Abstract
Aim:
To investigate the effects of safflor yellow A (SYA), a flavonoid extracted from Carthamus tinctorius L, on cultured rat cardiomyocytes exposed to anoxia/reoxygenation (A/R).
Methods:
Primary cultured neonatal rat cardiomyocytes were exposed to anoxia for 3 h followed by reoxygenation for 6 h. The cell viability was measured using MTT assay. The releases of lactate dehydrogenase (LDH) and creatine kinase (CK), level of malondialdehyde (MDA), and activities of glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) were analyzed. Hoechst 33258 staining and changes in Bcl-2/Bax ratio and caspase 3 activity were used to examine A/R-induced apoptosis.
Results:
The A/R exposure markedly decreased the viability of cardiomyocytes, suppressed the activities of SOD, GSH, CAT and GSH-Px, and Bcl-2 protein expression. Meanwhile, the A/R exposure markedly increased the release of LDH and CK, and MDA production in the cardiomyocytes, and increased the rate of apoptosis, caspase 3 activity, Bax protein expression. Pretreatment with SYA (40, 60 and 80 nmol/L) concentration-dependently blocked the A/R-induced changes in the cardiomyocytes. Pretreatment of the cardiomyocytes with the antioxidant N-acetylcysteine (NAC, 200 μmol/L) produced protective effects that were comparable to those caused by SYA (80 nmol/L).
Conclusion:
SYA protects cultured rat cardiomyocytes against A/R injury, maybe via inhibiting cellular oxidative stress and apoptosis.
Keywords: safflor yellow A, N-acetylcysteine, antioxidant, cardiomyocyte, anoxia/reoxygenation, oxidative stress, apoptosis
Introduction
In developed countries, cardiovascular diseases are the major cause of disability and mortality1. Myocardial ischemia and reperfusion is a major source of cardiomyocyte injury within the context of a host of clinical pathologies2. Ischemia/reperfusion (I/R) injury includes a series of events that may occur together or separately: reperfusion arrhythmias, myocardial stunning in “reversible mechanical dysfunction”, microvascular damage and cell death3,4,5,6,7. Oxidative stress and apoptosis have been reported to play significant roles in the progression of I/R8,9,10,11. Inhibition of oxidative stress and/or a direct intervention in apoptotic pathways at the membrane, at the mitochondria or at nuclear activation sites could provide molecular targets for potential therapeutic treatments12. The search for the most effective therapeutic drugs for I/R injury has long presented a problem. In the past few decades, there has been an increase in the use of complementary treatments, such as herbal remedies, in the treatment of diseases in the whole world. Many traditional plants have been reported to be useful for the control of problems due to ischemia and associated pathologies13.
Carthamus tinctorius L, a member of the Compositae, has been used as a Chinese medicine in the clinic to treat cardiovascular disease with anti-myocardial ischemia14,15. The chemical constituents in Carthamus tinctorius L are reported to include lignans16, flavonoids17, triterpene alcohols18, and polysaccharides19. Research shows that the main effective constituent of Carthamus tinctorius L is safflower yellow (SY), which contains several related flavonoids. SY consists of hydroxysafflor yellow A (HSYA), safflor yellow A (SYA), safflower yellow B (SYB), and other chemicals20,21,22,23,24. Many studies have demonstrated that SY possesses various physiological and pharmacological activities, including anticoagulant, antioxidant, antiapoptotic and calcium antagonist. However, the component responsible for these protective effects remains unknown.
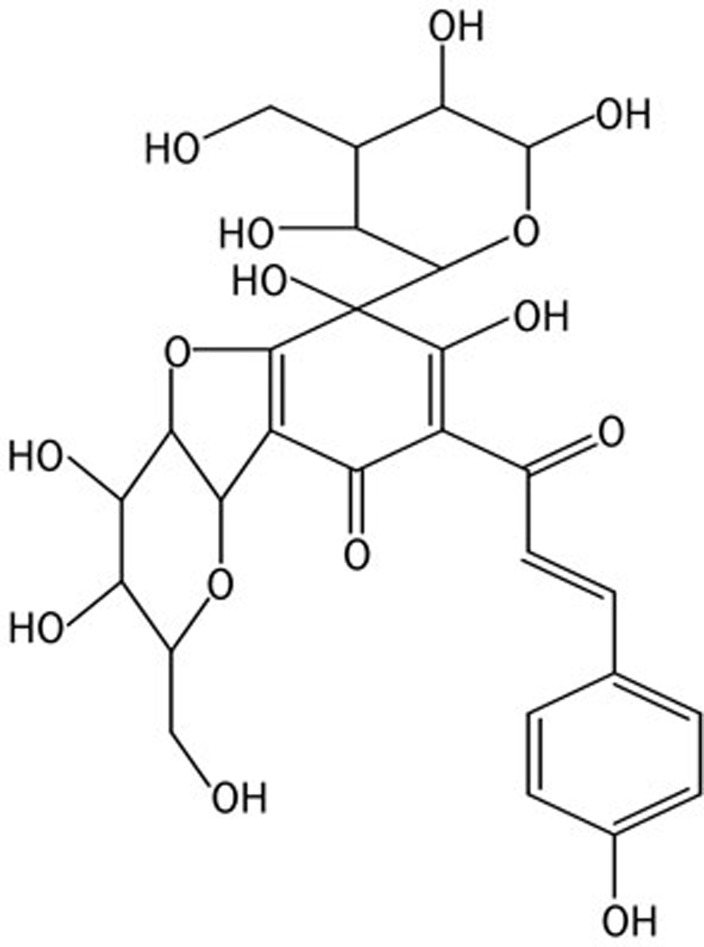
Like SYB and HSYA, SYA (Figure 1), a natural compound from the flower of Carthamus tinctorius L, is a water-soluble monomer of SY. Increasing evidence demonstrates that SYB and HSYA provide myocardial protection by acting at multiple target points, such as via antioxidant and antiapoptotic activity25,26,27,28, but there is little research on the effect of SYA on myocardial preservation. The aim of our study is to investigate whether SYA is an effective component of Carthamus tinctorius L with cardioprotective action.
Figure 1.
Chemical structure of safflor yellow A. Molecular formula: C27H29O15; molecular weight: 593.15.
Materials and methods
Materials
SYA (purity >98%) was purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological products (Beijing, China). Dulbecco's modified Eagle's medium (DMEM) was obtained from GIBCO (Life Technologies, Grand Island, NY, USA), and fetal bovine serum (FBS) was provided by Hangzhou Sijiqing Biological Engineering Materials Co Ltd (Hangzhou, China). N-acetylcysteine (NAC), MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide], type I collagenase and Western blot reagents were purchased from Sigma (St Louis, MO, USA). The kits for the determination of LDH, CK, MDA, GSH-Px, CAT, SOD, and GSH were obtained from Jiancheng Bioengineering Institute (Nanjing, China). Anti-β-actin, anti-Bax, and anti-Bcl-2 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The fluorescent kit for Hoechest 33258 was obtained from Roche (Mannheim, Germany). The caspase 3 assay kit was purchased from Chemicon International Inc (Temecula, CA, USA).
Animals
All animals used in this study were cared for in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health, and the local Animal Ethics Committee approved all procedures. This study used 1- to 3-d-old Sprague-Dawley rats (gender was not determined).
Primary cardiomyocyte culture
Isolation of cardiac myocytes was performed as previously described29. Briefly, the hearts were harvested and placed in PBS. Then, the hearts were minced and dissociated with 1 mg/mL type I collagenase. Cardiomyocytes were enriched by preplating for 90 min to eliminate fibroblasts. More than 90% of the cells were identified as cardiac myocytes by immunocytochemistry with anti-sarcomeric β-actin (Sigma). The cells were then cultured in DMEM with 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin and maintained in 5% CO2 at 37 °C. The cells were seeded at a density of 4×104 cells/mL in 96-well microplates for MTT assays or at a density of 3×105 cells/mL in 6-well microplates for LDH, CK, MDA activity assays and antioxidant enzyme assays, and for the measurement of other biochemical indicators. The culture medium was changed every second day. After 72 h, cultured cardiomyocytes were used in subsequent experiments. Cardiac myocytes were randomly divided into six groups: 1) a control group without any treatment; 2) a model group, which was cultured under anoxic conditions for 3 h and then under reoxygenation conditions for 6 h; 3) a NAC treatment group, which was pretreated with 200 μmol/L NAC for 24 h before anoxia/reoxygenation (A/R) injury; 4) SYA-40 treatment group, which were pretreated with SYA at concentration of 40 nmol/L for 24 h before A/R injury; 5) SYA-60 treatment group, which were pretreated with SYA at concentration of 60 nmol/L for 24 h before A/R injury; 6) SYA-80 treatment group, which were pretreated with SYA at concentration of 80 nmol/L for 24 h before A/R injury.
In vitro A/R injury model
The in vitro model of A/R used in the present study was similar to the model previously described by Koyama et al30. To mimic an A/R model (via anoxia in the medium), cells were placed in a hypoxia solution and incubated for 3 h at 37 °C in a hypoxic chamber (HEPA CELL 100, Germany) filled with 95% N2 and 5% CO2. The hypoxia solution contains (in mmol/L: NaCl 98.5, KCl 10, MgSO4 1.2, CaCl2 1.0, HEPES 20, and sodium lactate 40, at pH 6.8, 37 °C). For A/R studies, the medium was then replaced by maintenance medium during the reoxygenation period, and cells were cultured for 6 h under normal conditions.
Cell viability assays
Cell viability was determined by MTT assay. Cells were seeded at a density of 4×104 cells/well in 96-well plates. After treatment, 20 μL of the MTT solution (5 mg/mL) was added to each well, resulting in a final concentration of 5 mg/mL, and kept for 4 h at 37 °C. Afterward, the medium was removed and DMSO (150 mL) was added to each well. The optical density (OD) was determined spectrophotometrically at 490 nm with a microplate reader (Infinite M200 PRO, Switzerland), and the cell survival ratio was expressed as a percentage of the control.
LDH and CK activities assays
Many experiments have reported that LDH and CK are two reliable markers of cardiac myocyte I/R injury31,32. After the A/R procedure, cell culture medium was collected to determine LDH and CK levels by measuring the conversion of pyruvate to lactate and creatine to phosphagen, respectively. The activities of LDH and CK were analyzed with microplates at 450 nm.
Measurement of MDA activity
MDA is a product of lipid peroxidation that can cause crosslinking polymerization of proteins, nucleic acids and some macromolecules, so the amount of MDA often reflects the degree of lipid peroxidation33,34. Culture medium was collected 6 h post reoxygenation, transferred to clean epoxy epoxide (EP) tubes and stored at −80 °C. Thiobarbituric acid reactive substances were assessed by measuring the MDA concentration at 532 nm with the thiobarbituric acid method, which is based on the reaction of MDA with thiobarbituric acid to form a stable chromophoric product.
Determination of cellular antioxidant enzyme activities
Determination of the activities of antioxidant enzymes was conducted according to the instructions provided in the assay kits (Jiancheng Bioengineering Institute, Nanjing, China). In brief, after 6 h of reoxygenation, the cells were washed twice in PBS, resuspended in 1 mL of 0.1 mol/L phosphate buffer (pH 7.4) and homogenized. The homogenate was centrifuged at 4000 rounds per minute for 10 min at 4 °C, and the supernatants were collected after centrifugation for subsequent tests. GSH activity was assayed at 420 nm and calculated on the basis of a GSH calibration curve. SOD activity was assayed at 560 nm on the basis of its ability to inhibit the oxidation of hydroxylamine by the superoxide anion from the xanthine oxidase system. GSH-Px activity was measured at 412 nm on the basis of the rate of oxidation of reduced glutathione to oxidized glutathione by H2O2 under the catalysis of GSH-Px. The principles of the assay for catalase are based on the determination of the H2O2 decomposition rate at 240 nm. The protein content in the cell lysate was determined with Coomassie blue staining solution.
Morphological assessment and quantification of apoptotic myocytes
Hoechst 33258 staining, which allows apoptotic and normal cells to be clearly distinguished on the basis of nuclear morphology (chromatin condensation and fragmentation), was used for quantification of apoptotic myocytes. Briefly, cells were washed in ice-cold PBS, fixed in 10% neutral buffered formalin for 10 min at room temperature, and washed again in ice-cold PBS. Then, the cells were exposed to Hoechst 33258 (2 μg/mL in PBS) and incubated for 20 min at room temperature. Cells were then washed three times in PBS and examined under a fluorescence microscope with an appropriate filter. The percentage of apoptotic cells displaying chromatin condensation and nuclear fragmentation was determined. Apoptosis in cardiomyocytes was quantified by the number of apoptotic nuclei in the total number of nuclei in 10 continuous microscopic fields under ×400 magnification by using the following formula: apoptosis index=(apoptotic nuclei/total nuclei)×100%.
Caspase 3 activity
Caspase 3 is an important component of the final pathway leading to cell death. Its activity was measured using a fluorometric assay kit according to the manufacturer's protocol. In brief, 4×105 control or A/R treated cells were lysed in 50 μL ice-cold lysis buffer, placed on ice for 30 min, and then centrifuged at 10 000×g for 5 min at 4 °C. The protein concentrations in the supernatants were quantified with the Coomassie blue staining solution. Reaction buffer (50 μL) and caspase 3 substrate (5 μL) were added to the cell lysates. After incubation at 37 °C for 4 h, the fluorescence was measured on a microplate reader with excitation at 400 nm.
Western blotting analysis
For Western blot, equal amounts of protein lysates were separated using 10% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel electrophoresis. The gels were blotted onto a nitrocellulose membrane and incubated with the indicated antibodies. Blots were developed by ECL according to the manufacturer's instructions. β-Actin was used as a loading control.
Statistical analysis
All values were expressed as the mean±SD. A one-way analysis of variance followed by Bonferoni's correction was carried out to test for any differences between the mean values of groups. A P value <0.05 was considered to be significant.
Results
SYA protected cells from A/R-induced cell viability loss
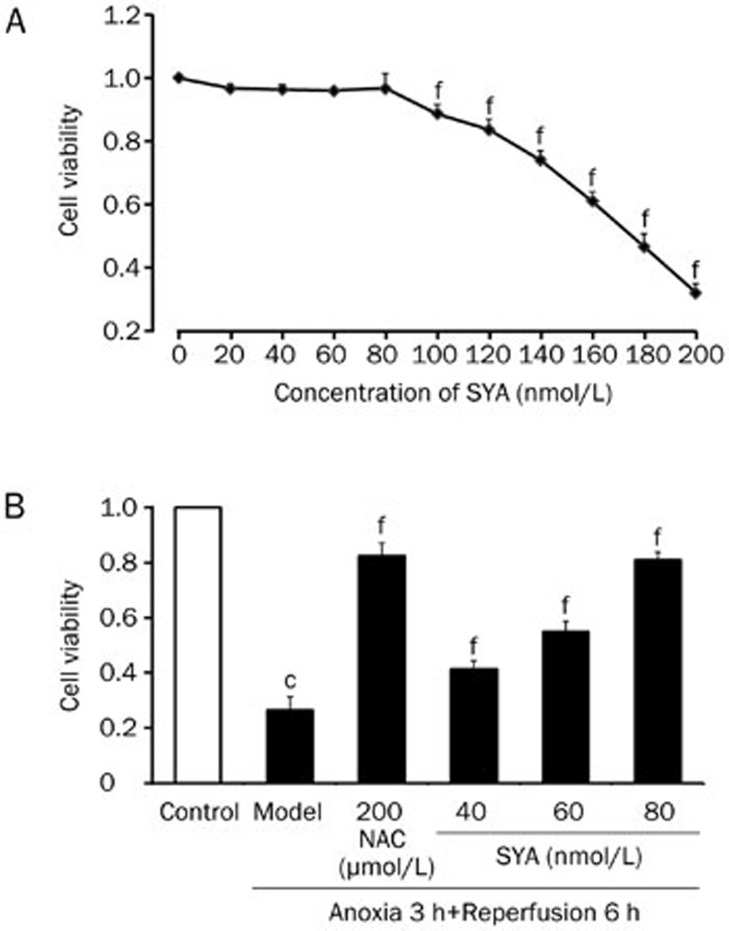
Cell viability was shown in Figure 2 using the MTT assay. We first examined the viability of cardiomyocytes after treatment with SYA (0–200 nmol/L). SYA at concentrations below 100 nmol/L did not significantly affect cell viability, so three concentrations (40, 60, and 80 nmol/L) were selected as the working concentrations. A/R caused a significant decrease in cell viability (P<0.01); the results in Figure 2B indicate that SYA protected cells against injury in a dose-dependent manner.
Figure 2.
The results of cell viability-MTT assay. (A) The toxic effect of SYA (0–200 nmol/L) was measured in neonatal rat cardiomyocytes after 4 h incubation by MTT. (B) Different concentrations of SYA (40, 60, and 80 nmol/L) were pretreated 24 h prior to A/R. The cells were divided into six groups: control, model (A/R), NAC (200 μmol/L+A/R), SYA-40 (40 nmol/L+A/R), SYA-60 (60 nmol/L+A/R), SYA-80 (80 nmol/L+A/R). Data were presented as mean±SD of three experiments. cP<0.01 as compared with control. fP<0.01 as compared with the model group.
Effect of SYA on LDH and CK activities
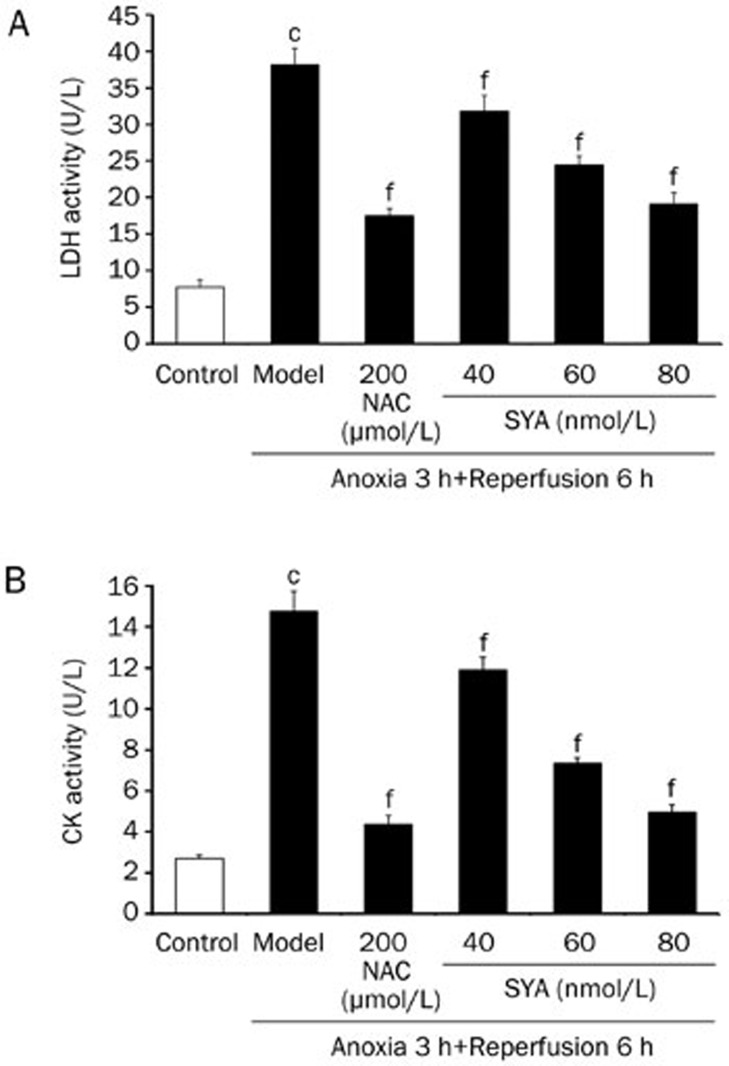
The release of LDH and CK was used as an index of cardiomyocyte injury. LDH and CK release in the model group was higher than that in the control group (P<0.01), and significant decreases in LDH and CK activities were observed after pretreatment with SYA (40, 60, and 80 nmol/L) or NAC (200 μmol/L) compared with the model group (P<0.01) (Figure 3). These results indicate that SYA is a potent cardioprotective agent against A/R injury.
Figure 3.
Effect of SYA on LDH and CK activities in the culture supernatant of cardiomyocytes subjected A/R injury. The cardiomyocytes were pretreated with SYA (40, 60, and 80 nmol/L) or NAC (200 μmol/L) for 24 h, and then subjected to 3 h anoxia followed by 6 h reoxygenation. The activities of LDH and CK were determined by the methods of corresponding assay kits. Data were presented as mean±SD of three experiments. cP<0.01 as compared with control. fP<0.01 as compared with the model group.
Effect of SYA on MDA content
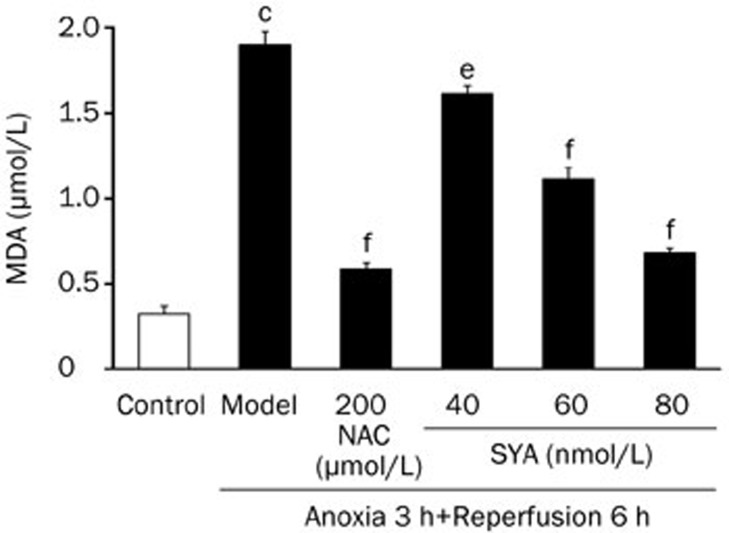
The concentration of MDA in the culture media was measured as a biochemical marker for lipid peroxidation. Cardiomyocytes subjected to A/R showed an increase in MDA content compared with untreated cells. Treatment with SYA (60 and 80 nmol/L) or NAC (200 μmol/L) produced a significant reduction in MDA content in cardiomyocytes undergoing A/R (P<0.01) (Figure 4).
Figure 4.
Effects of SYA on A/R induced change of MDA contents in cardiomyocytes. The cardiomyocytes were pretreated with SYA (40, 60, and 80 nmol/L) or NAC (200 μmol/L) for 24 h, and subjected to A/R as described in Methods section. The content of MDA in different groups of cardiomyocytes was detected by the method in the detection kit. Data were presented as mean±SD of three experiments. cP<0.01 as compared with control. eP<0.05, fP<0.01 as compared with the model group.
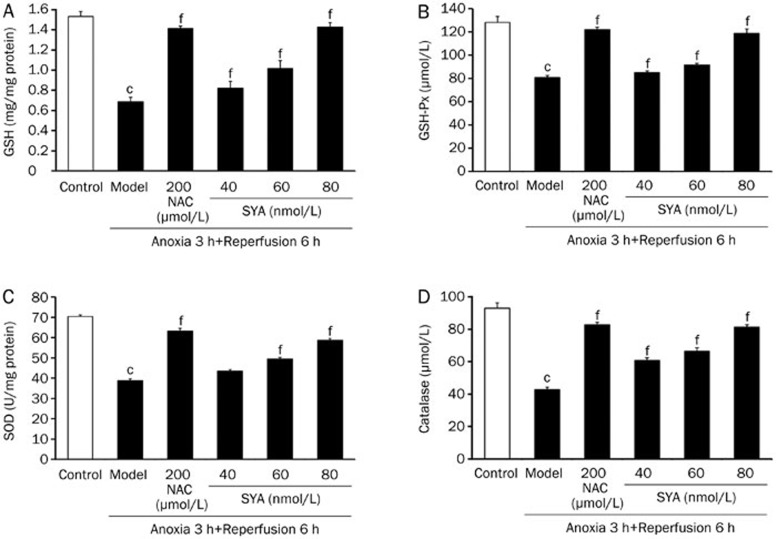
Effect of SYA on antioxidant enzyme activities
GSH, glutathione peroxidase (GSH-Px), SOD, and catalase are important antioxidant enzymes, and the activities of these four enzymes were examined in this study. When the cardiomyocytes were damaged, the activities of these enzymes (GSH, GSH-Px, catalase, and SOD) were depressed compared with their activities in normal cells (P<0.01). These changes demonstrated that the antioxidant system was disrupted by A/R. Treatment with SYA or NAC significantly increased the activities of the antioxidant enzymes (P<0.01) and protect cardiomyocytes from oxidative damage (Figure 5).
Figure 5.
Effect of SYA on antioxidant enzyme activities. The cardiomyocytes were pretreated with SYA (40, 60, and 80 nmol/L) or NAC (200 μmol/L) for 24 h, and subjected to A/R as described in Methods section. The cells lysate was centrifugated, and then the supernatant was assayed for activities of GSH (A), GSH-Px (B), SOD (C), and catalase (D). Data were presented as mean±SD of three experiments. cP<0.01 as compared with control. fP<0.01 as compared with the model group.
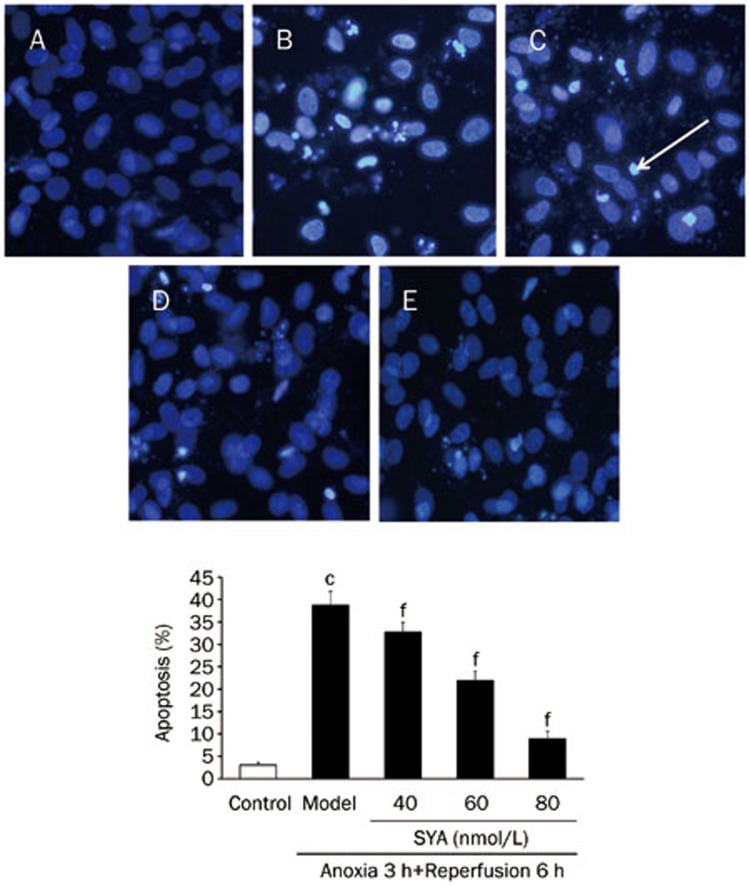
Effect of SYA on apoptosis
Nuclear morphological change was observed by Hoechst 33258 staining, which illustrated that the control cells exhibited uniformly dispersed chromatin, normal organelles and intact cell membranes. After A/R injury, myocytes featured typical characteristics of apoptosis, including shrinkage of the nuclei, chromatin condensation and the appearance of a few apoptotic bodies. However, with SYA pretreatment, the morphological changes were significantly attenuated, and the number of cells with nuclear condensation and fragmentation was significantly decreased (P<0.01) (as shown in Figure 6).
Figure 6.
Effect of SYA on the apoptosis rate of cardiac myocytes after A/R injury (×200). (A) Control group; (B) Model group; (C) SYA-40 (40 nmol/L)+A/R; (D) SYA-60 (60 nmol/L)+A/R; (E) SYA-80 (80 nmol/L)+A/R. White arrow head indicates the typical positive cells. Data were presented as mean±SD of three independent experiments. cP<0.01 as compared with control. fP<0.01 as compared with the model group.
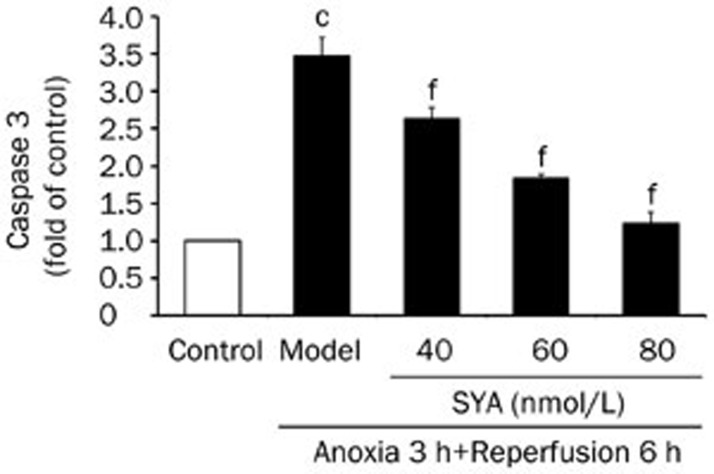
Effect of SYA on caspase 3 activity
To further characterize the inhibitory effect of SYA on myocardial cell apoptosis, we examined whether SYA could inhibit caspase 3 activity. In this study, caspase 3 activity was triggered by A/R (P<0.01) and could be inhibited by SYA pretreatment in a dose-dependent manner (Figure 7).
Figure 7.
Effect of SYA on the caspase 3 activity of cardiac myocytes after A/R injury. Caspase 3 activity was measured using a fluorometric assay kit according to the manufacturer's protocol. Data were presented as mean±SD of three independent experiments. cP<0.01 as compared with control. fP<0.01 as compared with the model group.
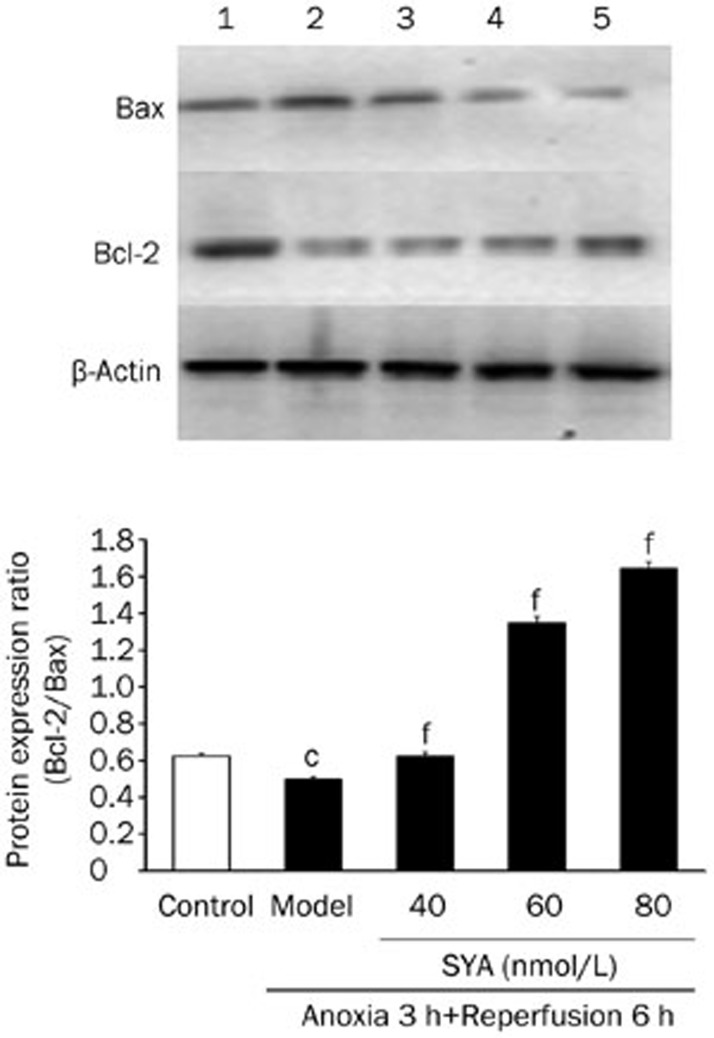
Effect of SYA on Bcl-2 and Bax expression
As shown in Figure 8, A/R potently inhibited Bcl-2 expression and promoted Bax expression so that the Bcl-2/Bax ratio was reduced in A/R-injured cardiomyocytes compared with control (P<0.01); however, SYA reversed the effects of A/R on Bcl-2 and Bax expression, increasing the Bcl-2/Bax ratio in a dose-dependent manner (P<0.01).
Figure 8.
Effects of SYA on the expression of Bcl-2 and Bax proteins in cardiac myocytes after A/R injury. Cardiomyocytes were pertreated with or without SYA for 24 h and then subjected to A/R injury. After quantified protein concentration, Western blot was performed using corresponding antibodies. 1, Control group; 2, Model group; 3, SYA-40 (40 nmol/L)+A/R; 4, SYA-60 (60 nmol/L)+A/R; 5, SYA-80 (80 nmol/L)+A/R. Data were presented as mean±SD of three independent experiments. cP<0.01 as compared with control. fP<0.01 as compared with the model group.
Discussion
Carthamus tinctorius L (safflower) is a widely used herb in the traditional medical systems of China. The major constituents of safflower are flavonoids, a group of polyhydroxy phenols. Flavonoids are good antioxidants, at least in vitro35. There is ample evidence to indicate the beneficial effects of flavonoids on ischemic reperfused hearts through in vitro application (when added to perfusates) or when administered to blood36,37,38,39,40,41, which could be of use in acute ischemia/reperfusion situations, such as for heart surgeries and transplants. SYA, a flavonoid, is a natural compound from the flower of the safflower plant, and there are few previous reports on its pharmacological activity. In this study, we investigated the effect of SYA on oxidative damage and apoptosis induced by A/R in cardiomyocytes. We utilized primary cardiomyocytes subjected to A/R in a hypoxic solution to simulate A/R injury in order to examine the mechanisms of SYA-mediated protection of cardiomyocytes against A/R injury. We used NAC, which has been reported to have antioxidant properties42,43,44, as the positive control to investigate the possible mechanism for SYA's protection of cardiomyocytes against A/R injury.
We monitored the cell survival rate through MTT assay. After 6 h of reperfusion, the percentage cell survival was decreased in the model group, and SYA protected cells in a dose-dependent manner. Three concentrations of SYA without toxic effect were chosen in the following experiments. LDH and CK are expressed constitutively in mammalian cells and are abundant in endochylema. They cannot transit through the cytoplasmic membrane in the normal physiological state, but when a cell is damaged or dead, LDH and CK are released from the cell45,46. Therefore, LDH activity and CK activity in the culture media represent the extent of cell injury induced by A/R. Our results showed that LDH activity and CK activity were significantly decreased following SYA treatment (vs model, P<0.01), implying that SYA has the ability to protect cardiomyocytes from A/R induced injury.
It is well-known that oxidative stress has been largely implicated in the pathogenesis of myocardial ischemia/reperfusion injury47,48. Reactive oxygen species (ROS) may interact degeneratively with cellular components, including nucleic acids, proteins, and lipids, to compromise structure and function49. In the heart, ROS-induced abnormalities include cytotoxicity, cardiac stunning, arrhythmias, alterations of Ca2+ homeostasis, and intracellular ATP depletion50,51. Cellular defense against free radical injury is mediated by several potent antioxidant enzymes, including SOD, catalase, and GSH-Px, all of which physiologically reduce ROS levels52,53. As one of the peroxidized products of polyunsaturated fatty acids, MDA concentration in cells reflects the degree of lipid peroxide and indirectly reflects the production of intracellular ROS. SOD has the ability to transform intracellular superoxide anions to H2O2, and the formed H2O2 is subsequently scavenged by catalase and GSH-Px through enzymatic reactions. Catalase is an enzyme located in the peroxisome and very efficiently promotes the conversion of H2O2 to H2O and O254. GSH-Px acts in conjunction with the tripeptide glutathione (GSH), which is present in cells in high (micromolar) concentrations. GSH-Px decomposes peroxides to water while simultaneously oxidizing GSH55.
Based on previous studies, we investigated whether the protection of SYA was attributed to its antioxidative activity. We determined MDA content and the activity of cellular antioxidants, including the activities of catalase, GSH-Px and SOD and the content of GSH. Our results clearly demonstrated that SYA decreased cellular MDA content and enhanced antioxidant enzyme activity and increased antioxidant agent content. These findings suggested that the cardioprotection of SYA could be partly attributed to enhanced antioxidant capacity in cardiomyocytes.
Accumulating evidence indicates that apoptosis, a gene controlled programmed cell death pathway, contributes significantly to post-ischemic cardiomyocyte death, suggesting that therapeutic interventions that inhibit apoptotic cell death may attenuate I/R-induced heart injury56. Apoptosis is characterized by cell shrinkage, chromatin condensation, DNA fragmentation, membrane blebbing, and the formation of apoptotic bodies12. In this work, an antiapoptotic effect was confirmed by Hoechst 33258 staining, and the results strongly suggested that SYA might exert a protective effect against apoptosis in response to A/R.
Further investigation was performed, focusing on the possible mechanisms involved in the antiapoptosis effect of SYA. Previous studies have indicated that an increase in proapoptotic Bax family proteins and a decrease in antiapoptotic Bcl-2 family proteins are involved in apoptosis57,58. Thus, the ratio of Bc1-2 to Bax may be a critical factor in the cellular threshold for apoptosis59,60,61,62,63. The caspase cascade also plays a key role in apoptosis: the activation of caspase 3 results in cleavage of cytoskeletal and nuclear proteins and nucleosomal fragmentation of DNA64.
Our data showed that A/R increased caspase 3 activity and Bax expression and that it inhibited Bcl-2 synthesis in neonatal rat cardiomyocytes so that the balance between Bcl-2 and Bax was broken. As a result, the cells fell into programmed cell death. SYA inhibited cell apoptosis by suppressing caspase 3 activity and Bax expression and increasing Bcl-2 synthesis, maintaining the Bcl-2/Bax balance. These results indicated that alleviating apoptosis during A/R might be one of the mechanisms of the cardioprotective effect of SYA. At the same time, the changes may be associated with an increase in antioxidant capacity and a decrease in MDA content. However, whether the alleviation of apoptosis was caused by enhanced antioxidant activity remains to be determined.
In conclusion, we cultured neonatal rat cardiomyocytes and demonstrated that SYA induced protection against A/R injury, as measured by the survival rate of cells and the leakage of LDH and CK into the culture media. SYA displays antioxidant activity by enhancing the activity of catalase, GSH-Px, and SOD and by elevating the level of GSH. Its antioxidant ability at 80 nmol/L may be as good as that at 200 μmol/L NAC. We also found that SYA had antiapoptotic activity. However, it is not clear whether the mechanisms are mutually independent or part of a cascade event; further study of the detailed mechanisms is now in progress. In this paper, we discussed the myocardial protection mediated by SYA and its promise as a therapeutic intervention for the treatment of myocardial ischemia. This study is a preliminary investigation of SYA, and much more work is required to develop it into a new drug. It also should be emphasized that the effective dose is close to the minimum toxic concentration, and safety should be tested in subsequent research.
Author contribution
Jia-lin DUAN, Yue GUAN, Ying YIN, and Guo WEI designed the research; Jing-wen WANG, Jia CUI, Dan ZHOU, Yan-rong ZHU, and Wei QUAN performed the research; Jia-lin DUAN, Yue GUAN, Ying YIN, and Guo WEI analyzed the data; Jia-lin DUAN wrote the paper; Ai-dong WEN and Miao-miao XI revised the paper.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No 81173514 and 81001673), the “13115” Technology Innovation Project of Shaanxi Province (No 2010ZDKG-62), Xijing Research Boosting Program (No XJZT10D02) and Excellent Civil Service Training Fund of the Fourth Military Medical University (No 4138C4IDK6). The authors thank colleagues in the New Drug Research and Development Center of Xijing Hospital for their technical assistance.
References
- Reiter RJ, Tan DX. Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res. 2003;58:10–9. doi: 10.1016/s0008-6363(02)00827-1. [DOI] [PubMed] [Google Scholar]
- Goldhaber JI, Weiss JN. Oxygen free radicals and cardiac reperfusion abnormalities. Hypertension. 1992;20:118–27. doi: 10.1161/01.hyp.20.1.118. [DOI] [PubMed] [Google Scholar]
- Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–34. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- Hess ML, Barnhart GR, Crute S, Komwatana P, Krause S, Greenfield LJ. Mechanical and biochemical effects of transient myocardial ischemia. J Surg Res. 1979;26:175–84. doi: 10.1016/0022-4804(79)90097-0. [DOI] [PubMed] [Google Scholar]
- Limbruno U, Zucchi R, Ronca-Testoni S, Galbani P, Ronca G, Mariani M. Sarcoplasmic reticulum function in the 'stunned' myocardium. J Mol Cell Cardiol. 1989;21:1063–72. doi: 10.1016/0022-2828(89)90804-3. [DOI] [PubMed] [Google Scholar]
- Jeroudi MO, Hartley CJ, Bolli R. Myocardial reperfusion injury: role of oxygen radicals and potential therapy with antioxidants. Am J Cardiol. 1994;73:2B–7B. doi: 10.1016/0002-9149(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Braunwald E, Kloner RA. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982;66:1146–9. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- Vatner DE, Kiuchi K, Manders WT, Vatner SF. Effects of coronary arterial reperfusion on beta-adrenergic receptor-adenylyl cyclase coupling. Am J Physiol. 1993;264:H196–H204. doi: 10.1152/ajpheart.1993.264.1.H196. [DOI] [PubMed] [Google Scholar]
- MacLellan WR, Schneider MD. Death by design. Circ Res. 1997;81:137–44. doi: 10.1161/01.res.81.2.137. [DOI] [PubMed] [Google Scholar]
- Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82:1111–29. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- Kaul N, Siveski-Iliskovic N, Hill M, Slezak J, Singal PK. Free radicals and the heart. J Pharmacol Toxicol Methods. 1993;30:55–67. doi: 10.1016/1056-8719(93)90008-3. [DOI] [PubMed] [Google Scholar]
- Gustafsson AB, Gottlieb RA. Mechanisms of apoptosis in the heart. J Clin Immunol. 2003;23:447–59. doi: 10.1023/b:joci.0000010421.56035.60. [DOI] [PubMed] [Google Scholar]
- Wua L, Qiao H. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine. 2007;14:652–8. doi: 10.1016/j.phymed.2007.07.060. [DOI] [PubMed] [Google Scholar]
- Li XZ, Liu JX, Shang XH, Fu JH. Protective effects of hydroxysafflor yellow A on acute myocardial ischemia in dogs. Chin Pharmacol Bull. 2006;6:533–7. [Google Scholar]
- Zheng WC, Chen DB, Li B, Zhang L. The preventive effect safflor yellow on myocardium injury of myocardium ischemic reperfusion in rats. Chin Pharmacol Bull. 2003;19:1032–4. [Google Scholar]
- Palter R, Lundin RE, Haddon WF. A cathartic lignan glycoside isolated from carthamus tinctorus. Phytochemistry. 1972;11:2871–4. [Google Scholar]
- Kazuma K, Takahashi T, Sato K, Takeuchi H, Matsumoto T, Okuno T. Quinochalcones and flavonoids from fresh floretsin different cultivars of Carthamus tinctorius L. Biosci Biotechnol Biochem. 2000;64:1588–99. doi: 10.1271/bbb.64.1588. [DOI] [PubMed] [Google Scholar]
- Akihisa T, Yasukawa K, Oinuma H, Kasahara Y, Yamanouchi S, Takido M, et al. Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry. 1996;43:1255–60. doi: 10.1016/s0031-9422(96)00343-3. [DOI] [PubMed] [Google Scholar]
- Hirokawa T, Hirokawa S, Yamauchi N, Kataoka T, Woo JT, Nagai K. Immunomodulating activities of polysaccharide fractions from dried safflower petals. Cytotechnology. 1997;25:205–11. doi: 10.1023/A:1007947329496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An XQ, Li YH, Chen J. Separation and identification of safflower yellow A and carthamin from Carthamus tinctorius L. Chin Tradit Herb Drugs. 1990;21:188–9. [Google Scholar]
- Meselhy MR, Kadota S, Momose Y, Hatakeyama N, Kusai A, Hattori M, et al. Two new quinochalcone yellow pigments from Carthamus tinctorius and Ca2+ antagonistic activity of tinctormine. Chem Pharm Bull. 1993;41:1796–802. doi: 10.1248/cpb.41.1796. [DOI] [PubMed] [Google Scholar]
- Yin HB, He ZS. A novel semi-quinone chalcone sharing a pyrrole ring C-glycoside from carthamus tinctorius. Tetrahedrom Lett. 2000;41:1955–8. [Google Scholar]
- Meselhy MR, Kadota S, Momose Y, Hattori M, Namba T. Tinctormine, a novel Ca2+ antagonist N-containing quinochalcone C-glycoside from Carthamus tinctorius L. Chem Pharm Bull. 1992;40:3355–7. doi: 10.1248/cpb.40.3355. [DOI] [PubMed] [Google Scholar]
- Onodera J, Obara H, Hirose R, Matsuba S, Sato N, Suzuki M. The structure of safflomin C, a constituent of safflower. Chem Lett. 1989;9:1571–4. [Google Scholar]
- Wang CY, Ma HM, Zhang SP. Safflor yellow B suppresses pheochromocytoma cell (PC 12) injury induced by oxidative stress via antioxidant system and Bcl-2 /Bax pathway. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:135–42. doi: 10.1007/s00210-009-0424-x. [DOI] [PubMed] [Google Scholar]
- Xue HY, Wei XB, Ding H. The protective effects of hydroxysafflower yellow A on hypoxia cardiomyocyte. Zhongguo Lao Nian Xue Za Zhi. 2007;7:140–2. [Google Scholar]
- Wei XB, Liu XQ. Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci Lett. 2005;386:58–62. doi: 10.1016/j.neulet.2005.05.069. [DOI] [PubMed] [Google Scholar]
- Liu SX, Zhang X, Wang YF, Li XC, Xiang MX, Bian C, et al. Upregulation of heme oxygenase-1 expression by hydroxysafflor yellow A conferring protection from anoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes. Int J Cardiol. 2012;160:95–101. doi: 10.1016/j.ijcard.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Foncea R, Andersson M, Ketterman A, Blakesley V, Sapag-Hagar M, Sugden PH, et al. Insulin-like growth factor-I rapidly activates multiple signal transduction pathways in cultured rat cardiac myocytes. J Biol Chem. 1997;272:19115–24. doi: 10.1074/jbc.272.31.19115. [DOI] [PubMed] [Google Scholar]
- Koyama T, Temma K, Akera T. Reperfusion-induced contracture develops with a decreasing [Ca2+] in single heart cells. Am J Physiol. 1991;261:H1115–22. doi: 10.1152/ajpheart.1991.261.4.H1115. [DOI] [PubMed] [Google Scholar]
- Nakano M, Knowlton AA, Dibbs Z, Mann DL. Tumor necrosis factor-alpha confers resistance to hypoxic injury in the adult mammalian cardiac myocyte. Circulation. 1998;97:1392–400. doi: 10.1161/01.cir.97.14.1392. [DOI] [PubMed] [Google Scholar]
- Wang HC, Zhang HF, Guo WY, Su H, Zhang KR, Li QX, et al. Hypoxic postconditioning enhances the survival and inhibits apoptosis of cardiomyocytes following reoxygenation: role of peroxynitrite formation. Apoptosis. 2006;11:1453–60. doi: 10.1007/s10495-006-7786-z. [DOI] [PubMed] [Google Scholar]
- Turguta G, Enlib Y. Changes in the levels of MDA and GSH in mice serum, liver and spleen after aluminum administration. East J Med. 2006;11:7–12. [Google Scholar]
- Botsoglou NA, Fletouris DJ. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J Agric Food Chem. 1994;42:1931–7. [Google Scholar]
- Rice-Evans C. Flavonoid antioxidants. Curr Med Chem. 2001;8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- Amorini AM, Lazzarino G, Galvano F, Fazzina G, Tavazzi B, Galvano G. Cyanidin-3-O-beta-glucopyranoside protects myocardium and erythrocytes from oxygen radical-mediated damages. Free Radic Res. 2003;37:453–60. doi: 10.1080/1071576021000055253. [DOI] [PubMed] [Google Scholar]
- Fantinelli JC, Schinella G, Cingolani HE, Mosca SM. Effects of different fractions of a red wine non-alcoholic extract on ischemia-reperfusion injury. Life Sci. 2005;76:2721–33. doi: 10.1016/j.lfs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- Hirai M, Hotta Y, Ishikawa N, Wakida Y, Fukuzawa Y, Isobe F, et al. Protective effects of EGCg or GCg, a green tea catechin epimer, against postischemic myocardial dysfunction in guinea-pig hearts. Life Sci. 2007;80:1020–32. doi: 10.1016/j.lfs.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Hotta Y, Huang L, Muto T, Yajima M, Miyazeki K, Ishikawa N, et al. Positive inotropic effect of purified green tea catechin derivative in guinea pig hearts: the measurements of cellular Ca2+ and nitric oxide release. Eur J Pharmacol. 2006;552:123–30. doi: 10.1016/j.ejphar.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med. 2004;10:55–62. doi: 10.2119/2004-00032.aneja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Xu Z, Criswell HE, Boysen PG. Propyl paraben inhibits voltage-dependent sodium channels and protects cardiomyocytes from ischemia-reperfusion injury. Life Sci. 2004;74:3043–52. doi: 10.1016/j.lfs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Tossios P, Bloch W, Huebner A, Raji MR, Dodos F, Klass O, et al. N-acetylcysteine prevents reactive oxygenspecies-mediated myocardial stress in patients undergoing cardiac surgery: Results of a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2003;126:1513–20. doi: 10.1016/s0022-5223(03)00968-1. [DOI] [PubMed] [Google Scholar]
- Orhan G, Yapici N, Yuksel M, Sargin M, Senay S, Yalçin AS, et al. Effects of N-acetylcysteine on myocardial ischemia-reperfusion injury in bypass surgery. Heart Vessels. 2006;21:42–7. doi: 10.1007/s00380-005-0873-1. [DOI] [PubMed] [Google Scholar]
- Abe M, Takiguchi Y, Ichimaru S, Tsuchiya K, Wada K. Comparison of the protective effect of N-Acetylcysteine by different treatments on rat myocardial ischemia-reperfusion injury. J Pharmacol Sci. 2008;106:571–7. doi: 10.1254/jphs.fp0071664. [DOI] [PubMed] [Google Scholar]
- Hyunsoo K, Sung CY, Tae YL, Daewon J. Discriminative cytotoxicity assessment based on various cellular damages. Toxicol Lett. 2009;184:13–7. doi: 10.1016/j.toxlet.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Corey MJ, Kinders RJ, Brown LG, Vessella RL. A very sensitive coupled luminescent assay for cytotoxicity and complement-mediated lysis. J Immunol Methods. 1997;207:43–51. doi: 10.1016/s0022-1759(97)00098-7. [DOI] [PubMed] [Google Scholar]
- Fu JJ, Huang HQ, Liu JJ, Pi RB, Chen JW, Liu PQ. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol. 2007;568:213–21. doi: 10.1016/j.ejphar.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Wu Y, Yuan BX. Effects of Qiangxin capsules on myocardial reperfusion arrhythmias in rats. North Phar J. 2001;16:23–4. [Google Scholar]
- Kehrer JP, Smith CV.In: Natural antioxidants in human health and disease. Free radicals in biology: sources, reactivates, and roles in the etiology of human diseases. San Diego: CA, USA; 1994. p32
- Das DK, Maulik N. Antioxidant effectiveness in ischemia/reperfusion tissue injury. Methods Enzymol. 1994;233:601–10. doi: 10.1016/s0076-6879(94)33063-8. [DOI] [PubMed] [Google Scholar]
- Lefer DJ, Granger DN. Oxidative stress and cardiac disease. Am J Med. 2000;109:315–23. doi: 10.1016/s0002-9343(00)00467-8. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Tengattini S, Russel J. Cardiovascular diseases: protective effects of melatonin. J Pineal Res. 2008;44:16–25. doi: 10.1111/j.1600-079X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Gottlib RA, Engler RL. Apoptosis in myocardial ischemia-reperfusion. Ann N Y Acad Sci. 1999;30:412–26. doi: 10.1111/j.1749-6632.1999.tb09255.x. [DOI] [PubMed] [Google Scholar]
- Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Lou HQ, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz. 2002;27:662–8. doi: 10.1007/s00059-002-2430-3. [DOI] [PubMed] [Google Scholar]
- Misao J, Hayakawa Y, Ohno M, Kato S, Fujiwara T, Fujiwara H. Expression of Bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation. 1996;94:1506–12. doi: 10.1161/01.cir.94.7.1506. [DOI] [PubMed] [Google Scholar]
- Hanada M, Aime-Sempe C, Sato T, Reed JC. Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem. 1995;270:11962–9. doi: 10.1074/jbc.270.20.11962. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–32. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Tamm I, Schriever F, Dörken B. Apoptosis: implications of basic research for clinical oncology. Lancet Oncol. 2001;2:33–42. doi: 10.1016/S1470-2045(00)00193-5. [DOI] [PubMed] [Google Scholar]
- Oubrahim H, Stadtman ER, Chock PB. Mitochondria play no roles in Mn(II) induced apoptosis in HeLa cells. Proc Natl Acad Sci U S A. 2001;98:9505–10. doi: 10.1073/pnas.181319898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal WZ, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–51. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DW, Ali A, Thornberry NA, Vai llancourt JP, Ding CK, Gallant M, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]