditioned media of treated cells (Figure 4B).
Figure 4.
Effect of BLM or BAPN on Cu concentrations in the media of cultured HLF cells. HLF cells were exposed to BLM or BAPN at indicated concentrations for 24 h. Media were collected and sent to detect Cu concentrations by Flame Atomic Absorption Spectrophotometry. Data shown are the mean±SD (n=3). bP<0.05, cP<0.01 compared with control.
Determination of Cu originally bound to BLM
To assess original Cu bound to BLM, various concentrations of BLM dissolved in double-distilled water were tested by Atomic Absorption Spectrophotometry. As shown in Table 1, BLM at 3, 10, and 30 μg/mL contained Cu equal 0.0012, 0.0022, and 0.0042 mg/L, respectively. These results indicate chelating Cu by BLM which is used in this study.
Table 1. Cu concentrations in various concentrations of BLM solution.
| Group | Cu (mg/L) |
|---|---|
| Control | 0.0001 |
| BLM (3 μg/mL) | 0.0012 |
| BLM (10 μg/mL) | 0.0022 |
| BLM (30 μg/mL) | 0.0042 |
Note: BLM was dissolved in dd-H2O at indicated concentrations. Cu concentrations in various concentrations of BLM solution were determined by Flame Atomic Absorption Spectrophotometry.
Discussion
In this study, we investigated the molecular and cellular events following cells exposure to BLM which may result in pulmonary fibrosis. Because of the critical role of LO, a Cu-dependent amino oxidase, in morphogenesis and tissue repair of the lung ECM, we studied changes in LO mRNA, protein, and catalytic activity levels in cultured human fetal lung fibroblasts (HLF) exposed to BLM. Here, we showed that BLM significantly enhanced LO catalytic activity in concomitant with elevation of Cu levels in the HLF-conditioned media.
Pulmonary fibrosis, especially idiopathic pulmonary fibrosis (IPF), has been thought as a result of an initial injury to the lung that causes the recruitment of inflammatory cells, release of cytokines and eventual increase of fibroblast activity leading to parenchymal remodeling19, 20. Although many progresses have been made in explanation of pathogenesis of IPF in recent years, the precise mechanism of IPF remains unclear and thus there is no effective treatment for IPF. Increasing evidence suggests that cytokines such as TGF-β21, 22, 23, 24, connective tissue growth factor (CTGF)25, 26, 27, platelet-derived growth factor (PDGF)28, 29, basic fibroblast growth factor (bFGF)30, 31, 32, epidermal growth factor (EGF)33, tumour necrosis factor +(TNF-α)34, insulin-like growth factors (IGFs)35, interferons (INF)36 and interleukins (IL)37 play an important role in the pathogenesis of pulmonary fibrosis. Release of these cytokines causes fibroblast proliferation, myofibroblast activation, collagen synthesis and excessive deposition of ECM6, 38. In all these molecular and cellular events, we think that collagen synthesis and excessive deposition of ECM catalyzed by LO may be a key and final event leading lung tissue repair, remodeling and fibrosis. In light of this view, we studied the BLM effect on LO catalytic activity and LO expression in cultured HLF cells.
Results reported here show that LO catalytic activity in conditioned medium is upregulated in cultured HLF cells exposure to BLM at final concentration range from 10 to 30 μg/mL. LO is a metalloenzyme requiring 1 mole of Cu at its active site per mole of enzyme. Cu binding to the proenzyme occurs in secretory vesicles such as the trans-Golgi apparatus16. Once feed of Cu, the resulting inactive apoenzyme was fully reactivated by reconstitution with Cu, but not by divalent Ni, Cd, Zn, Co, Fe, Hg, Mg, etc. Severe Cu deficiency resulted in decreased crosslinking of connective tissue, consistent with decreased function of LO39. Enzyme activity levels were decreased in the skin of weanling rats fed with a copper deficient diet40. Exogenously added Cu has been shown to elevate LO activity and LO mRNA levels in cultured RFL6 cells41.
Upregulation of LO activity as shown in this paper may be caused by Cu increase. So, we tested the Cu concentration in the medium of cultured HLF cells. Consistently, the Cu concentrations in conditioned media of cultured HLF cells exposed to BLM were increased with increases of BLM ranging from 10 to 30 μg/mL (final concentrations in media). The bleomycins currently employed clinically are a mixture of the two Cu-chelating peptides, bleomycins A2 and B2. In chemistry, the bleomycins form equimolar complexes with metal ions, including Cu2+ and Fe2+42. Further Table 1 shows Cu bound to the BLM samples which are used in this study. This may explain the phenomenon of increases of Cu concentrations in the media of cultured HLF cells exposure to BLM and upregulation of LO activity.
It should be noted that low concentration of BLM apparently upregulated LO transcription and translation under the specified conditions. Apparently, activation of LO gene transcription by BLM with a low dose was an early cellular event. BLM bound Cu may also acts as a critical factor for LO gene transactivation. Cu has been shown to regulate transcription of several genes by activation of metal response element (MRE) and antioxidant response element (ARE)43. The cloned rat LO gene contains at least two MREs and one ARE in the promoter region -804/-1 upstream of ATG which displayed the maximal promoter activity44.
We don't know why high concentration of BLM reduced LO expression at protein or mRNA levels. Interestingly, BLM at 30 μg/mL which significantly increased LO activity decreased mRNA level of LO to 20.7 fold of the control, while BAPN at 100 μmol/L which inhibits LO activity increased LO mRNA level to 16.4 fold of the control.
In brief, our studies have revealed that BLM upregulates LO activity in association of increasing copper concentration in conditioned media of cultured HLF cells. Some literature has reported increased LO activity is associated with fibrotic disease such as lung13. So, BLM induces upregulation of lysyl oxidase in HLF cells, which may be the mechanism of bleomycin-induced pulmonary fibrosis.
Author contribution
Li-jun CHEN designed research; Xin-wen SU, Guang-yun LIN, and Shi-feng LI performed research; Li-jun CHEN and Wan-de LI analyzed data; Li-jun CHEN and Wan-de LI wrote the paper; Yi-jun HUANG and Guang-mei YAN supported in technical assistance.
Acknowledgments
This work was supported by research grants from Chinese Ministry of Education for returnee and National Natural Science foundation of China (No 30772606). We thank Da An Gene Co Ltd of Sun Yat-Sen University for technical assistance of Real-time RT-PCR.
References
- Cross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–25. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, et al. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci USA. 2000;15:1778–83. doi: 10.1073/pnas.97.4.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N, O'Connor R. Idiopathic pulmonary fibrosis: current understanding of the pathogenesis and the status of treatment. CMAJ. 2004;171:153–60. doi: 10.1503/cmaj.1030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Slutsky AS. Idiopathic pulmonary fibrosis — new insights. N Engl J Med. 2007;356:1370–2. doi: 10.1056/NEJMcibr070490. [DOI] [PubMed] [Google Scholar]
- Travis WD, King TE, Bateman ED, Jr, Lynch DA. American Thoracic Society/European respiratory Society International Multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- Zhang K, Phan SH. Cytokines and pulmonary fibrosis. Biol Signals. 1996;5:232–9. doi: 10.1159/000109195. [DOI] [PubMed] [Google Scholar]
- Vaillant P, Menard O, Vignaud JM, Martinet N, Martinet Y. The role of cytokines in human lung fibrosis. Monaldi Arch Chest Dis. 1996;51:145–52. [PubMed] [Google Scholar]
- Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of Inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2008;10:287–301. doi: 10.1089/ars.2007.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–51. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- Selman M, Pardo A. The epithelial/fibroblastic pathway in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:S93–7. [PubMed] [Google Scholar]
- Kagan HM.Characterization and regulation of lysyl oxidaseIn Mecham, P R (Ed), Biology of the Extracellular Matrix, Vol I: Regulation of matrix accumulation. Academic Press, Orlando, FL. 1986 p 321–98.
- Boak AM, Roy R, Berk J, Taylor L, Polgar P, Goldstein RH, et al. Regulation of lysyl oxidase expression in lung fibroblasts by transforming growth factor-β1 and prostaglandin E2. Am J Respir Cell Mol Biol. 1994;11:751–5. doi: 10.1165/ajrcmb.11.6.7946403. [DOI] [PubMed] [Google Scholar]
- Counts DF, Evans JN, Dipetrillo TA, Sterling KM, Jr, Kelley J. Collagen lysyl oxidase activity in the lung increases during bleomycin-induced lung fibrosis. J Pharmacol Exp Ther. 1981;219:675–8. [PubMed] [Google Scholar]
- Siegel RC, Chen KH, Greenspan JS, Aguiar JM. Biochemical and immunochemical study of lysyl oxidase in experimental hepatic fibrosis in the rat. Proc Natl Acad Sci USA. 1978;75:2945–9. doi: 10.1073/pnas.75.6.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan HM. Lysyl oxidase: mechanism, regulation and relationship to liver fibrosis. Path Res Pract. 1994;90:910–9. doi: 10.1016/S0344-0338(11)80995-7. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. J Cell Biochemistry. 2003;88:660–72. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Chou DK, Zhao Y, Gao S, Chou IN, Toselli P, Stone P, et al. Pertubation of copper (Cu) homeostasis and expression of Cu-binding proteins in Cadmium-resistant lung fibroblasts. Toxicol Sci. 2007;99:267–76. doi: 10.1093/toxsci/kfm158. [DOI] [PubMed] [Google Scholar]
- Palamakumbura AH, Trackman PC. A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal Biochem. 2002;300:245–51. doi: 10.1006/abio.2001.5464. [DOI] [PubMed] [Google Scholar]
- Rogliani P, Mura M, Assunta Porretta M, Saltini C. New perspectives in the treatment of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2008;2:75–93. doi: 10.1177/1753465808089363. [DOI] [PubMed] [Google Scholar]
- Sime PJ, O' Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol. 2001;99:308–19. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc. 2006;3:413–7. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olman MA, Matthay MA. Transforming growth factor-beta induces fibrosis in immune cell-depleted lungs. Am J Physiol Lung Cell Mol Physiol. 2003;285:L522–6. doi: 10.1152/ajplung.00110.2003. [DOI] [PubMed] [Google Scholar]
- Gauldie J, Sime PJ, Xing Z, Marr B, Tremblay GM. Transforming growth factor-beta gene transfer to the lung induces myofibroblast presence and pulmonary fibrosis. Curr Top Pathol. 1999;93:35–45. doi: 10.1007/978-3-642-58456-5_5. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR. Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast. Curr Med Chem. 2009;16:1400–17. doi: 10.2174/092986709787846497. [DOI] [PubMed] [Google Scholar]
- Atamas SP, White B. Cytokine regulation of pulmonary fibrosis in scleroderma. Cytokine growth Factor Rev. 2003;14:537–50. doi: 10.1016/s1359-6101(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998;11:1218–21. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]
- Trojanowska M. Role of PDGF in fibrotic diseases and systemic sclerosis. Rheumatology (Oxford) 2008;47 suppl5:v2–4. doi: 10.1093/rheumatology/ken265. [DOI] [PubMed] [Google Scholar]
- Marinelli WA, polunovsky VA, Harmon KR, Bitterman PB. Role of platelet-derived growth factor in pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5:503–4. doi: 10.1165/ajrcmb/5.6.503. [DOI] [PubMed] [Google Scholar]
- Inoue Y, King TE, Barker E, Jr, Daniloff E, Newman LS. Basic fibroblast growth factor and its receptors in idiopathic pulmonary fibrosis and lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2002;166:765–73. doi: 10.1164/rccm.2010014. [DOI] [PubMed] [Google Scholar]
- Sannes PL, Khosla J, Johnson S, Goralska M, McGahan C, Menard M. Basic fibroblast growth factor in fibrosing alveolitis induced by oxygen stress. Chest. 1996;109:44s–45s. doi: 10.1378/chest.109.3_supplement.44s. [DOI] [PubMed] [Google Scholar]
- Henke C, Marineili W, Jessurun J, Fox J, Harms D, Peterson M, et al. Macrophage production of basic fibroblast growth factor in the fibroproliferative disorder of alveolar fibrosis after lung injury. Am J Pathol. 1993;143:1189–99. [PMC free article] [PubMed] [Google Scholar]
- Burgel PR, Nadel JA. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur Respir J. 2008;32:1068–81. doi: 10.1183/09031936.00172007. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Collart MA, Grau GE, Sappino AP, Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990;344:245–7. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- Krein PM, Winston BW. Roles for insulin-like growth factor 1 and transforming growth factor-beta in fibrotic lung disease. Chest. 2002;122:289s–93s. doi: 10.1378/chest.122.6_suppl.289s. [DOI] [PubMed] [Google Scholar]
- Bouros D, Antoniou KM, Tzouvelekis A, Siafakas NM. Interferon-gamma 1b for the treatment of idiopathic pulmonary fibrosis. Expert Opin Biol Ther. 2006;6:1051–60. doi: 10.1517/14712598.6.10.1051. [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Brody AR. IL-9 and lung fibrosis: a Th2 good guy. Am J Respir Cell Mol Biol. 2001;24:365–7. doi: 10.1165/ajrcmb.24.4.f205. [DOI] [PubMed] [Google Scholar]
- Martinet Y, Menard O, Vaillant P, Vignaud JM, Martinet N. Cytokines in human lung fibrosis. Arch Toxicol Supp. 1996;18:127–39. doi: 10.1007/978-3-642-61105-6_14. [DOI] [PubMed] [Google Scholar]
- Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16:387–98. doi: 10.1016/s0945-053x(98)90012-9. [DOI] [PubMed] [Google Scholar]
- Rucker RB, Romero-Chapman N, Wong T, Lee J, Steinberg FM, McGee C, et al. Modulation of lysyl xoidase by dietary copper in rats. J Nutr. 1996;126:51–60. doi: 10.1093/jn/126.1.51. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Gao S, Chou I-N, Toselli P, Stone P, Li W. Inhibition of the expression of lysyl oxidase and its substrates in cadmium-resistant rat fetal lung fibroblasts. Toxicol Sci. 2006;90:478–89. doi: 10.1093/toxsci/kfj112. [DOI] [PubMed] [Google Scholar]
- Hardman JG, Limbird LE.Goodman & Gilman's the Pharmacological Basis of Therapeutics11th edition). New York: McGraw-Hill Companies, 2006
- Mattie MD, Freedman JH. Copper-inducible transcription: Regulation by metal- and oxidative stress responsive pathways. Am J Physio Cell Physio. 2004;286:C293–C301. doi: 10.1152/ajpcell.00293.2003. [DOI] [PubMed] [Google Scholar]
- Gao S, Zhao Y, Kong L, Toselli P, Chou IN, Stone P, et al. Cloning and characterization of the rat lysyl oxidase gene promoter: Identification of core promoter elements and functional nuclear factor I binding sites. J Bio Chem. 2007;282:25322–37. doi: 10.1074/jbc.M610108200. [DOI] [PubMed] [Google Scholar]