Abstract
Aim:
To investigate the immunosuppressive effect of gossypol in mice both in vitro and in vivo.
Methods:
The in vitro effect of gossypol on the proliferation of lymphocytes isolated from lymph nodes of BALB/c mice was determined by CFSE staining and by an MTS assay. Lymphocyte activation and lymphoblastic transformation were evaluated with immunostaining. Cell apoptosis was detected by Annexin-V and Hoechst 33342 staining. The in vivo immunosuppressive effect of gossypol on the DTH reaction was evaluated using a mouse DTH model induced by 2,4-dinitro-1-fluorobenzene (DNFB). The thickness of the ears was measured, and the histological changes of the mouse auricles were observed after hematoxylin-eosin staining. The proliferation capacity of lymphocytes from DTH mice was also assayed.
Results:
In vitro, gossypol could significantly inhibit the proliferation of mouse lymphocytes stimulated with phorbol ester plus ionomycin in a dose-dependent manner. Although the expression of the early activation antigen CD69 was not affected, the lymphoblastic transformation of both T and B lymphocyte subsets was significantly suppressed by gossypol. Moreover, gossypol could induce apoptosis of lymphocytes, and the effect was time- and dose-dependent. In vivo, the DTH reaction in mice was markedly alleviated by gossypol injected intraperitoneally. Lymphocytes from drug-treated DTH mice had a reduced proliferation capacity as compared with lymphocytes from untreated DTH mice. Gossypol treatment also markedly reduced the number of infiltrated lymphocytes in the auricles of DTH mice.
Conclusion
Gossypol exhibited immunosuppressive effects in mice, probably by inhibition of lymphocyte proliferation and by induction of cell apoptosis.
Keywords: gossypol, lymphocytes, cell proliferation, apoptosis, delayed-type hypersensitivity
Introduction
Gossypol, a yellow polyphenolic compound originally extracted from cottonseed, possesses antifertility activity and has been used as a male contraceptive drug for many years1. In recent years, gossypol has also been reported to exhibit a variety of other biological activities, including anti-tumor, anti-bacterial, anti-oxidant, and anti-inflammatory activities, as well as antiviral activity against a number of enveloped viruses such as the human immunodeficiency virus (HIV)2, 3. It has also been found to be a protein kinase C (PKC) inhibitor, and it suppresses the T cell activation in vitro normally seen in response to polyclonal activators4. As expression of NF-κB−regulated genes plays important roles in oncogenesis and inflammatory responses5, 6, gossypol is presumed to exhibit anti-tumor activity through the suppression of NF-κB activity and NF-κB−related gene expression7. Considering the previously reported anti-inflammatory activity of gossypol8, 9, this reagent has been suggested to be a potential drug for the treatment of psoriasis10. Further investigation of the effects of gossypol on lymphocytes is still required to better understand its targets at the cellular and molecular levels. The aim of this study is to investigate the immunosuppressive effects of gossypol both in vitro and in vivo in mice. Our results indicate that gossypol exhibited an immunosuppressive effect in mice, probably by inhibition of lymphocyte proliferation and by induction of cell apoptosis.
Materials and methods
Animals and reagents
Female BALB/c mice, 6–8 weeks of age, were supplied by the Experimental Animal Center of Southern Medical University (Guangzhou, China). Gossypol (98% purity), Hoechst 33342, phorbol 12,13-dibutyrate (PDB), ionomycin (Ion) and dimethyl sulfoxide (DMSO) were purchased from Sigma (USA). RMPI-1640 and fetal bovine serum (FBS) were obtained from GibcoBRL (USA). Carboxyfluorescein diacetate succinimidyl ester (CFSE) was a product of Molecular Probes (USA). 2,4-Dinitro-1-fluorobenzene (DNFB) was obtained from Kasei Kogyo (Japan). A CellTiter96 Aqueous One Solution Cell Proliferation Assay kit (MTS) was purchased from Promega (USA). Phycoerythrin (PE)-conjugated Annexin V (Annexin V-PE) and 7-amino-actinomycin D (7-ADD) were obtained from Becton Dickinson (USA). Fluorescent labeled monoclonal antibodies against CD4 (FITC), CD8 (APC), CD19 (PerCP-Cy5.5), and CD69 (PE) were purchased from eBioscience (USA).
Isolation of lymphocytes and cell culture
BALB/c mice were sacrificed and the lymph nodes were isolated (2–3 mice were sacrificed in each experiment). A single-cell suspension was prepared by passing the tissue through a 200-μm nylon mesh screen. The cells were washed twice with PBS, counted and resuspended in RPMI-1640 medium containing 10% FBS (complete medium). Lymphocytes at a density of 2×106/mL were added to a 96-well plate (200 μL/well) and incubated at 37 °C in a humidified atmosphere of 5% CO2. Cells were stimulated with PDB (1×10−7 mol/L) plus Ion (0.5 μg/mL) in the presence or absence of different concentrations of gossypol. Gossypol was dissolved in DMSO (the final concentration of DMSO was less than 0.025%).
Flow cytometry analysis
The lymphocytes were collected after 24 h of PDB plus Ion stimulation and were labeled with fluorescent antibodies against CD4, CD8, CD19, and CD69 at 4 °C for 20 min. The cells were then washed with PBS, fixed with 4% paraformaldehyde in PBS, and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, USA).
CFSE labeling assay
CFSE-labeling was performed as described previously11. Briefly, lymphocytes at 2×107cells/mL were stained with CFSE (2 μmol/L) for 10 min at 37 °C, washed twice with PBS, and resuspended in RPMI-1640 medium containing 10% FBS. Lymphocytes at 2×106cells/mL were seeded in a 24-well plate (500 μL/well) and harvested after 48 h of incubation. The fluorescence intensity of the lymphocytes was analyzed on a FACSCalibur flow cytometer.
MTS assay
Lymphocytes in 96-well plates were treated with various concentrations of gossypol and then cultured for 48 h at 37 °C in a 5% CO2 incubator. Twenty microliters of the MTS solution was added to each well, and the plates were incubated for another 4 h. The absorbance at 490 nm was measured using a microplate reader (Bio-Rad, USA).
Apoptosis analysis
After incubation with gossypol for 8–48 h, lymphocytes were harvested and washed twice with cold PBS. The cells were resuspended in 100 μL of binding buffer and incubated with 5 μL of Annexin V-PE and 5 μL of 7-AAD in the dark for 15 min. Finally, 400 μL of binding buffer was added to each tube, and the cells were analyzed by a flow cytometer.
Nuclear morphology
Lymphocytes were seeded in 24-well plates and treated with different concentrations of gossypol for 24 h. After washing twice with PBS, the cells were stained with Hoechst 33342 solution (final concentration 2.5 μg/mL) and then incubated at 37 °C for 30 min, spun at 1500 r/min for 5 min, and transferred to a slide covered with a cover slip. The nuclear morphology was observed under a confocal microscope (Perkin Elmer, USA) using ultraviolet light. The percentage of apoptotic cells was calculated from ten randomly selected microscopic fields (400×magnification).
Delayed-type hypersensitivity (DTH)
A DTH assay was performed as previously described12, 13. Five BALB/c mice in each group were sensitized by applying 20 μL of 0.5% DNFB dissolved in a mixture of acetone and olive oil (4:1) on the shaved abdomen. Five days after the initial sensitization, the animals were challenged with 10 μL of 0.2% DNFB on the left ear. The right ear of each mouse was treated with acetone–olive oil vehicle. Gossypol (25 mg·kg−1·d−1) was administered (ip) for 7 consecutive days from the day of sensitization. The thickness of the ears was measured with an engineer's micrometer at 48 h after challenge. The mice were then sacrificed and the thymuses and spleens were dissected out. The weight of the organs was recorded. The proliferation ability of the lymphocytes isolated from the lymph nodes of five mice in each group was analyzed by an MTS assay after stimulation with PBD plus Ion for 48 h. The auricles were fixed in 10% phosphate-buffered formalin and embedded in paraffin wax. Sections of 5 μm were stained with hematoxylin-eosin and observed under a microscope.
Statistical analysis
Data are expressed as mean±SD. Statistical analysis was performed using the unpaired Student's t-test. P<0.05 was considered statistically significant.
Results
Inhibition of in vitro lymphocyte proliferation by gossypol
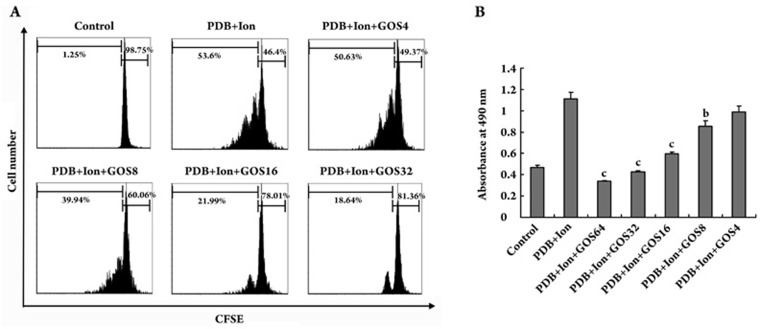
Initially, we examined the effect of gossypol on the proliferation of lymphocytes in response to polyclonal activators using a CFSE-staining assay. The fluorescence intensity of CFSE-labeled cells decreased by one half after each cell division. Thus, the cell proliferation (or division) rate can be analyzed by flow cytometry. As shown in Figure 1A, no cell proliferation was observed in the control group without activators, whereas more than 50% of the lymphocytes underwent cell division at least once when they were stimulated with PDB plus ionomycin (Ion) for 48 h. Gossypol (8 μmol/L or higher) could significantly inhibit cell division and the proliferation rate of the lymphocytes activated by PDB plus Ion in a dose-dependent manner. Meanwhile, an MTS assay was performed to confirm the effect of gossypol. This assay also showed that gossypol inhibited lymphocyte proliferation in a dose-dependent manner (Figure 1B).
Figure 1.
Inhibitory effect of gossypol on the proliferation of mouse lymphocytes. (A) Flow cytometry analysis of CFSE-labeled cells. Lymphocytes were stained with CFSE and stimulated with PDB+Ion for 48 h in the presence of various concentrations of gossypol. (B) MTS assay. Cells were stimulated with PDB+Ion for 48 h in the presence of various concentrations of gossypol. At the end of the culturing period, 20 μL of the MTS solution was added to each well, and then the cells were incubated for another 4 h. The absorbance at 490 nm was measured using a microplate reader. Values are expressed as the mean±SD. bP<0.05, cP<0.01 vs PDB+Ion group. GOS: gossypol; GOS64: 64 μmol/L GOS; GOS32: 32 μmol/L GOS; GOS16: 16 μmol/L GOS; GOS8: 8 μmol/L GOS; GOS4: 4 μmol/L GOS.
Gossypol suppressed lymphoblastic transformation
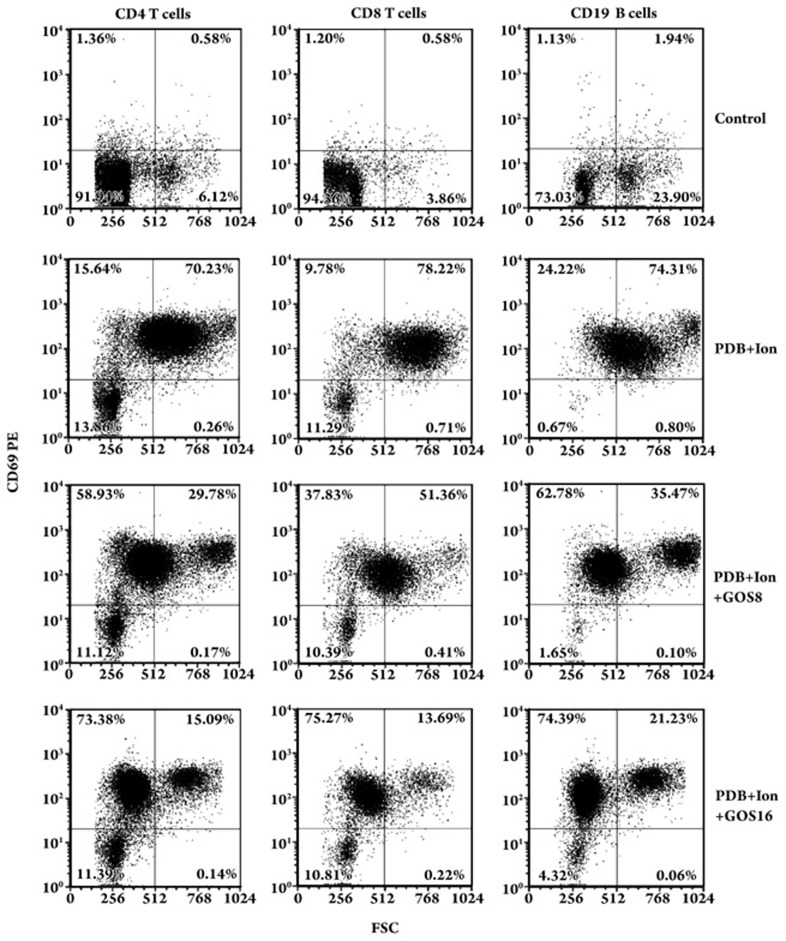
Next, flow cytometry was used to analyze whether gossypol had a selective effect on the activation and lymphoblastic transformation of T and B lymphocyte subsets. As shown in Figure 2, gossypol at concentrations of 8 μmol/L and 16 μmol/L had no significant effect on the expression of the early activation antigen CD69 (the cells in the upper quadrants) on CD4 T cells, CD8 T cells, or CD19 B cells, suggesting that at these two doses gossypol did not inhibit lymphocyte activation. However, the lymphoblastic transformation [the sizes of lymphocytes were proportional to values of the forward scatter (FSC)] of CD4 T cells, CD8 T cells, and CD19 B cells was markedly inhibited by 16 μmol/L of gossypol when compared with the lymphoblastic transformation of the lymphocytes treated only with PDB plus Ion (10%–20% vs 70%–80% in the upper right quadrants). Meanwhile, the aggregated colony sizes of gossypol-treated lymphocytes were also significantly smaller than the aggregated colony sizes of cells in the PDB plus Ion group (Figure S1). As the lymphoblastic transformation and aggregated colony formation were positively correlated with subsequent cell proliferation, this result suggests that gossypol could inhibit proliferation of both T and B lymphocytes without any selective effect on the lymphocyte subsets.
Figure 2.
Flow cytometry analysis of the effect of gossypol on CD69 expression and on lymphoblastic transformation of lymphocytes stimulated by PDB plus Ion for 24 h. One representative dot plot of each triplicate is presented. GOS: gossypol; GOS16: 16 μmol/L GOS; GOS8: 8 μmol/L GOS.
Figure S1.
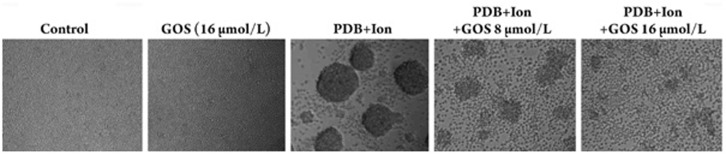
Effect of gossypol on the formation of cell aggregated colony.
Gossypol induced apoptosis in lymphocytes
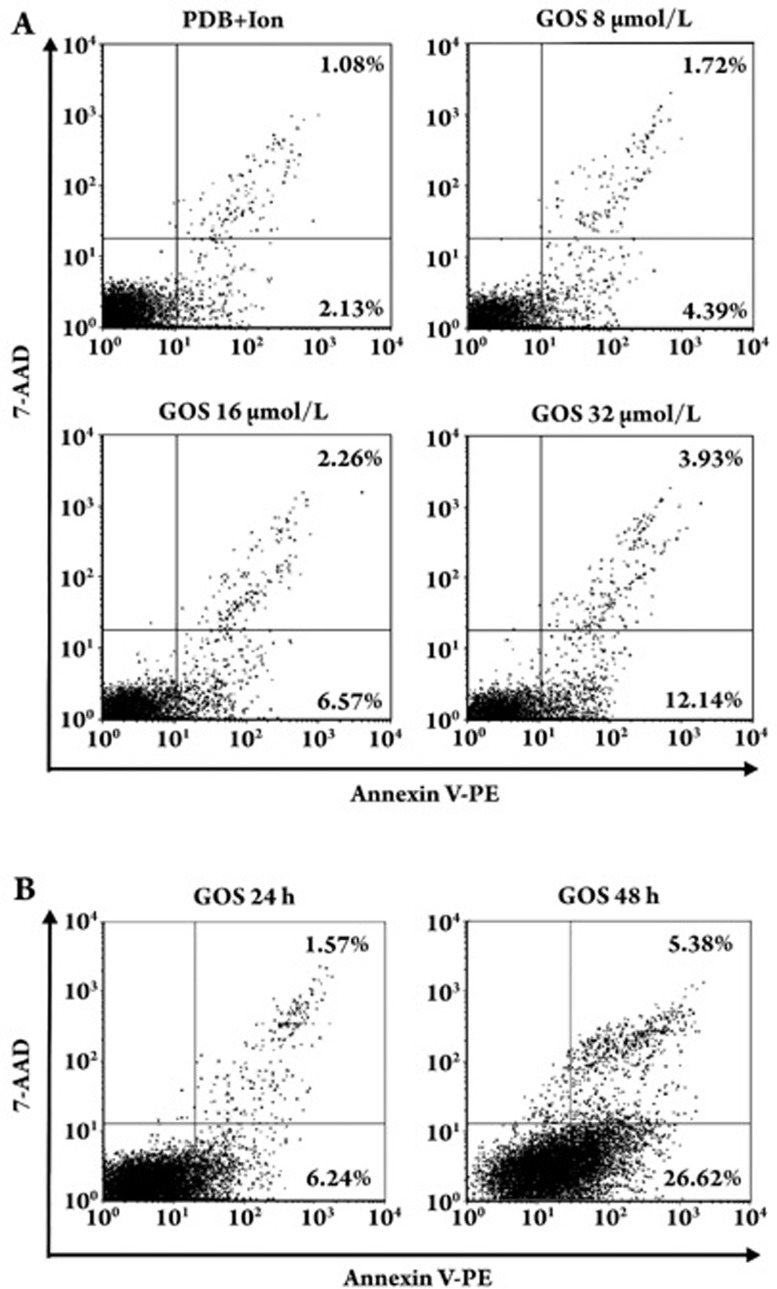
We examined whether gossypol inhibited lymphocyte function by induction of cell apoptosis. Apoptotic cells were identified by Annexin V-PE positive/7-AAD negative staining. As shown in Figure 3A, gossypol could induce apoptosis in lymphocytes activated with PDB plus Ion as early as 8 h after the start of the incubation, and this effect was dose-dependent. The apoptotic proportions of lymphocytes treated with 8, 16, and 32 μmol/L of gossypol were 4.39%, 6.57%, and 12.14%, respectively, and the PDB plus Ion control value was 2.13%. The proportion of apoptotic cells increased as the incubation time increased, suggesting that the effect of gossypol was also time-dependent (Figure 3B).
Figure 3.
Flow cytometry analysis of apoptosis in mouse lymphocytes using Annexin-V staining. (A) Cells were stimulated with PDB+Ion for 8 h in the presence of various concentrations of gossypol. (B) Cells were stimulated with PDB+Ion for 24 and 48 h in the presence of 8 μmol/L gossypol. Values are the ratios of the percentage of cells in early apoptosis (Annexin V–PE positive/7-AAD negative) to the percentage of cells in late apoptosis (Annexin V–PE positive/7-AAD positive). One representative experiment of three independent experiments is presented.
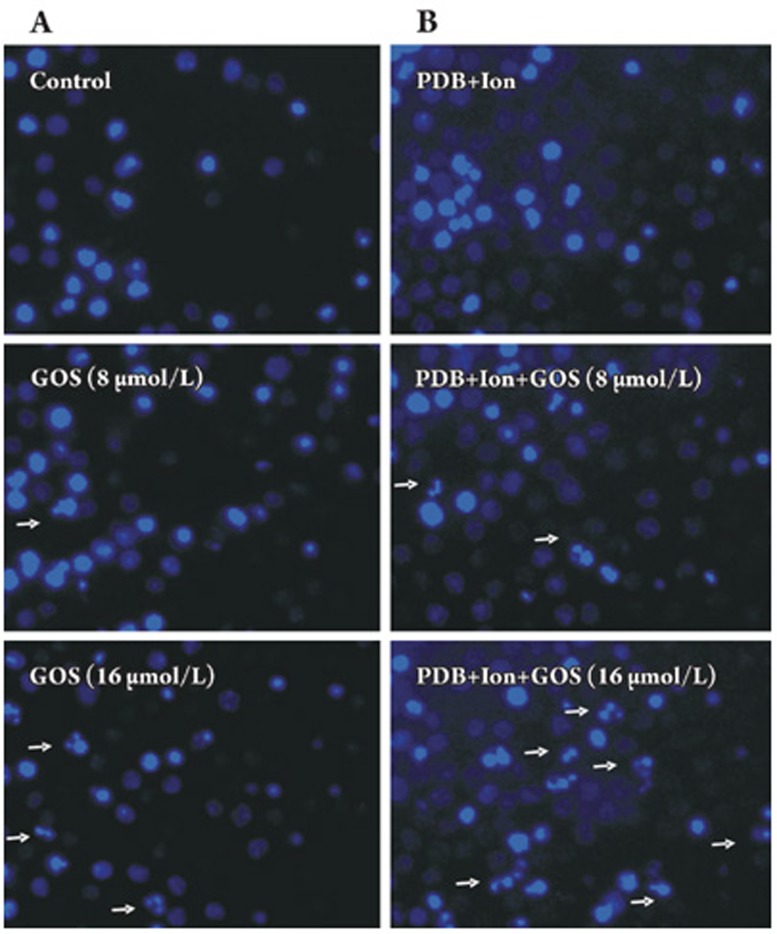
Hoechst 33342 staining can reveal apoptotic cells with fragmented or condensed nuclei. As shown in Figure 4, the nuclei of the control lymphocytes were uniformly blue, whereas some nuclei of the gossypol-treated cells contained small bright blue dots representing nuclear fragmentation and/or chromatin condensation, the typical characteristics of apoptotic nuclei. When treated with 16 μmol/L of gossypol for 24 h, the activated lymphocytes had a significantly higher proportion of apoptotic nuclei as compared with the resting lymphocytes [(16.5±2.1)% vs (10.3±1.9)% the percentage of apoptotic cells was calculated from ten random microscope fields at 400×magnification] (P<0.05).
Figure 4.
The nuclear morphology of mouse lymphocytes stained with Hoechst 33342 (400×magnification). (A) Resting lymphocytes treated with gossypol for 24 h. (B) PDB+Ion-activated lymphocytes treated with gossypol for 24 h. Arrows show the apoptotic cells. GOS: gossypol.
Effect of gossypol on the DTH reaction
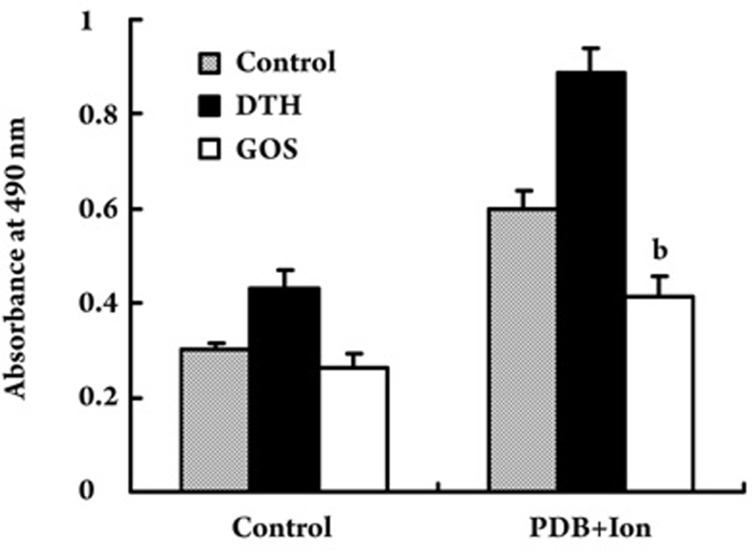
In the DTH mouse model, DNFB induced a pronounced inflammatory reaction in the challenged ears as compared with the control ears. When treated with gossypol, ear swelling was markedly reduced, whereas the body weight was not affected (Table 1). Meanwhile, the thymus and spleen indexes of the gossypol-treated group also decreased (P<0.05) (Table 2). In addition, the MTS results showed that the proliferation rate of the lymphocytes from the gossypol-treated group was significantly lower than the proliferation rate in the DTH group without any treatment (P<0.05) (Figure 5). These results suggest that gossypol exhibited an in vivo immunosuppressive effect in mice, probably through inhibiting lymphocyte proliferation.
Table 1. Comparison of ear thickness and body weight of mice in different groups. Mean±SD. n=5. bP<0.05 vs DTH group.
| Group |
Body weight (g) |
Difference in ear thickness |
|
|---|---|---|---|
| 1 day | 8 day | (left ear-right ear) (mm) | |
| Control | 16.65±0.79 | 17.68±0.76 | 0.02±0.02 |
| DTH | 15.53±1.77 | 16.46±1.25 | 0.28±0.04 |
| GOS-treated | 17.33±1.63 | 16.0±1.21 | 0.13±0.03b |
Table 2. Comparison of thymus indexes and spleen indexes for different groups. The indexes of the thymus and spleen were calculated as the ratio of organ weight (mg) to total body weight (g). Mean±SD. n=5. bP<0.05 vs control group.
| Group | Thymus index | Spleen index |
|---|---|---|
| Control | 2.14±0.6 | 3.5±0.7 |
| DTH | 2.81±0.5 | 4.23±0.9 |
| GOS-treated | 1.45±0.3b | 2.35±0.6b |
Figure 5.
Inhibitory effect of gossypol on the proliferation of lymphocytes from DTH model mice. Gossypol (25 mg·kg−1·d−1) was administered for 7 days by ip injection before the mice were sacrificed. Lymphocytes were isolated from lymph nodes and were stimulated with PDB plus Ion and then cultured for 48 h at 37°C in a humidified atmosphere of 5% CO2. At the end of the culturing period, 20 μL of MTS solution was added to each well, and then the cells were incubated for another 4 h. The absorbance at 490 nm was measured using a microplate reader. Data are presented as the mean ±SD. n=6. bP<0.05 vs DTH group. GOS: gossypol.
Histological analysis of auricle tissue from DTH mice
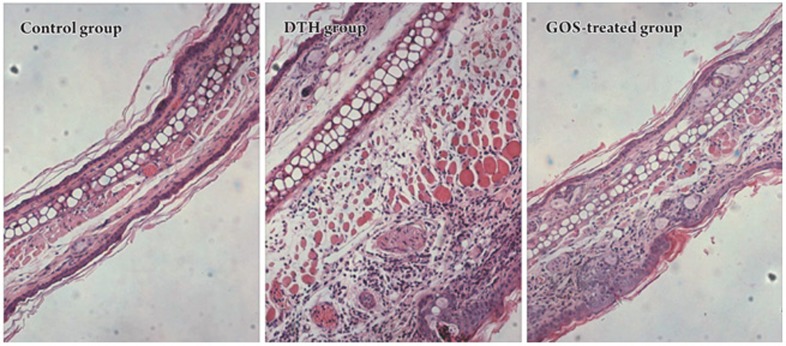
Histological sections of auricle tissue showed that the DNFB-challenged ears of DTH mice suffered from edema and had been infiltrated with a large number of inflammatory cells. The gossypol treatment reduced ear edema and leukocyte infiltration (Figure 6). This was consistent with the measurement of ear thickness (Table 1). Together, these results suggest that gossypol could significantly inhibit the DNFB-induced DTH reaction.
Figure 6.
Hematoxylin-eosin staining of mouse auricle tissue sections (×100). See Material and Methods for details. GOS: gossypol.
Discussion
In the present study, we used both lymphocytes cultured in vitro and an in vivo DTH mouse model to evaluate the immunosuppressive effect of gossypol. In the in vitro assays, we showed that gossypol inhibited the proliferation of mouse lymphocytes stimulated with polyclonal activators in a dose- and time-dependent manner and that gossypol could also induce cell apoptosis in both resting and activated lymphocytes. It appeared that the inhibitory activity of gossypol was not confined to one of the lymphocyte subsets as the lymphoblastic transformation of all lymphocyte subsets was suppressed, although the expression of the early activation marker CD69 was not blocked by this reagent. Moreover, the in vivo study using the DTH model revealed that gossypol could suppress inflammatory ear edema and infiltration of lymphocytes in the DNFB-treated ears. The target on which gossypol exerted its effect might exist in lymphocytes because the lymphocytes from gossypol-treated mice had a reduced proliferation capacity as compared with lymphocytes from control mice. Taken together, our data suggest that gossypol exhibits an immunosuppressive effect on mice, probably by inhibiting proliferation and inducing apoptosis of lymphocytes.
The exact target of gossypol in lymphocytes is still unknown. It has been reported that gossypol is an inhibitor of protein kinase C (PKC)14, which has been shown to markedly reduce the expression of interleukin-2 and interferon-γ in activated human T lymphocytes in response to polyclonal activators15. Pharmacologic activators of PKC, such as PDB, which enters the cells and directly activates PKC, and calcium ionophores, such as ionomycin, which raises the cytosolic free calcium ion concentration, can act together to stimulate lymphocyte activation, interleukin-2 synthesis and lymphocyte proliferation. Consistent with a previous study on human lymphocytes15, the current research also showed that gossypol significantly inhibited the proliferation of mouse lymphocytes in response to PDB plus ionomycin. It seems that gossypol may inhibit lymphocyte proliferation by blocking PKC activity and the calcium signaling pathway. However, the expression of CD69, an early activation marker of lymphocytes that is upregulated by PKC activation16, was not affected at the doses of gossypol used in this study. We presume that gossypol might act on multiple targets besides PKC or that low concentrations of gossypol might not effectively block PKC activity and CD69 expression induced by PDB, while it could still act on the other components of the signaling pathway, such as calcium signaling, thereby inhibiting lymphocytic transformation and proliferation. Other potential action sites of gossypol remain to be identified in lymphocytes.
Over the past several decades, gossypol has been demonstrated to induce apoptosis in several tumor cell lines in vitro and has been suggested to be a potential anti-tumor drug17, 18, 19. The mechanism of gossypol's anti-tumor activity may involve cell cycle arrest in the G0/G1 phase by upregulating p51 and p2120, 21, 22. Recent studies have also shown that gossypol-induced apoptosis is followed by a caspase-dependent and -independent processes that involves the release of AIF from the mitochondria to the cytosol, a process that is the result of inhibiting the heterodimerization of Bcl-XL/Bcl-2 with pro-apoptosis molecules23, 24, 25. Gossypol also induces the truncation of the Bid protein, the loss of mitochondrial membrane potential (ΔΨm), the release of cytochrome c from mitochondria into the cytosol, and the activation of caspases 3, 8, and 926. In the present study, we showed that gossypol could induce the apoptosis of mouse lymphocytes in a time- and dose-dependent manner, but higher concentrations of gossypol may cause largely necrotic cell death (data not shown). Our results are in accordance with a previous study in which the concentrations of gossypol required to induce apoptosis of human lymphocytes without causing necrosis were between 25 and 50 μmol/L27. The doses of gossypol we used to induce lymphocyte apoptosis were lower than 25 μmol/L and caused only a minor proportion of necrosis. Further studies are warranted to determine whether gossypol induces apoptosis in mouse lymphocytes by a similar mechanism as it does in tumor cells, in which gossypol binds to and inhibits Bcl-XL and Bcl-224, 25.
Gossypol is suggested to be a potential reagent for the treatment of psoriasis10, as it has been found that gossypol possesses anti-proliferative activity against keratinocytes at doses that are non-toxic to humans28 and that lack mutagenicity29. Psoriasis is a common chronic inflammatory skin disease characterized by a marked inflammatory infiltrate and by the hyperproliferation of keratinocytes. T lymphocytes play a crucial role in the immunopathogenesis of this disease30. In the present study, our data suggest that gossypol might suppress the mouse DTH reaction by reducing the proliferation and infiltration of lymphocytes. Thus our data also imply that gossypol may exhibit an effect beneficial for the treatment of psoriasis. As systemic injection of gossypol markedly decreased the thymus and spleen indexes, which suggested that this reagent has potential in vivo toxicity to the immune organs at the doses we used in this study. Local administration at the site of the lesions, such as those caused by psoriasis, may be the preferred approach for the treatment of some inflammatory diseases. Other routes, such as oral administration, are also worth testing for gossypol treatment.
In summary, our results suggest that gossypol not only could inhibit the proliferation of mouse lymphocytes and induce apoptosis in vitro but also could alleviate the DNFB-induced DTH reaction in vivo. These results suggest that gossypol may exert beneficial effects in the treatment of inflammatory diseases. Further investigation is warranted to identify the potential targets of gossypol's immunosuppressive effect.
Author contribution
Wen-bin XU and Xian-hui HE designed research; Wen-bin XU, Li-hui XU, Hong-song LU, Huan-jing SHI, and Jing-fang DI performed research; Wen-bin XU, Xian-hui HE, and Dong-yun OU-YANG analyzed data; Wen-bin XU, Xian-hui HE, and Dong-yun OU-YANG wrote the paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 30572199, No 30230350, No 30371651) and the Key Subject of Biochemistry and Molecular Biology of Guangdong Province.
We thank Prof Qi-xuan XIE for her kind help in capturing images; Dr Xi-chao WANG for performing the flow cytometry analysis; Chun-yong LIU, Xiao-ying WANG, He GUO, Qi GAO, and Yu-ting LI for their excellent technical assistance; and Zhi YANG and Man-lin YAO for kindly helping with preparation of the manuscript.
References
- Wu D. An overview of the clinical pharmacology and therapeutic potential of gossypol as a male contraceptive agent and in gynaecological disease. Drugs. 1989;38:333–41. doi: 10.2165/00003495-198938030-00001. [DOI] [PubMed] [Google Scholar]
- Dodou K. Investigations on gossypol past and present developments. Expert Opin Investig Drugs. 2005;14:1419–34. doi: 10.1517/13543784.14.11.1419. [DOI] [PubMed] [Google Scholar]
- Dao VT, Gaspard C, Mayer M, Georges HW, Suong NN, Rogert JM. Synthesis and cytotoxicity of gossypol related compounds. Eur J Med Chem. 2000;35:805–13. doi: 10.1016/s0223-5234(00)00165-3. [DOI] [PubMed] [Google Scholar]
- He XH, Zeng YZ, Li Z, Xu LH, Sun H, Zeng JM. Inhibitory effects of gossypol on the activation of human T-lymphocytes stimulated with polyclonal activators. Chin J Pathophysiol. 2001;17:510–4. [Google Scholar]
- Sharma HW, Narayanan R. The NF-κB transcription factor in oncogenesis. Anticancer Res. 1996;16:589–96. [PubMed] [Google Scholar]
- Albert S, Baldwin , Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Moon DO, Kim MO, Lee JD, Kim GY. Gossypol suppresses NF-κB activity and NF-κB-related gene expression in human leukemia U937 cells. Cancer Lett. 2008;264:192–200. doi: 10.1016/j.canlet.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Benhaim P, Mathes SJ, Hunt TK, Scheuenstuh H, Benz CC. Induction of neutrophil Mac-1 integrin expression and superoxide production by the medicinal plant extract gossypol. Inflammation. 1994;18:443–58. doi: 10.1007/BF01560692. [DOI] [PubMed] [Google Scholar]
- Yu BZ, Rogers J, Ranadive G, Baker S, Wilton DC, Apitz CR, et al. Gossypol modification of Ala-1 of secreted phospholipase A2: a probe for the kinetic effects of sulfate glycoconjugates. Biochemistry. 1997;36:12400–11. doi: 10.1021/bi962972i. [DOI] [PubMed] [Google Scholar]
- Kalliopi D, Rosaleen JAW, John L, David APS, Michael DS, Paul WG. Synthesis of gossypol atropisomers and derivatives and evaluation of their anti-proliferative and anti-oxidant activity. Bioorg Med Chem. 2005;13:4228–37. doi: 10.1016/j.bmc.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Zhao JX, Zeng YY, He XH, Wang N, Di JF, Zeng S. Application of vital dye CFDA-SE to study lymphocytic proliferation. Chin J Cell Mol Immunol. 2003;19:109–11. [PubMed] [Google Scholar]
- Corsini AC, Bellucci SB, Costa MG. A simple method of evaluating delayed type hypersensitivity in mice. J Immunol Meth. 1979;30:195–200. doi: 10.1016/0022-1759(79)90093-0. [DOI] [PubMed] [Google Scholar]
- Gad SC. The mouse ear swelling test (MEST) in the 1990. Toxicology. 1994;93:33–46. doi: 10.1016/0300-483x(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Nakadate T, Jeng AY, Blumberg PM. Comparison of protein kinase C functional assays to clarify mechanisms of inhibitor action. Biochem Pharmacol. 1988;37:1541–5. doi: 10.1016/0006-2952(88)90016-0. [DOI] [PubMed] [Google Scholar]
- He XH, Zeng YY, Xu LH, Sun H, Li Z, Di JF. Influences of protein kinase C inhibitors on the expression of IL-2 and IFN-γ by human T-lymphocytes. Chin J Pathophysiol. 2002;18:522–25. [Google Scholar]
- Testi R, Phillips JH, Lanier LL. T cell activation via Leu-23 (CD69) J Immunol. 1989;143:1123–8. [PubMed] [Google Scholar]
- Tuszynski GP, Cossu G. Differential cytotoxicity of gossypol on human melonoma, colon carcinoma, and other tissue culture cell lines. Cancer Res. 1984;44:768–71. [PubMed] [Google Scholar]
- Gilbert NE, O'Reilly JE, Chang CJ, Lin YC, Brueggemeier RW. Antiproliferative activity of gossypol and gossypolone on human breast cancer cells. Life Sci. 1995;57:61–7. doi: 10.1016/0024-3205(95)00243-y. [DOI] [PubMed] [Google Scholar]
- Wang XH, Wang J, Wong SC, Chow LS, Nicholls JM, Wong YC, et al. Cytotoxic effect of gossypol on colon carcinoma cells. Life Sci. 2000;67:2663–71. doi: 10.1016/s0024-3205(00)00857-2. [DOI] [PubMed] [Google Scholar]
- Thomas M, Von HV, Moustafa Y, Montmasson MP, Monet JD. Effects of gossypol on the cell cycle phases in T-47D human breast cancer cells. Anticancer Res. 1991;11:1469–75. [PubMed] [Google Scholar]
- Van PC, Seidman AD, Reidenberg MM, Moasser MM, Sklarin N, Van ZK, et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat. 2001;66:239–48. doi: 10.1023/a:1010686204736. [DOI] [PubMed] [Google Scholar]
- Ligueros M, Jeoung D, Tang B, Hochhauser D, Reidenberg MM, Sonenberg M. Gossypol inhibition of mitosis, cyclin D1 and Rb protein in human mammary cancer cells and cyclin-D1 transfected human fibrosarcoma cells. Br J Cancer. 1997;76:21–8. doi: 10.1038/bjc.1997.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MC, Liu HP, Tian ZK, Griffith BN, Ji M, Li QT. Gossypol induces apoptosis in human PC-3 prostate cancer cells by modulating caspase-dependent and caspase-independent cell death pathways. Life Sci. 2007;80:767–74. doi: 10.1016/j.lfs.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K, Wierda WG, Keating MJ, Gandhi V. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2008;112:1971–80. doi: 10.1182/blood-2007-12-126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q, et al. Natural BH3 mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther. 2008;7:2192–202. doi: 10.1158/1535-7163.MCT-08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou DX, Uto T, Tong XH, Takeshita T, Tanigawa S, Imamura I, et al. Involvement of reactive oxygen species-independent mitochondrial pathway in gossypol-induced apoptosis. Arch Biochem Biophys. 2004;428:179–87. doi: 10.1016/j.abb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Yurtcu E, Ergun MA, Menevse A. Apoptotic effect of gossypol on human lymphocytes. Cell Biol Int. 2003;27:791–94. doi: 10.1016/s1065-6995(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Coutinho EM. Gossypol: a contraceptive for men. Contraception. 2002;65:259–63. doi: 10.1016/s0010-7824(02)00294-9. [DOI] [PubMed] [Google Scholar]
- Li YF, Booth GM, Seegmiller RE. Evidence for embryotoxicity of gossypol in mice and chicks with no evidence of mutagenic activity in the ames test. Reprod Toxicol. 1989;3:59–62. doi: 10.1016/0890-6238(89)90039-7. [DOI] [PubMed] [Google Scholar]
- Barker JN. Psoriasis as a T cell-mediated autoimmune disease. Hosp Med. 1998;59:530–3. [PubMed] [Google Scholar]