Abstract
Aim:
Catecholamine-induced vascular smooth muscle cell (VSMC) proliferation is one of the major events in the pathogenesis of atherosclerosis and vascular remodeling. The calcineurin-NFAT pathway plays a role in regulating growth and differentiation in various cell types. We investigated whether the calcineurin-NFAT pathway was involved in the regulation of phenylephrine-induced VSMC proliferation.
Methods:
Proliferation of VSMC was measured using an MTT assay and cell counts. Localization of NFATc1 was detected by immunofluorescence staining. NFATc1-DNA binding was determined by EMSA and luciferase activity analyses. NFATc1 and calcineurin levels were assayed by immunoprecipitation.
Results:
Phenylephrine (PE, an α1-adrenoceptor agonist) increased VSMC proliferation and cell number. Prazosin (an α1-adrenoceptor antagonist), cyclosporin A (CsA, an inhibitor of calcineurin) and chelerythrine (an inhibitor of PKC) decreased PE-induced proliferation and cell number. Additional treatment of VSMC with CsA or chelerythrine further inhibited proliferation and cell number in the chelerythrine-pretreatment group and the CsA-pretreatment group. CsA and chelerythrine alone had no effect on either absorbance or cell number. CsA decreased PE-induced calcineurin levels and activity. NFATc1 was translocated from the cytoplasm to the nucleus upon treatment with PE. This translocation was reversed by CsA. CsA decreased the PE-induced NFATc1 level in the nucleus. PE increased NFAT's DNA binding activity and NFAT-dependent reporter gene expression. CsA blocked these effects.
Conclusion:
CsA partially suppresses PE-induced VSMC proliferation by inhibiting calcineurin activity and NFATc1 nuclear translocation. The calcineurin-NFATc1 pathway is involved in the hyperplastic growth of VSMC induced by phenylephrine.
Keywords: phenylephrine, calcineurin, nuclear factor of activated T cells, proliferation, vascular smooth muscle cells
Introduction
Vascular smooth muscle cells (VSMCs) are able to proliferate and display different phenotypes in response to altered environmental stimuli1. Abnormal proliferation of VSMCs is a key event in the early stages of the arterial wall thickening that occurs during the pathogenesis of atherosclerosis, vascular remodeling and restenosis. During the processes of arteriosclerosis development, vascular injury and vessel response to stress, a number of molecules with a broad spectrum of biological activities are produced, including catecholamines. These bioactive molecules provide a permissive milieu for VSMCs to undergo proliferation. These proliferated VSMCs have lost their ability to contract, having reverted to an embryonic phenotype, and secrete a large amount of extracellular matrix (ECM). Migration of VSMCs from the medium to the intima and proliferation within the intima contribute to the progression of vessel lesions2 3 4. Thus, investigating the molecular mechanisms involved in the proliferation of VSMCs is important for understanding vascular disease and may lead to therapeutic developments.
Catecholamine regulation of VSMCs growth may provide a physiological means by which vessel wall mass can be coupled with the level of sympathetic stimulation and contractile requirements. It may also link pathological vessel wall growth processes to enhanced sympathetic activity. In cultured VSMCs, catecholamines modulate DNA synthesis through specific adrenergic receptors5. Accumulating evidence suggests that the calcineurin-nuclear factor of activated T cells (NFAT) signaling pathway plays a role in regulating growth and differentiation in several cell types and thereby contributes to skeletal muscle differentiation6, cardiac hypertrophy7 and blood vessel development8. Inhibition of NFAT is responsible for cardiac hypertrophy after α1-adrenoceptor stimulation9. Calcineurin-NFAT is implicated in VSMCs proliferation and migration induced by platelet-derived growth factor-BB (PDGF-BB) and thrombin10, 11. However, whether the calcineurin-NFAT pathway is involved in the regulation of catecholamine-induced VSMCs proliferation is unclear.
Materials and methods
Reagents
Phenylephrine (PE, P-6126), prazosin (P-7791), timolol (T-6394), cyclosporin A (CsA, C-3662), chelerythrine (C-2932) and [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) were purchased from Sigma-Aldrich Corp (St Louis, MO, USA). [γ-32P]ATP (3000 Ci/mmol) was obtained from Beijing Fu Rui Biotechnology Company (Beijing, China). Consensus double-stranded NFATc1 binding oligonucleotide, 5′-CGCCCAAAGAGGAAAATTTGTTTCATA-3′, 3′-GCGGGTTTCTCCTTTTAAACAAAGTAT-5′, was synthesized by Invitrogen Corporation. T4 polynucleotide kinase was procured from Promega (Madison, WI). The biotinylated protein ladder detection pack was from Cell Signaling Technology, Inc (Beverly, MA, USA). The calcineurin assay kit was manufactured by the Nanjing Jiancheng Bioengineering Institute (NJBI; Nanjing, China). FuGENE 6 transfection reagent was obtained from Roche Applied Science. Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco Life Technologies (Grand Island, NY, USA). The primary antibodies used in Western blotting, mouse anti-NFATc1 monoclonal antibody (sc-7294), rabbit anti-β actin polyclonal antibody (sc-1616) and goat anti-calcineurin Aα affinity-purified polyclonal antibody (sc-6123) were all purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA).
Cell culture
Primary VSMCs were isolated from the medium of thoracic aortas of 100- to 150-g male Sprague-Dawley rats. The purity of the VSMCs was verified by immunostaining with a monoclonal antibody against smooth muscle-specific α-actin at passage 3. Cells of 95% purity were used. Cells of passage 3–4 were growth arrested by incubation in DMEM containing 0.1% fetal bovine serum for 72 h, synchronized at the G0 phase and then used to perform the experiments.
Cell proliferation assay
After VSMCs were incubated at 37 °C for 48 h, stock [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) solution was added and incubated with the cells for 4 h. The medium was then completely removed without disturbing the formazan crystals that had formed within the cells. After Me2SO (Merck, Darmstadt, Germany) was added to the wells, the plate was shaken for a short time, and optical density was measured at 570 nm. In parallel experiments, growth-arrested VSMCs were treated with the various combinations of chemicals described below for 48 h. Cell numbers were determined by the Trypan Blue dye exclusion assay using a hemocytometer.
Calcineurin enzymatic activity
The activity of calcineurin was determined using a calcineurin activity assay kit following the manufacturer's protocol. The RII phosphopeptide (BioMol) was used as a highly specific substrate for calcineurin. The detection of free inorganic phosphate released from RII by calcineurin was based on the malachite green dye reaction. Reactions were terminated after 30 min, and absorption was read on an ultraviolet spectroscope at 660 nm. The activity was corrected for protein concentration. Calcineurin activity was expressed as a percentage compared with the control group.
Immunofluorescence staining
VSMCs were fixed in 4.0% formaldehyde for 15 min at room temperature and washed in PBS three times for 5 min. Immunocytochemical staining for NFATc1 was performed using indirect immunofluorescence. Cells were incubated with the anti-NFATc1 monoclonal antibody at a dilution of 1:100. Signals of NFATc1 were detected using the anti-mouse FITC-conjugated secondary antibody (ICN Biomedicals) at a dilution of 1:100 for 45 min. After being washed in PBS three times for 5 min each, cells were examined under a fluorescence microscope and photographed.
Nuclear extraction
For nuclear extraction, cells were washed with PBS, centrifuged at 250×g for 5 min, and resuspended in 5 packed cell volumes (PCV) of hypotonic buffer [buffer A: 10 mmol/L HEPES-KOH (pH 7.9), 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.2 mmol/L PMSF, 0.5 mmol/L DTT], followed by centrifugation at 1850×g for 5 min. The supernatant was discarded and the pellets were resuspended in 3 PCV of hypotonic buffer at 4 °C for 10 min. Swollen cells were homogenized 15 times with a glass homogenizer and centrifuged at 6000×g for 15 min. The pellet containing the nuclei was resuspended for 30 min at 4 °C in one PCV of high salt buffer [buffer B: 20 mmol/L HEPES-KOH (pH 7.9), 25 % glycerol, 1.5 mmol/L MgCl2, 420 mmol/L NaCl, 0.2 mmol/L EDTA, 0.2 mmol/L PMSF, 0.5 mmol/L DTT] and then centrifuged at 14 000×g for 30 min. The supernatant corresponded to the nuclear extract and was stored at −80 °C. Nucleic protein concentration was determined using a BCA kit.
Electrophoretic mobility shift assay
Quiescent VSMCs were treated with or without PE (10 μmol/L) in the presence or absence of CsA (0.5 μg/mL) for 48 h, and nuclear extracts were prepared as described above. Protein-DNA complexes were formed by incubating 10 μg of nuclear protein in a total volume of 20 μL consisting of 20 mmol/L HEPES-KOH (pH 7.8), 75 mmol/L KCl, 2.5 mmol/L MgCl2, 1 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L dithiothreitol, 4.5 μg of bovine serum albumin, 2 μg of poly(dI-dC), 10% glycerol, and 100 000 cpm of [γ-32P]ATP labeled double-stranded NFAT-binding oligonucleotide probe for 30 min on ice. Protein-DNA complexes were resolved on a 6% polyacrylamide gel using 1×Tris glycine-EDTA buffer [25 mmol/L Tris-HCl (pH 8.5), 200 mmol/L glycine, 0.1 mmol/L EDTA]. Double-stranded oligonucleotides (NFATc1, 5′-CGCCCAAAGAGGAAAATTTGTTTCATA-3′, 3′-GCGGGTTTCTCCTTTTAAACAAAGTAT-5′) were labeled with [γ-32P]ATP using the T4 polynucleotide kinase kit according to the manufacturer's protocol (Promega).
Luciferase assay
VSMCs were plated evenly on 35 mm dishes and grown in DMEM supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 units/mL penicillin and 100 μg/mL streptomycin. At 50%−80% confluence, the medium was replaced with DMEM containing 0.1% fetal bovine serum and cells were transfected with the pT8NFAT-Luc plasmid using the Geneporter transfection reagent according to the manufacturer's instructions (Roche Applied Science). Luciferase was controlled by an interleukin-2 (IL-2) promoter containing three tandem NFAT-activator protein-1 enhancer sequences. Approximately 1 μg of DNA was transfected into 2×105 cells in 30 μL RPMI with 6 μL Geneporter and 130 μL unsupplemented RPMI. After 48 h of transfection, VSMCs were treated with or without PE (10 μmol/L) in the presence or absence of CsA (0.5 μg/mL) for 48 h. Cells were then lysed in 0.5% Nonidet P-40 lysis buffer, and luciferase activity was measured using the Luciferase Assay System (Promega) and a Turner Luminometer (TD-20/20).
Western blotting analysis
The cells were washed twice with phosphate-buffered saline (PBS) before being lysed in RIPA buffer [1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L PMSF, 2 mg/L leupeptin] for 30 min. Insoluble debris was removed by centrifugation at 14 000×g at 4 °C for 20 min and the supernatant (cytoplasmic extract) was stored at −80 °C. Protein concentration was determined using a microassay kit from Bio-Rad with bovine serum albumin as the standard. Samples containing an equal amount of protein (40 μg) were mixed with 2×SDS loading buffer and electrophoresed on a 10% SDS-PAGE gel. The proteins on the gel were then transferred onto a nitrocellulose membrane (Hybond, Amersham Biosciences). The membranes were incubated with specific primary antibodies overnight at 4 °C at a 1:200 dilution. The membranes were then washed three times with PBST and incubated for 2 h with HRP-conjugated secondary antibodies. The protein was visualized with chemiluminescence, and a densitometric scanner was used to determine the density of the band. All experiments were repeated at least five times independently.
Statistics
The results are expressed as means±SD. All data were analyzed with SPSS 11.5. ANOVA, post-hoc analysis and the Newman-Keuls test were used to compare differences among groups. P-values less than 0.05 were considered statistically significant.
Results
PE stimulated VSMC proliferation via α1 adreno-ceptors
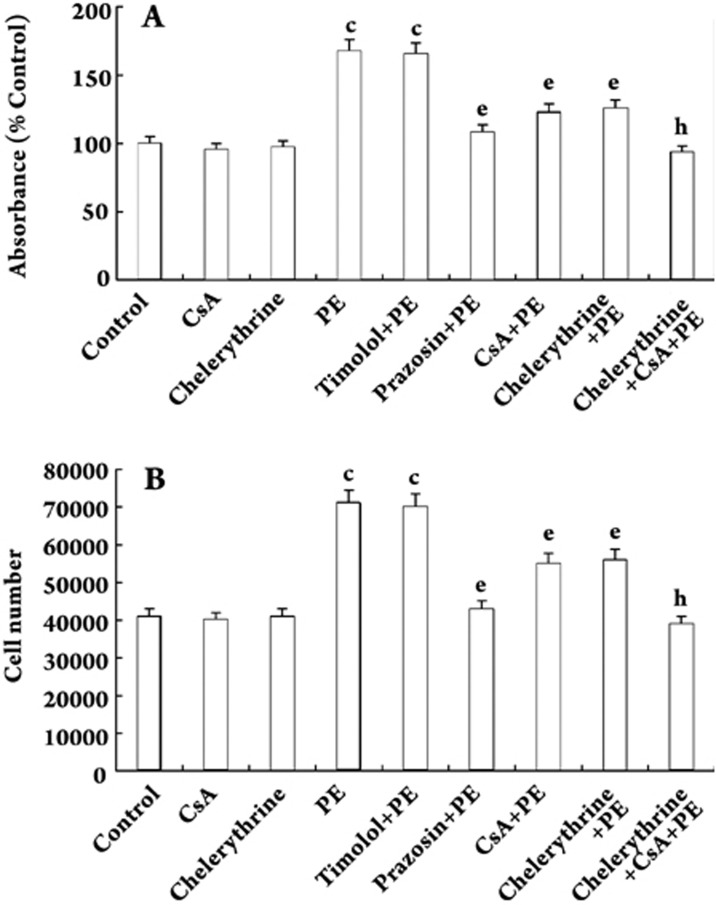
Quiescent (growth-arrested) VSMCs were incubated in the absence (control) or presence of PE (10 μmol/L), prazosin (10 μmol/L), timolol (1 mg/mL), CsA (0.5 μg/mL) or chelerythrine (20 μmol/L), or various combinations for 48 h. Optical density was then measured. PE increased the absorbance of VSMCs by 67.6% compared with the control group (P<0.01). Prazosin, CsA and chelerythrine decreased PE-induced proliferation of VSMCs by 35.4%, 26.7%, and 25.2%, respectively. Additional treatment with CsA or chelerythrine further inhibited VSMCs proliferation by 25.4% or 23.8% compared with the chelerythrine-pretreatment group or the CsA-pretreatment group (all P<0.05, Figure 1A). To confirm these findings, cell number was measured. PE increased VSMCs number by 73.2% compared with the control group (P<0.01). Prazosin, CsA and chelerythrine decreased PE-induced VSMCs numbers by 39.4%, 22.5%, and 21.1%, respectively. Additional treatment with CsA or chelerythrine further decreased VSMCs numbers by 30.4% or 29.1% compared with the chelerythrine-pretreatment or CsA-pretreatment group (all P<0.05, Figure 1B). Timolol did not alter the increased absorbance or cell number induced by PE. CsA and chelerythrine alone had no significant effect on either absorbance or cell number.
Figure 1.
PE-induced proliferation in VSMCs. Quiescent (growth arrested) VSMCs were stimulated in the absence (control) or presence of PE (10 μmol/L), prazosin (10 μmol/L), timolol (1 mg/mL), CsA (0.5 μg/mL) and chelerythrine (20 μmol/L), or various combinations of these for 48 h. Optical density and cell numbers were measured. n=5 experiments. Mean±SD. cP<0.01 vs control; eP<0.05 vs PE; hP<0.05 vs chelerythrine+PE group or CsA+PE group.
Calcineurin was activated in response to PE
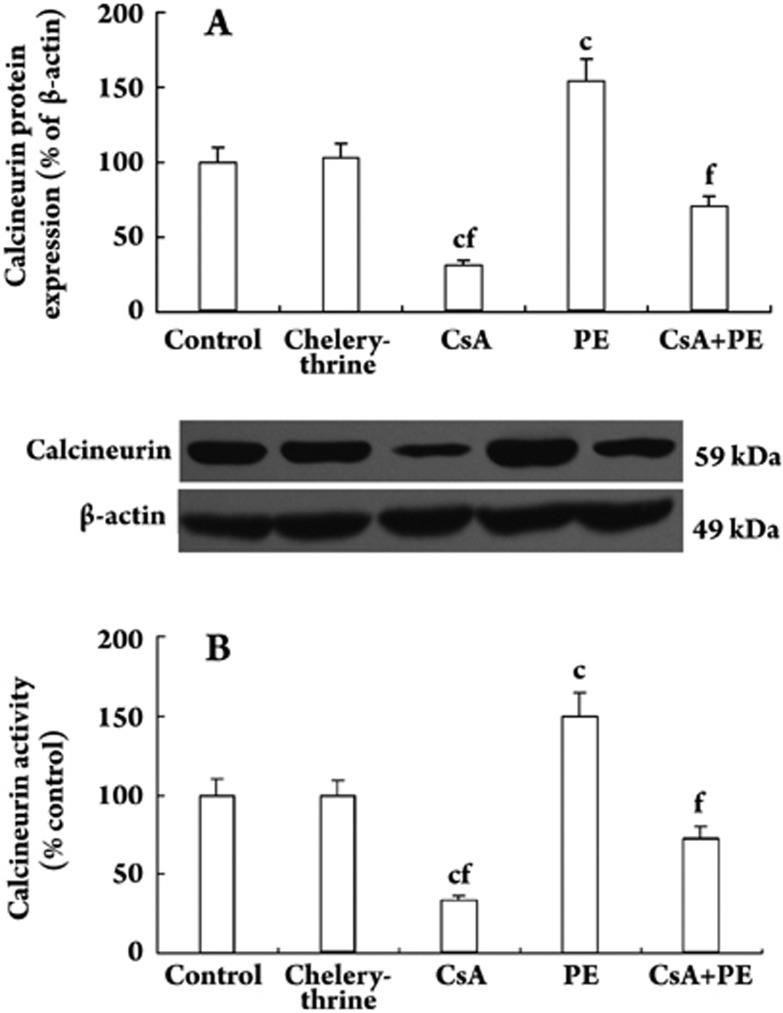
Quiescent VSMCs were treated with or without PE (10 μmol/L) in the presence or absence of CsA (0.5 μg/mL) and chelerythrine (20 μmol/L) for 48 h. PE increased calcineurin levels and activity significantly, whereas CsA decreased calcineurin levels and activity. CsA decreased PE-induced calcineurin levels and activity by 54.2% (Figure 2A) and 51.6% (Figure 2B, all P<0.01), respectively. Chelerythrine had no effect on calcineurin level or activity. The relative expression levels of the protein were normalized with β-actin.
Figure 2.
Effect of PE and CsA on calcineurin protein level and activity in VSMCs. Quiescent VSMCs were treated with or without PE (10 μmol/L) in the presence or absence of CsA (0.5 μg/mL) and chelerythrine (20 μmol/L) for 48 h. Calcineurin protein expression and activity were measured by Western blot and a calcineurin assay kit. The relative expression level in control was set at 1.0. n=5 experiments. Mean±SD. cP<0.01 vs control; fP<0.01 vs PE group.
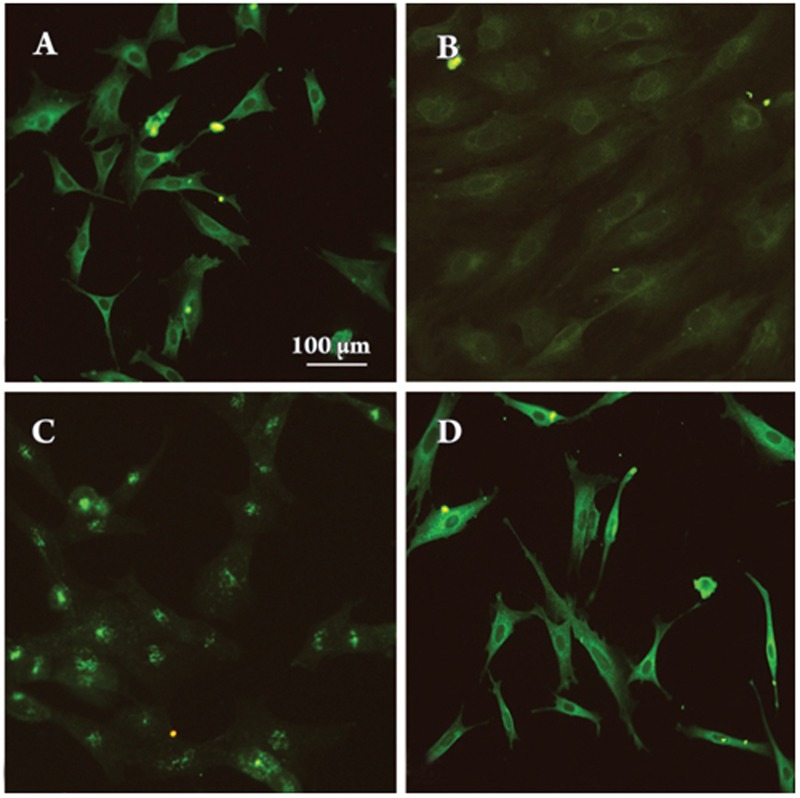
PE induced NFATc1 translocation into the nucleus
When cells were growth-arrested, NFATc1 was scattered inside the cytoplasm and absent from the nucleus (Figure 3A). After treatment with PE (10 μmol/L) for 9 h, NFATc1 was found near the nucleus (Figure 3B); after treatment for 24 h, NFATc1 was found almost exclusively within the nucleus (Figure 3C). This translocation was reversed by CsA (0.5 μg/mL) (Figure 3D), which blocks calcineurin.
Figure 3.
Subcellular localization of NFATc1. Quiescent VSMCs (A, control) were treated with PE (10 μmol/L) for 9 h (B), 24 h (C) or after pretreatment with PE for 24 h and then incubated with CsA (0.5 μg/mL) for another 24 h (D). All cells at the various times were subjected to immunofluorescence staining using an anti-NFATc1 monoclonal antibody (sc-7294).
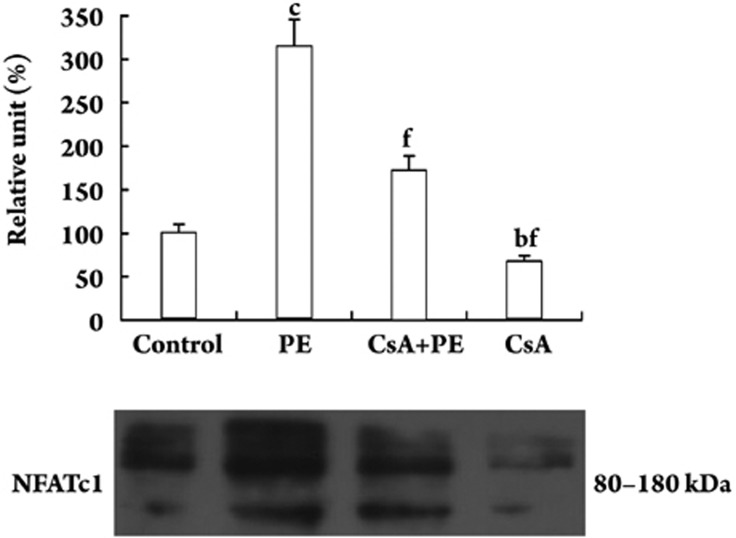
PE increased the level of NFATc1 in the nucleus
Quiescent VSMCs were treated with or without PE (10 μmol/L) in the presence or absence of CsA (0.5 μg/mL) for 48 h and nuclear extracts were prepared. A total of 20 μg of nucleic protein from each treatment group was analyzed by Western blotting for NFATc1 levels. The NFATc1 level was much higher in the PE-treated VSMCs compared with the control group. CsA decreased PE-induced NFATc1 expression by 45.4% (P<0.01, Figure 4).
Figure 4.
Nuclear protein expression of NFATc1 in VSMCs. Quiescent VSMCs were treated with or without PE (10 μmol/L) in the presence or absence of CsA (0.5 μg/mL) for 48 h and nuclear extracts were prepared. Nucleic protein samples (20 μg) were subjected to Western blotting using the anti-NFATc1 antibody as indicated. n=5 experiments. Mean±SD. bP<0.05, cP<0.01 vs control; fP<0.01 vs PE.
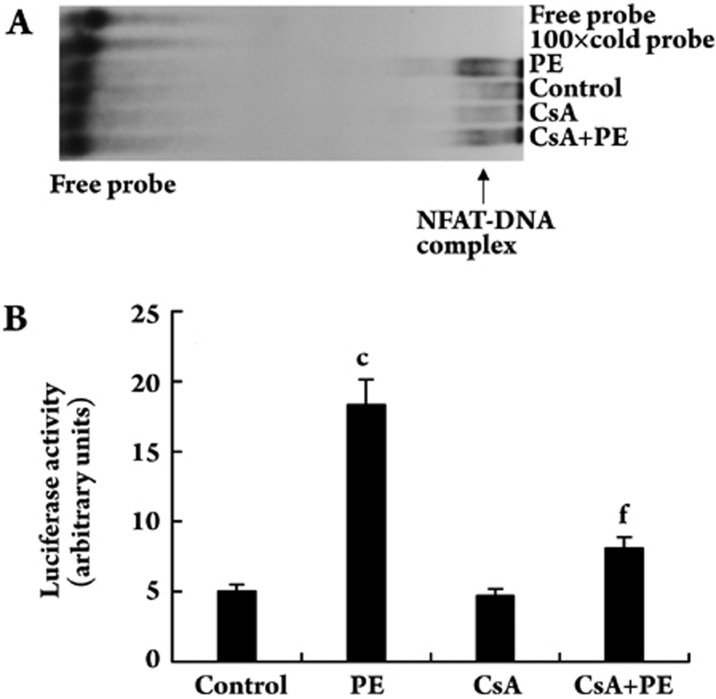
PE up-regulates NFAT-DNA binding activity in VSMCs
Quiescent VSMCs were treated with or without PE (10 μmol/L) in the presence or absence of CsA (0.5 μg/mL) for 48 h and nuclear extracts were prepared. Nuclear extracts containing 10 μg of protein from the control group and each treatment group were incubated with 100 000 cpm of [γ-32P]ATP-labeled NFAT sequence and the protein-DNA complexes were separated by electrophoresis on 6% polyacrylamide gels. Luciferase activity was measured using the Luciferase Assay System (Promega) and a Turner Luminometer (TD-20/20). PE increased NFAT-DNA binding activity compared with the control. CsA significantly blocked the NFAT-DNA binding activity induced by PE (Figure 5A). Consistent with the NFAT-DNA binding activity, NFAT-dependent luciferase activity was also increased by about three-fold in PE-treated VSMCs compared with the control. CsA blocked the PE-induced increase in luciferase activity by 55.7% (P<0.01). CsA alone had no effect on basal luciferase activity (Figure 5B).
Figure 5.
Effect of PE and CsA on NFATc1-DNA binding activity in VSMCs. (A) Quiescent VSMCs were treated with or without PE (10 μmol/L) in the presence or absence of CsA (0.5 μg/mL) for 48 h and nuclear extracts were prepared. Nuclear extracts containing an equal amount of protein from the control and each treatment group were analyzed for NFAT-DNA binding activity using a [γ-32P]ATP-labeled sequence double-stranded NFAT-binding oligonucleotide probe. (B) Quiescent VSMCs were transfected with pT8NFAT-Luc plasmid DNA for 48 h, and then treated with or without PE (10 μmol/L) in the presence or absence of CsA (0.5 μg/mL) for 48 h. Cell extracts containing equal amounts of protein from the control and each treatment group were assayed for luciferase activity. cP<0.01 vs control; fP<0.01 vs PE.
Discussion
Catecholamines aggravate atherosclerosis in animals and humans and may be involved in cell growth12. The G0 or G1 phase of the cell cycle may be the point of catecholamine action. Catecholamines stimulate VSMCs proliferation via the activation of α1-adrenoceptors, which trigger phosphoinositide hydrolysis and activate the protein kinase C (PKC)/MAPK pathway, leading to phosphorylation of transcription factors and leads to DNA synthesis and cell proliferation. Fetal calf serum-induced VSMCs proliferation is enhanced by α-adrenergic and inhibited by β-adrenergic stimulation. α1-adrenoceptor blockade reduces rabbit aortic intimal hyperplasia induced by balloon angioplasty13. Phenylephrine (PE), a specific α1-adrenoceptor agonist, stimulates Ca2+ oscillations and cell growth14, 15. Infusion of PE induces cardiac hypertrophy and fibrosis in rat hearts16. On the other hand, prazosin, a selective α1-adrenoceptor antagonist, reduces intimal hyperplasia in rabbit abdominal aortas17 and reduces VSMCs DNA synthesis in the medium of rat aortas stimulated by angiotensin II18. In our study, PE increased VSMCs proliferation (as measured by MTT) and cell counts. Prazosin completely suppressed the increases in absorbance and cell number induced by PE, whereas timolol had no marked effect on VSMCs proliferation. CsA and chelerythrine (a competitive inhibitor of PKC) inhibited this PE-induced VSMCs growth. The inhibitory effect of chelerythrine could be enhanced by CsA. PE increased but CsA decreased calcineurin levels and activity in VSMCs. CsA inhibited PE-induced calcineurin expression and activity. Therefore, we conclude that PE stimulates VSMCs proliferation via α1-adrenoceptors. Calcineurin is involved in PE-induced VSMCs proliferation. CsA partially inhibits PE-induced proliferation of VSMCs by regulating calcineurin expression and its activity.
Calcineurin (PP2B) is a calcium/calmodulin-activated, serine-threonine phosphatase and is a downstream target of intracellular Ca2+ signaling19. It is a multifunctional regulator of various downstream signaling pathways20. One of the downstream effectors is NFAT. Calcineurin is activated by the binding of calcium and calmodulin and transmits signals to the nucleus through the dephosphorylation and translocation of NFAT. NFAT, which is functionally related to the Rel/NF-κB family of transcriptional activators, is a family of transcription factors composed of five proteins: NFAT1 (NFATp, NFATc2), NFAT2 (NFATc, NFATc1), NFAT3 (NFATc4), NFAT4 (NFATx, NFATc3) and NFAT5 [TonEBP: tonicity element binding protein or OREBP (osmotic response element binding protein)]21, 22. NFAT5 differs from the others in its lack of cooperativity with the Fos and Jun proteins and is involved in the regulation of tonicity-responsive genes23. All isoforms of NFAT are highly homologous in their N-terminal region, which corresponds to the regulatory element. Their C-terminal region includes the DNA binding domain. Several specific sequences are located in the N-terminal domain, including the calcineurin fixation site, the nuclear localization signal (NLS), the nuclear exportation site (NES) and numerous serine/threonine phosphorylation sites. NFAT has been described as a signal integrator, interlinking Ca2+ signaling and other signaling pathways and inducing specific genetic programs. The vasoconstrictive effect of PE was associated with CsA-dependent calcineurin activation and nuclear translocation of NFAT in pulmonary artery smooth muscle24. The calcineurin-NFAT signaling pathway plays a role in regulating growth, differentiation and cell cycle progression in various cell types25, 26. In cultured VSMCs, NFAT activation increased migration and proliferation27. Blockade of NFAT signaling reduces neointima formation in a rat carotid artery injury model28, 29. NFAT may also play a role in smooth muscle differentiation via regulation of smooth muscle-specific markers, such as smooth muscle-myosin heavy chain (SM-MHC) and α-actin30, 31. The immunosuppressant CsA can bind to calcineurin as a stable complex with cyclophilin and inhibit calcineurin's ability to dephosphorylate NFAT. CsA blunts platelet-derived growth factor-BB (PDGF-BB) and thrombin-induced VSMCs proliferation 32.
NFAT activation is regulated primarily through control of its subcellular localization33. In unstimulated cells, NFAT is a hyperphosphorylated cytosolic protein. Activated calcineurin dephosphorylates multiple serine residues within the regulatory region of NFAT, inducing a conformational change in NFAT that exposes the NLS and allows the importation of NFAT into the nucleus34. Calcineurin also plays a role in promoting the nuclear retention of NFAT by masking nuclear export signals recognized by the exporting protein, Crm-1, and by maintaining NFAT in a dephosphorylated state. In smooth muscle cells, NFATc1 is the most expressed isoform35, and although there is a small amount of NFATc3, NFATc2 and NFATc4 are not detectable by Western blot. NFATc1 but not NFATc2 or NFATc3 is translocated from the cytoplasm to the nucleus upon treatment of VSMCs with PDGF-BB or thrombin10. To test the role of NFATc1 in PE-induced proliferation, we looked for and detected subcellular localization of NFATc1 by indirect immunofluorescence staining. As shown in Figure 3, NFATc1 was widely scattered in the cytoplasm in quiescent cells, but after treatment with PE for 9 h it was found close to and around the nucleus. After 24 h NFATc1 was found almost exclusively within the nucleus. This translocation was reversed by CsA. The calcineurin inhibitor prevented NFATc1 nuclear translocation. Consistent with this effect, NFATc1 levels were also higher in the nuclear fractions of VSMCs treated with PE than in the control group. CsA decreased the enhanced protein level of NFATc1 previously induced by PE in the nucleus (Figure 4). These findings suggest that NFATc1 is translocated from the cytoplasm to the nucleus in response to PE. A calcineurin inhibitor prevents this PE-induced NFATc1 nuclear translocation.
In the nucleus, activated NFAT binds to a consensus DNA sequence (GGAAAAT) in the promoter regions of genes via its Rel homology region (RHR) as a monomer or dimer and then activates transcription. To test the role of calcineurin-NFATc1 signaling in regulating PE-induced proliferation of VSMCs, we measured its activity in response to PE. As shown in Figure 5A, PE increased the NFAT DNA binding activity compared with the control. CsA significantly attenuated PE-induced NFAT DNA binding activity. To determine whether the increased NFAT DNA binding activity led to corresponding increases in NFAT-dependent transcription, VSMCs were transfected with a plasmid (pT8NFAT-Luc) in which the expression of a luciferase gene is controlled by three tandem NFAT-activator protein-1 enhancer sequences present in the IL-2 promoter. Consistent with the increased NFAT DNA binding activity, luciferase activity was also increased by about 3-fold in PE-treated VSMCs compared with the control group. CsA completely blocked this PE-induced increase in luciferase activity (Figure 5B). CsA alone had no effect on basal luciferase activity. One mechanism for the up-regulated activity of NFATc1-DNA binding may be an increase in the quantity of NFATc1 itself in the nucleus.
In conclusion, our study demonstrates that calcineurin activation is required for the hyperplastic growth of VSMCs induced by PE. CsA partially suppresses PE-induced VSMCs proliferation by inhibiting calcineurin activity and NFATc1 nuclear translocation. The calcineurin-NFATc1 pathway is therefore involved in the phenylephrine-induced proliferation of VSMCs.
Author contribution
Prof Ning-ling SUN designed the research; Dr Xiao PANG performed the research and wrote the paper.
Acknowledgments
We would like to thank Prof Chun-yan ZHOU and Ms Yi-nan LIU for their help with the experiments. We also thank Prof Chao-shu TANG for his kind help in preparing the manuscript.
References
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Kalmes A, Vesti BR, Daum G, Abraham JA, Clowes AW. Heparin blockade of thrombin-induced smooth muscle cell migration involves inhibition of epidermal growth factor (EGF) receptor transactivation by heparin-binding EGF-like growth factor. Circ Res. 2000;87:92–8. doi: 10.1161/01.res.87.2.92. [DOI] [PubMed] [Google Scholar]
- Duan C, Bauchat JR, Hsieh T. Phosphatidylinositol 3-kinase is required for insulin-like growth factor-I-induced vascular smooth muscle cell proliferation and migration. Circ Res. 2000;86:15–23. doi: 10.1161/01.res.86.1.15. [DOI] [PubMed] [Google Scholar]
- Chai YC, Howe PH, Dicorleto PE, Chisolm GM. Oxidized low density lipoprotein and lysophosphatidylcholine stimulate cell cycle entry in vascular smooth muscle cells. Evidence for release of fibroblast growth factor-2. J Biol Chem. 1996;271:17791–7. doi: 10.1074/jbc.271.30.17791. [DOI] [PubMed] [Google Scholar]
- Nakaki T, Nakayama M, Yamamoto S, Kato R. Alpha 1-adrenergic stimulation and beta 2-adrenergic inhibition of DNA synthesis in vascular smooth muscle cells. Mol Pharmacol. 1990;37:30–6. [PubMed] [Google Scholar]
- Delling U, Tureckova J, Lim HW, De Windt LJ, Rotwein P, Molkentin JD. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol. 2000;20:6600–11. doi: 10.1128/mcb.20.17.6600-6611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–75. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci USA. 2002;99:11363–8. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellaturu CR, Ghosh SK, Rao RK, Jennings LK, Hassid A, Rao GN. A potential role for nuclear factor of activated T-cells in receptor tyrosine kinase and G-protein-coupled receptor agonist-induced cell proliferation. Biochem J. 2002;368:183–90. doi: 10.1042/BJ20020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Kukreja RS, Datta BN, Chakra Varti RN. Catecholamine-induced aggravation of aortic and coronary atherosclerosis in monkey. Atherosclerosis. 1981;40:291–8. doi: 10.1016/0021-9150(81)90139-8. [DOI] [PubMed] [Google Scholar]
- O'Malley MK, McDermott EWM, Mehigan D, O'Higgins NJ. Role of prazosin in reducing the development of rabbit intimal hyperplasia after endothelial denudation. Br J Surg. 1989;76:936–8. doi: 10.1002/bjs.1800760921. [DOI] [PubMed] [Google Scholar]
- Hamada H, Damron DS, Hong SJ, Van Wagoner DR, Murray PA. Phenylephrine-induced Ca2+ oscillations in canine pulmonary artery smooth muscle cells. Circ Res. 1997;81:812–23. doi: 10.1161/01.res.81.5.812. [DOI] [PubMed] [Google Scholar]
- Shaw L, O'Neill S, Jones CJ, Austin C, Taggart MJ. Comparison of U46619-, endothelin-1- or phenylephrine-induced changes in cellular Ca2+ profiles and Ca2+ sensitisation of constriction of pressurised rat resistance arteries. Br J Pharmacol. 2004;141:678–88. doi: 10.1038/sj.bjp.0705647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farivar RS, Crawford DC, Chobanian AV, Brecher P. Effect of angiotensin II blockade on the fibroproliferative response to phenylephrine in the rat heart. Hypertension. 1995;25:809–13. doi: 10.1161/01.hyp.25.4.809. [DOI] [PubMed] [Google Scholar]
- Chobanian AV. Can antihypertensive drugs reduce atherosclerosis and its clinical complications. Am J Hypertens. 1994;7:119s–125s. doi: 10.1093/ajh/7.10.119s. [DOI] [PubMed] [Google Scholar]
- van Kleef EM, Smits JFM, De Mcy JGR, Cleutjens JPM, Lombardi DM. α1-Adrenoreceptor blockade reduces the angiotensinII-induced vascular smooth muscle cell DNA synthesis in the rat thoracic aorta and carotid artery. Circ Res. 1992;70:1122–7. doi: 10.1161/01.res.70.6.1122. [DOI] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Shibasaki F, Hallin U, Uchino H. Calcineurin as a multifunctional regulator. J Biochem (Tokyo) 2002;131:1–15. doi: 10.1093/oxfordjournals.jbchem.a003063. [DOI] [PubMed] [Google Scholar]
- Macian F, Rodriguez CL, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–89. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- Feske S, Okamura H, Hogan PG, Rao A. Ca2+/calcineurin signalling in cells of the immune system. Biochem Biophys Res Commun. 2003;311:1117–32. doi: 10.1016/j.bbrc.2003.09.174. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez C, Antos CL, Shelton JM, Richardson JA, Lin F, Novobrantseva TI, et al. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci USA. 2004;101:2392–7. doi: 10.1073/pnas.0308703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghi A, Sims SM. Constrictor-induced translocation of NFAT3 in human and rat pulmonary artery smooth muscle. Am J Physiol. 2005;289:L1061–L1074. doi: 10.1152/ajplung.00096.2005. [DOI] [PubMed] [Google Scholar]
- Lipskaia L, Lompre AM. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol Cell. 2004;96:55–68. doi: 10.1016/j.biolcel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Viola JP, Carvalho LD, Fonseca BP, Teixeira LK. NFAT transcription factors: from cell cycle to tumor development. Braz J Med Biol Res. 2005;38:335–44. doi: 10.1590/s0100-879x2005000300003. [DOI] [PubMed] [Google Scholar]
- Liu Z, Dronadula N, Rao GN. A novel role for nuclear factor of activated T cells in receptor tyrosine kinase and G protein-coupled receptor agonist induced vascular smooth muscle cell motility. J Biol Chem. 2004;279:41218–26. doi: 10.1074/jbc.M406917200. [DOI] [PubMed] [Google Scholar]
- Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, et al. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97:488–95. doi: 10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang C, Dronadula N, Li Q, Rao GN. Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury induced neointima formation in a rat carotid artery model. J Biol Chem. 2005;280:14700–08. doi: 10.1074/jbc.M500322200. [DOI] [PubMed] [Google Scholar]
- Gonzalez Bosc LV, Layne JJ, Nelson MT, Hill-Eubanks DC. Nuclear factor of activated T cells and serum response factor cooperatively regulate the activity of an α-actin intronic enhancer. J Biol Chem. 2005;280:26113–20. doi: 10.1074/jbc.M411972200. [DOI] [PubMed] [Google Scholar]
- Wada H, Hasegawa K, Morimoto T, Kakita T, Yanazume T, Abe M, et al. Calcineurin- GATA-6 pathway is involved in smooth muscle-specific transcription. J Cell Biol. 2002;156:983–91. doi: 10.1083/jcb.200106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Sliedregt-Bol K, Overkleeft H, van der Marel GA, van Berkel TJ, Biessen EA. Therapeutic potential of a synthetic peptide inhibitor of nuclear factor of activated T cells as antirestenotic agent arterioscler. Thromb Vasc Biol. 2006;26:1531–7. doi: 10.1161/01.ATV.0000225286.30710.af. [DOI] [PubMed] [Google Scholar]
- Zhu J, McKeon F. Nucleocytoplasmic shuttling and the control of NF-AT signaling. Cell Mol Life Sci. 2000;57:411–20. doi: 10.1007/PL00000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H, Aramburu J, Garcia-Rodriguez C, Viola JP, Raghavan A, Tahiliani M, et al. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell. 2000;6:539–50. doi: 10.1016/s1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Boss V, Abbott KL, Wang XF, Pavlath GK, Murphy TJ. The cyclosporin A-sensitive nuclear factor of activated T cells (NFAT) proteins are expressed in vascular smooth muscle cells. Differential localization of NFAT isoforms and induction of NFAT-mediated transcription by phospholipase C-coupled cell surface receptors. J Biol Chem. 1998;273:19664–71. doi: 10.1074/jbc.273.31.19664. [DOI] [PubMed] [Google Scholar]