Abstract
Pneumonia, the most typical and frequent lower respiratory tract infection (LRTI), is a leading cause of health problems in the United States. Bacteria represent the most prevailing cause of pneumonia in both children and adults. Although pneumonia with a single bacterial infection is common, a significant portion of patients with pneumonia is polymicrobial. This infection is often complexed with other physiological factors such as cytokines and growth factors. Nontypeable Haemophilus influenzae (NTHi) is the most frequently recovered Gram-negative bacterial pathogen in the respiratory system and induces strong inflammatory responses. NTHi also synergizes with other respiratory pathogens, such as Streptococcus pneumoniae and respiratory viruses and pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α). It is noteworthy that NTHi not only synergizes with growth factors such as transforming growth factor-beta (TGF-β), but also utilizes growth factor receptors such as TGF-β receptor and epidermal growth factor receptor (EGFR), to enhance inflammatory responses. Although appropriate inflammation is a protective response against invading pathogens, an uncontrolled inflammatory response is often detrimental to the host. Thus, inflammation must be tightly regulated. The human immune system has evolved strategies for controlling overactive inflammatory response. One such important mechanism is via regulation of negative feedback regulators for inflammation. CYLD, a multifunctional deubiquitinase, was originally reported as a tumor suppressor, but was recently identified as a negative regulator for nuclear factor-kappa B (NF-κB) signaling. It is induced by NTHi and TNF-α via a NF-κB-dependent mechanism, thereby serving as an inducible negative feedback regulator for tightly controlling inflammation in NTHi infection.
Keywords: CYLD, negative regulation, NF-κB, nontypeable Haemophilus influenzae, synergistic regulation
Introduction
Lower respiratory tract infection (LRTI) is a leading healthcare crisis that accounts for more than 50 million deaths per year worldwide.1 Community-acquired pneumonia, a prevalent form of LRTI, results in more than 10 million doctors visits annually in the United States.2 The most common bacteria responsible for community-acquired pneumonia include Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis.1, 3 Not only can these bacteria wreak havoc on the host via toxins and injury, but they can also induce a strong inflammatory response that can further damage the lung tissue and cause morbidity.4 However, rarely do these bacteria cause inflammation in an isolated circumstance. Combined with the primary infection, other secondary pro-inflammatory agonists, such as host-secreted cytokines, growth factors and viral and/or bacterial infections, can synergistically increase the total inflammatory response beyond the additive value of the individual agonists.5, 6, 7, 8, 9 In order to maintain an adequate level of inflammation, the immune system has developed negative regulatory mechanisms, like deubiquitinases such as CYLD and A20, for the tight regulation of inflammatory responses to prevent host damage.10, 11, 12, 13, 14 The goal of this review is to discuss the current knowledge of the synergistic activation of lung inflammation by bacteria, such as nontypeable Haemophilus influenzae (NTHi), combined with other co-inducers and the role of deubiquitinases, such as CYLD, in negative feedback regulation of inflammation.
Synergistic upregulation of NTHi-induced lung inflammation
NTHi and Inflammation
NTHi is a Gram-negative bacterium that is the leading cause of exacerbation of chronic obstructive pulmonary disease (COPD) in adults, a major form of LRTI, as well as otitis media (OM) in children.15, 16, 17 A hallmark of NTHi infection is the host's prolonged immune response against the pathogen, leading to debilitating inflammation. This response is primarily due to the release of pro-inflammatory mediators, such as interleukin-8 (IL-8), that signal leukocyte migration to the area of infection. However, this inflammatory response can often intensify the symptoms of the disease, and lead to increased host damage. Therefore, it is imperative that the host's inflammatory response is tightly regulated.
Inflammation is the body's primary response to harmful stimuli and is a critical part of the innate immune response, acting to signal the host to any bodily insult.18 The classical hallmarks of inflammation include redness, heat, pain, swelling and loss of function. After the initial trigger, pro-inflammatory mediators such as tumor necrosis factor-alpha (TNF-α), IL-1β and IL-8 are released and act to attract leukocytes, such as neutrophils, dendritic cells and macrophages, to the site of injury. This cascade promotes the elimination of the original antigen and ultimately results in repairing and healing the host.19 However, an overactive inflammatory response can cause severe tissue damage, and can lead to debilitating diseases such as COPD and OM.
Although NTHi makes up part of the nasopharyngeal flora in most of the population, it can cause disease in both children and adults. In children, it is the leading cause of OM. OM is the most common bacterial infection, as well as the primary cause of conductive hearing loss, in children and is responsible for nearly 30 million doctor visits each year in the United States, costing approximately five billion dollars in patient care.20, 21 NTHi is also the primary bacterium associated with exacerbation of COPD in adults. COPD is a progressive deterioration of the lungs that leads to respiratory distress. It is predominantly caused by extended exposure to cigarette smoke or other lung irritants like air pollution or chemical fumes. It is currently the fourth leading cause of death in the United States, and is projected to be the third leading cause of death in adults worldwide by 2030.22, 23, 24
Synergistic upregulation of inflammation by NTHi and cytokines
While NTHi has been shown to individually upregulate inflammation through the NF-κB signaling pathway, Watanabe et al. demonstrated that pro-inflammatory mediators, such as IL-8, TNF-α and IL-1β, were significantly upregulated when human airway epithelial cells were treated with both NTHi and TNF-α.9 This synergistic activation occurred through both the NIK–IKK–IκBα and the MEKK1–MKK3/5-p38 MAPK pathways. NF-κB activation increased twofold more than through stimulation with NTHi alone, and threefold more than treating with only TNF-α. A similar phenomenon can be seen in IL-8 and IL-1β upregulation.9
Predictably, synergistic activation by NTHi and endogenous factors are not limited to the lungs. Moon et al. showed that NTHi and IL-1α can synergistically upregulate β-defensins, molecules with antimicrobial properties, in human middle ear epithelial cells via the p38 MAPK pathway. While this synergistic activation was limited to the middle ear, the specificity can be possibly attributed to the difference in surface receptors each cell type expresses.25
Interestingly, in response to bacterial infection, the NTHi-treated cells initially secreted both TNF-α and IL-1α in order to alert the host of a potential biological threat. The secretion of these cytokines produced an autocrine feedback loop that further enhanced the inflammatory response in conjunction with the already existing NTHi stimuli in a synergistic manner.
Synergistic upregulation of inflammation by NTHi and growth factors
In addition to coupregulating lung inflammation with cytokines, NTHi has been shown to synergistically enhance host inflammatory response with growth factors. Ishinaga et al. reported that transforming growth factor-beta (TGF-β) synergistically enhanced NTHi-induced inflammation by inducing acetylation of the NF-κB p65 subunit.5 While more commonly known as a key factor in regulating important cellular processes such as cell differentiation and proliferation, TGF-β, when cotreated with NTHi, significantly increased NF-κB activity in epithelial cells in vitro and inflammation in lung tissues of mice.5, 26, 27 This synergistic upregulation was shown to be due to the TGF-β induction of p65 acetylation through the Smad3/4–PKA–p300-dependent signaling pathway.5 It is important to note that traditionally, TGF-β is known to be an anti-inflammatory mediator; therefore, its role in synergistically enhancing NTHi-induced inflammation is particularly unique.28
Recently, Xu et al. demonstrated that the epidermal growth factor receptor (EGFR) signaling pathway also plays a role in upregulating NTHi-induced inflammation.29 By perturbing EGFR signaling using AG1478, an EGFR tyrosine kinase inhibitor, NTHi-induced NF-κB activation in A549 cells was markedly attenuated. Similar results were also seen in cells in which EGFR expression was significantly reduced with EGFR knockdown via siRNA. In mice, pre-treatment with AG1478 significantly inhibited NTHi-induced upregulation of pro-inflammatory mediators such as TNF-α, IL-1β and macrophage inflammatory protein-2. Furthermore, EGFR signaling was shown to mediate NTHi-induced NF-κB activation through the PI3k–Akt as well as the MKK3/6–p38 pathways.29 Previously, Jiang et al. suggested that EGFR signaling leads to NF-κB activation through a caspase recruitment domain-containing scaffold molecule, CARMA3, and resulted in increased tumor growth in vivo.30
Synergistic upregulation of inflammation by NTHi and other microbial pathogens
Although it is well established that NTHi alone can induce potent inflammation in controlled in vitro and in vivo settings, polymicrobial infections of NTHi with other bacterial and viral pathogens are more prevalent in vivo.31 The tissue damage caused by COPD makes the lungs susceptible to numerous bacterial infections in addition to NTHi, most commonly Streptococcus pneumoniae and Moraxella catarrhalis.1, 3, 32 Kweon et al. previously reported that NTHi and S. pneumoniae synergize to upregulate NF-κB activity through the IKKβ–IκBα and the p38–MAPK signaling pathways in airway epithelial A549 and NHBE cells.6 When the cells were cotreated with both species, NF-κB-dependent transcription of proinflammatory mediators such as TNF-α, IL-1β and IL-8 was significantly upregulated in comparison with cells that were treated with each bacteria strain separately.6 Lim et al. has also shown that the combinatorial treatment of NTHi and S. pneumoniae can synergistically induce inflammation via upregulating TLR-2 in vitro and in vivo.7 In mice treated intratracheally with both NTHi and S. pneumoniae, IL-1β mRNA expression was significantly increased in comparison with mice inoculated with each individual strain. Similar to what Kweon et al. and Lim et al. reported, Ratner et al. also demonstrated a synergistic increase in IL-8 due to a combinatorial infection by NTHi and S. pneumoniae via the p38 MAPK pathway. Work done in human epithelial cells stimulated by both strains exhibited a significant NF-κB-dependent and p38 MAPK-dependent upregulation of IL-8.8
In addition to bacterial co-infections, COPD lungs are often susceptible to additional viral infections, such as influenza virus and human rhinovirus. Wilkinson et al. examined 56 patients with COPD exacerbations, and determined that in cases where patients were infected with both Haemophilus influenzae as well as human rhinovirus, there was significantly more IL-6 in the patient serum, suggesting increased inflammation.32 Co-infections of H. influenzae and viruses have been shown to increase mortality. Lee et al. period developed a mouse model for NTHi/influenza virus co-infection that showed a 100% lethality rate in mice co-infected with NTHi and virus in a manner that is not completely dependent on TNF-α, IL-6 or TLR-4, but is dependent on the host's innate immunity. Concurrently, the mechanism seems to be T-cell- and B-cell-independent, suggesting an unknown innate immunity dependent pathway. Interestingly, the mortality rate of the co-infections was critically dependent on the duration of time between the influenza and NTHi exposure, with incubations between 3 and 4 days showing the maximum lethality.33
Negative feedback regulation of lung inflammation
Deubiquitinase, CYLD, as a negative feedback regulator in lung inflammation
Although inflammatory responses against invading microbial pathogens are critical mechanisms for survival, dysregulated inflammatory responses are detrimental to the host. Tight control of inflammation, therefore, is critical for the survival of the host. Immune systems have evolved multiple strategies to regulate and maintain an adequate level of inflammation, such as deubiquitinases like CYLD and A20.10, 11, 12, 13, 14, 34, 35 Among them, the role of CYLD has been the most extensively investigated in respiratory bacterial infections, especially by NTHi.
While NTHi and other coagonists can synergistically induce strong levels of inflammation in the lungs, the deubiquitinase, CYLD, is a multitasker that can negatively regulate many of the pathways induced during infection. The tumor suppressor gene, CYLD, was first reported when it was discovered that a mutation in the gene leads to the formation of benign tumors in skin appendages.36 CYLD is a deubiquitinating enzyme that has been implicated in the downregulation of NF-κB.37, 38, 39 This enzyme contains ubiquitin carboxy-terminal hydrolases that can bind to ubiquitin and detach it from a target protein. Ubiquitin is a small regulatory protein that is present in all eukaryotic cells. When attached to a pre-existing protein, it can direct its further processing, leading to either activation or degradation. Polymerization of ubiquitin typically occurs at one of its seven lysine residues. Conjugation at Lysine 48 (K48) directs the tagged protein, such as IκBα and p100, towards degradation, whereas Lysine 63 (K63)-linked polymerization has been implicated in the activation of proteins.14, 40, 41, 42 CYLD specifically targets ubiquitin chains that are linked by K63 and removes them from the protein, leading to its inactivation.42 Recently, it has been proposed that CYLD complexes with the E3 ligase, Itch, to regulate inflammation via Tak1 in macrophages.43 While it has been previously suggested that CYLD deubiquitinates Tak1's K63-linked polyubiquitin chain, Ahmed et al. showed that after CYLD removes the K63-linked ubiquitin, Itch catalyzes the polymerization of K48-linked ubiquitin to Tak1, signaling its degradation.43, 44 Along with being a well-known tumor suppressor in eukaryotic cells, CYLD has also been linked to other critical functions, such as the regulation of osteogenesis and spermatogenesis and the development of T cells, B cells and lung tissue.45, 46, 47, 48, 49, 50, 51 Trompouki et al. implicated CYLD as critical in lung maturation. Mice carrying a homozygous deletion of Cyld exon 9 died soon after birth due to underdeveloped lungs, stemming from an overly thick mesenchyme that prohibited the formation of the alveolocapillary barrier.52 It is interesting to note that the lethality of this mutation was not seen in other Cyld inactivated mouse models. The authors suggested that this phenotype was due to the deletion of exon 9 as opposed to exons 2 and 3 in the mouse models presented by Reiley et al., Massoumi et al. and Zhang et al., as well as the expression and scaffolding activity discrepancies between these different mutations.53, 54, 55
Known CYLD targets within the NF-κB regulatory pathway include TRAF 2/6/7, NEMO and Tak1.56 Interestingly, Jono et al. had shown that NF-κB is also critical in the upregulation of CYLD, thereby unveiling a novel negative feedback loop in regulating inflammation in NTHi infection.57 CYLD was further determined to have anti-inflammatory effects in mice exposed to NTHi by downregulating NF-κB activation via TRAF6/7.13 Lim et al. showed that in CYLD−/− mice, lung tissue had increased leukocyte infiltration in response to NTHi infection compared to the wild-type (WT) mice. Concurrently, CYLD−/− mice also had upregulated proinflammatory cytokines in response to NTHi.13
In addition to playing a critical role in regulating NTHi-induced inflammation, CYLD's role expands to regulate inflammation and lung injury induced by other bacteria as well. In Escherichia coli-induced pneumonia, CYLD is critical in regulating the inflammatory response. Expression of IL-1β and IL-6 was enhanced in CYLD−/− mice, along with increased neutrophil infiltration suggested CYLD's function as a negative regulator of the innate immune response.12 In Klebsiella pneumoniae infection, an important Gram-negative bacteria implicated in community-acquired pneumonia, CYLD is upregulated through the pattern-recognition receptor NOD1 in order to dampen NF-κB- and MAPK phosphatase 1 (MKP-1)-dependent inflammation. By decreasing the cell's ability to generate an inflammatory response, K. pneumoniae is better able to evade the host's immune response.58 Interestingly, in response to severe S. pneumoniae infection, CYLD seems to play a critical role in enhancing tissue injury and death in WT mice.59 This detrimental role of CYLD in acute lung injury during severe S. pneumonia infection is mainly attributed to CYLD's role in negatively regulating the p38 MAPK pathway, leading to the downregulation of type 1 plasminogen activator inhibitor in WT mice, which causes severe tissue injury and alveolar hemorrhaging.59
Additional deubiquitinases may also play a role in negatively regulating inflammation
While CYLD has been well documented in playing a critical role in negatively regulating the host's innate immune response, it is joined by numerous other deubiquitinases that have demonstrated anti-inflammatory roles. A20, another well-defined deubiquitinase, also targets ubiquitin polymerized at K63 and has been shown to negatively regulate NF-κB signaling. A20−/− mice exhibited an inability to negatively regulate TNF-α expression while still maintaining regulatory control of IL-1β, suggesting a TNF-α pathway-dependent regulation of NF-κB.60 Cezanne, a member of the A20 deubiquitinating family of enzymes, has been shown to be capable of regulating NF-κB-controlled IL-8 expression in TNF-α-treated cells.61
Conclusion
In recent studies, the classical dogma of having one etiological agent responsible for one pathological symptom has been replaced by the idea that multiple inducers can combine to synergistically increase host responses in an in vivo setting under physiological conditions. Currently, it is becoming more apparent that the regulation of lung inflammation is not simply a linear cause and effect, but rather a complicated skein of combinatorial signals that upregulate the host's innate immune system. While pathological bacteria, such as NTHi, can induce the host's inflammatory response, the presence of other co-inducers, such as cytokines, growth factors and other bacteria and viruses, can synergistically enhance this response, causing further damage to the host. Concurrently, deubiquitinases, such as CYLD, are upregulated during inflammation in order to tightly control the immune response in the hopes of limiting host damage. As we continue to investigate the mechanisms and regulation NTHi-induced lung inflammation, it becomes increasingly clear that other pro-inflammatory agonists play a vital role in this response. Understanding how this synergistic activation of inflammation occurs and is regulated will be critical in developing novel therapies in the devastating diseases it causes, such as COPD and other LRTI.
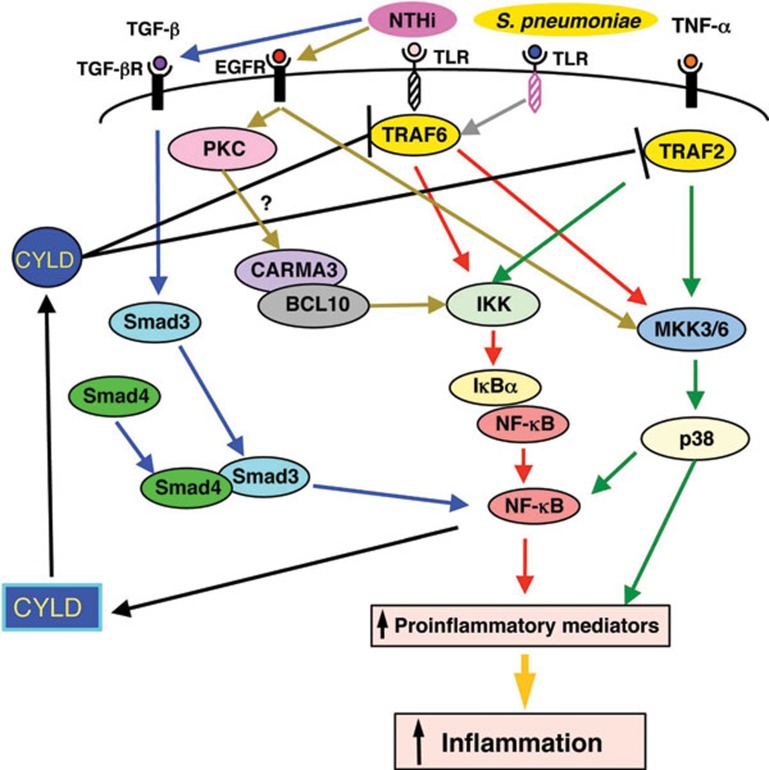
Figure 1.
Schematic diagram illustrating synergistic and feedback mechanisms that regulate inflammation in respiratory infections. NTHi induces inflammation in a TRAF6–IKK–NF-κB- and TRAF6–MKK3/6-p38-dependent manner. Bacterial pathogen Streptococcus pneumoniae and pro-inflammatory cytokine TNF-α synergize with NTHi to induce inflammation by synergistically activating both NF-κB and p38 MAPK signaling pathways. TGF-β synergizes with NTHi to induce inflammation via enhancing Smad3/4–PKA-mediated p65 acetylation. EGFR-mediated activation of MKK3/6–p38 signaling and possibly PKC–CARMA3/BCL10 signaling leads to synergistic activation of NF-κB and the subsequent inflammatory responses. NTHi and TNF-α induce CYLD expression in a NF-κB-dependent manner, which in turn serves as a negative feedback regulator for inflammation by deubiquitinating and inactivating TRAF6 or TRAF2. BCL10, B-cell lymphoma/leukemia 10; CARMA3, caspase recruitment domain (CARD) and membrane-associated guanylate kinase-like domain protein 3; CYLD, cylindromatosis; EGFR, epidermal growth factor receptor; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cell inhibitor alpha; IKK, IκB kinase; MKK3/6, mitogen-activated protein kinase kinase 3/6; NF-κB, nuclear factor-kappa B; NTHi, nontypeable Haemophilus influenzae; PKC, protein kinase C; TGF-β, transforming growth factor-beta; TNF-α, tumor-necrosis factor-alpha; TRAF2, TNF receptor-associated factor 2; TRAF6, TNF receptor-associated factor 6; S. pneumoniae, Streptococcus pneumoniae.
Acknowledgments
This work was supported by grants from National Institute of Health DC005843, DC005843-S1, DC004562 and AI073374 (to J-DL) and AHA 10SDG2630077 (to JHL).
References
- Nicolau D. Clinical and economic implications of antimicrobial resistance for the management of community-acquired respiratory tract infections. J Antimicrob Chemother. 2002;50 (Suppl S1:61–70. doi: 10.1093/jac/dkf809. [DOI] [PubMed] [Google Scholar]
- Brixner DI. Clinical and economic outcomes in the treatment of lower respiratory tract infections. Am J Manag Care. 2004;10:S400–S407. [PubMed] [Google Scholar]
- Guthrie R. Community-acquired lower respiratory tract infections: etiology and treatment. Chest. 2001;120:2021–2034. doi: 10.1378/chest.120.6.2021. [DOI] [PubMed] [Google Scholar]
- Khair OA, Davies RJ, Devalia JL. Bacterial-induced release of inflammatory mediators by bronchial epithelial cells. Eur Respir J. 1996;9:1913–1922. doi: 10.1183/09031936.96.09091913. [DOI] [PubMed] [Google Scholar]
- Ishinaga H, Jono H, Lim JH, Kweon SM, Xu H, Ha UH, et al. TGF-beta induces p65 acetylation to enhance bacteria-induced NF-kappaB activation. EMBO J. 2007;26:1150–1162. doi: 10.1038/sj.emboj.7601546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon SM, Wang B, Rixter D, Lim JH, Koga T, Ishinaga H, et al. Synergistic activation of NF-kappaB by nontypeable H. influenzae and S. pneumoniae is mediated by CK2, IKKbeta–IkappaBalpha, and p38 MAPK. Biochem Biophys Res Commun. 2006;351:368–375. doi: 10.1016/j.bbrc.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Jono H, Ha UH, Xu H, Ishinaga H, Morino S, et al. Streptococcus pneumoniae synergizes with nontypeable Haemophilus influenzae to induce inflammation via upregulating TLR2. BMC Immunol. 2008;9:40. doi: 10.1186/1471-2172-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner AJ, Lysenko ES, Paul MN, Weiser JN. Synergistic proinflammatory responses induced by polymicrobial colonization of epithelial surfaces. Proc Natl Acad Sci USA. 2005;102:3429–3434. doi: 10.1073/pnas.0500599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Jono H, Han J, Lim DJ, Li JD. Synergistic activation of NF-kappaB by nontypeable Haemophilus influenzae and tumor necrosis factor alpha. Proc Natl Acad Sci USA. 2004;101:3563–3568. doi: 10.1073/pnas.0400557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Ha UH, Woo CH, Xu H, Li JD. CYLD is a crucial negative regulator of innate immune response in Escherichia coli pneumonia. Cell Microbiol. 2008;10:2247–2256. doi: 10.1111/j.1462-5822.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- Lim JH, Jono H, Koga T, Woo CH, Ishinaga H, Bourne P, et al. Tumor suppressor CYLD acts as a negative regulator for non-typeable Haemophilus influenza-induced inflammation in the middle ear and lung of mice. PLoS ONE. 2007;2:e1032. doi: 10.1371/journal.pone.0001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2010;17:25–34. doi: 10.1038/cdd.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyd J, Cripps A. Nontypeable Haemophilus influenzae: challenges in developing a vaccine. J Biotechnol. 1999;73:103–108. doi: 10.1016/s0168-1656(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Li JD. Exploitation of host epithelial signaling networks by respiratory bacterial pathogens. J Pharmacol Sci. 2003;91:1–7. doi: 10.1254/jphs.91.1. [DOI] [PubMed] [Google Scholar]
- Murphy TF, Bakaletz LO, Smeesters PR. Microbial interactions in the respiratory tract. Pediatr Infect Dis J. 2009;28:S121–S126. doi: 10.1097/INF.0b013e3181b6d7ec. [DOI] [PubMed] [Google Scholar]
- Ferrero-Miliani L, Seidelin JB, Nielsen OH. Regulation of cytokine production in inflammatory bowel disease. Ugeskr Laeger. 2006;168:1847–1850. Danish. [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Chartrand SA, Pong A. Acute otitis media in the 1990s: the impact of antibiotic resistance. Pediatr Ann. 1998;27:86–95. doi: 10.3928/0090-4481-19980201-06. [DOI] [PubMed] [Google Scholar]
- Gates GA. Cost-effectiveness considerations in otitis media treatment. Otolaryngol Head Neck Surg. 1996;114:525–530. doi: 10.1016/S0194-59989670243-7. [DOI] [PubMed] [Google Scholar]
- Minino AM, Arias E, Kochanek KD, Murphy SL, Smith BL. Deaths: final data for 2000. Natl Vital Stat Rep. 2002;50:1–119. [PubMed] [Google Scholar]
- Moghaddam SJ, Ochoa CE, Sethi S, Dickey BF. Nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease and lung cancer. Int J Chron Obstruct Pulmon Dis. 2011;6:113–123. doi: 10.2147/COPD.S15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein AL, Irazola VE, Bazzano LA, Sobrino E, Calandrelli M, Lanas F, et al. Detection and follow-up of chronic obstructive pulmonary disease (COPD) and risk factors in the Southern Cone of Latin America: the pulmonary risk in South America (PRISA) study. BMC Pulm Med. 2011;11:34. doi: 10.1186/1471-2466-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SK, Lee HY, Pan H, Takeshita T, Park R, Cha K, et al. Synergistic effect of interleukin 1 alpha on nontypeable Haemophilus influenzae-induced up-regulation of human beta-defensin 2 in middle ear epithelial cells. BMC Infect Dis. 2006;6:12. doi: 10.1186/1471-2334-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Hill CS. New insights into TGF-beta–Smad signalling. Trends Biochem Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Wahl SM. Transforming growth factor beta: the good, the bad, and the ugly. J Exp Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XS, Steere RR, Fedorchuk CA, Pang J, Lee JY, Lim JH, et al. Activation of epidermal grwoth factor receptor is required for NTHi-induced NF-κB-dependent inflammation. PLoS ONE. 2011;6:e28216. doi: 10.1371/journal.pone.0028216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Grabiner B, Zhu YF, Jiang CY, Li HX, You Y, et al. CARMA3 is crucial for EGFR-induced activation of NF-kappaB and tumor progression. Cancer Res. 2011;71:2183–2192. doi: 10.1158/0008-5472.CAN-10-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozyilmaz E, Akan OA, Gulhan M, Ahmed K, Nagatake T. Major bacteria of community-acquired respiratory tract infections in Turkey. Jpn J Infect Dis. 2005;58:50–52. [PubMed] [Google Scholar]
- Wilkinson TM, Hurst JR, Perera WR, Wilks M, Donaldson GC, Wedzicha JA, et al. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;129:317–324. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LN, Dias P, Han D, Yoon S, Shea A, Zakharov V, et al. A mouse model of lethal synergism between influenza virus and Haemophilus influenzae. . Am J Pathol. 2010;176:800–811. doi: 10.2353/ajpath.2010.090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–2055. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- Metcalf D, Mifsud S, Di Rago L, Nicola NA, Hilton DJ, Alexander WS. Polycystic kidneys and chronic inflammatory lesions are the delayed consequences of loss of the suppressor of cytokine signaling-1 (SOCS-1) Proc Natl Acad Sci USA. 2002;99:943–948. doi: 10.1073/pnas.022628499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- Kovalenko A, Chable-Bessia C, Cantarella G, Israël A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G, et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, et al. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoumi R. Ubiquitin chain cleavage: CYLD at work. Trends Biochem Sci. 2010;35:392–399. doi: 10.1016/j.tibs.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Zeng M, Sinha I, Polin L, Wei WZ, Rathinam C, et al. The E3 ligase Itch and deubiquitinase Cyld act together to regulate Tak1 and inflammation. Nat Immunol. 2011;12:1176–1183. doi: 10.1038/ni.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204:1475–1485. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G. Tumor suppressor CYLD: negative regulation of NF-kappaB signaling and more. Cell Mol Life Sci. 2008;65:1123–1132. doi: 10.1007/s00018-007-7465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Chang M, Paul EM, Babu G, Lee AJ, Reiley W, et al. Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. J Clin Invest. 2008;118:1858–1866. doi: 10.1172/JCI34257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Reiley WR, Lee AJ, Wright A, Wu X, Zhang M, et al. Deubiquitinating enzyme CYLD regulates the peripheral development and naive phenotype maintenance of B cells. J Biol Chem. 2007;282:15884–15893. doi: 10.1074/jbc.M609952200. [DOI] [PubMed] [Google Scholar]
- Lee AJ, Zhou X, Chang M, Hunzeker J, Bonneau RH, Zhou D, et al. Regulation of natural killer T-cell development by deubiquitinase CYLD. EMBO J. 2010;29:1600–1612. doi: 10.1038/emboj.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204:1475–1485. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A, Reiley WW, Chang M, Jin W, Lee AJ, Zhang M, et al. Regulation of early wave of germ cell apoptosis and spermatogenesis by deubiquitinating enzyme CYLD. Dev Cell. 2007;13:705–716. doi: 10.1016/j.devcel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Reiley WW, Zhang MY, Jin W, Losiewicz M, Donohue KB, Norbury CC, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7:411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
- Trompouki E, Tsagaratou A, Kosmidis SK, Dolle P, Qian J, Kontoyiannis DL, et al. Truncation of the catalytic domain of the cylindromatosis tumor suppressor impairs lung maturation. Neoplasia. 2009;11:469–476. doi: 10.1593/neo.81424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley WW, Zhang M, Jin W, Losiewicz M, Donohue KB, Norbury CC, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7:411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
- Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Zhang J, Stirling B, Temmerman ST, Ma CA, Fuss IJ, Derry JMJ, et al. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Invest. 2006;116:3042–3049. doi: 10.1172/JCI28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Jono H, Kai H, Li JD. The tumor suppressor cylindromatosis (CYLD) acts as a negative regulator for Toll-like receptor 2 signaling via negative cross-talk with TRAF6 and TRAF7. J Biol Chem. 2005;280:41111–41121. doi: 10.1074/jbc.M509526200. [DOI] [PubMed] [Google Scholar]
- Jono H, Lim JH, Chen LF, Xu H, Trompouki E, Pan ZK, et al. NF-kappaB is essential for induction of CYLD, the negative regulator of NF-kappaB: evidence for a novel inducible autoregulatory feedback pathway. J Biol Chem. 2004;279:36171–36174. doi: 10.1074/jbc.M406638200. [DOI] [PubMed] [Google Scholar]
- Regueiro V, Moranta D, Frank CG, Larrarte E, Margareto J, March C, et al. Klebsiella pneumoniae subverts the activation of inflammatory responses in a NOD1-dependent manner Cell Microbiol01113135–153. [DOI] [PubMed] [Google Scholar]
- Lim JH, Stirling B, Derry J, Koga T, Jono H, Woo CH, et al. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity. 2007;27:349–360 (2007). doi: 10.1016/j.immuni.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- Enesa K, Zakkar M, Chaudhury H, Luong le A, Rawlinson L, Mason JC, et al. NF-kappaB suppression by the deubiquitinating enzyme Cezanne: a novel negative feedback loop in pro-inflammatory signaling. J Biol Chem. 2008;283:7036–7045. doi: 10.1074/jbc.M708690200. [DOI] [PubMed] [Google Scholar]