Abstract
Background
Prkcz has been identified as a gene whose expression is positively correlated with ethanol consumption in mice and is also induced by ethanol. Two proteins are produced from Prkcz: protein kinase M zeta (PKMζ), which is expressed in the nervous system and protein kinase C zeta (PKCζ), which is expressed in other tissues. We examined Prkcz−/− mice that lack PKCζ and PKMζ to investigate the role of this gene in behavioral responses to ethanol.
Methods
Male Prkcz−/− and wild-type littermates were tested for ethanol consumption using four procedures: 24-hour intermittent access, 4-hour limited intermittent access, 4-day drinking-in-the-dark (DID), and 24-hour continuous access. We also assessed the acute hypnotic effect of ethanol, ethanol reward, and taste preference for sweet-, bitter-, salty-, and umami-flavored solutions. Finally, we determined whether ethanol could increase PKMζ and PKCζ transcripts and protein expression in wild-type mice using quantitative PCR and western blot analysis.
Results
Prkcz−/− mice consumed more ethanol than their wild-type littermates in both intermittent access procedures, but not in the DID or 24-hour continuous access procedures. Ethanol exposure increased Prkcz transcripts in cultured PC12 cells and intermittent ethanol consumption increased PKMζ protein in the ventral striatum of wild-type mice.
Conclusions
Absence of PKMζ in the brain is associated with increased ethanol intake during procedures that incorporate intermittent consumption sessions every other day. Our data suggest that ethanol induces PKMζ, which acts in a negative feedback loop to limit binge-like ethanol consumption.
Keywords: PKC zeta, PKM zeta, knockout mice, alcohol consumption, reward, ventral striatum
INTRODUCTION
Prkcz transcript abundance has been positively correlated with ethanol consumption in mice. Mouse strains that consume high levels of ethanol have higher levels of Prkcz transcripts in the brain compared with strains that consume low levels of ethanol (Mulligan et al., 2006). In addition, Prkcz can be induced by ethanol, as a single episode of ethanol intake increases Prkcz transcripts in C57BL/6 mouse brain (Mulligan et al., 2011). It is not yet known if Prkcz regulates behavioral responses to ethanol.
The Prkcz gene encodes for protein kinase C zeta (PKCζ) and protein kinase M zeta (PKMζ). PKMζ mRNA is transcribed from an internal start site in the Prkcz gene and is a gene product that is independent from PKCζ (Hernandez et al., 2003). PKCζ and PKMζ have complementary expression patterns, with PKCζ primarily expressed outside of the nervous system and PKMζ mainly expressed in the brain (Hernandez et al., 2003). Together with protein kinase C iota (PKCι), PKCζ and PKMζ comprise the atypical subfamily of PKCs (Hirai and Chida, 2003). Among the PKCs, PKMζ is unique as it lacks a regulatory domain and is thus constitutively active (Hernandez et al., 2003). PKMζ has been implicated in the maintenance of long-term memories (Hardt et al., 2010; Pastalkova et al., 2006; Shema et al., 2007; Serrano et al., 2008), including reward memory for cocaine and morphine (Li et al., 2011; Shabashov et al., 2012). In this study, we used homologous recombination to generate Prkcz−/− mice that lack both PKCζ and PKMζ to investigate the role of Prkcz in behavioral responses to ethanol.
MATERIALS AND METHODS
Generation of Prkcz−/− mice
A vector targeting exon 9 of the mouse Prkcz gene was generated using a 129S6/SvEvTac mouse BAC clone from the RPC1-22 library and the plasmid pK-11 Frt-PGKNeo-Frt-LoxP-pBSSK (Meyers et al., 1998). All junctions in the final construct were confirmed by sequencing and by Southern blot analysis of restriction digests. The AatII-linearized vector was electroporated into 129S6/SvEvTac ES cells, which were selected with neomycin. Surviving ES cell clones were screened by PCR and Southern analysis to confirm the presence of the two loxP sites and properly targeted 5′ and 3′ ends. Positive clones were injected into C57BL/6J blastocysts to generate chimeric mice. Chimeras were mated with transgenic C57BL/6J mice homozygous for Flpase (Jackson Laboratories, Sacramento, CA, USA) to remove the neomycin selection cassette, and progeny were crossed with C57BL/6 CMV-Cre (Jackson Laboratories, Sacramento, CA, USA) mice to delete exon 9. Hybrid C57BL/6 X 129S6_SvEvTac wild-type and Prkcz−/− littermates were genotyped for the wild-type and mutant alleles using a forward primer (GGTATAGTAGGCAGCTATTGCG) located in the long arm of the construct and a reverse primer (TCCTGCCTCAGCCAGAAAACAAACCACACGG) located outside of the construct in genomic DNA.
All mice were housed in standard ventilated cages under a 12-h light: 12h dark cycle, with lights on at 6 AM and off at 6 PM. Food was freely available for all experiments. All studies used naïve male wild-type and Prkcz−/− littermates at a minimum age of 8 weeks old. All procedures were conducted in accordance with guidelines of the NIH and the Gallo Center Institutional Animal Care and Use Committee.
24-hour intermittent access ethanol consumption (Hwa et al., 2011)
Naïve mice were singly housed in double grommet cages in a light-dark cycle room with lights on at 6 AM and off at 6 PM for 3 days prior to beginning the procedure. During the first week, mice were provided water with increasing concentrations of ethanol. On Monday morning, mice were presented with two bottles, one containing 3% ethanol in tap water and the other tap water alone, for 24 hours. On Wednesday morning, the concentration in the ethanol bottle was increased to 6% and on Friday morning to 10%. Beginning with the second week, mice were provided 2 bottles containing either 20% ethanol or water every Monday, Wednesday and Friday for 24 hours per session, for 3 weeks. Bottles were weighed after every session and the positions of the bottles were alternated every session to account for side preferences. The mice were weighed every Monday morning. Two separate cohorts of mice were tested and data were combined for the analysis. Data were analyzed by ANOVA with a repeated measure for session and a between subjects factor for genotype.
4-hour limited, intermittent access ethanol consumption
Ethanol naïve mice were singly housed in single grommet cages and acclimated to a reverse light-dark cycle (lights off at 10 AM, on at 10 PM) for 2 weeks prior to the start of ethanol consumption. On day 1, the mice were weighed and a single bottle of tap water was presented for four hours, starting two hours into the dark cycle (12 noon-4 PM) to measure baseline water consumption. Subsequently, a single bottle of 20% ethanol in tap water was presented on Monday, Wednesday and Friday, 12 pm to 4pm, for nine drinking sessions. Mice were provided a water bottle at all other times. The weight of the 20% ethanol bottle was recorded at the end of each session. Three separate cohorts of mice were tested and data were combined for analysis by ANOVA with a repeated measure for time and between subjects factor for genotype, followed by a Student-Newman-Keuls post-hoc test.
Drinking-in-the-dark (DID) procedure (Rhodes et al., 2005)
Ethanol naïve mice were singly housed in single grommet cages and acclimated to a reverse light-dark cycle (lights off at 10 AM, on at 10 PM) for 2 weeks prior to the start of binge ethanol consumption. The day before the start of the test, the weights of the mice were recorded. On days 1-3, mice were presented with a single bottle of 20% ethanol in tap water for 2 hours, from 1-3 PM. On day 4, mice were presented with the 20% ethanol bottle for 4 hours, from 1-5 PM. The bottles were weighed after each session. Two separate cohorts of mice were tested and data combined for analysis. Data were analyzed by ANOVA with a repeated measure for session and a between subjects factor for genotype.
Continuous access, 24-hour, two-bottle choice ethanol consumption
Naïve mice were singly housed in double grommet cages under a normal light-dark cycle (lights on at 6 AM, off at 6 PM). Mice were presented with a bottle of tap water and a bottle of ethanol in tap water. The ethanol concentrations presented were 3%, 6%, 10%, 14% and 20% with four days access to each concentration. Water and ethanol bottles were weighed every 2 days, and the bottle positions were alternated after weighing to account for side preferences. Mice were weighed every week. Three separate cohorts of mice were tested and data were combined for analysis by ANOVA with a repeated measure for ethanol concentration and a between subjects factor for genotype.
Ethanol conditioned place preference (CPP)
Naïve mice were tested in an unbiased procedure using a two-chamber apparatus (Med Associates, St. Albans, VT, USA) with custom acrylic floors of differing textures and a central partition with a manual door. On habituation day (Monday), mice were injected with saline and placed in the apparatus with access to both chambers for 15 minutes. On Tuesday through Friday and the following Monday through Thursday (8 sessions), mice were injected once each day with either 2 g/kg i.p. ethanol or an equivalent volume of saline, and confined to one chamber for 5 minutes. The mice received the opposite injection paired with the alternate context the next day and the pairings were alternated each day. The order of the injections and the paired chamber were randomized. Test day occurred on Friday (session 9), when all mice were injected with saline and allowed to explore both chambers for 15 minutes. The total time spent in the ethanol-paired chamber during test day was recorded. Data were analyzed by a Student's t-test. The second cohort of mice that was tested in the 24-hour intermittent access procedure was tested for ethanol CPP after the conclusion of the consumption procedure. All aspects of the conditioning and testing were similar to those used for the ethanol naïve group.
Loss of righting reflex (LORR)
Ethanol naïve mice were injected with 3.6 g/kg ethanol i.p. and placed alone in clean cages. The time at which the mouse lost the righting reflex was considered the start of LORR. The mouse was considered to have recovered the righting reflex when it could right itself three times within 30 seconds, which signaled the end of LORR. The total duration of LORR was recorded for each mouse. Data were analyzed by a Student's t-test.
Blood ethanol clearance
Ethanol naïve mice were injected with 4.0 g/kg ethanol i.p. and placed alone in clean cages. At 30, 60, 90, 120 and 180 minutes after injection, 20 μl of tail vein blood was collected from each mouse. Ethanol concentrations in the blood samples were measured using a β-nicotinamide adenine dinucleotide - alcohol dehydrogenase enzymatic assay (Zapata et al., 2006).
Taste preference assay
Naïve mice were singly housed in double grommet cages under a normal light-dark cycle (lights on at 6AM and off at 6PM). On Monday, the mice were weighed prior to presentation of one bottle containing the tastant and one bottle of tap water. Each tastant was presented for a total of 4 days at 2 different concentrations, each presented for 2 days (concentration 1: Monday-Tuesday, concentration 2: Wednesday-Thursday). The bottles were weighed daily and positions were alternated to account for side preferences. The mice were then provided two bottles of tap water for the next three days (Friday-Sunday). The next tastant was presented the following week. Tastants were presented in the following order: saccharin (1.5 mM and 15 mM), quinine (0.01 mM and 0.1 mM), sucrose (10 mM and 100 mM), NaCl (20 mM and 200 mM), glucose (10 mM and 100 mM) and monosodium glutamate (MSG, 10 mM and 100 mM). A preference score was calculated by dividing the amount of flavored water consumed by total fluid consumed. Three cohorts of mice were tested and data combined for analysis. Data were analyzed by ANOVA with a repeated measure for concentration and a between subjects factor for genotype, followed by a Student-Newman-Keuls post-hoc test.
Western blotting
Wild-type mice from cohort 1 of the 24-hour intermittent access procedure, and cohort 3 of the 24-hour continuous access procedure, and ethanol-naïve control mice were sacrificed, and the ventral and dorsal striatum were dissected and frozen on dry ice for processing. The mice from both procedures were sacrificed immediately after completing the procedure. The brain protein samples (50 μg) were separated in pre-cast NuPage 4-12% Bis-Tris gels (Life Technologies, Grand Island, NY) and transferred onto Hybond C nitrocellulose membranes (Fisher Scientific, Asheville, NC). PKCζ and PKMζ samples from the intermittent access procedure were incubated with anti-PKCζ (from T. Sacktor, SUNY Downstate, NY) at a dilution of 1:2000 in 5% PBS with 0.1% Tween 20 (PBS-T) overnight at 4°C. Protein samples from the continuous access procedure were incubated with anti-PKCζ from Cell Signaling (C24E6) at a dilution of 1:1000 in 5% TBS with 0.1% Tween 20 (TBS-T). Membranes were incubated with horseradish peroxidase-conjugated donkey anti-rabbit antibodies from Jackson Immuno Research Labs, Inc. (West Grove, PA) for 1 hour at room temperature. Immunoreactivity was detected by enhanced chemiluminescence (Thermo Fisher Scientific, Rockford, IL). Immunoreactive bands on autoradiograms of blots were scanned and quantified using ImageJ (http://rsbweb.nih.gov/ij/). PKCζ and PKMζ samples were normalized to GAPDH for the intermittent access procedure samples or total protein measured by Coomassie staining for the continuous access procedure samples, both from a second identically loaded gel. Data were expressed relative to the mean immunoreactivity determined in wild-type samples, and analyzed by a Student's t-test.
Quantitative PCR
PC12 cells were cultured in Dulbecco's Modified Eagle Medium supplemented with 5% fetal bovine serum, 10% heat-inactivated horse serum, 100 U/ml penicillin and 100 μg/ml streptomycin. The cells were maintained at 37°C in a humidified 10% CO2 incubator in uncoated flasks. The medium was replaced every 3-5 days. RNA was isolated from PC12 cells using an RNeasy Plus Miniprep kit (Qiagen, Los Angeles, CA). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using reverse transcription reagents from Applied Biosystems (Life Technologies, Grand Island, NY). TaqMan and SYBR Green quantitative polymerase chain reactions (QPCR) were performed using standard thermal cycling conditions on an ABI PRISM 7900 Sequence Detection System (Life Technologies, Grand Island, NY). To detect rat PKMζ transcripts, a custom probe and primer set that detects the unique 5′ UTR of PKMζ were used. The forward primer was GCA CAG AGA CGC TTG TTT TCG, the reverse primer CAC TAA AAC GCG GCA TGG AT and the probe TGA CGT CAG CTC CTC. To detect rat PKCζ and PKCι, we designed primers for use with the SYBR Green detection system. The PKCι forward primer was TAT GGC TTC AGC GTT GAC TG and was located between exons 13 and 14, and the reverse primer was TGG GTC CTT GTT GAG GAA AC located between exons 15 and 16. The PKCζ forward primer was GGA CCT CTG TGA GGA AGT GC located in exon 2, and the reverse primer was CTT GGG AAA ACG TGG ATG AT was located between exons 3 and 4. A rodent GAPDH probe and primer set (Life Technologies, Grand Island, NY) was used as a housekeeping gene control. Negative controls with no cDNA were performed for all reactions, and all reactions were repeated 3-6 times and averaged for a single data point. Relative amplicon quantification was calculated by normalizing Ct values to GAPDH. Relative gene expression between treatment groups was calculated by normalizing these values to a control sample. Data were analyzed by ANOVA with factors for time and ethanol concentration.
Statistical analysis
Ethanol consumption (g/kg) was calculated using the weight of fluid consumed multiplied by percent concentration of ethanol and the density of ethanol, divided by the mouse weight. Preference was calculated as the weight of the ethanol fluid consumed divided by the weight of total fluid consumed. All data were shown as mean ± SEM values. Outliers were identified by the Grubb's test. Data were tested for normality and analyzed with Prism 5.0 (GraphPad Software, La Jolla, CA) or SigmaPlot 11 (Systat Software Inc., San Jose, CA). Other statistical tests are listed with each method above. Post-hoc tests were run if the interaction between factors was significant, and data points that were statistically different are marked with asterisks in the figures. Differences between mean values were considered significant when P<0.05.
RESULTS
Increased ethanol consumption by Prkcz−/− mice during intermittent access procedures
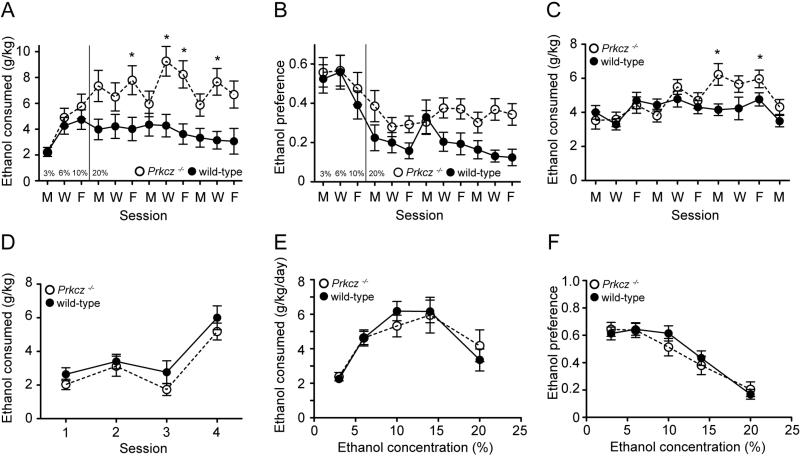
Prkcz−/− mice showed normal behaviors in a home cage environment and did not have any observable morphological abnormalities. We tested if Prkcz−/− mice showed altered ethanol consumption compared with wild-type mice by examining their drinking in four different procedures. We first used a 24-hour intermittent ethanol consumption procedure that produces high levels of ethanol consumption (Hwa et al., 2011). Mice were presented with 20% ethanol and water for 24 hours every Monday, Wednesday and Friday (Hwa et al., 2011). Prkcz−/− mice consumed more ethanol over time [Fgenotype X time (11,440)=3.196, P=0.0003, Fgenotype (1, 440)=8.776, P=0.005, Ftime (11, 440)=5.650, P<0.0001, Fig 1A] and had a greater preference for ethanol compared with wild-type littermates [Fgenotype X time (11,440)=1.369, P=0.18, Fgenotype (1,440)=4.705, P=0.04, Ftime (11,440)=11.38, P<0.0001, Fig. 1B]. We next tested a 4-hour limited access, intermittent ethanol consumption procedure, under which the mice were presented with a single bottle of 20% ethanol for 4 hours every Monday, Wednesday and Friday. Similar to the 24-hour intermittent access procedure, Prkcz−/− mice consumed more ethanol [Fgenotype X session (9, 374)=2.472, P=0.009, Fgenotype (1, 374)=1.961, P=0.17, Fsession (9, 374)=6.393, P<0.001, Fig. 1C] compared with wild-type mice. By contrast, there was no genotype difference in consumption in the DID procedure where mice were presented with a single bottle of 20% ethanol for 2 hours, for 3 days in a row, followed by a 4 hour presentation on the fourth day [Fgenotype X session (3,117)=0.278, P=0.841, Fgenotype (1,117)=1.908, P=0.175, Fsession (3,117)=26.31, P<0.0001, Fig. 1D]. We also did not observe a genotype difference in 24-hour, continuous access, two-bottle choice ethanol consumption [Fgenotype X concentration (4,180)=0.7442, P=0.56, Fgenotype (1,180)=0.0016, P=0.97, Fconcentration (4,180)=18.78, P<0.0001, Fig. 1E] or ethanol preference [Fgenotype X concentration (4,180)=0.9286, P=0.45, Fgenotype (1,180)=0.1353, P=0.71, Fconcentration (4,180)=40.02, P<0.0001, Fig. 1F].
Figure 1.
Prkcz−/− mice (n=22) show greater (A) ethanol consumption and (B) preference in the 24-hour intermittent access procedure compared with wild-type mice (n=20). *P<0.05 by a Bonferroni post-hoc test. (C) Prkcz−/− mice (n=23) show greater ethanol consumption in the 4-hour limited access procedure than wild-type mice (n=26). *P<0.05 by a Student-Newman-Keuls post-hoc test. Monday, Wednesday and Friday are represented on the x-axis as M, W, F. (D) Ethanol consumption in the DID procedure. Prkcz−/− mice=22, wild-type mice=19. (E) Ethanol consumption and (F) preference in the 24-hour continuous access procedure. Prkcz−/− mice=21, wild-type mice=26.
Bitter taste reactivity is altered in Prkcz−/− mice
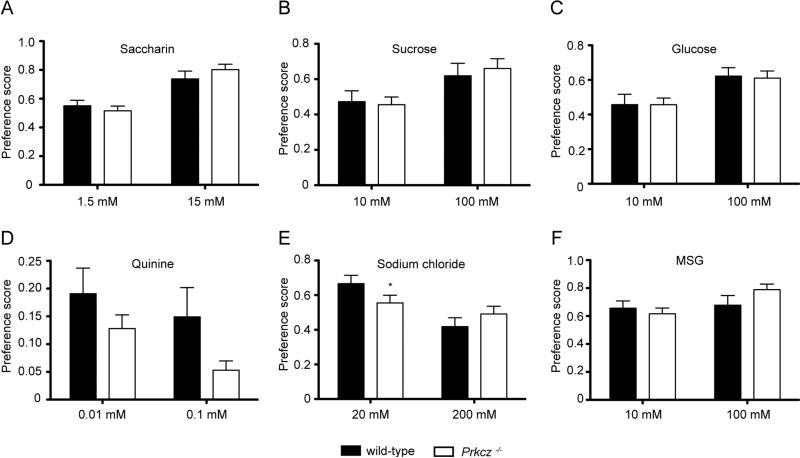
The preference for sweet tasting solutions can influence alcohol consumption (Brasser et al., 2010; Sinclair et al., 1992). To determine if Prkcz−/− mice have altered taste perception, we presented a series of different flavored solutions to Prkcz−/− and wild-type mice (Fig. 2). Prkcz−/− and wild type mice showed similar preference for sucrose [Fgenotype X concentration (1,47)=0.3822, P=0.54, Fgenotype (1,47)=0.034, P=0.85, Fconcentration (1,47)=13.40, P<0.001], saccharin [Fgenotype X concentration (1,47)=1.816, P=0.18, Fgenotype (1,47)=0.112, P=0.74, Fconcentration (1,47)=41.07, P<0.0001] and glucose [Fgenotype X concentration (1,47)=0.0166, P=0.90, Fgenotype (1,47)=0.017, P=0.90, Fconcentration (1,47)=12.85, P<0.001]. Since ethanol also has a bitter taste (Kiefer and Mahadevan, 1993), we examined quinine consumption and found a significant effect of genotype indicating that Prkcz−/− mice show greater aversion to quinine compared with wild-type mice [Fgenotype X concentration (1,47)=0.2161, P=0.64, Fgenotype (1,47)=5.497, P=0.02, Fconcentration (1,47)=2.684, P=0.11]. To determine if general taste reactivity was altered we also examined consumption of solutions containing sodium chloride or monosodium glutamate (MSG). Wild-type mice consumed more sodium chloride than Prkcz−/− mice at the lower salt concentration [Fgenotype X concentration (1,47)=4.308, P=0.04, Fgenotype (1,47)=0.1470, P=0.70, Fconcentration (1,47)=12.40, P=0.001]. Both genotypes showed similar preference for MSG-containing solutions [Fgenotype X concentration (1,47)=2.827, P=0.10, Fgenotype (1,47)=0.417, P=0.52, Fconcentration (1,47)=4.724, P=0.03].
Figure 2.
Preference for (A) saccharin, (B) sucrose, (C) glucose, (D) quinine, (E) sodium chloride and (F) MSG solutions in Prkcz−/− mice (n=21) and wild-type mice (n=11). *P<0.05 compared with wild-type mice by a Student-Newman-Keuls post-hoc test.
Conditioned place preference for ethanol is similar in Prkcz−/− and wild-type mice
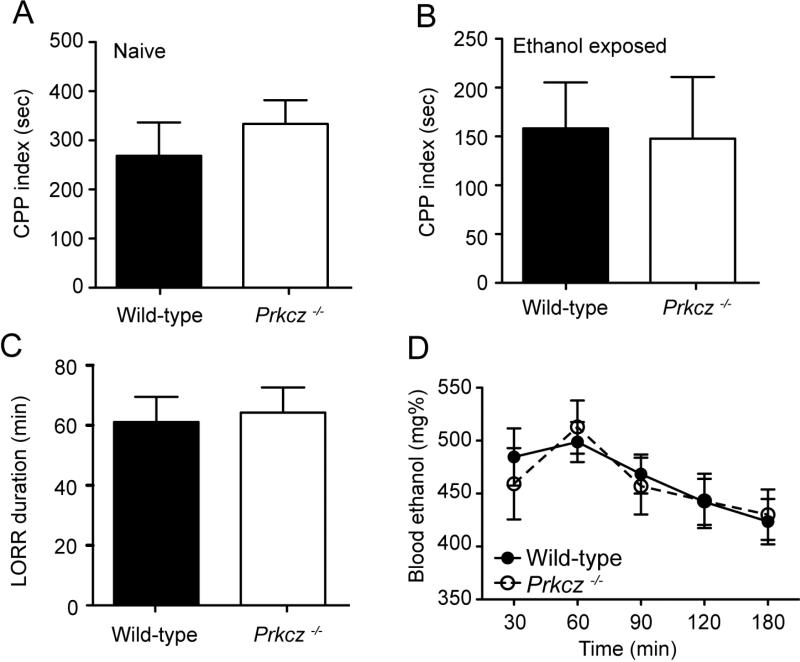
We tested if naïve Prkcz−/− mice had altered ethanol reward by assessing ethanol CPP. Prkcz−/− (t=6.849, P=0.001) and wild-type mice (t=3.950, P=0.002) spent significantly more time in the ethanol-paired chamber after conditioning, indicating that ethanol was rewarding for both genotypes. There was no genotype difference in the magnitude of ethanol CPP (t=0.7254, P=0.48, Fig. 3A). Since Prkcz−/− mice consumed more ethanol than wild-type mice in the MWF intermittent access procedures, we tested if ethanol reward was altered in Prkcz−/− mice that had been consuming ethanol intermittently for several weeks. We measured ethanol CPP immediately following completion of the 24-hour intermittent access procedure in the second cohort of Prkcz−/− and wild-type mice. In this cohort, Prkcz−/− mice consumed more ethanol [Fgenotype X time (11,242)=3.804, P<0.0001, Fgenotype (1,242)=11.15, P=0.03, Ftime (11,242)=4.727, P<0.0001] and showed greater ethanol preference on Wednesday of week 4 compared with wild-type mice [Fgenotype X time (11,242)=2.326, P=0.009, Fgenotype (1,242)=1.570, P=0.22, Ftime (11,242)=7.636, P<0.0001; P<0.05, Bonferroni post-hoc, Supplementary Fig. 1]. Again, both Prkcz−/− (t=2.339, P=0.04) and wild-type mice (t=3.370, P=0.006) spent more time in the ethanol-paired chamber after conditioning, and there was no genotype difference in the magnitude of ethanol CPP (t=0.1357, P=0.89, Fig. 3B).
Figure 3.
Ethanol CPP is similar in (A) ethanol naïve Prkcz−/− (n=9) and wild-type mice (n=12), and in (B) Prkcz−/− (n=11) and wild-type mice (n=13) that previously consumed ethanol under an intermittent access procedure. (C) There were no genotype differences in the duration of ethanol-induced loss of righting reflex after injection of 3.6 g/kg i.p. ethanol, n=9 per genotype, (D) or in the clearance of 4.0 g/kg i.p. ethanol, n=10 per genotype.
Ethanol-induced loss of righting and ethanol clearance are similar in Prkcz−/− and wild-type mice
For several lines of knockout mice there has been observed an inverse relationship between the level of ethanol consumption and the acute effect of ethanol as measured by the duration of ethanol-induced loss of the righting reflex (Hodge et al., 1999; Spanagel et al., 2002; Thiele et al., 1998; Thiele et al., 2000; Weinshenker et al., 2000), such that mutants that consume more ethanol often show a shorter duration of the LORR than wild type animals. To determine if such an inverse relationship was present in Prkcz−/− mice, we administered 3.6 g/kg ethanol i.p. and measured the duration of the ethanol LORR. Prkcz−/− and wild-type mice showed a similar duration of the ethanol LORR (t=0.2622, P=0.80, Fig. 3C). We also observed that blood ethanol clearance after a 4.0 g/kg i.p. ethanol injection was similar between wild-type and Prkcz−/− mice [FgenotypeXtime (4,72)=1.145, P=0.34, Fgenotype (1,72)=0.009, P=0.93, Ftime (4,72)=17.20, P<0.0001, Fig. 3D].
Ethanol increases PKMζ transcription and protein expression
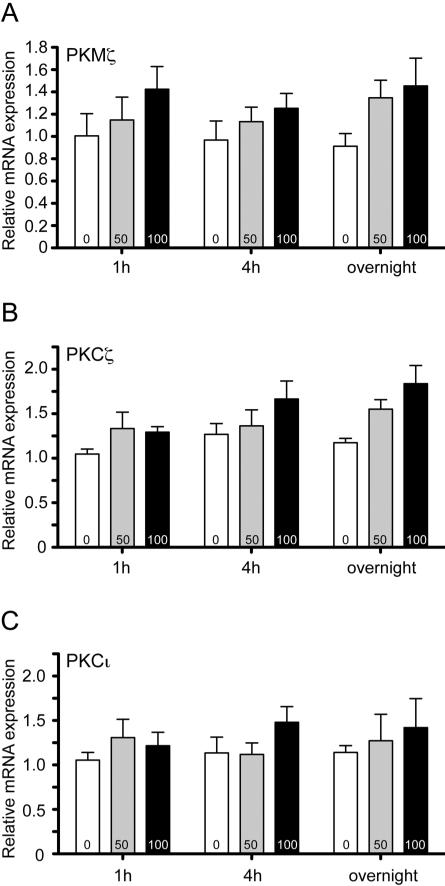
A recent study found that a single session of ethanol consumption increases Prkcz transcripts in C57BL/6 mouse brain (Mulligan et al., 2011), but did not distinguish between PKMζ and PKCζ transcripts. We tested if ethanol increases the transcription of all three atypical PKCs in vitro, using PC12 cells treated with 0, 50 and 100 mM ethanol for 1 hour, 4 hours and overnight. For PKMζ, there was a significant effect of ethanol concentration [Fethanol X time (4,36)=0.270, P=0.90, Fethanol (2,36)=4.071, P=0.03, Ftime (2,36)=0.343, P=0.71, Fig. 4A], and for PKCζ, there was a significant effect of ethanol concentration and time [Fethanol X time (4,36)=0.838, P=0.51, Fethanol (2,36)=6.948, P=0.003, Ftime (2,36)=3.400, P=0.04, Fig. 4B]. There was no effect of ethanol on PKCζ transcripts [Fethanol X time (4,36)=0.330, P=0.86, Fethanol (2,36)=1.300, P=0.28, Ftime (2,36)=0.139, P=0.87, Fig. 4C]. These data indicate that ethanol increases the levels of PKMζ and PKCζ transcripts, but does not affect PKCι transcripts, indicating that the effect of ethanol is specific for the Prkcz gene.
Figure 4.
(A) PKMζ, (B) PKCζ and (C) PKCι transcript levels in PC12 cells after different incubation periods with 0 (empty bars), 50 mM (grey bars) and 100 mM ethanol (black bars). n=5 data points per transcript.
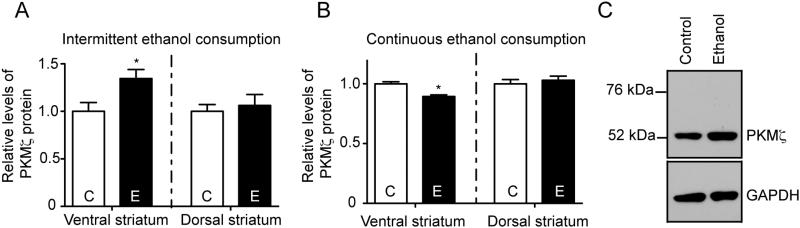
We next tested if chronic ethanol consumption could increase PKMζ protein levels in the brain, by using western blot analysis to differentiate between PKMζ and PKCζ. In brain samples from wild-type mice that completed the 24-hour intermittent access ethanol consumption procedure, PKMζ levels were increased in the ventral striatum (t=2.557, P=0.03) but not in the dorsal striatum (t=0.4203, P=0.68, Fig. 5A), compared with wild-type mice that were not exposed to ethanol. In contrast, wild-type mice that completed the 24-hour continuous access consumption procedure had slightly lower levels of PKMζ in the ventral striatum (t=4.845, P<0.0001) but not in the dorsal striatum (t=0.5984, P=0.56, Fig. 5B) compared with wild-type mice that were not exposed to ethanol. PKMζ was detectable at 52 kDa, but PKCζ (76 kDa) could not be detected (Fig. 5C).
Figure 5.
PKMζ protein levels from control, ethanol-naïve mice (“C”) and ethanol-consuming mice (“E”) from the (A) intermittent access procedure (“C” n=6, “E” n=8) and (B) continuous access procedure (“C” n=10, “E” n=12). (C) Representative western blot showing PKMζ immunoreactivity in striatal samples from a control mouse and a mouse that consumed ethanol under the 24-hour access, intermittent procedure. PKMζ is detectable at 52 kDa whereas PKCζ (76 kDa) is not. *P<0.05 by Student's t-test.
DISCUSSION
In this study, we assessed the role of the Prkcz gene in ethanol consumption using Prkcz−/− mice. We initially hypothesized that Prkcz−/− mice would consume less ethanol than wild-type mice, since Mulligan and colleagues (Mulligan et al., 2006) had found that mouse strains that consume greater amounts of ethanol have higher expression of Prkcz transcripts in the brain. We were surprised to find that Prkcz−/− mice consumed more ethanol than wild-type mice and only in procedures that incorporated repeated consumption sessions every other day. The increased ethanol consumption in Prkcz−/− mice also cannot be explained by altered sweet taste preference, as Prkcz−/− mice did not show differences in saccharin, sucrose or glucose preference, which were presented during the first, third and fifth week of taste testing. Nor can their increased ethanol consumption be explained by a reduced perception of bitter taste since Prkcz−/− mice showed greater aversion than wild type littermates to quinine. Ethanol reward also did not appear to be altered in Prkcz−/− mice since there was no genotype difference in ethanol CPP at baseline or after chronic intermittent ethanol consumption. Since we tested only male mice, our findings may not extend to female mice. We utilized an intermittent consumption procedure that produces an escalation of consumption over time in male C57BL/6J mice (Hwa et al., 2011); however, other lines of mice, such as heterogeneous HS/Npt mice, do not show an escalation in consumption (Crabbe et al., 2012). We did not observe an escalation of ethanol consumption in our hybrid C57BL/6 X 129S6_SvEvTac wild-type mice. Together, these data suggest that escalation of consumption in the intermittent ethanol procedure is influenced by genetic background and not all strains of mice show this effect. Genetic background also influences the total amount of ethanol consumed in different procedures. Hybrid C57BL/6 X 129S6_SvEvTac mice consume significantly less ethanol compared with C57BL/6 mice in the 24-hour continuous access procedure and the 4-hour limited access procedure (Lim et al., 2012). The level of consumption in our hybrid C57BL/6 X 129S6_SvEvTac wild-type mice was less than that seen with C57BL/6J mice in the 24-hour intermittent ethanol procedure (Hwa et al., 2011; Crabbe et al., 2012).
PKMζ is implicated in learning and maintenance of long-term memory, including drug-reward memory (Li et al., 2011; Shabashov et al., 2012). However, the role of PKMζ in memory maintenance has recently been questioned (Lee et al., 2013; Volk et al., 2013; Lisman, 2012; Wu-Zhang et al., 2012). We have found no deficits in learning and memory in Prkcz−/− mice in a variety of procedures, including cocaine conditioned place preference (Lee et al., 2013). This suggests that the differences in ethanol consumption that we observed in Prkcz−/− mice are not due to a deficit in learning and memory. Since Prkcz−/− mice lack PKMζ throughout development, it is possible that compensatory factors contributed to their ethanol drinking phenotype. We did not find a compensatory increase in the expression of other PKCs in the brains of Prkcz−/− mice (Lee et al., 2013). However, it is possible that in addition to the lack of PKMζ, other unknown compensatory factors influenced their behavior.
We confirmed that the abundance of Prkcz transcript was increased by ethanol exposure, and showed that this effect was specific for Prkcz, as ethanol exposure did not increase transcript abundance for Prkci, the other atypical PKC, in cultured PC12 cells. In C57BL/6 mice, the level of Prkcz transcript in the brain is increased after a single episode of ethanol consumption (Mulligan et al., 2011) and it is likely that the induced transcript is translated into PKMζ, not PKCζ, since PKCζ is undetectable in the brain (Hernandez et al., 2003).
Particularly striking was our observation that increased consumption was apparent only when animals were tested using intermittent (Monday, Wednesday, Friday) procedures and not when allowed daily access to ethanol. This finding suggested that a period of withdrawal and abstinence lasting up to 24 hours is important to recruit Prkcz into regulation of ethanol drinking. In support of this conclusion, we found that PKMζ was increased in the ventral striatum of wild-type mice only after intermittent, but not after continuous ethanol drinking. Taken together, our findings suggest that cycles of ethanol consumption and withdrawal induce PKMζ, which then acts as a negative feedback signal to limit ethanol consumption.
In vitro, PKMζ is induced during long-term potentiation (LTP) and PKMζ can increase synaptic strength by increasing the number of glutamate receptor subunit 2 (GluR2)-containing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in the post-synaptic density (Sacktor, 2011; Yao et al., 2008). Chronic ethanol consumption and withdrawal generate a hyper-glutamatergic state, which is thought to promote further ethanol consumption (Spanagel, 2009). In mice, ethanol withdrawal increases glutamate levels in the striatum (Hinton et al., 2012). We hypothesize that repeated ethanol withdrawal induces PKMζ in the striatum and strengthens a neural circuit that limits ethanol consumption. This neural circuit may include brain-derived neurotrophic factor (BDNF). BDNF and its target receptor, TrkB, are expressed at glutamatergic synapses, and are important in the development of LTP (Minichiello, 2009). BDNF may be upstream of PKMζ, as BDNF can facilitate the increase in PKMζ levels after LTP in hippocampal brain slices (Mei et al., 2011). BDNF expression is induced by acute ethanol application in primary hippocampal cultures (McGough et al., 2004) and is increased after ethanol self-administration in rat brain (Jeanblanc et al., 2009). Importantly, BDNF also negatively regulates ethanol consumption – injecting BDNF into rat brain reduces ethanol self-administration (Jeanblanc et al., 2009), and decreasing BDNF, either by viral-mediated RNA interference in rats (Jeanblanc et al., 2009) or genetically as in Bdnf+/− mice (McGough et al., 2004), increases ethanol consumption. Similar to PKMζ, BDNF expression is induced during withdrawal from chronic ethanol exposure in rats (Tapia-Arancibia et al., 2001). We propose that repeated withdrawal from chronic ethanol increases BDNF and PKMζ expression which together act to limit further ethanol consumption. Further study of circuits through which they act could lead to a better understanding of how ethanol consumption is regulated.
In summary, our data suggest that PKMζ is part of a protective mechanism that limits high levels of ethanol consumption. PKMζ is increased by chronic, intermittent ethanol consumption in mice, and this increased PKMζ expression appears to limit further ethanol consumption. Elucidating the mechanisms by which PKMζ limits ethanol consumption may uncover novel targets for the development of drugs to treat alcohol use disorders.
Supplementary Material
Acknowledgments
Support: This work was supported by grant AA017072 from NIAAA to ROM, a CIHR post-doctoral fellowship to AML, and by funds provided by the State of California for medical research on alcohol and substance abuse through UCSF.
Footnotes
Current affiliation: Robert O Messing, M.D., University of Texas at Austin, College of Pharmacy, 107 West Dean Keeton, BME, 3.510E, Austin, Texas, 78712.
REFERENCES
- Brasser SM, Norman MB, Lemon CH. T1r3 taste receptor involvement in gustatory neural responses to ethanol and oral ethanol preference. Physiol Genomics. 2010;41:232–243. doi: 10.1152/physiolgenomics.00113.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, et al. Intermittent availability of ethanol does not always lead to elevated drinking in mice. Alcohol Alcohol. 2012;47:509–517. doi: 10.1093/alcalc/ags067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt O, et al. PKMzeta maintains 1-day- and 6-day-old long-term object location but not object identity memory in dorsal hippocampus. Hippocampus. 2010;20:691–695. doi: 10.1002/hipo.20708. [DOI] [PubMed] [Google Scholar]
- Hernandez AI, et al. Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem. 2003;278:40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Hinton DJ, et al. Ethanol withdrawal-induced brain metabolites and the pharmacological effects of acamprosate in mice lacking ENT1. Neuropharmacology. 2012;62:2480–2488. doi: 10.1016/j.neuropharm.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem. 2003;133:1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- Hodge CW, et al. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hwa LS, et al. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, et al. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer SW, Mahadevan RS. The taste of alcohol for rats as revealed by aversion generalization tests. Chem Sens. 1993;18:509–522. [Google Scholar]
- Lee AM, et al. Prkcz null mice show normal learning and memory. Nature. 2013;493:416–419. doi: 10.1038/nature11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, et al. Inhibition of PKMzeta in nucleus accumbens core abolishes long-term drug reward memory. J Neurosci. 2011;31:5436–5446. doi: 10.1523/JNEUROSCI.5884-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JP, et al. Responses to ethanol in C57BL/6 versus C57BL/6 × 129 hybrid mice. Brain Behav. 2012;2:22–31. doi: 10.1002/brb3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Memory erasure by very high concentrations of ZIP may not be due to PKM-zeta. Hippocampus. 2012;22:648–649. doi: 10.1002/hipo.20980. [DOI] [PubMed] [Google Scholar]
- McGough NN, et al. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, et al. BDNF facilitates L-LTP maintenance in the absence of protein synthesis through PKMzeta. PLoS One. 2011;6:e21568. doi: 10.1371/journal.pone.0021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, et al. Molecular profiles of drinking alcohol to intoxication in C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:659–670. doi: 10.1111/j.1530-0277.2010.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, et al. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, et al. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Sacktor TC. How does PKMzeta maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Serrano P, et al. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabashov D, Shohami E, Yaka R. Inactivation of PKMzeta in the NAc Shell Abolished Cocaine-Conditioned Reward. J Mol Neurosci. 2012;47:546–553. doi: 10.1007/s12031-011-9671-7. [DOI] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, et al. Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol. 1992;9:155–160. doi: 10.1016/0741-8329(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spanagel R, et al. The neuronal nitric oxide synthase gene is critically involved in neurobehavioral effects of alcohol. J Neurosci. 2002;22:8676–8683. doi: 10.1523/JNEUROSCI.22-19-08676.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Arancibia L, et al. Effects of alcohol on brain-derived neurotrophic factor mRNA expression in discrete regions of the rat hippocampus and hypothalamus. J Neurosci Res. 2001;63:200–208. doi: 10.1002/1097-4547(20010115)63:2<200::AID-JNR1012>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Thiele TE, et al. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thiele TE, et al. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk LJ, et al. PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature. 2013;493:420–423. doi: 10.1038/nature11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, et al. Ethanol-associated behaviors of mice lacking norepinephrine. J Neurosci. 2000;20:3157–3164. doi: 10.1523/JNEUROSCI.20-09-03157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Zhang AX, et al. Cellular pharmacology of protein kinase Mzeta (PKMzeta) contrasts with its in vitro profile: implications for PKMzeta as a mediator of memory. J Biol Chem. 2012;287:12879–12885. doi: 10.1074/jbc.M112.357244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, et al. PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci. 2008;28:7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.