Abstract
Trogocytosis is a process which involves the transfer of membrane fragments and cell surface proteins between cells. Various types of T cells have been shown to be able to acquire membrane-bound proteins from antigen-presenting cells and their functions can be modulated following trogocytosis. However, it is not known whether induced regulatory T cells (iTregs) can undergo trogocytosis, and if so, what the functional consequences of this process might entail. In this study, we show that iTregs can be generated from CD80−/−CD86−/− double knockout (DKO) mice. Using flow cytometry and confocal fluorescence microscopy, we demonstrate that iTregs generated from DKO mice are able to acquire both CD80 and CD86 from mature dendritic cells (mDCs) and that the acquisition of CD86 occurs to a higher extent than that of CD80. Furthermore, we found that after co-incubation with iTregs, dendritic cells (DCs) downregulate their surface expression of CD80 and CD86. The trogocytosis of both CD80 and CD86 occurs in a cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), CD28 and programmed death ligand-1 (PDL1)-independent manner. Importantly, we showed that iTregs that acquired CD86 from mDCs expressed higher activation markers and their ability to suppress naive CD4+ T-cell proliferation was enhanced, compared to iTregs that did not acquire CD86. These data demonstrate, for the first time, that iTregs can acquire CD80 and CD86 from mDCs, and the acquisition of CD86 may enhance their suppressive function. These findings provide novel understanding of the interaction between iTregs and DCs, suggesting that trogocytosis may play a significant role in iTreg-mediated immune suppression.
Keywords: CD80, CD86, CTLA-4, iTregs, trogocytosis
Introduction
Regulatory T cells (Tregs) are a vital subpopulation of T cells and are essential for maintaining peripheral tolerance, preventing autoimmune diseases and limiting chronic inflammatory diseases.1 Natural CD4+CD25+FoxP3+ Tregs (nTregs) develop in the thymus, while adaptive or induced Tregs (iTregs) are generated in the periphery from conventional CD4+CD25−FoxP3− T cells.2 CD4+CD25+Foxp3+ iTregs can also be generated in vitro by stimulation of naive CD4 T cells in the presence of transforming growth factor-β (TGF-β),3, 4 which induces Foxp3 expression.5 Once induced, iTregs can regulate immune responses both in vitro and in vivo.5, 6, 7, 8, 9 Whereas the nature and the mechanisms involved in nTreg-mediated suppression have been studied extensively, iTreg development and function remain elusive.
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) expressed on Tregs is a key molecule involved in controlling their suppressive function, and together with CD28, helps regulate immune responses. Both CTLA-4 and CD28 can bind to the B7 family members CD80 and CD86 expressed on the surface of antigen-presenting cells (APCs). CD28 enhances T-cell proliferation by increasing the transcription and the mRNA stability of IL-2, as well as by upregulating the anti-apoptotic protein Bcl-xL.10 CTLA-4 restricts T-cell activation by inhibiting IL-2 production and cell cycle progression.11 While both CD28 and CTLA-4 can bind CD80 and CD86, CD80 is the major ligand mediating CTLA-4 localization and CD86 is the main ligand for CD28 concentration at the immunological synapse.12 Dendritic cells (DCs) are professional APCs and their costimulatory molecule expression levels, including CD80 and CD86, are critical for eliciting T-cell responses. One of the mechanisms by which Tregs suppress immune responses is to inhibit DCs. Onishi et al.13 found that T-cell receptor (TCR)-stimulated nTregs from DO11.10 transgenic mice were able to downregulate CD80 and CD86 expression on DCs in a lymphocyte function-associated antigen-1 (LFA-1)- and CTLA-4-dependent manner. Qureshi et al.14 demonstrated that both effector T cells and Tregs can downregulate CD80 and CD86 expression by CTLA4-mediated trans-endocytosis. Whether iTregs can downregulate CD80 and CD86 expression through trogocytosis is not known.
Trogocytosis is the transfer of membrane-associated proteins together with lipid fractions of the cell membrane. It is a widespread phenomenon which endows acceptor cells with novel functions.15 Trogocytosis has been demonstrated by a variety of cell types,16 with both stimulatory and suppressive effects on immune responses. So far, most of the work on trogocytosis has been carried out on effector T cells. To the best of our knowledge, no previous study has analyzed CD80 and CD86 acquisition by iTregs. In this study, we sought to better characterize iTreg generation, trogocytosis and function. To examine trogocytosis of CD80 and CD86, we generated iTregs from CD80−/−CD86−/− double knockout (DKO) mice. We demonstrate that iTregs can acquire both CD80 and CD86 from mature bone marrow-derived dendritic cells (BMDCs) and express the acquired molecules on their surface. Co-incubation of mature dendritic cells (mDCs) with iTregs resulted in downregulation of their CD80 and CD86 expression. Furthermore, trogocytosis of CD80 and CD86 occurs independently of CTLA-4 or CD28 expression on iTregs. More importantly, we found that iTregs that are able to acquire CD86 showed enhanced suppressive function. These data reveal, for the first time, that trogocytosis of costimulatory molecules from APCs may have biological significance in relation to Treg function.
Materials and methods
Mice
C57BL/6 (Thy1.2+), B6.PL-Th1a/CyJ (congenic to C57BL/6, Thy1.1+) and B6.SJL-PtprcaPepcb/BoyJ (CD45.1+) mice were obtained from The Jackson Laboratory (Bar Harbor, Maine). CD80−/−CD86−/− DKO mice and CD80−/− knockout (KO) mice (all on C57BL/6 background) were kindly provided by Dr David Kelvin (University Health Network). All mice were housed in specific pathogen-free conditions at the University Health Network mouse facility (Toronto, Canada). All experiments were approved by the University Health Network Animal Care Committee.
Antibodies (Abs) and reagents
Reagents used in this study included: recombinant murine (rm) granulocyte–macrophage colony-stimulating factor (GM-CSF; ProSpec, East Brunswick, New Jersey), RPMI-1640 media (GIBCO, Burlington, Ontario), lipopolysaccharide (LPS; Sigma, Oakville, Ontario), antiphycoerythrin (anti-PE) microbeads (Miltenyi Biotec, Boston, Massachusetts), LD columns (Miltenyi Biotec), IL-2 (Chiron Corp., Emeryville, California), TGF-β (R&D Systems, Burlington, Ontario), propidium iodide (Sigma-Aldrich), eFluor 450 (eBioscience, San Diego, California), PKH26 (Sigma-Aldrich) and carboxyfluorescein diacetate, succinimidyl ester (CFSE; Invitrogen, Burlington, Ontario). Cells were fixed and permeabilized using BD Cytofix/Cytoperm Plus Fixation/Permeabilization kit (BD) and FoxP3 Staining Buffer Set (eBioscience).
The following Abs were purchased from BioLegend (San Diego, California): PE-conjugated anti-CD8 (53-6.7), PE-anti-CD25 (PC61), PE-anti-CD11b (M1/70), PE-anti-NK1.1 (PK136), PE-anti-TER119 (TER-119), PE-anti-CD4 (GK1.5), PE-anti-CD11c (N418), PE-CD274/programmed death ligand-1 (PDL1) (10F.9G2), fluorescein isothiocyanate (FITC)-conjugated anti-CD25 (PC61), FITC-anti-CD80 (16-10A1), FITC-anti-CD86 (GL-1), FITC-anti-CD11c (N418), allophycocyanin (APC)-conjugated anti-CD90.1/Thy1.1 (OX-7), APC-anti-CD11c (N418), PE/Cy5-conjugated anti-CD4 (GK1.5), Alexa Fluor 488-conjugated anti-CD3 (17A2), Alexa Fluor 647-conjugated anti-CD11c (N418), purified antimouse CD28, anti-CD3 (2C11), blocking LEAF purified antimouse CD152/CTLA-4 (UC10-4B9), blocking LEAF purified antimouse CD28 and LEAF purified antimouse CD274/PDL1 (10F.9G2), purified syrian hamster IgG isotype control. The following Abs were purchased from eBioscience: PE-anti-B220 (RA3-6B2), PE-anti-Foxp3 (FJK-16s), PE/Cy7-anti-CD3 (145-2C11), PE-anti-CD80 (16-10A1), PE-CTLA-4 (UC10-4B9), PE-CD69 (H1.2F3), PE-CD44 (IM7) and APC-CD45.1 (A20). PE-conjugated anti-CD86 (GL-1) and blocking purified anti-CTLA4 (UC10-4F10-11) were purchased from BD Biosciences Pharmingen (Mississauga, Ontario).
BMDCs
C57BL/6 wild-type (WT) mice, CD45.1+ mice or CD80−/− single KO mice were used to isolate BMDCs. Briefly, femur and tibia bones were dissected and flushed with RPMI media. Red blood cells were lysed by incubation in red blood cell Lysis buffer (0.14 M NH4Cl, 0.02 M Tris Base, pH 7.2) and the remaining cells were pelleted at 400g for 5 min. Cells were plated at 2×106 cells per 100 mm dish in 10 ml RPMI-1640 complete medium containing 100–200 U/ml (=20 ng/ml) rm GM-CSF. At day 3, another 10 ml complete medium containing 100–200 U/ml rm GM-CSF were added to the plates. At days 6 and 8, a 50% media swap was done with fresh media containing 100–200 U/ml rm GM-CSF. LPS (0.1 µg/ml) was added to the complete medium for the last 24 h.
In vitro induction of iTregs
C57BL/6 WT or CD80−/−CD86−/− DKO mice were used for the isolation of CD4+CD25− T cells. The cells were then induced in vitro to produce CD4+CD25+Foxp3+ cells (iTregs) as previously described.8 Briefly, splenocytes and lymph node cells were incubated with PE-conjugated anti-CD8, PE-anti-CD25, PE-anti-B220, PE-anti-CD11b, PE-anti-NK1.1 and PE-anti-TER119 Abs for 20 min at 4 °C and washed once. Cells were then resuspended in buffer (0.5% bovine serum albumin, 2 mM EDTA in phosphate-buffered saline, pH 7.2) and incubated with anti-PE microbeads for 20 min at 4 °C and washed once. CD4+CD25− cells were purified by LD column. For the induction of iTregs, these cells were cultured in 24-well plates coated with 10 µg/ml anti-CD3 mAb, in the presence of 2 µg/ml soluble anti-CD28 Ab, 200 U/ml IL-2 and 5 ng/ml TGF-β. Cells were cultured for 5 days and the expression of Foxp3 and CD25 was evaluated by flow cytometry.
Acquisition experiments
WT or DKO iTregs (1.5×105 cells/well) were cocultured with BMDCs, at a DC/iTreg ratio of 2∶1, in 96-well round bottom plates with 50 U/ml IL-2. At various time points after coculture, cells were collected and immunostained and used either for flow cytometry or confocal microscopy analysis. To examine acquisition of CD80 and CD86 by iTregs after coculture with DCs, plots were gated on PI−CD11c−CD4+ cells and CD80 and CD86 staining within this gate was examined. In blocking assays, iTregs were pre-incubated with either 20 µg/ml anti-CTLA-4 Ab, 20 µg/ml anti-CD28 Ab or 20 µg/ml anti-PDL1 Ab, 1 h prior to coculture.
Flow cytometry
Samples were stained with the appropriate Abs and sample acquisition was performed on a Becton Dickinson LSRII and a Beckman Coulter Cytomics FC500 cytometer and data were analyzed using FlowJo software (TreeStar Inc., Ashland, Oregon).
Cell sorting
DKO iTregs were cocultured with CD80−/− BMDCs in 96-well round bottom plates with 50 U/ml IL-2 for 24 h. Cells were collected and stained with FITC-conjugated anti-CD11c, PE-conjugated anti-CD86 and PE/Cy5-conjugated anti-CD4. The DKO iTregs which expressed a high level of acquired CD86 and the iTregs which did not acquire CD86 were sorted by using a Becton Dickinson FACS Aria II cell sorter.
Confocal microscopy
Cells were washed with phosphate-buffered saline and stained with Alexa Fluor 488-conjugated anti-CD3 (17A2), Alexa Fluor 647-conjugated anti-CD11c (N418) and PE-conjugated anti-CD80 (16-10A1) or CD86 (GL1) Abs. Cells were plated onto chamber slides and visualized live using an Olympus Fluoview 1000 Laser Scanning Confocal Microscope.
Suppression assay
CD4+CD25− T cells (Thy1.1+) were purified from naive B6.PL-Th1a/CyJ mice, labeled with CFSE, and cultured (1×105 cells/well) with 1 µg/ml anti-CD3 Ab in the presence of irradiated syngeneic splenocytes (2×104 cells/well). Varying numbers of CD4+CD25+Foxp3+ iTreg from WT or DKO C57BL/6 mice were added to the culture at suppressor to responder ratios 1∶0.5, 1∶1, 1∶2, 1∶4 and 1∶8. After 4 days of culture, proliferation of responder cells was measured by gating on Thy1.1+ cells and assessing their CFSE signal by flow cytometry.
Statistical analysis
Data are presented as the mean±SEM per group. Statistical analysis was made for multiple comparisons using Student's t-test. A P value of <0.05 was considered to be statistically significant.
Results
Functional CD4+CD25+Foxp3+ iTregs can be generated in vitro from CD80−/−CD86−/− DKO mice
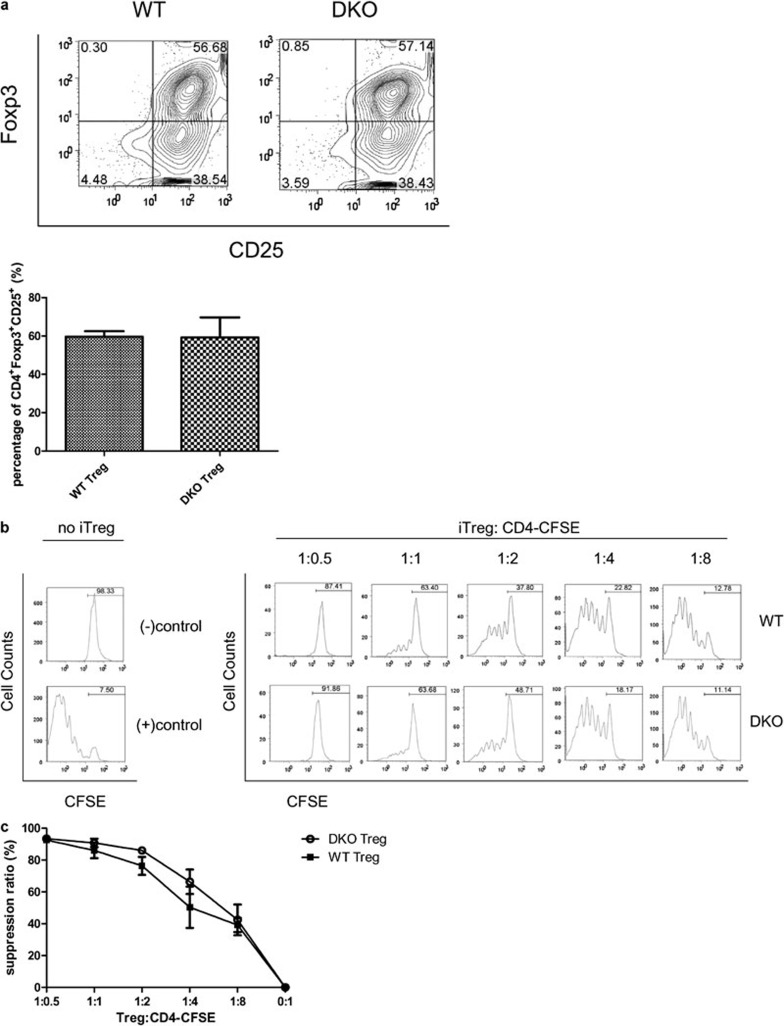
It is known that T cells can express CD80 and CD86 on their surface after activation.17 In order to avoid the potential influence of endogenously expressed CD80 and CD86, we chose to study iTreg trogocytosis of CD80 and CD86 using CD80−/−CD86−/− DKO mice. However, mice that lack CD80 and CD86 show a dramatic reduction in nTreg frequency in the thymus,18 indicating that these molecules are important for nTreg development. Whether iTreg generation and function would be affected by the CD80/CD86 DKO was not known. To address this question, we first determined whether equivalent numbers of functional CD4+CD25+Foxp3+ iTregs could be generated from CD4+CD25− T cells obtained from CD80−/−CD86−/− DKO mice and WT mice. We found that similar percentages of CD4+CD25+Foxp3+ T cells could be generated from WT and DKO naive CD4+ T cells, and on average, 60% of cells in culture were CD4+CD25+Foxp3+ (Figure 1a). To evaluate if the DKO iTregs were functional, iTregs were generated from WT and DKO mice (Thy1.2+) and cocultured with purified naive Thy1.1+CD4+ T cells at varying suppressor to responder ratios (Figure 1b). Suppression of proliferation of Thy1.1+CD4+ T cells was measured by flow cytometry. As shown in Figure 1b and c, both WT and DKO iTregs could profoundly suppress the proliferation of responder cells in a dose-dependent manner. The results demonstrate that, unlike that seen for nTregs, CD80 and CD86 expression does not affect iTreg generation and function.
Figure 1.
In vitro generation of CD4+CD25+Foxp3+ T cells from murine naive T cells. (a) The expression of Foxp3 and CD25 was evaluated in CD4+CD25− cells isolated from either C57BL/6 (WT) mice or from CD80−/−CD86−/− DKO mice, activated for 5 days with plate-bound anti-CD3 Ab, soluble anti-CD28 Ab and IL-2 in the presence of TGF-β. Left: WT iTreg. Right: CD80−/−CD86−/− DKO iTreg. (b) Dose-dependent suppression of T-cell proliferation by CD4+CD25+Foxp3+ iTreg cells. CD4+CD25− T cells (Thy1.1+) were purified from naive B6.PL-Th1a/CyJ mice, labeled with CFSE, and cultured (1×105 cells/well) with 1 µg/ml anti-CD3 Ab in the presence of irradiated syngeneic splenocytes (2×104 cells/well). Varying numbers of CD4+CD25+Foxp3+ iTreg from WT or DKO C57BL/6 mice were added to the culture at suppressor to responder ratios 1∶0.5, 1∶1, 1∶2, 1∶4 and 1∶8. After 4 days of culture, proliferation of responder cells was measured by gating on Thy1.1+ cells and assessing their CFSE signal by flow cytometry. Numbers represent the percentage of undivided cells. (−) control: CFSE labeled CD4+CD25− T cells without any stimulation or CD4+CD25+Foxp3+ T cells. (+) contol: CFSE labeled CD4+CD25− T cells were cultured with anti-CD3 Ab in the presence of irradiated APCs without CD4+CD25+Foxp3+ T cells. (c) Undivided populations were analyzed by determining the suppression ratio obtained with the formula: suppression ratio=(experiment group−positive control)/experiment group×100%. The data shown are representative of at least three different experiments. Ab, antibody; APC, antigen-presenting cell; CFSE, carboxyfluorescein diacetate, succinimidyl ester; DKO, double knockout; iTreg, induced regulatory T cell; WT, wild-type.
iTregs acquire CD80 and CD86 by trogocytosis after coculture with mDCs
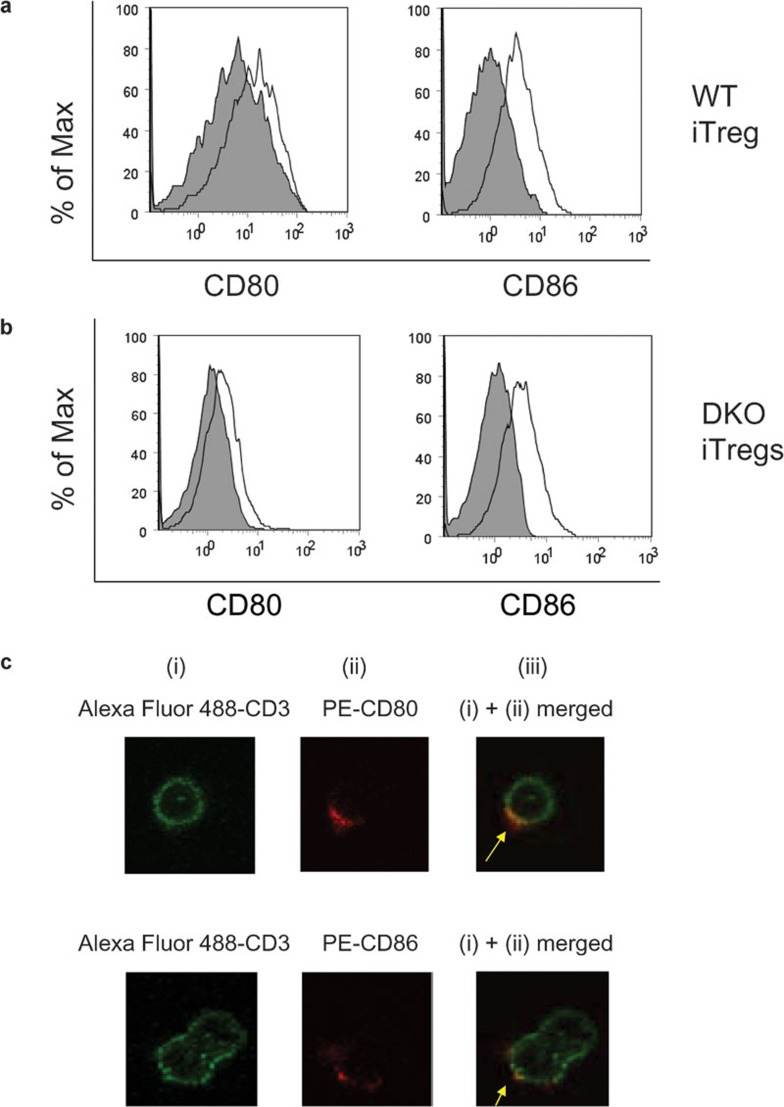
Several reports have shown that effector T cells are able to undergo trogocytosis of costimulatory molecules after coculture with APCs.19, 20, 21, 22 To determine if iTregs shared this ability, iTregs were cocultured with mDCs and the level of CD80 and CD86 expression was measured on iTregs cultured alone, or after coculture with mDCs. To examine CD80 and CD86 acquisition by iTregs, plots were gated on PI−CD11c−CD4+ cells. As shown in Figure 2a, the amounts of CD80 and CD86 on WT iTregs were increased after co-incubation with mDCs. To rule out endogenous upregulation of CD80 and CD86 in WT iTregs after coculture with mDCs, we used iTregs generated from CD80−/−CD86−/− DKO mice. As shown in Figure 2b, the expression of CD80 and CD86 on DKO iTregs was also increased after co-incubation with mDCs, suggesting acquisition of these molecules.
Figure 2.
Flow cytometry analysis of CD80 and CD86 acquired by (a) WT iTregs or (b) DKO iTregs. iTregs were isolated from C57BL/6 (WT) mice or from CD80−/−CD86−/− DKO mice and were cultured alone or cocultured with mDCs from C57BL/6 mice at a DC/iTreg ratio of 2∶1. Cells were stained with PI, CD11c, CD4, CD80 and CD86. Shaded graph: CD80 or CD86 expressed on iTregs alone. Line graph: CD80 or CD86 expressed on iTregs after co-incubation with BMDCs. The plots shown are gated on PI−CD11c−CD4+ cells and are representative of at least nine different experiments. (c) Confocal microscopy analysis of CD80 and CD86 acquired by DKO iTregs after co-incubation with BMDCs. (i) Green: Alexa Fluor 488-conjugated anti-CD3. (ii) Red: PE-conjugated anti-CD80 (top) or CD86 (bottom). (iii) Merged image of (i) and (ii). BMDC, bone marrow-derived dendritic cell; DC, dendritic cell; DKO, double knockout; iTreg, induced regulatory T cell; mDC, mature dendritic cell; WT, wild-type.
To further confirm this finding, we examined this phenomenon by using confocal microscopy. DKO iTregs and mDCs were cocultured for 24 h and stained with Alexa Fluor 647-anti-CD11c, Alexa Fluor 488-anti-CD3 and PE-anti-CD80 or CD86. DKO iTregs showed diffuse staining for CD3 while only a small membrane patch stained positive for either CD80 or CD86 (Figure 2c). Superimposition of the red PE signal on the green Alexa Fluor 488 signal resulted in yellow staining on DKO iTregs, suggesting colocalization of CD3 and CD80 or CD86. These results demonstrate that CD80 and CD86 are physically acquired by DKO iTregs from mDCs through trogocytosis.
To further understand the nature of iTreg trogocytosis, we investigated the time course of CD80 and CD86 acquisition by iTregs. DKO iTregs were cocultured with mDCs, and CD80 and CD86 expression on iTregs was measured by flow cytometry at various time points after coculture (Figure 3a). After 2 h of incubation, DKO iTregs had acquired both CD80 and CD86; however, a higher amount of CD86 than CD80 was present on iTreg membranes (Figure 3b). During the 24-h time course, DKO iTregs continued to acquire both CD80 and CD86, with higher acquisition of CD86 observed at all time points examined.
Figure 3.
(a) Dynamics of CD80 and CD86 acquisition by DKO iTregs after co-incubation with BMDCs. DKO iTregs were cultured alone or cocultured with mDCs during a time course from 2 to 24 h. Shaded graph: CD80 or CD86 expressed on DKO iTregs alone. Line graph: CD80 or CD86 expressed on DKO iTregs after coculture with BMDCs. (b) The MFI percentage increase of CD80 and CD86 acquired by DKO iTregs after co-incubation with BMDCs. Percentage increase of MFI=(MFIafter co-incubation−MFIpre-incubation )/MFIpre-incubation×100%. (c) Amount of PKH26 acquired by DKO iTregs after co-incubation with PKH26-labeled BMDCs. MFI percent change of acquired PKH26 is shown. BMDC, bone marrow-derived dendritic cell; DKO, double knockout; iTreg, induced regulatory T cell; mDC, mature dendritic cell; MFI, median fluorescence intensity.
Because trogocytosis is known to involve transfer of both proteins and membrane fragments, we prestained mDCs with the membrane dye PKH26 prior to coculture with iTregs, and then measured the amount of dye transferred to iTregs after coculture. In contrast to the steady increase in CD80 and CD86 on iTregs, transfer of membrane fragments from DCs to iTregs was very high at 6 h of coculture, but then decreased from 6 to 24 h of coculture (Figure 3c). This suggests that CD80 and CD86 are not passively transferred from DCs to iTregs along with membrane fragments, but that a specific interaction may be involved.
CD80 and CD86 acquisition by iTregs is not CTLA-4-, CD28- or PDL1-dependent
Tregs express high levels of CTLA-423 which is known to bind to CD80 and CD86, and is critical for Treg function.24 Qureshi et al.14 recently showed that CTLA-4 is critical for human and mouse CD4+CD25+ Tregs to acquire CD86 from APCs through trans-endocytosis. However, we found that nTregs isolated from ctla4 gene KO mice could acquire both CD80 and CD86 and express these acquired molecules on their surface at the same levels as seen in WT B6 mice (Figure 4a), suggesting that CTLA-4 is not required for nTregs to acquire CD80/CD86 from mDCs. Because CTLA-4 is required for the induction of iTregs,25 it was not possible to examine iTregs from these mice. Therefore, we assessed the role of CTLA-4 in iTreg trogocytosis by blocking studies. To this end, iTregs were pre-incubated with anti-CTLA-4 mAbs to block CTLA-4, and then cocultured with mDCs. We found that this treatment did not prevent acquisition of CD80/CD86 by iTreg (Figure 4b and d). CD28 is another major molecule that is expressed on iTregs and can bind to CD80/CD86, and has been shown to be involved in trogocytosis of CD80 by effector T cells.19 Again, we found that trogocytosis of CD80 and CD86 still occurred in the presence of anti-CD28 mAbs (Figure 4c and d). Therefore, in our system, the acquisition of CD80 and CD86 by iTregs occurs by a CTLA-4- and CD28-independent mechanism. Since PDL1 has been shown to be expressed on Tregs26 and to interact with CD80,27 we tested the role of this protein in iTreg acquisition. The use of a PDL1 blocking Ab on iTregs prior to coculture with DCs did not block acquisition of either CD80 or CD86 (Figure 4d), suggesting PDL1 is not involved in our system. Although expression of CTLA-4, CD28 and PDL1 could be easily detected on iTregs (Figure 4e), none of the blocking Abs affected trogocytosis.
Figure 4.
(a) Acquisition of CD80 and CD86 by CTLA-4−/− nTregs. Naturally occurring CD4+CD25+Foxp3+ nTregs were isolated from CTLA-4 KO mice and cultured alone or cocultured with mDCs from C57BL/6 mice. The amount of CD80 and CD86 on nTregs cultured alone (shaded graph) or after coculture with mDCs (line graph) was evaluated by flow cytometry. (b) CD4+CD25+Foxp3+ iTregs were incubated with 20 µg/ml CTLA-4 Ab or (c) 20 µg/ml CD28 Ab to block the function of either CTLA-4 or CD28, respectively, 1 h prior to coculture with mDCs. Surface and intracellular levels of CD86 and CD80 on iTregs cultured alone (dark gray shaded graph) or on iTregs cultured with DCs without blocking Ab (solid line), with blocking Ab (dotted line) or with isotype Ab (light gray shaded graph) are shown. Data are representative of 3–5 experiments with three mice per experiment. (d) MFI values of CD86 and CD80 expressed on iTregs after culture with DCs, with and without blocking Abs and isotype (iso) control Abs. The error bars represent SEM. None of the blocking Abs produced a significant change in the level of CD80 and CD86 acquired by iTregs (one-way ANOVA with Bonferroni's post hoc test). (e) Levels of CTLA-4, CD28 and PDL1 were analyzed on iTregs (line graph). Shaded graph=FMO control. Ab, antibody; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; DC, dendritic cell; FMO, fluorescence-minus-one; iTreg, induced regulatory T cell; KO, knockout; mDC, mature dendritic cell; MFI, median fluorescence intensity; nTreg, natural regulatory T cell; PDL1, programmed death ligand-1.
iTregs which acquired CD86 show stronger suppression and increased levels of activation markers than iTregs which did not acquire CD86
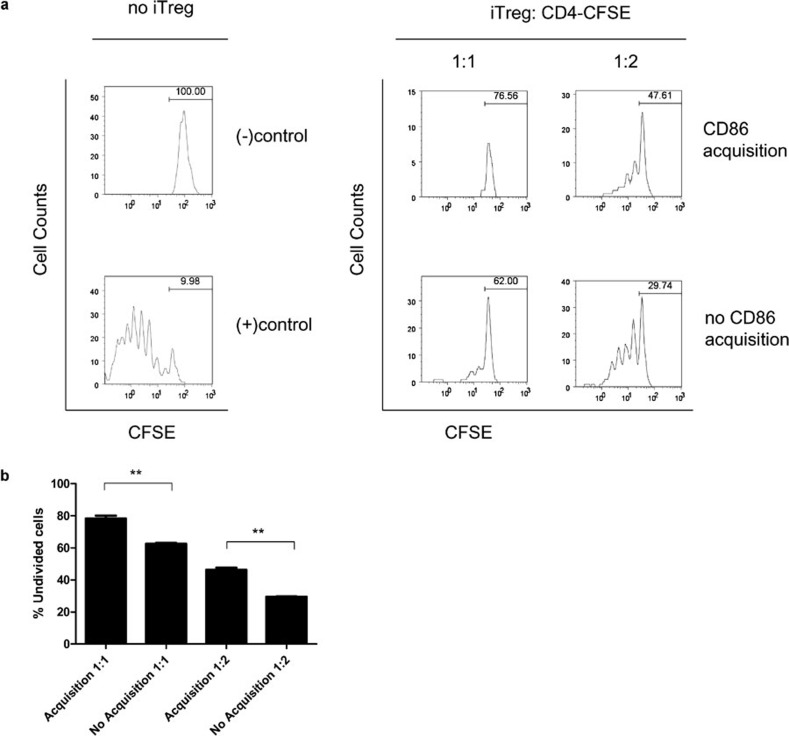
Next, we determined the consequence of trogocytosis on iTregs. Although both CD80 and CD86 could be acquired by DKO iTregs, CD86 was acquired to a higher extent than CD80 (Figure 3). To better characterize the differences between the iTregs which acquired and those that did not acquire CD86, we compared the suppressive capacity of each group. DKO iTregs were incubated with CD80−/− mDCs and then sorted according to the level of CD86 acquisition. To evaluate the suppressive capacity of each group towards responder T cells, a suppression assay was carried out using purified Thy1.1+ naive CD4+ T cells as responders and the sorted iTregs (Thy1.2+) as suppressors. As shown in Figure 5a, both the iTregs which acquired CD86 and the iTregs which did not acquire CD86 were able to suppress the proliferation of responder cells. Interestingly, iTregs which had acquired CD86 displayed a greater suppressive capacity towards CD4+ T cells than iTregs which had not acquired CD86 (Figure 5b). These data suggest that acquisition of CD86 from mDCs can enhance iTreg suppressive function.
Figure 5.
(a) Comparison of suppressive capacity between iTregs which had acquired CD86 and iTregs which had not acquired CD86. DKO iTregs were cocultured with CD80−/− mDCs and then sorted according to the level of CD86 acquisition. CD4+CD25− T cells (Thy1.1+) were purified from naive B6.PL-Th1a/CyJ mice, labeled with CFSE, and cultured (1×105 cells/well) with 1 µg/ml anti-CD3 Ab in the presence of irradiated syngeneic splenocytes (2×104 cells/well). Varying numbers of the sorted DKO iTreg were added to the culture at suppressor to responder ratios 1∶1 and 1∶2. After 4 days of culture, proliferation of responder cells was measured by gating on Thy1.1+ cells and assessing their CFSE signal by flow cytometry. Numbers represent the percentage of undivided cells. (−) control: CFSE labeled CD4+CD25− T cells without any stimulation or iTregs. (+) contol: CFSE labeled CD4+CD25− T cells were cultured with anti-CD3 Ab in the presence of irradiated APC without iTregs. (B) The percent of undivided cells is shown from suppression assays carried out in (a). The data shown are representative of two different experiments, each carried out in duplicate, using 2–3 mice in each experiment. **P<0.05, one-way ANOVA and Bonferroni's multiple comparison test. Ab, antibody; APC, antigen-presenting cell; CFSE, carboxyfluorescein diacetate, succinimidyl ester; DKO, double knockout; iTreg, induced regulatory T cell; mDC, mature dendritic cell.
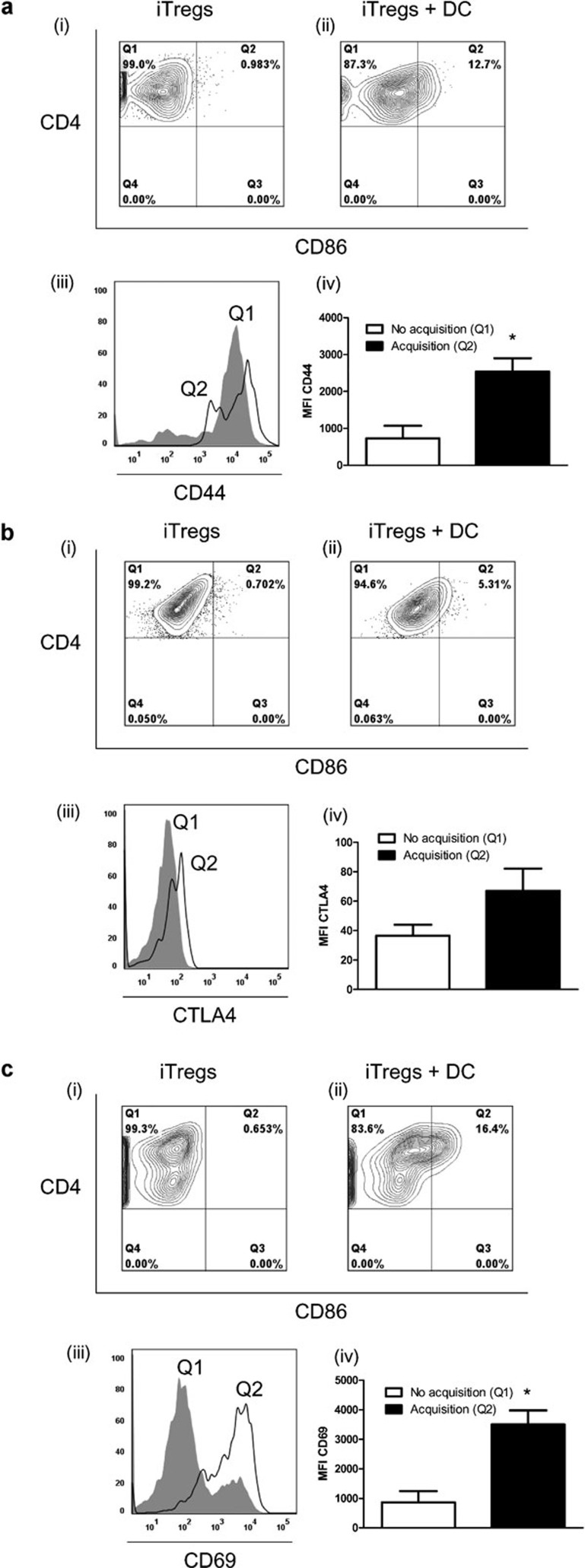
To examine the basis for the difference in suppressive capacity observed between the two groups, we compared levels of activation markers between iTregs which acquired CD86 with those that did not acquire CD86 (Figure 6). Interestingly, the population of iTregs which showed acquisition of CD86 displayed significantly higher levels of CD44 and CD69 (Figure 6a and c) than the population of iTregs which did not acquire CD86. Levels of CTLA4 were also increased on iTregs which acquired CD86 (Figure 6b) compared to iTregs which did not acquire CD86; however, this increase did not reach statistical significance.
Figure 6.
Characterization of activation markers on iTregs following acquisition of CD86. iTregs were cultured alone (i) or cocultured with mDCs from CD45.1+ mice (ii), and the level of CD4 and CD86 were analyzed by flow cytometry. Cells were gated on eF450−CD45.1−CD4+ cells. Cells that stained positive for CD4 only (Q1) after coculture represent iTregs that did not acquire CD86, while cells that were double positive for CD4 and CD86 (Q2) after coculture represent iTregs that acquired CD86. (iii) The level of (a) CD44, (b) CTLA-4 and (c) CD69 was measured on iTregs after coculture with mDCs. Histograms show levels of activation markers on iTregs that did not acquire CD86 (Q1 from (ii), shaded graph) and on iTregs that did acquire CD86 (Q2 from (ii), line graph). (iv) The MFI from three independent experiments, each with three pooled mice, for activation markers on iTregs that acquired and that did not acquire CD86 is shown. The error bars represent SEM. *P<0.05, One-way ANOVA with Bonferroni's post hoc test. iTreg, induced regulatory T cell; mDC, mature dendritic cell; MFI, median fluorescence intensity.
Downregulation of CD80 and CD86 on mDCs after coculture with iTregs
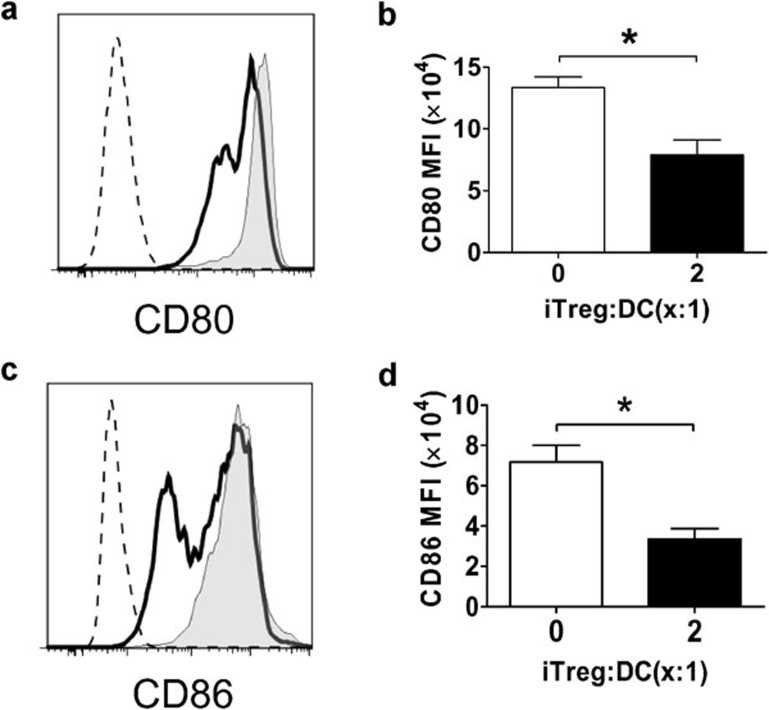
nTregs have been shown to be able to inhibit maturation of DCs13 or downregulate CD80 and CD86 expression on mDCs through trans-endocytosis.14 To determine whether iTregs have any effect on mDC CD80 and CD86 expression, iTregs were cocultured with bone marrow-derived, LPS-matured DCs, which express high levels of CD80 and CD86. CD80 and CD86 expression on mDCs was determined by flow cytometry after co-incubation. As shown in Figure 7, significant downregulation of both CD80 and CD86 on mDCs was observed after 24 h of coculture with iTregs. These data indicate that iTregs can downregulate CD80 and CD86 expression on mDCs.
Figure 7.
Downregulation of CD80 and CD86 expression on DCs by iTregs. BM-derived LPS-matured DCs from B6 mice, which express high levels of CD80 and CD86, were cultured alone or cocultured together with iTregs from B6 mice at iTreg/DC ratio=2∶1. After 24 h, cultures were stained with PI, anti-CD4, anti-CD11c, anti-CD80 and anti-CD86 mAbs, and then analyzed by flow cytometry. (a, c) The plots shown are gated on PI−CD4−CD11c+ cells (shaded graph: CD80 or CD86 expression on DCs cultured alone; solid line: CD80 or CD86 expression on DCs after coculture with iTregs; dotted line: isotype control). These experiments were repeated at least three times each with two or three mice and similar results were obtained. The bar graphs show the MFI of CD80 (b) and CD86 (d). The error bars represent SEM. 0.01<*P<0.05, Student's t-test. BM, bone marrow; DC, dendritic cell; iTreg, induced regulatory T cell; LPS, lipopolysaccharide; mAb, monoclonal antibody; MFI, median fluorescence intensity.
Discussion
CD80 and CD86 have been shown to be important for the thymic development and peripheral homeostasis of nTregs in mice.28 In contrast, CD4+CD25+Foxp3+ iTregs could be induced from CD80−/−CD86−/− DKO cells to a similar extent as from WT cells (Figure 1a). It was previously shown that Tregs cocultured with mDCs from CD80-/CD86-deficient mice showed reduced survival, and B7 expression on DCs was deemed necessary for Treg maintenance.18 However, this same study showed that normal thymic development and peripheral distribution of conventional T cells were not affected in CD80-/CD86-deficient mice. In accordance with this, we were able to generate functional iTregs from CD80−/−CD86−/− DKO mice (Figure 1b and c), indicating that, unlike nTregs, the induction and function of iTregs is not dependant on these molecules.
Intercellular transfer of cell surface membrane fragments and proteins has been shown to result in altered cell functions,19, 29, 30 and thus could play an important role in modulating immune responses. Several cell types, including T and B lymphocytes, natural killer cells and γδ T cells15, 16 have been shown to acquire cell surface molecules, including peptide–major histocompatibility complex (MHC), costimulatory and adhesion molecules.31 However, to the best of our knowledge, the ability of iTregs to undergo this process was not known. In this study, we found that after coculture of iTregs with mDCs, iTregs expressed CD80 and CD86 on their surface (Figure 2a). The observed expression of CD80 and CD86 on iTregs was not due to upregulation of these molecules as iTregs that were generated from CD80−/−CD86−/− DKO mice also expressed these molecules after co-incubation with mDCs (Figure 2b). Confocal microscopy for cell surface expression of CD80 and CD86 indicated that both proteins were expressed on small localized areas on iTregs from DKO mice, further confirming trogocytosis as the mechanism of expression. These data provide clear evidence that iTregs are capable of acquiring CD80 and CD86 from mDCs. Since pretreatment of iTregs with an endocytosis inhibitor did not alter iTreg trogocytosis (data not shown), this suggests that the acquired molecules were not internalized by this process. Furthermore, we found that CD80 and CD86 were acquired by iTregs very quickly and iTreg acquisition increased from 2 to 24 h of coculture with mDCs. In addition, CD86 was acquired to a greater extent by iTregs than CD80 (Figure 3b). The reason for this difference is not known. Although CD80 and CD86 share the same receptors, they have distinct binding affinities and kinetics18 and have been shown to have differential functions.32, 33 Levels of both proteins were equally expressed on mDCs prior to coculture (data not shown), ruling out increased availability of CD86 for trogocytosis.
Transfer of cell surface proteins between cells has been shown to occur by various mechanisms, including through several cell surface receptors.22 We found that the kinetics of CD80 and CD86 acquisition by iTregs were different to that of the membrane dye PKH26. Whereas the CD80 and CD86 expression levels on iTregs continued to rise, the PKH26 level declined with time (Figure 3c). These data suggest that the transfer of CD80 and CD86 from mDCs to iTregs might be mediated through a specific receptor–ligand interaction rather than by passive transfer of membrane fragments. Previous studies have described that stimulation of TCR significantly increased T-cell trogocytosis.19, 34 We found that addition of anti-CD3 mAb to iTreg–DC coculture resulted in only a slight increase in iTreg trogocytosis of CD80 and CD86 (data not shown). Since the induction of iTregs with anti-CD28 and anti-CD3 Abs produces already activated T cells, and adding anti-CD3 Ab during coculture did not have a significant impact on iTreg trogocytosis, this suggests that anti-CD3 stimulation may be important for T-cell activation rather than having a direct effect on T-cell trogocytosis.
iTregs express high levels of CTLA-4 and CD28, and both can bind to CD80 and CD86. There have been conflicting reports regarding the role of these receptors in the acquisition of CD80 and CD86 by recipient cells. Sabzevari et al.19 demonstrated a TCR- and CD28-dependent, but CTLA-4-independent mechanism of acquisition of CD80 by CD4+ T cells. Onishi et al.13 reported that nTregs downregulate CD80/CD86 expression in a CTLA-4- and LFA-1-dependent manner. Qureshi et al.14 showed a CD28-independent, but CTLA4-dependent mechanism of acquisition of CD80 and CD86 by both T effectors and Tregs. We found that nTregs generated from CTLA-4-deficient mice were able to acquire high levels of both CD80 and CD86 from mDCs (Figure 4a), indicating that CTLA-4 is not required for nTregs to acquire CD80 and CD86. Because CTLA-4 is required for the induction of iTregs,25 we could not use CTLA-4-deficient mice to examine the role of CTLA-4 in iTreg trogocytosis. Alternatively, we addressed this question by blocking studies and found that blocking CTLA-4 on iTreg with anti-CTLA-4 Ab did not affect iTreg trogocytosis of CD80 and CD86 (Figure 4b). In the report by Qureshi et al.,14 the authors describe a trans-endocytosis of CD80 and CD86 and have deemed this process to be different from trogocytosis, as they did not detect cell surface acquisition. In our study, however, iTreg acquisition of CD80/CD86 was not affected when an endocytosis inhibitor was used (data not shown), indicating that it is unlikely that endocytosis is a mechanism of acquisition by iTregs. Because the study by Qureshi et al.14 used transfected cells overexpressing costimulatory molecules, the mechanisms involved may differ from our system where naturally occurring levels of CD80 and CD86 were examined on primary cells. Furthermore, co-incubation with anti-CD28 (Figure 4c) or anti-PDL1 (Figure 4d) Abs, which show clear binding to iTregs (Figure 4e) also failed to block acquisition of CD80 and CD86 by iTregs. These data suggest that iTregs may acquire CD80 and CD86 via a different mechanism which requires further study.
The functional consequences of trogocytosis can have a stimulatory or suppressive effect on immune responses depending on the cell type and acquired protein involved. Sabzevari et al.19 have shown that naive CD4+ T cells which acquired CD80 upon activation were capable of acting as APCs. In another study, CD4+ T cells could differentiate into suppressive cells when they acquired HLA-G.35 Previously, we36 and others37 have shown that both mouse and human CD3+CD4−CD8− double negative Tregs were able to acquire MHC–peptides from APCs and use the acquired MHC–peptides to trap and kill activated CD4+ or CD8+ T effectors in an antigen-specific manner. Here, we found that the ability of iTregs to suppress naive CD4+ T-cell proliferation was greatly enhanced after iTregs acquired CD86 from mDCs compared to iTregs that did not acquire CD86 (Figure 5). Thus, trogocytosis by iTregs enhances the suppressive function of these cells. Because there are likely several cell surface proteins that are transferred during trogocytosis, we cannot rule out acquisition of other molecules in playing a role in increasing iTreg suppression.
To examine the basis for the difference in suppressive capacity observed between iTregs which had acquired and iTregs which had not acquired CD86, several activation markers were examined (Figure 6). Levels of activation markers were increased in the group of iTregs which had acquired CD86, indicating that these cells were in a more activated functional state. In support of our findings, Rosenits et al.38 showed that trogocytosis-positive T cells displayed higher levels of CD69 and a more activated phenotype than trogocytosis-negative T cells. Differences in cell activation status may contribute to the functional differences in suppressive capacity observed between the two groups of cells. Although we do not know the exact mechanism of trogocytosis-enhanced iTreg suppression in our system, it is possible that acquisition of costimulatory molecules may facilitate cell-to-cell contact and lead to enhanced suppression. Zhou et al.39 have demonstrated that nTregs with acquired peptide–MHC-II molecules show enhanced suppression to target CD4+ T cells. They postulated that acquired ligands on nTregs can attract target cells to facilitate suppression mediated by other effector molecules. In our study, the increased levels of CD44, which plays a role in cell adhesion, on iTregs which had acquired CD86, may also contribute to the subsequent enhanced suppressive function.
In addition to enhancing iTreg suppressive function, we also observed a significant downregulation of CD80/CD86 expression on DCs after coculture with iTregs (Figure 7). As the levels of surface expression of these costimulatory molecules are critical for mDCs to activate T cells, these data suggest that iTregs may suppress immune responses via controlling the function of DCs. Whether trogocytosis of CD80/CD86 by iTregs and downregulation of CD80 and CD86 on mDCs is a cause–effect or an epiphenomenon requires further investigation.
In conclusion, the studies reported here demonstrate for the first time that iTregs are able to acquire CD80 and CD86 from mDCs in a CTLA-4- and CD28-independent manner and the acquired costimulatory molecules confer increased suppressive function and may play an important role in regulating immune responses by iTregs.
References
- Vignali D. How many mechanisms do regulatory T cells need. Eur J Immunol. 2008;38:908–911. doi: 10.1002/eji.200738114. [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor. Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+. . J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+. . J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+. . J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P, et al. Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671–680. doi: 10.1136/gut.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+CD25+ regulatory cells from murine naive T cells. Nat Protoc. 2007;2:1789–1794. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly E, Hudrisier D. What is trogocytosis and what is its purpose. Nat Immunol. 2003;4 doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- Ahmed KA, Munegowda MA, Xie Y, Xiang J. Intercellular trogocytosis plays an important role in modulation of immune responses. Cell Mol Immunol. 2008;5:261–269. doi: 10.1038/cmi.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Maiti PK, Nandi D. Role of CD80, CD86, and CTLA4 on mouse CD4+ T lymphocytes in enhancing cell-cycle progression and survival after activation with PMA and ionomycin. J Leukoc Biol. 2002;72:921–931. [PubMed] [Google Scholar]
- Zeng M, Guinet E, Nouri-Shirazi M. B7-1 and B7-2 differentially control peripheral homeostasis of CD4+CD25+Foxp3+ regulatory T cells. Transpl Immunol. 2009;20:171–179. doi: 10.1016/j.trim.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Sabzevari H, Kantor J, Jaigirdar A, Tagaya Y, Naramura M, Hodge J, et al. Acquisition of CD80 (B7-1) by T cells. J Immunol. 2001;166:2505–2513. doi: 10.4049/jimmunol.166.4.2505. [DOI] [PubMed] [Google Scholar]
- Tatari-Calderone Z, Semnani RT, Nutman TB, Schlom J, Sabzevari H. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J Immunol. 2002;169:6162–6169. doi: 10.4049/jimmunol.169.11.6162. [DOI] [PubMed] [Google Scholar]
- Zhou J, Tagaya Y, Tolouei-Semnani R, Schlom J, Sabzevari H. Physiological relevance of antigen presentasome (APS), an acquired MHC/costimulatory complex, in the sustained activation of CD4+ T cells in the absence of APCs. Blood. 2005;105:3238–3246. doi: 10.1182/blood-2004-08-3236. [DOI] [PubMed] [Google Scholar]
- Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, et al. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137–1148. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Domenig C, Habicht A, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. . J Immunol. 2005;174:3408–3415. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- Cox JH, McMichael AJ, Screaton GR, Xu XN. CTLs target Th cells that acquire bystander MHC class I-peptide complex from APCs. J Immunol. 2007;179:830–836. doi: 10.4049/jimmunol.179.2.830. [DOI] [PubMed] [Google Scholar]
- LeMaoult J, Krawice-Radanne I, Dausset J, Carosella ED. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci USA. 2004;101:7064–7069. doi: 10.1073/pnas.0401922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- Lumsden JM, Roberts JM, Harris NL, Peach RJ, Ronchese F. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J Immunol. 2000;164:79–85. doi: 10.4049/jimmunol.164.1.79. [DOI] [PubMed] [Google Scholar]
- Balbo P, Silvestri M, Rossi GA, Crimi E, Burastero SE. Differential role of CD80 and CD86 on alveolar macrophages in the presentation of allergen to T lymphocytes in asthma. Clin Exp Allergy. 2001;31:625–636. doi: 10.1046/j.1365-2222.2001.01068.x. [DOI] [PubMed] [Google Scholar]
- Bahcheli D, Hay V, Nadeau JL, Piccirillo CA. Transfer of cell membrane components via trogocytosis occurs in CD4+Foxp3+CD25+ regulatory T-cell contact-dependent suppression. Autoimmunity. 2011;44:607–615. doi: 10.3109/08916934.2011.571730. [DOI] [PubMed] [Google Scholar]
- LeMaoult J, Caumartin J, Daouya M, Favier B, Le RS, Gonzalez A, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040–2048. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med. 2000;6:782–789. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, et al. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4−CD8− double-negative regulatory T cells. Blood. 2005;105:2828–2835. doi: 10.1182/blood-2004-07-2583. [DOI] [PubMed] [Google Scholar]
- Rosenits K, Keppler SJ, Vucikuja S, Aichele P. T cells acquire cell surface determinants of APC via in vivo trogocytosis during viral infections. Eur J Immunol. 2010;40:3450–3457. doi: 10.1002/eji.201040743. [DOI] [PubMed] [Google Scholar]
- Zhou G, Ding ZC, Fu J, Levitsky HI. Presentation of acquired peptide–MHC class II ligands by CD4+ regulatory T cells or helper cells differentially regulates antigen-specific CD4+ T cell response. J Immunol. 2011;186:2148–2155. doi: 10.4049/jimmunol.1002917. [DOI] [PubMed] [Google Scholar]