Abstract
Aim:
To investigate the effects of advanced glycation end products (AGEs) on calcification in human aortic smooth muscle cells (HASMCs) in vitro and the underlying mechanisms.
Methods:
AGEs were artificially prepared. Calcification of HASMCs was induced by adding inorganic phosphate (Pi, 2 mmol/L) in the media, and observed with Alizarin red staining. The calcium content in the supernatant was measured using QuantiChrome Calcium Assay Kit. Expression of the related mRNAs and proteins was analyzed using real-time PCR and Western blot, respectively. Chromatin immunoprecipitation (ChIP) assay was used to detect the binding of NF-κB to the putative IGF1R promoter.
Results:
AGEs (100 μg/mL) significantly enhanced Pi-induced calcification and the levels of osteocalcin and Cbfα1 in HASMCs. Furthermore, the treatment decreased the expression of insulin-like growth factor 1 receptor (IGF1R). Over-expression of IGF1R in HASMCs suppressed the AGEs-induced increase in calcium deposition. When IGF1R expression was knocked down in HASMCs, AGEs did not enhance the calcium deposition. Meanwhile, AGEs time-dependently decreased the amounts of IκBα and Flag-tagged p65 in the cytoplasmic extracts, and increased the amount of nuclear p65 in HASMCs. In the presence of NF-κB inhibitor PDTC (50 μmol/L), the AGEs-induced increase in calcium deposition was blocked. Over-expression of p65 significantly enhanced Pi-induced mineralization, but suppressed IGF1R mRNA level. Knockdown of p65 suppressed the AGEs-induced increase in calcium deposition, and rescued the IGF1R expression. The ChIP analysis revealed that NF-κB bound the putative IGF1R promoter at position −230 to −219 bp. The inhibition of IGF1R by NF-κB was abolished when IGF1R reporter plasmid contained mutated binding sequence for NF-κB or an NF-κB reporter vector.
Conclusion:
The results demonstrate that AGEs promote calcification of human aortic smooth muscle cells in vitro via activation of NF-κB and down-regulation of IGF1R expression.
Keywords: vascular calcification, advanced glycation end products, human aortic smooth muscle cell, osteocalcin, Cbfα1, insulin-like growth factor 1 receptor (IGF1R), nuclear factor-kappa B, PDTC
Introduction
Vascular calcification (VC) is common in many diseases, including end-stage renal disease, diabetes, and atherosclerosis, and naturally progresses, finally leading to increased mortality1,2,3,4. Approaches to prevent vascular calcification are far from ideal, partly because the precise mechanism is complex and heterogeneous. Growing evidence has suggested that VC is not a passive precipitation of calcium phosphate, but an active process involving the transdifferentiation of vascular smooth muscle cells (VSMCs) into osteoblast-like cells, changes in the expression of bone-associated proteins, and apoptosis5,6. However, the detailed mechanisms that control vascular calcification are still unclear.
In recent years, hyperglycemia and many hyperglycemia-related factors, including advanced glycation end products (AGEs), have been linked to diabetic complications7,8. AGEs are formed by the Maillard reaction, a complicated, non-enzymatic and irreversible process that links reducing sugar groups to proteins, lipids or nucleic acids. Evidence from studies has indicated that AGEs contribute to the development of atherosclerosis and microvascular diseases9,10. The interaction between AGEs and its major receptor, the receptor for advanced glycation end products (RAGE), has been established as a pivotal pathogenic factor for promoting cell dysfunction11. A previous study has suggested that AGEs induce VSMCs calcification by osteoblast-like differentiation of smooth muscle cells through RAGE/p38 MAPK, but there are most likely other mechanisms for VSMCs calcification12. In our previous study, we show that AGEs can increase Pi-induced calcification of VSMCs by inhibiting IGF1R expression, which has been shown to modulate calcification13. However, the role of IGF1R expression in AGE-accelerated calcification is currently unclear. Here, we show that AGEs inhibit IGF1R expression through NF-κB in HASMCs and accelerate Pi-induced calcification. Targeting NF-κB/IGF1R may help prevent vascular calcification.
Materials and methods
Materials
Dulbecco's modified Eagle's medium, fetal bovine serum, and penicillin-streptomycin were purchased from GIBCO (Grand Island, NE, USA). The NF-κB inhibitor PDTC and alizarin red stain were purchased from Sigma Chemicals (St Louis, MO, USA). TRIzol was purchased from Invitrogen (Carlsbad, CA, USA). Antibodies against IGF1R, p65, osteocalcin, CBFα1, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); an antibody against IκBα was purchased from Millipore (Billerica, MA, USA).
Preparation of AGEs
BSA (Sigma, St Louis, MO, USA) was incubated with 500 mmol/L glucose in PBS for 10 weeks at 37 °C in the presence of 1.5 mmol/L phenylmethylsufonyl fluoride, 0.5 mmol/L EDTA, penicillin (100 U/mL) and streptomycin (100 U/mL) under sterile conditions as described previously14. The unincorporated sugars were removed using dialysis of the PBS. Control, unmodified BSA was prepared under the same conditions without adding sugar. Endotoxin levels were checked using an endotoxin testing kit. The concentration of the AGE-BSA solution used in this study was 100 μg/mL. The AGE-BSA solution was confirmed to be endotoxin free (<0.5 U/mL of endotoxin).
Cell culture
Human aortic smooth muscle cells (HASMCs) were obtained from Invitrogen and grown at 37 °C in humidified air containing 5% CO2. They were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS, penicillin (100 U/mL) and streptomycin (100 U/mL). This medium was replaced with fresh medium every 2 days.
Calcification assay
At 80% confluence, the HASMCs were switched to calcification medium, which was prepared by adding 2 mmol/L of inorganic phosphate (Pi) to the growth media (GM). The GM and calcification medium were changed every 2 days.
Quantification of calcium deposition
Cells grown on 48-well plates were washed twice with PBS and decalcified with 0.6 mol/L HCl for 24 h. The calcium content of the supernatants was measured using the QuantiChrome Calcium Assay Kit (Gentaur, Hayward, CA, USA). After decalcification, the cells were solubilized using a solution of 0.1 mol/L NaOH and 0.1% sodium dodecyl sulfate, and the protein content of the samples was measured using a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). The calcium content of the cells was normalized to the protein content and expressed as mg/mg protein.
Alizarin red staining
The cells were incubated with 10% neutral-buffered formalin for 15 min, rinsed and incubated with 2% aqueous alizarin red solution (pH 4.0–4.2) for 10–15 min. After staining, the cells were washed twice with distilled water and once with 70% ethanol.
Vector construction
Full-length IGF1R and p65 mRNA from the human genome were amplified by qPCR and then sub-cloned into a pCDH lentiviral vector. The IGF1R and p65 siRNA were subcloned into the pLKO lentiviral vector. For viral infection, HASMCs were plated overnight and then infected with lentiviruses in the presence of polybrene (6 mg/mL, Sigma-Aldrich) for 24 h. The target sequences for the IGF1R and p65 siRNA were as follows: IGF1R siRNA, 5′-CAA TGA GTA CAA CTA CCG C-3′ p65 siRNA, 5′-GGA TTG AGG AGA AAC GTA A-3′.
IGF1R reporter assay and chromatin immunoprecipitation
The 5′ flanking region of the IGF1R gene was cloned into the pGL3 luciferase reporter (Geneart, Regensburg, Germany), and an IGF1R pGL3 construct containing a mutated binding site for nuclear factor (NF)-κB (DNF-κB) was generated. HASMCs were co-transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) with pGL3-IGF1R and the transfection control β-gal expression vector. After 6 h, the transfection media was replaced with DMEM containing 10% FBS. On the following day, the cells were treated with the vehicle or AGE-BSA for 24 h. The control of NF-κB activation was a pNF-κB-luciferase vector. The cell lysates were harvested 24 h after AGE-BSA treatment. The luciferase activity in the cell lysates was assayed using the Dual Luciferase Reporter kit (Promega, Madison, WI, USA).
For the chromatin immunoprecipitation (ChIP) assay, cells were subjected to AGE-BSA and stimulated for 24 h. After separation, the chromatin was incubated for 4 h with 5 mg of anti–NF-κB p65 (Abcam) or IgG (Santa Cruz Biotechnology, Cambridge, UK) as a negative control.
Real-time reverse transcription polymerase chain reaction
The total RNA was isolated using TRIzol reagent according to the manufacturer's instructions and stored at −80 °C. A quantitative polymerase chain reaction (qPCR) kit (DyNAmo Flash SYBR Green; Finnzymes Oy, Espoo, Finland) was used according to the manufacturer's instructions. The primer sequences were as follows: IL-8 sense 5′-ATG ACT TCC AAG CTG GCC GTG-3′, anti-sense 5′-TCT CAG CCC TCT TCA AAA ACT-3′ TNF sense 5′-CGA GTG ACA AGC CTG TAG CC-3′, anti-sense 5′-CAT ACC AGG GCT TGG CCT CA-3′ IGF1R sense 5′-CGA TGT GTG AGA AGA CCA CCA-3′, anti-sense 5′-ACA TTT TCT GGC AGC GGT TT-3′ β-actin sense 5′-GGA CTT CGA GAC GGA GAT GG-3′, anti-sense 5′-GCA CCG TGT TGG CGT AGA GG-3′.
Western blot
The total cell proteins were initially extracted by centrifugation in RIPA lysis buffer. Equal amounts of the protein samples were loaded onto 10% SDS-PAGE gels and then transferred to a polyvinylidene difluoride (PVDF) membrane. The non-specific proteins were blocked with 5% non-fat dried milk for 1 h. The membranes were incubated with the primary antibodies anti-osteocalcin, anti-CBFα1, anti-IGF1R, anti-p65, and anti-β-actin overnight at 4 °C and then with secondary antibody (HRP-conjugated IgG) for 1 h. HRP-conjugated secondary antibodies were used in conjunction with an ECL chemiluminescence detection system. Protein expression was analyzed by Gel-Pro Analyzer 4 software and normalized to β-actin. The Western blots were repeated 3–5 times, and qualitatively similar results were obtained.
Statistical analysis
The experimental data were expressed as the means±SD. The group means were compared by Student's t test using the GraphPad Prism software system (GraphPad San Diego, CA, USA) and the statistical software program SPSS 13.0 for Windows (Chicago, IL, USA). Pearson correlation tests were also performed. P values <0.05 were considered to be significant in all cases.
Results
AGEs promote Pi-induced calcification in HASMCs
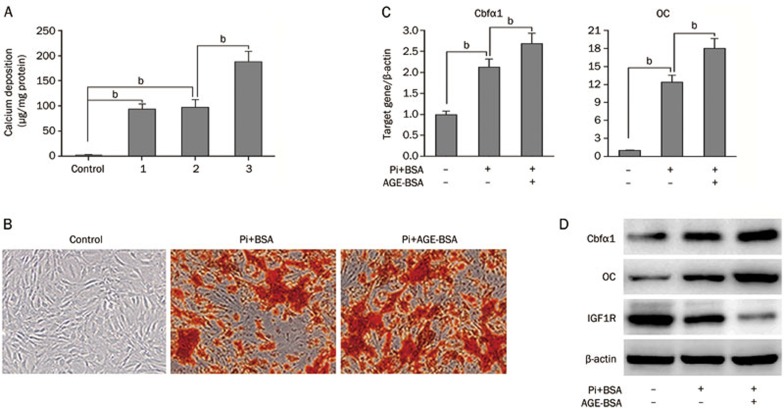
To investigate the effects of AGEs on calcification progression, we introduced a widely used cell model by treating HASMCs with Pi (2 mmol/L), Pi+BSA, or Pi+AGE-BSA (100 μg/mL) in the media. BSA alone did not accelerate Pi-induced HASMC calcification after 7 days of incubation, but Pi+AGE-BSA significantly accelerated Pi-induced calcium deposition compared to Pi alone (Figure 1A). Also, AGE-BSA treatment significantly promoted calcium nodule formation, as revealed by alizarin red staining (Figure 1B). Accordingly, qPCR revealed that the mRNA levels of bone-related molecules, Cbfα1 and OC, were significantly increased with AGE-BSA added to Pi (Figure 1C). The protein expression of Cbfα1 and OC were similarly altered (Figure 1D). Because vascular calcification can be modulated by positive and negative mechanisms, we also examined the expression of IGF1R, which can modulate VSMC calcification. Notably, IGF1R expression was reduced with the addition of AGE-BSA (Figure 1D).
Figure 1.
AGEs accelerate the progression of calcification in HASMCs. (A) HASMCs were cultured in the following four groups for 7 days: control group (growth medium), group 1 (calcification medium only), group 2 (calcification medium+BSA), and group 3 (calcification medium+AGE-BSA). Extracellular calcium measurements were performed using the QuantiChrome Calcium Assay, and the data were normalized using the total protein concentration. (B) Representative images of alizarin red staining in microscopic views (×100) are shown. (C) qPCR analysis of mRNA levels of bone-related molecules, Cbfα1 and OC, in HASMCs treated with AGEs for 7 d. (D) Western blot analysis of OC, Cbfα1, and IGF1R protein expression; β-actin was used as a control for protein loading. For the bar graphs, data from three independent experiments were included for statistical analysis. bP<0.05.
AGEs promote HASMC calcification by inhibiting IGF1R expression
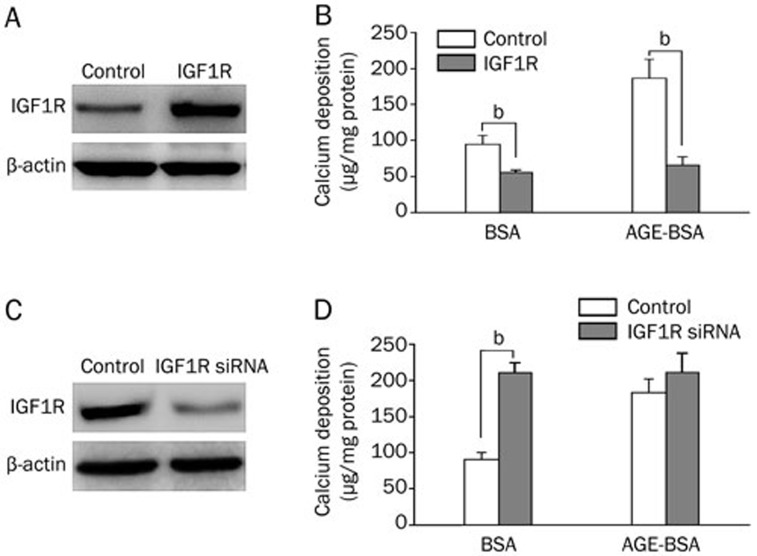
IGF1R has been shown to modulate calcium-induced calcification in VSMCs. To explore whether AGEs promote vascular calcification by inhibiting IGF1R expression, we examined whether over-expression of IGF1R can inhibit AGE-enhanced vascular calcification in HASMCs by lentiviral transduction (Figure 2A). AGE-BSA potently enhanced Pi-induced mineralization in HASMCs expressing an empty vector, but mineralization was significantly suppressed in HASMCs expressing IGF1R (Figure 2B). In addition, AGE-BSA failed to further increase calcium deposition when IGF1R expression was knocked down using IGF1R siRNA (Figures 2C and 2D).
Figure 2.
IGF1R expression is inhibited in AGE-promoted calcification in HASMCs. (A) Western blot analysis identified the overexpression of IGF1R by a lentiviral vector in HASMCs. (B) HASMCs stably transfected with IGF1R were treated with 2 mmol/L Pi in the presence or absence of 100 μg/mL AGE-BSA for 7 d, and extracellular calcium measurements were performed. (C) Western blot analysis confirmed IGF1R knockdown via the lentiviral vector in HASMCs. (D) HASMCs stably transfected with IGF1R siRNA were treated with 2 mmol/L Pi in the presence or absence of 100 μg/mL AGE-BSA for 7 d, and extracellular calcium measurements were performed. The data are presented as the mean±SD of three independent experiments performed in duplicate. bP<0.05.
NF-κB activation is essential for AGE-accelerated HASMC calcification
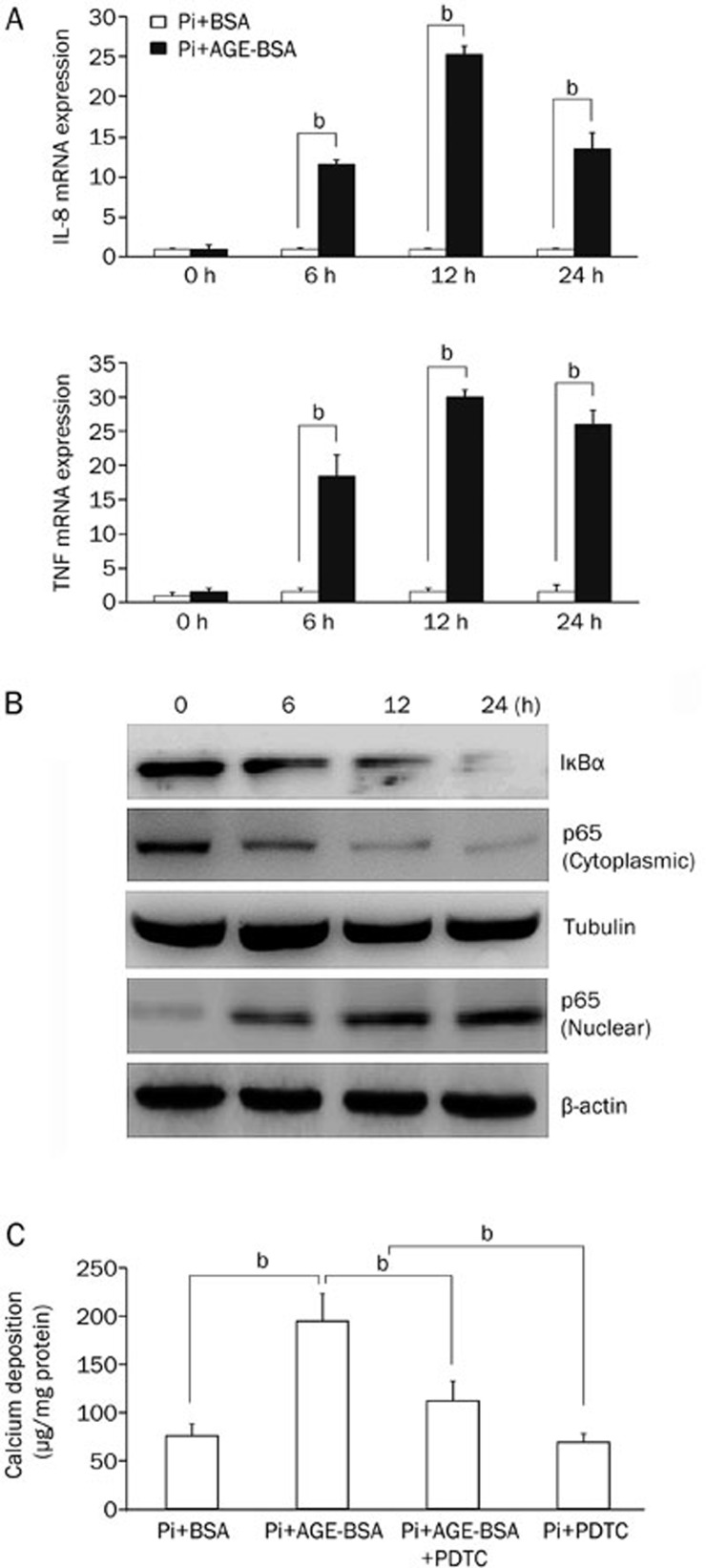
We then tested the role of nuclear factor-κB (NF-κB) signaling in AGEs-accelerated calcium deposition in HASMCs. The amounts of IL-8 and TNF increased with an increasing time of AGE-BSA treatment, as shown by the qPCR data (Figure 3A). In the cytoplasmic extracts, the amounts of IκBα and p65 decreased over time with AGE-BSA treatment, whereas the amount of nuclear p65 increased (Figure 3B). Then, we used the NF-κB inhibitor PDTC (50 μmol/L) to investigate the role of NF-κB in HASMC calcification. We found that PDTC suppressed an AGE-induced increase in calcium deposition (Figure 3C).
Figure 3.
AGEs promote inorganic phosphate (Pi)-induced HASMC calcification by activating NF-κB. (A) Real-time PCR analysis of the mRNA levels of interleukin 8 (IL-8) and TNF. (B) Western blots were used to measure the amounts of IκBα and p65 from cytoplasmic extracts and p65 from nuclear extracts. (C) Extracellular calcium measurements were performed in the presence or absence of PDTC. The data are presented as the mean±SD of three independent experiments performed in duplicate. bP<0.05.
AGE activation of NF-κB decreases IGF1R expression and promotes HASMC calcification
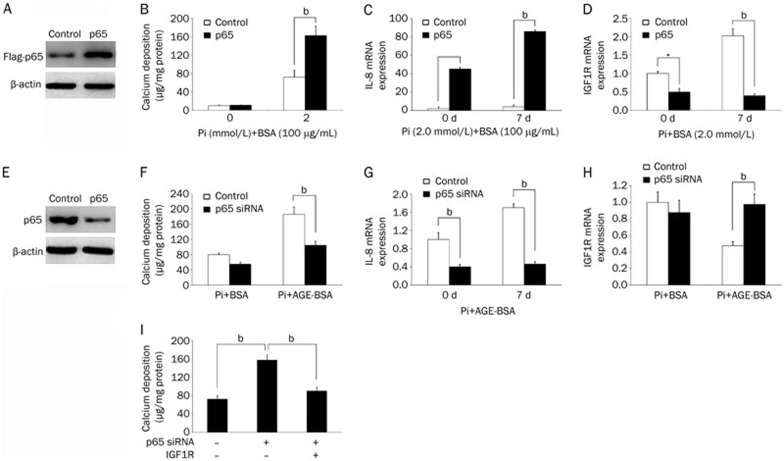
To determine whether AGE-activated NF-κB was sufficient to promote calcification, we overexpressed Flag-tagged p65 in HASMCs by lentiviral transduction (Figure 4A). As a result, p65 overexpression significantly enhanced Pi-induced mineralization and increased IL-8 mRNA expression (Figures 4B and C) but repressed IGF1R expression (Figure 4D). To determine whether NF-κB is essential for AGEs-enhanced mineralization, we depleted p65 in HASMCs using lentivirus-mediated expression of siRNA and confirmed the knockdown by western blot analysis (Figure 4E). In contrast to p65 overexpression, p65 knockdown significantly reduced AGE-augmented mineralization and IL-8 expression in Pi-treated HASMCs (Figures 4F and 4G). In accordance, p65 knockdown rescued the AGE-mediated decrease in IGF1R expression (Figure 4H). IGF1R overexpression in HASMCs prevented p65-augmented calcium deposition (Figure 4I). Therefore, AGEs may activate NF-κB to promote calcification by downregulating IGF1R expression.
Figure 4.
NF-κB signaling mediates the effects of AGEs on IGF1R expression and calcification in HASMCs. (A) Western blot analysis confirmed the overexpression of Flag-tagged p65 via a lentiviral vector in HASMCs. (B) HASMCs stably expressing p65 or a control empty vector were treated with BSA or AGE-BSA (100 μg/mL) in calcification medium for 7 d (calcium content assay for calcium deposition). (C, D) HASMCs stably expressing p65 or a control vector were treated with BSA or AGE-BSA (100 μg/mL) in calcification medium for 7 d (q-PCR analysis of IL-8 and IGF1R mRNA levels). (E) Western blot analysis confirmed p65 knockdown via a lentiviral vector in HASMCs. (F) HASMCs stably expressing p65 or control siRNA were treated with BSA or AGE-BSA (100 μg/mL) in calcification medium for 7 d (calcium content assay for calcium deposition). (G, H) HASMCs stably expressing p65 or control siRNA were treated with BSA or AGE-BSA (100 μg/mL) in calcification medium for 7 d (q-PCR analysis of IL-8 and IGF1R mRNA levels). (I) HASMCs stably expressing p65 and/or IGF1R via a lentiviral vector were grown in calcification medium for 7 d (calcium content assay for calcium deposition). The data are presented as the mean±SD of three independent experiments performed in duplicate. bP<0.05.
NF-κB influences IGF1R promoter activity
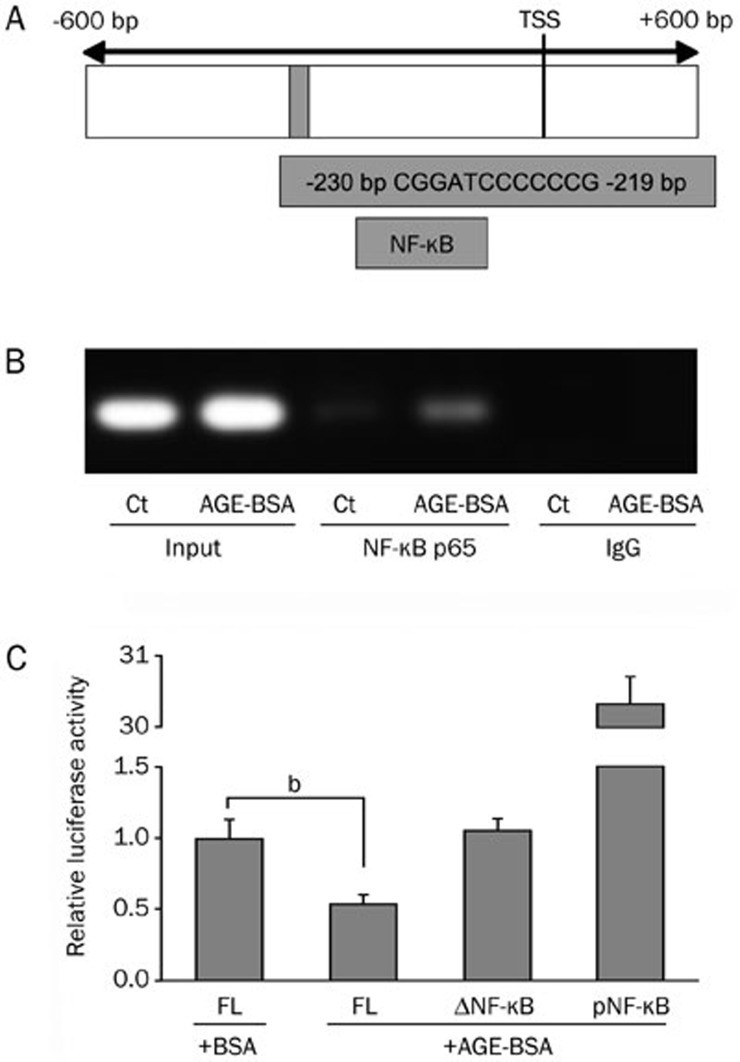
To investigate the mechanism responsible for transcriptional repression of IGF1R by NF-κB, we turned our attention to the 5′ upstream site of the IGF1R gene containing the putative IGF1R promoter. Available public databases and analysis of full-length cDNA (Genbank NM_000875) identified the transcription start site of IGF1R at position −611 relative to the first codon (Figure 5A). Further analysis of the putative IGF1R promoter region revealed the existence of a potential binding site for NF-κB (core sequence 5′-CGGATCCCCCCG-3′) at position −230 to −219 relative to the transcription start site.
Figure 5.
IGF1R promoter activity is reduced through nuclear factor (NF)-κB. (A) Schematic of the putative IGF1R promoter region containing one potential binding sequence for NF-κB and luciferase constructs containing the full-length IGF1R promoter. (B) A chromatin immunoprecipitation (ChIP) assay was used to examine the NF-κB p65 subunit binding to the putative human IGF1R promoter in HASMCs after exposure to AGE-BSA. Controls included a polymerase chain reaction performed with whole HASMC cell genomic DNA (input), NF-κB p65 antibody, and IgG antibody. (C) HASMCs were transfected with the IGF1R reporter plasmid (3000 bp), an IGF1R reporter plasmid containing the mutated binding sequence for NF-κB (ΔNF-κB) or an NF-κB reporter vector (pNF-κB) and exposed to AGE-BSA before the relative luciferase activity was determined. The data are presented as the mean±SD of three independent experiments performed in duplicate. bP<0.05. Ct, control; FL, full length; TSS, transcription start site.
To identify possible binding of NF-κB to the putative IGF1R promoter, we used ChIP analysis. For this purpose, we stimulated HASMCs with AGE-BSA and amplified NF-κB binding with primers spanning the characteristic sequences. This analysis in HASMCs revealed a prominent band at 215 bp in the nuclei derived from AGE-BSA-stimulated cells, identifying the binding of NF-κB at position −230 to −219 (Figure 5B). To further identify the functional implications of NF-κB binding to the putative IGF1R promoter, we used a firefly luciferase reporter vector. We found significantly decreased luciferase activity in the full-length construct. This repression was attenuated in the construct with a targeted mutation of the central NF-κB core sequence. Luciferase activity was significantly attenuated in the mutated reporter plasmid, demonstrating no repression in HASMCs (Figure 5C).
Discussion
A significant predictor for cardiovascular events, vascular calcification is present in patients with atherosclerosis, chronic kidney disease, diabetic vasculopathy, and aging. Vascular calcification is a complex process associated with the expression of proteins that initiate or inhibit mineralization in vessel walls15,16. Our study provides a novel mechanism for AGEs exacerbating HASMC calcification by activating NF-κB. AGE-activated NF-κB suppressed IGF1R expression, leading to inhibition of calcification. We provide a direct link between AGEs and the osteogenic program attributed to vascular calcification.
Advanced glycation end products are a heterogeneous group of bioactive molecules that have been implicated in the aging of tissue proteins and the development of diabetes, atherosclerosis, chronic kidney disease, and other diseases17,18,19. Recent data have demonstrated that AGEs accelerate the progression of diabetic atherosclerosis and vascular calcification20,21,22. AGEs act through their receptor RAGE, and increase apoptosis and the induction of alkaline phosphatase (ALP) and osteogenic genes12,23, including CBFα1, a key regulatory transcription factor critical for osteoblast differentiation, and osteocalcin, which is a very specific protein indicative of osteoblast activity. CBFα1 knockout mice fail to form mineralized bone24 and exhibit low ALP activity and osteocalcin expression. However, how inhibitors of calcification are regulated by AGEs is less defined. From a mechanistic viewpoint, our data provide further evidence that AGEs contribute to vascular calcification by regulating the osteogenic program through mediators and regulators. AGEs increased calcium deposition and decreased IGF1R expression in the presence of the calcification inducer Pi.
Insulin-like growth factor-1 (IGF1) receptor (IGF1R) signals are vital for the proliferation, survival, and migration of many cells, including VSMCs25,26. Both IGF1 and IGF1R are expressed in tissues with vascular pathology27,28. IGF1 is also expressed in bones and promotes bone formation29. Recently, IGF1 signaling has been implicated in the regulation, proliferation, and osteoblastic differentiation of calcifying vascular cells30. Moreover, IGF1R expression and activity has been shown to inhibit calcium-induced calcification13. In our study, we demonstrated that AGEs markedly repressed IGF1R expression and promoted calcification in HASMCs. To our knowledge, the present study is the first to report that AGEs decrease IGF1R expression in HASMCs. In addition, the restoration of IGF1R expression greatly decreased AGEs-induced calcification. Furthermore, AGEs could not further increase Pi-induced calcification after IGF1R knockdown using lentivirus-mediated expression of siRNA, indicating that IGF1R may play a great role in HASMC calcification.
What is the potential signaling pathway that mediates AGEs, IGF1R expression and HASMC calcification? Previous reports have shown that AGEs can activate NF-κB, which stimulates MMP-9 gene expression31. Moreover, Wang et al showed that TNF-mediated NF-κB activation aggravates human vascular calcification via repression of ANKH, which is an endogenous mineralization inhibitor32. In this study, we demonstrated that NF-κB activation is essential for AGE-promoted HASMC calcification. This finding is supported by the AGE-induced increase in IL-8 and TNF expression, promoting IκBα degradation and p65 nuclear translocation; knocking down p65 using siRNA significantly reduced Pi-induced calcium deposition in HASMCs. Moreover, AGE activation of NF-κB decreased IGF1R expression and promoted HASMC calcification. Studies on the putative IGF1R promoter identified a nuclear factor-κB–dependent mechanism to be involved in IGF1R repression. These results suggest that AGEs promote Pi-induced calcification by regulating NF-κB and IGF1R pathways.
In summary, our finding that AGE enhanced vascular calcification in HASMCs by inhibiting IGF1R expression suggests that IGF1R may be a crucial regulator for AGE-directed vascular calcification. In addition, our study showed that the NF-κB pathway is responsible for an AGE-mediated decrease in IGF1R expression in HASMCs. These data may improve our understanding of the complexity of vascular calcification in patients with chronic kidney disease, diabetes, or atherosclerosis.
Acknowledgments
The present study was supported by the Natural Science Foundation of Shanghai, China (No 09ZR1425200).
References
- Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–42. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014–21. doi: 10.1093/ndt/15.7.1014. [DOI] [PubMed] [Google Scholar]
- London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–40. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- Reaven PD, Sacks J. Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes. Diabetologia. 2005;48:379–85. doi: 10.1007/s00125-004-1640-z. [DOI] [PubMed] [Google Scholar]
- Covic A, Kanbay M, Voroneanu L, Turgut F, Serban DN, Serban IL, et al. Vascular calcification in chronic kidney disease. Clin Sci (Lond) 2010;119:111–21. doi: 10.1042/CS20090631. [DOI] [PubMed] [Google Scholar]
- Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–7. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008;45:1–16. doi: 10.1159/000115118. [DOI] [PubMed] [Google Scholar]
- Chen XF, Lin WD, Lu SL, Xie T, Ge K, Shi YQ, et al. Mechanistic study of endogenous skin lesions in diabetic rats. Exp Dermatol. 19:1088–95. doi: 10.1111/j.1600-0625.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Stern DM. The dark side of glucose. Nat Med. 1995;1:1002–4. doi: 10.1038/nm1095-1002. [DOI] [PubMed] [Google Scholar]
- Vlassara H. Advanced glycation end-products and atherosclerosis. Ann Med. 1996;28:419–26. doi: 10.3109/07853899608999102. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Nakamura K, Matsui T, Noda Y, Imaizumi T. Receptor for advanced glycation end products (RAGE): a novel therapeutic target for diabetic vascular complication. Curr Pharm Des. 2008;14:487–95. doi: 10.2174/138161208783597416. [DOI] [PubMed] [Google Scholar]
- Tanikawa T, Okada Y, Tanikawa R, Tanaka Y.Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK J Vasc Res 200946572–80.19571577 [Google Scholar]
- Di Bartolo BA, Schoppet M, Mattar MZ, Rachner TD, Shanahan CM, Kavurma MM. Calcium and osteoprotegerin regulate IGF1R expression to inhibit vascular calcification. Cardiovasc Res. 2011;91:537–45. doi: 10.1093/cvr/cvr084. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Illmer T, Kasper M, Luther T, Quehenberger P, Tritschler H, et al. Advanced glycation end product (AGE)-mediated induction of tissue factor in cultured endothelial cells is dependent on RAGE. Circulation. 1997;96:2262–71. doi: 10.1161/01.cir.96.7.2262. [DOI] [PubMed] [Google Scholar]
- Duer MJ, Friscic T, Proudfoot D, Reid DG, Schoppet M, Shanahan CM, et al. Mineral surface in calcified plaque is like that of bone: further evidence for regulated mineralization. Arterioscler Thromb Vasc Biol. 2008;28:2030–4. doi: 10.1161/ATVBAHA.108.172387. [DOI] [PubMed] [Google Scholar]
- Wallin R, Wajih N, Greenwood GT, Sane DC. Arterial calcification: a review of mechanisms, animal models, and the prospects for therapy. Med Res Rev. 2001;21:274–301. doi: 10.1002/med.1010. [DOI] [PubMed] [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Yamagishi S. Involvement of toxic AGEs (TAGE) in the pathogenesis of diabetic vascular complications and Alzheimer's disease. J Alzheimers Dis. 2009;16:845–58. doi: 10.3233/JAD-2009-0974. [DOI] [PubMed] [Google Scholar]
- Yamagishi S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol. 2011;46:217–24. doi: 10.1016/j.exger.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Forbes JM, Yee LT, Thallas V, Lassila M, Candido R, Jandeleit-Dahm KA, et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004;53:1813–23. doi: 10.2337/diabetes.53.7.1813. [DOI] [PubMed] [Google Scholar]
- Ren X, Shao H, Wei Q, Sun Z, Liu N. Advanced glycation end-products enhance calcification in vascular smooth muscle cells. J Int Med Res. 2009;37:847–54. doi: 10.1177/147323000903700329. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Matsui T. Smooth muscle cell pathophysiology and advanced glycation end products (AGEs) Curr Drug Targets. 2010;11:875–81. doi: 10.2174/138945010791320827. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiang Y, Liu N, Ren L, Zhu Y, An Y, et al. Advanced glycation end-product Nepsilon-carboxymethyl-Lysine accelerates progression of atherosclerotic calcification in diabetes. Atherosclerosis. 2012;221:387–96. doi: 10.1016/j.atherosclerosis.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Kavurma MM, Figg N, Bennett MR, Mercer J, Khachigian LM, Littlewood TD. Oxidative stress regulates IGF1R expression in vascular smooth-muscle cells via p53 and HDAC recruitment. Biochem J. 2007;407:79–87. doi: 10.1042/BJ20070380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinis B, Baserga R. IGF-I receptor signalling in transformation and differentiation. Mol Pathol. 2001;54:133–7. doi: 10.1136/mp.54.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:435–44. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- Kavurma MM, Bennett MR. Expression, regulation and function of trail in atherosclerosis. Biochem Pharmacol. 2008;75:1441–50. doi: 10.1016/j.bcp.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Kasukawa Y, Baylink DJ, Wergedal JE, Amaar Y, Srivastava AK, Guo R, et al. Lack of insulin-like growth factor I exaggerates the effect of calcium deficiency on bone accretion in mice. Endocrinology. 2003;144:4682–9. doi: 10.1210/en.2003-0745. [DOI] [PubMed] [Google Scholar]
- Radcliff K, Tang TB, Lim J, Zhang Z, Abedin M, Demer L. Insulin-like growth factor-I regulates proliferation and osteoblastic differentiation of calcifying vascular cells via extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase pathways. Circ Res. 2005;96:398–400. doi: 10.1161/01.RES.0000157671.47477.71. [DOI] [PubMed] [Google Scholar]
- Zhu P, Ren M, Yang C, Hu YX, Ran JM, Yan L. Involvement of RAGE, MAPK and NF-κB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp Dermatol. 2012;21:123–9. doi: 10.1111/j.1600-0625.2011.01408.x. [DOI] [PubMed] [Google Scholar]
- Zhao G, Xu MJ, Zhao MM, Dai XY, Kong W, Wilson GM, et al. Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int. 2012;82:34–44. doi: 10.1038/ki.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]