Abstract
Aim:
To investigate the anti-asthmatic mechanisms of the traditional Chinese medicine Pericarpium citri reticulatae (PCR).
Methods:
The alkaloid section (AS) of PCR was extracted using an ion exchange resin, separated, and purified into different fractions by semi-preparative HPLC. These fractions were screened for beta2-adrenergic receptor (β2AR) agonistic activity using rat β2AR-transfected CHO-CRE-EGFP cells. AS and its isolated components were characterized by ultra-performance liquid chromatography/quadrupole time-of-flight MS (UPLC/Q-Tof MS) and were evaluated for their spasmolytic and antitussive activities both in vitro and in vivo in a guinea pig model.
Results:
We demonstrated that the AS component responsible for activating β2AR signaling was synephrine. Both AS and synephrine showed significant spasmolytic effects on acetylcholine chloride (ACh)-induced contractions in isolated guinea pig trachea, and they protected against histamine-induced experimental asthma by prolonging the latent period. We further identified stachydrine as the antitussive component that could significantly reduce citric acid–induced coughing. The combination of these two bioactive compounds had a more potent spasmolytic activity in comparison with the single use of synephrine or stachydrine.
Conclusion:
We conclude that synephrine and stachydrine are the key components of AS that mediate asthma relief due to their synergism when used in combination.
Keywords: Pericarpium citri reticulatae (PCR), asthma, beta2-adrenergic receptor (β2AR) agonists, bronchodilator, antitussive, synephrine, stachydrine
Introduction
Many beneficial natural foods used in traditional Chinese medicine (TCM), such as citrus fruits, are being analyzed to identify the components responsible for their therapeutic responses. Citrus herbs (Citrus aurantium L) were documented as effective anti-asthmatic remedies thousands of years ago in “Huangdi Nei Jing”, and a number of citrus species are currently recorded in the Chinese Pharmacopoeia as appropriate for medicinal use. One such species is Pericarpium citri reticulatae (PCR), which is the dried rind of ripe Citrus reticilata Blancon fruits, and which is commercially referred to as chenpi. This is a widely planted and consumed species in southern China that has various therapeutic properties, including reducing fevers, soothing asthma, stimulating the appetite, and enhancing immune system function1, 2. Due to its complex ingredients, the active components and pharmacological mechanisms of PCR-mediated reduction of asthmatic symptoms have not been thoroughly investigated.
Asthma is a treatable but incurable chronic inflammatory disease characterized by reversible airway obstruction, airway remodeling, and nonspecific airway hyper-responsiveness3. Asthma epidemiological studies indicate that it is the most common chronic human disease. The total prevalence is estimated to be ∼10% in adults and ∼35% in children, and its prevalence increases >50 % every ten years4. Beta2-adrenergic receptor (β2-AR) agonists relieve airway bronchoconstriction by activating β2-ARs on the surface of airway smooth muscle cells5. They have been used for the relief and prophylactic treatment of asthmatic symptoms for many years. While research on β2-AR agonists has extensively focused on chemosynthetic drugs, TCM agents are also effective in asthma treatments, and research focused on identifying the responsible agents is warranted6, 7.
The use of β2-AR-transfected Chinese hamster ovary (CHO) cells expressing a cAMP response element (CRE) and enhanced green fluorescence protein (EGFP) is a useful screening assay for β2-AR activity. β2-AR activation elicits a variety of cAMP-dependent processes that allow protein kinase A catalytic subunits to move into the nucleus and phosphorylate CRE binding proteins (CREBs)8. These CREBs bind to the CRE of the EGFP promoter, which results in increased EGFP transcription and green fluorescence emission9, 10. The measurement of CRE-regulated reporter gene activity provides a sensitive assay for assessing intracellular changes in cAMP levels11, 12, 13 in order to study ligand affinity and efficacy for β2-AR.
Here, we sought to uncover the mechanism(s) that PCR mediates to relieve asthma. The alkaloid section (AS) of PCR was extracted and purified into different fractions. These fractions were screened for β2-AR agonistic or other anti-asthmatic activities using rat β2-AR-transfected CHO-CRE-EGFP cells. AS and its isolated components were also evaluated for their spasmolytic activity and in vivo antitussive activity using a histamine-induced experimental asthma guinea pig model and a citric acid-induced cough guinea pig model.
Materials and methods
Materials
CHO cells expressing CRE-EGFP were constructed by our laboratory. Salbutamol and propranolol hydrochloride were obtained from Sigma Chemical Co (St Louis, MO, USA). Cell culture media and reagents were obtained from GibcoBRL Life Technologies (Gaithersburg, MD, USA). The traditional Chinese herbs of Pericarpium citri reticulatae (chenpi) were purchased from Qi'ao Pharmaceutical Company (Anguo, China, Lot No 70135191). Standards for synephrine and stachydrine hydrochloride were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Citric acid and analytic grade heptafluorobutyric acid (HFBA) were obtained from Afar Aesar (St Louis, MO, USA). Pentoxyverine was obtained from Lisheng Pharmaceutical Corporation, Ltd (Tianjin, China). HPLC-grade acetonitrile was obtained from Tedia (Fairfield, Ohio, USA). Water was deionized using a Milli-Q water purification system (Millipore Bedford, MA, USA). Additional reagents used in this study were of analytical grade and commercially available.
Animals
Female guinea pigs (Dunkin-Hartley, body weight 250−350 g) were obtained from the Academy of Military Medical Science (Beijing, China). The animals were housed in groups of 6 per cage under a 12 h light-dark cycle, with food and water continuously available. All procedures and experiments were carried out in accordance with the internationally accepted guidelines for the care and use of laboratory animals and were approved by the local Institutional Animal Care and Use Committee.
Extraction and preparation of AS from PCR
The AS was obtained from PCR as follows. Twenty grams of PCR was powdered and soaked in 180 mL of HCl (0.02 mol/L) overnight. The supernatant was then passed through a cation exchange resin column (2 cm×20 cm, washed with water and eluted with 5% aqueous ammonia), and then the eluted alkaloid solution was passed through anion exchange resin and S8 microporous resin columns. The purified solution was collected and dried by vacuum, and the extract was lyophilized. A solid mass (0.94% w/w with respect to dry starting material) of the AS complex was obtained.
The bioactive compound-containing complex was dissolved in water and filtered through a 0.45-μm membrane. It was then separated by semi-preparative HPLC at room temperature on a 10 A VP-HPLC system (Shimadzu Co, Kyoto, Japan) using a stainless-steel column filled with Kromasil C18 (250 mm×10 mm, id, 10 μm, Eka chemicals, Bohus, Sweden). A mobile phase composition of 95% (v/v) HFBA solution (12 mm) and 5% (v/v) acetonitrile was used under isocratic conditions. The pH was adjusted with formic acid to 4.0, the flow rate was set to 3.0 mL/min, and UV detection was performed at 275 nm. The sample injection volume was 200 μL, and fractions were collected every 3 min. Ten fractions were obtained, and these fractions were concentrated to remove the organic solvent. The fractions were then evaluated for their bio-reactivity.
β2-AR agonistic activity assay
CHO-β2-AR-CRE-EGFP cells were constructed from the parental CHO cell line by our laboratory14 to express CRE-mediated EGFP and stably and sensitively express the rat β2-AR gene. CHO-β2-AR-CRE-EGFP cells were seeded into 96-well plates at a density of 5×104 cells/well and were incubated at 37 °C in 5% CO2 for 12 h in culture medium. The aforementioned HPLC fractions were diluted 1000-fold with RPMI-1640 medium containing fetal bovine serum (10%), and was added to the 96-well plate at 100 μL/well . Salbutamol, a β2-AR agonist, was used as a positive control. After a 10-h incubation, the medium was discarded, PBS was added to each well, and the cells were observed by an inverted fluorescence microscope (Olympus BX51, JAPAN) for EGFP expression. The fluorescence intensity was analyzed with Image Pro Plus 5.1 software (Media Cybernetics, Inc, MD, USA) and quantified using GraphPad Prism 4.0 software.
In vivo antitussive activity tests
Unanesthetized and unrestrained guinea pigs were individually placed in a transparent glass chamber (30 cm×15 cm×15 cm) and acclimatized for 5 min prior to citric acid challenge. Cough responses were induced by exposure to an aqueous citric acid aerosol (17.5%) for 20 s. The aerosol was generated with an ultrasonic nebulizer at a nebulization rate of 0.8 mL/min. The same nebulizer was used throughout the experiment. During the 5 min exposure, beginning with the initial irritant challenge, the animals were watched continuously by a trained observer, and the number of coughs was counted manually. The coughs could easily be distinguished from sneezes, since there was a clear difference in the sound and behavior of the animals16. Animals with >30 or <10 coughs in 5 min were not used for subsequent studies. After a 24 h recovery period, selected guinea pigs were randomly divided into several groups (n≥6 animals per group) for different treatments.
The above protocol was performed 5 min after the animals were exposed to aerosolized test solutions for 10 s (n=6). The number of coughs produced 5 min after the administration of test agents (Ct) was compared with the number of control coughs (Cc) on the first day. The antitussive effect was expressed as the percentage inhibition of the number of control coughs [(Cc-Ct)/Cc×100%].
Identification of the bioactive compounds from AS
According to the results of two screening courses for β2-adrenergic and antitussive activities, bioactive compounds were characterized by ultra-performance liquid chromatography (UPLC)/quadrupole time-of-flight mass spectrometry (Q-ToF-MS). A Waters Acquity UPLC system, equipped with a binary solvent delivery system, an autosampler, and a tunable UV (TUV) detector, was used for UPLC. Chromatography was performed on a 5 cm×2.1 mm Waters Acquity C18 1.7 μm column, with an injection volume of 10 μL. The mobile phase consisted of 12 mmol/L of aqueous HFBA/formic acid (A) and acetonitrile (B) pumped at a flow rate of 0.2 mL/min. The elution gradient was 95% (A) or 5% (B) and was kept isocratic.
Mass spectrometric analysis was performed on a Q-ToF premier mass spectrometer (Waters Co, USA) equipped with an electrospray ionization (ESI) source and a mass range of up to m/z 2000. The positive ion mode was employed, with spray and capillary voltages of 4.5 kV and 5.0 V, respectively, and the temperature was maintained at 220 °C. The solvent was nebulized using N2 as the sheath gas, at a flow rate of 0.80 L/min, and the auxiliary gas was used at a flow rate of 0.08 L/min.
Spasmolytic activity tests of isolated trachea in vitro
The animals were sacrificed by intraperitoneal injection of an overdose of sodium pentobarbital (75 mg/kg). The chest was opened to obtain the section of the trachea between the larynx and sternum, which was transferred to a dish containing warm Krebs-Henseleit buffer (NaCl 124 mmol/L, KCl 5 mmol/L, MgSO4 1.3 mmol/L, CaCl2 2.5 mmol/L, NaHCO3 25 mmol/L, NaH2PO4 0.6 mmol/L, and glucose 10 mmol/L). After removing excess connective tissue and fat, the trachea was dissected into 6−8 small rings of about a 2 mm width containing two or three cartilaginous segments15. The tracheal rings were mounted vertically in a 20 mL water-jacketed organ bath that was filled with Krebs-Henseleit buffer and aerated with a mixture of 95% O2 and 5% CO2 at 37 °C.
The bottom end of the ring was fixed to an L-shaped hook, and the top end was tied to a stainless steel wire connected to a force-displacement transducer. Changes in the isometric tension were recorded. Before each experiment, each tissue was subjected to a load of 1 g and allowed to equilibrate for at least 1 h, with frequent changes of Krebs-Henseleit buffer until a stable baseline tension was obtained17. The tissue was washed thoroughly with Krebs-Henseleit buffer immediately after the peak tension developed and was kept unstimulated until a stable baseline tension was obtained again. In all experiments, 200 μL of each drug was added into the 20 mL organ bath. All drug concentrations indicated the final concentration in the organ bath.
Maximum contraction in each tracheal ring was induced by treatment with acetylcholine chloride (ACh; 1×10−3 mol/L or 1×10−2 mol/L) at the beginning of the experiment. The tissue was immediately and thoroughly washed with Krebs-Henseleit buffer. Propranolol was added 10 min before the samples were placed in the organ bath, and ACh (1×10−2−1×10−8 mol/L) was added 10 min after. The peak contractile response was taken as 100 %, and the tension was expressed as the percentage of this peak response. Concentration-response (CR) curves were obtained, and the concentration producing 50% of the maximal response (EC50) was determined17.
In vivo bronchial hyperreactivity test
Thirty-five male Hartley strain guinea pigs (250−350 g) were divided into 5 groups. The animals were placed in an inhalation cage consisting of 3 boxes, designated A−C. In box A, test drugs were administered using an ultrasonic nebulizer that atomized the drugs at a rate of 0.8 mL/min. The animals were kept in box A for 1 min with spontaneous breathing and were administered atomized test drugs or normal saline. Box B served as a sluice through which the animal passed into box C. In box C, the animal was exposed to 0.1% histamine solutions for 20 s at an atomized rate of 0.8 mL/min. The animal was then withdrawn from the inhalation cage, and the time for asphyxial convulsions to occur was measured and defined as the latent period of asthma. The animals were initially stimulated by histamine alone, and the latent periods were recorded. Two days later, the animals were exposed to histamine again following their exposure to atomized drugs or normal saline in box A. The increasing rate of the latent period was calculated18.
Curve fitting and statistical analysis
Using Prism 4 (GraphPad Software Inc., San Diego, CA), the agonist CR curves were fitted by a least-squares, nonlinear, iterative regression to the following form of the Hill equation: E=Emin+[(Emax–Emin)/1+10(pEC50+p[A])n], where E is the effect, Emin and Emax are the lower and upper asymptotes (ie, the basal response and maximal agonist-induced response, respectively), p[A] is the log molar concentration of agonist, pEC50 is a location parameter equal to the log molar concentration of agonist producing Emax/2, and n is the gradient of the CR curve at the pEC50 level.
Data are presented as the mean±SEM. Multiple comparisons were analyzed by analysis of variance (ANOVA) using a Dunnett's test19. Alternatively, a paired t-test was used when appropriate. A P-value less than 0.05 was considered statistically significant.
Results
Determination of the active fraction based on the β2-AR agonistic activity assay and the experimental cough model
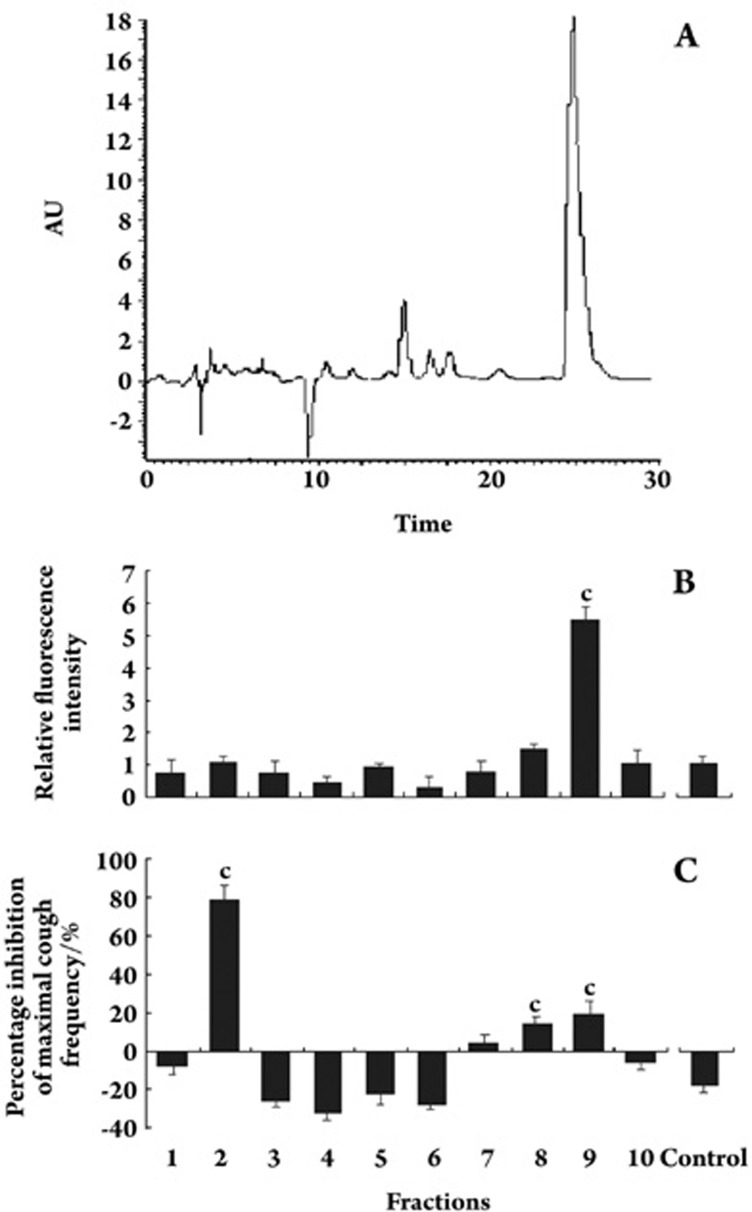
To identify the PCR components able to activate β2-AR, the AS was separated by semi-preparative HPLC (Figure 1A), and each HPLC fraction was subsequently tested for its ability to activate β2-AR using the CHO-β2-AR-CRE-EGFP cell line model (Figure 1B). The ninth fraction showed the highest activity, which was a ∼6-fold induction of EGFP activity over the basal level.
Figure 1.
Semi-HPLC performance (A) and determination of the active fraction based on fluorescence assays using CHO-β2-AR-CRE-EGFP cells (B) and the experimental cough model (C). The relative fluorescence intensity was measured as the relative intensity compared with the background. Each bar represents the mean±SEM. n=6, cP<0.01 vs control.
To identify the effective antitussive components, the 10 fractions were tested for their antitussive activity using the guinea pig citric acid–induced coughing model. As shown in Figure 1C, the second fraction showed a much higher antitussive activity compared with those of the control and other AS fractions.
Structural determination of the bioactive compounds using UPLC/Q-ToF-MS
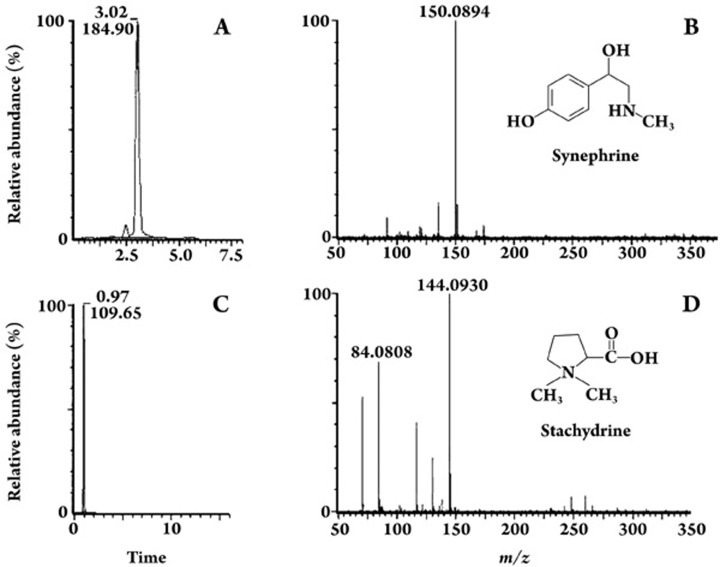
To identify the bioactive components of AS, a UPLC/Q-ToF-MS assay was used. The mass spectra and structure of the responsible compounds are shown in Figure 2. The ninth fraction, which showed the greatest ability to activate β2-AR, had an intense [M-OH]+ signal at m/z 150.1 in the positive-ion ESI-MS mode. The selected ion recording mode (SIR) chromatogram, which was used to monitor the analyte and the abundant ions at m/z 150.1, had a retention time that was the same as the standard synephrine (Figure 2A, 2B). Therefore, the active compound in the ninth fraction was identified as synephrine. The second fraction of antitussive activity showed a strong [M+H]+ signal at m/z 144.1, which was the same molecular weight and retention time as that of stachydrine (Figure 2C, 2D).
Figure 2.
UPLC-MS-selected ion recording (SIR) chromatograms and positive-ion ESI-MS spectra. UPLC-MS-SIR chromatograms for synephrine at m/z 150.1 (A) and stachydrine at m/z 144.1 (C). The positive-ion ESI-MS spectra and the structure of synephrine (B) and stachydrine (D).
β2-AR activating ability of AS and synephrine
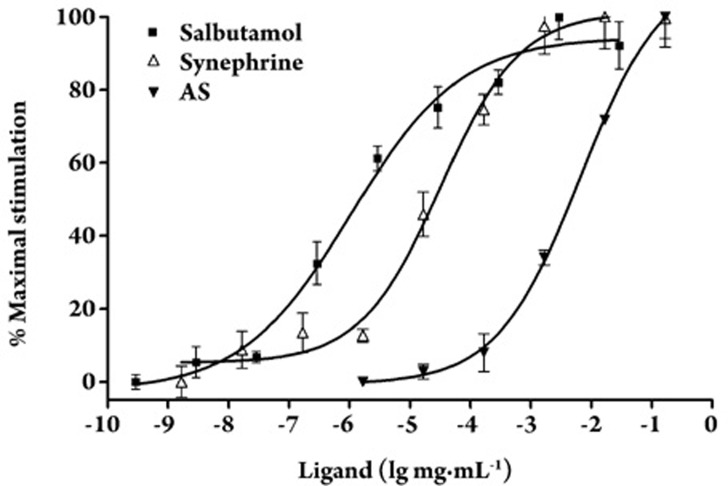
To affirm the β2-AR activating ability of synephrine, CHO-β2-AR-CRE-EGFP cells were treated with various concentrations of synephrine, AS, or the β2-adrenoceptor agonist salbutamol. Dose-dependent activity response curves for the samples are shown in Figure 3. The EC50 values of AS, synephrine, and salbutamol were 6.598×10−3, 3.191×10−5, and 1.056×10−6 mg/mL, respectively.
Figure 3.
Dose response curves of the activity of AS and synephrine in CHO-β2-AR-CRE-GFP cells. Data are expressed as the mean percentage of the maximal response induced by the respective ligands. Each bar represents mean±SEM. n=3.
Antitussive effects of AS and stachydrine in vivo
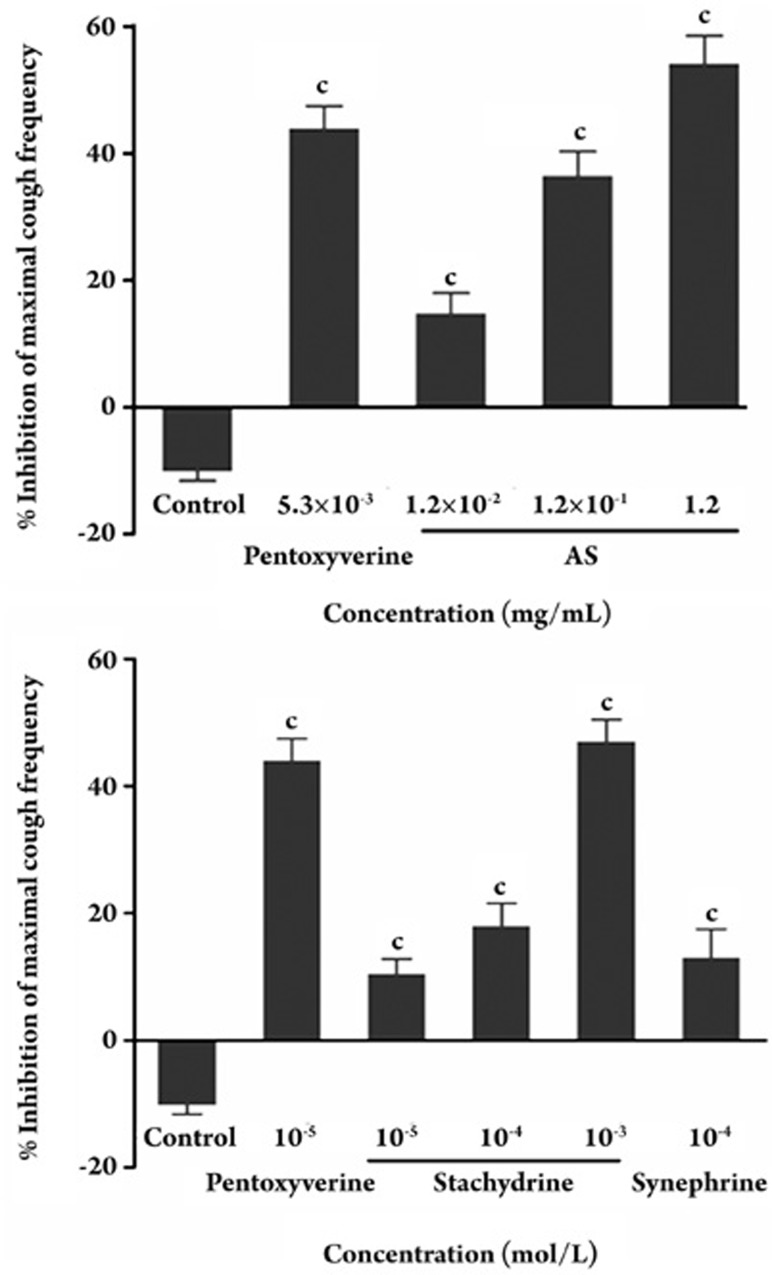
Since stachydrine is the most active component of AS, we evaluated the antitussive activities of AS and stachydrine hydrochloride using the guinea pig citric acid-induced coughing model. In all of the groups treated with AS and stachydrine, the cough response was inhibited in a dose-dependent manner with maximal inhibition of 10% (Figure 4). The concentration of stachydrine in AS, as determined by HPLC and MS analysis, was 4.53% (data not shown). Treatment with 0.012 to 1.2 mg/mL of AS (corresponding to a molar concentration of strychnine from 1×10−5 to 1×10−3 mol/L), inhibited the maximal cough frequency from 14.75%−54.16% during a 5-min monitoring period (Figure 4A). Treatment with 1×10−4, 1×10−5, and 1×10−6 mol/L of stachydrine decreased the percentage of coughs to 10.43 %±2.37%, 17.93%±3.65%, and 46.95%±7.57%, respectively (Figure 4B). We conclude that stachydrine contributed to the antitussive properties of the AS extract.
Figure 4.
Effects of AS (A) and stachydrine (B) on citric acid-induced coughing in guinea pigs. Percent inhibition of maximal cough frequency from the negative control frequency. Pentoxyverine was used as a positive control. Each bar represents the mean±SEM. n=6, cP<0.01 vs control.
Spasmolytic effect of AS and synephrine in vitro
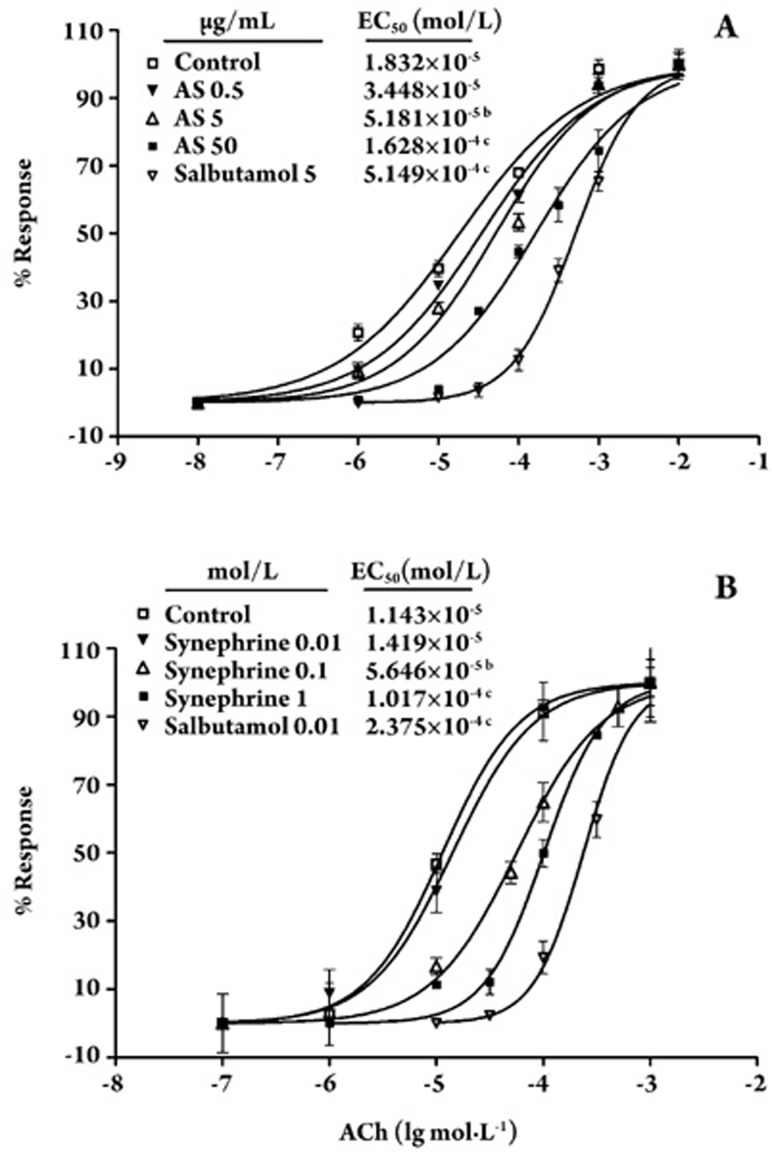
The abilities of synephrine and AS to relax the acetylcholine chloride (ACh)-induced contraction of guinea pig tracheae were compared with that of the positive control drug salbutamol. The bronchodilatory effects (shown as EC50) of AS and synephrine on ACh-contracted isolated guinea pig tracheal rings were statistically different from the control. The concentration of synephrine in AS was 32.5%, as determined by HPLC (data not shown). As shown in Figure 5, the AS and synephrine significantly inhibited ACh-induced tracheal contractions in a dose-dependent manner. AS at 1.4×10−2 or 1.4×10−3 mg/mL (corresponding to a molar concentration of synephrine at 1×10−4 or 10−5 mol/L) and synephrine at 1×10−4 or 1×10−5 mol/L significantly shifted the ACh dose-response curve to the right, while 1.4×10−4 mg/mL AS and 1×10−6 mol/L synephrine did not alter the curve. All EC50 values obtained from the dose-response curves are presented in Figure 5.
Figure 5.
Effect of AS and synephrine on the concentration-response curves of acetylcholine chloride (ACh). Tensions are expressed as percentages of peak contractile response, which is defined as 100%. A vertical line indicates mean±SEM. In each curve, n=6. bP<0.05, cP<0.01 vs control group.
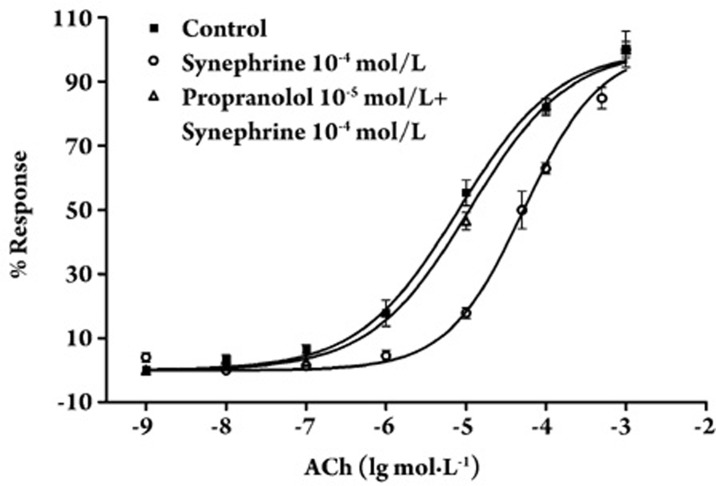
As shown in Figure 6, propranolol (1×10−5 mol/L), a blocker of β-ARs, significantly affected the inhibitory effect of 1×10−4 mol/L synephrine on ACh-induced tracheal contractions, and shifted the dose-response curve of ACh to the left. The EC50 value for ACh in rings treated with 1×10−4 mol/L of synephrine (5.262×10−5±0.36×10−5 mol/L) significantly differed from that of propranolol-treated strips co-treated with 1×10−4 mol/L of synephrine (1.103×10−5±0.38×10−5 mol/L). In conclusion, synephrine activated βARs to inhibit tracheal contractions, based on the observation that the propranolol-mediated inhibition of β-ARs significantly altered the relaxant effect induced by synephrine.
Figure 6.
Effect of 10−5 mol/L propranolol on the concentration–response curves of ACh in the presence of 10−4 mol/L of synephrine. The vertical line indicates mean±SEM. In each curve, n=3.
Spasmolytic effects of AS and synephrine in vivo
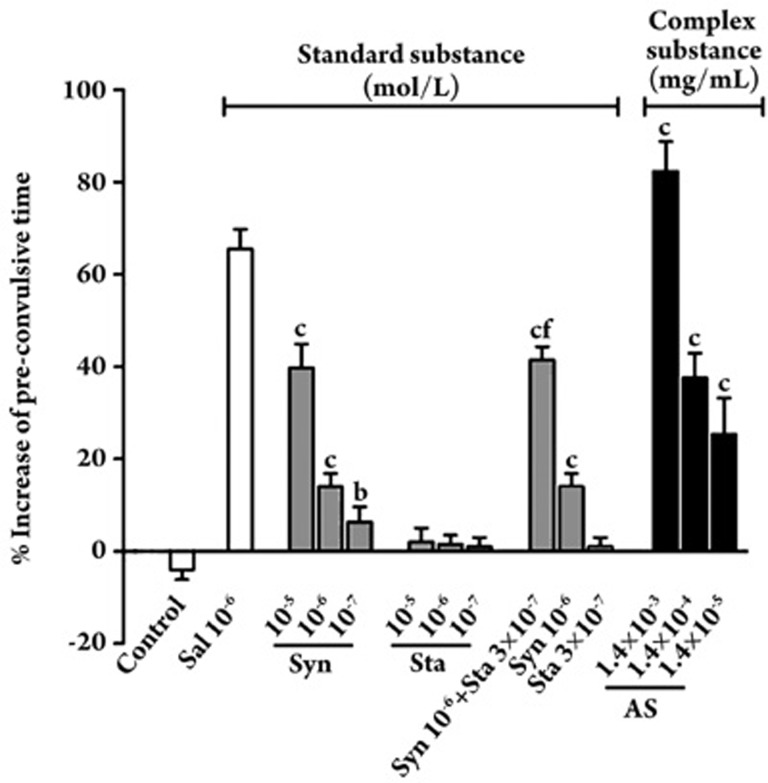
We next examined the effect of AS and its extracted synephrine on sensitive guinea pigs exposed to a 0.1% histamine spray (Figure 7). The positive control salbutamol (1×10−5 mol/L) prolonged the latent period by more than 62.0%. In the standard substance groups, treatment with 1×10−7, 1×10−6, and 1×10−5 mol/L of synephrine increased the pre-convulsive time by 6.4%±3.2%, 14%±2.83%, and 39.75%±5.12%, respectively. All dosage groups for synephrine effectively prolonged the pre-convulsive time in a dose-dependent manner, which significantly differed from that of the control group (P<0.01). However, there was no significant difference in the group that was treated with stachydrine alone. Therefore, we can conclude that synephrine was bronchoprotective and markedly reduced the severity of bronchoconstriction.
Figure 7.
The effect of the AS, synephrine (Syn), stachydrine (Sta) and the combination on the guinea pig asthma model induced by sprayed histamine. Values are presented as mean±SEM. n=6. bP<0.05 cP<0.01 vs control (saline inhalation group). fP<0.01 vs 10−6 mol/L synephrine (Syn).
In the complex substance group, which combined synephrine (1×10−6 mol/L) and stachydrine (3×10−7 mol/L), the latent period was prolonged to 41.2%, which significantly differed from the latent period resulting from the same dose of synephrine (P<0.01). This result was also confirmed in the case of 1.4×10−4 mg/mL of AS, which corresponded to concentrations of 1×10−6 mol/L for synephrine and 3×10−7 mol/L for stachydrine. When the guinea pigs were treated with 1.4×10−3, 1.4×10−4, and 1.4×10−5 mg/mL of AS, the percent increase in pre-convulsive time was 25.4%±7.8%, 37.6%±5.3%, and 82.2%±6.6%, respectively. We concluded that synephrine and stachydrine were the two major bronchodilating agents in PCR and that they reduced the severity of bronchoconstriction through synergistic action.
Discussion
Here, we successfully used a screening system based on the β2-AR signaling pathway to identify the β2-AR agonist synephrine in the crude extract of PCR by applying UPLC/Q-ToF-MS. Synephrine-induced tracheal relaxation was inhibited by the βAR blocker drug propranolol, indicating that PCR relieves airway bronchoconstriction by simulating βAR. Synephrine is a phenolic amine that has been used pharmacologically as a vascoconstrictor and bronchiectatic agent19. It promotes sympathomimetic activity that has been associated with signaling by β1, β2, β3, and α-adrenoceptors20. In this paper, the PCR extract, AS, significantly reduced ACh-induced contractions in isolated guinea pig tracheal rings by activating β2 adrenoceptors. It also protected against histamine-induced experimental asthma in guinea pigs by prolonging the latent period of asthma.
Airway constriction and cough are common symptoms of respiratory disease, and the relationship between these symptoms is complex. Nevertheless, effective asthma treatment is usually associated with reduced coughing22. In addition to its spasmolytic effects, which are listed under its description in ancient pharmacopeias, PCR also has significant antitussive effects. However, there are few published data explaining the mechanisms and specific components responsible for this activity. To specifically identify the antitussive compounds, we applied the guinea pig citric acid-induced coughing model to screen the fractions purified from AS. Stachydrine was identified from the PCR extract as having potent antitussive effects (Figure 4). Stachydrine (N, N-dimethylproline or Proline betaine) is a glycine betaine analogue. It is synthesized by many plants, especially alfalfa (Medicago sativa)23 and Capparis species24. However, to the best of our knowledge, there are few reports demonstrating that stachydrine has antitussive activity in the respiratory system. Further bioactivity studies demonstrated that synephrine (1×10−7 to 1×10−5 mol/L) induced dose-dependent relaxation effects in the tracheal strips isolated from a histamine-induced experimental asthma guinea pig model. Stachydrine (3×10−7 mol/L) had no effect when administered alone, but it increased the relaxing effects of synephrine to a greater extent than that obtained with a 10-fold higher concentration of synephrine alone (Figure 7). After comparing the effects of synephrine, stachydrine and the combination in vivo, we found that stachydrine had little spasmolytic effect alone, and the effects of the combination were more potent than administering synephrine alone. Therefore, the more potent spasmolytic effects induced by AS as compared with synephrine alone may be due to the synergistic effect of synephrine and stachydrine.
The antitussive effect of stachydrine was similar to that of pentoxyverine; however, we could not define the exact mechanism of the antitussive response from the present study. There is increasing evidence that the sensory airway nerves mediate the asthmatic response. Nerve endings are exposed by the continual epithelial shedding that occurs with asthma, and as a consequence, they may become hyper-sensitized and activated by inflammatory mediators. These activated nerve endings can release neuropeptides that spread and amplify inflammatory processes in the airways, leading to a vicious inflammatory cycle. Cough is believed to result from sensory nerve activation25. In this study, synephrine acted as a bronchodilator and showed little antitussive effects. βAR agonists act as bronchodilators because they loosen the muscles around the bronchial tubes, which can soothe spasms and suppress the asthmatic cough. This may explain, to some extent, why bronchodilating agents function as cough suppressors.
The anti-asthmatic alkaloid fraction was successfully obtained from PCR extract through a series of pharmacological evaluations in vitro and in vivo. Our results confirm that PCR AS may protect against wheezing, cough, and dyspnea that is induced during asthmatic attacks, and these effects are specifically mediated by synephrine and stachydrine in PCR. Bronchodilation induced by PCR and synephrine in vitro appears to be related to increased β-adrenergic activity. Stachydrine displayed significant antitussive effects in vivo. These bronchodilatory and antitussive effects of PCR might explain its traditional use in TCM as an anti-asthmatic remedy.
Author contribution
Gang BAI designed research; Qian SHI, Ze LIU, Yang YANG, and Peng GENG performed research; Fang BAI contributed new analytical tools and reagents; Yuan-yuan ZHU analyzed data; Qi ZHANG wrote the paper.
Acknowledgments
The work was supported in part by the National High Technology Research and Development Program of China (863 Program, No 2006AA020502), and Grant No 07JCZDJC05000 from the Natural Science Foundation of Tianjin, China.
References
- The Public Health Department of People's Republic of China. Pharmacopoeia of the People's Republic of China. Beijing: Chemical Industry Press; 2000. vol 1: p 148–9.
- Editor Committee for the Encyclopedia of Chinese traditional medicines. Encyclopedia of Chinese traditional medicines. Beijing: Encyclopedia of China Publishing House; 1999. vol 4: p 886–91.
- Kaur M, Chivers JE, Giembycz MA, Newton R. Long-acting β2-adrenoceptor agonists synergistically enhance glucocorticoid-dependent transcription in human airway epithelial and smooth muscle cells. Mol Pharmacol. 2008;73:203–14. doi: 10.1124/mol.107.040121. [DOI] [PubMed] [Google Scholar]
- Annesi-Maesano I. Asthma epidemiology. Rev Prat. 2005;55:1295–8. [PubMed] [Google Scholar]
- Rabe KF, Schmidt DT. Pharmacological treatment of asthma today. Eur Respir J Suppl. 2001;34:34s–40s. doi: 10.1183/09031936.01.00252501. [DOI] [PubMed] [Google Scholar]
- Fang SP, Tanaka T, Tago F, Okamoto T, Kojima S. Immunomodulatory effects of gyokuheifusan on INF-gamma/IL-4 (Th1/Th2) balance in ovalbumin (OVA)-induced asthma model mice. Biol Pharm Bull. 2005;28:829–33. doi: 10.1248/bpb.28.829. [DOI] [PubMed] [Google Scholar]
- Lee SK, Wong CK, Sung RY, Leung TF, Fung KP, Leung PC, et al. In vitro anti-allergic activities of a newly concocted traditional Chinese medicine-the wheeze-relief formula. J Ethnopharmacol. 2006;103:406–12. doi: 10.1016/j.jep.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Hill SJ, Baker JG, Rees S. Reporter-gene systems for the study of G-protein-coupled receptors. Curr Opin Pharmacol. 2001;1:526–32. doi: 10.1016/s1471-4892(01)00091-1. [DOI] [PubMed] [Google Scholar]
- Montminy MR, Gonzalez GA, Yamamoto KK. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990;13:184–8. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- Lalli E, Sassone-Corsi P. Signal transduction and gene regulation: the nuclear response to cAMP. J Biol Chem. 1994;269:17359–62. [PubMed] [Google Scholar]
- Chen J, Bai G, Yang Y, Geng P, Cao Y, Zhu Y. Identifying glucagon-like peptide-1 mimetics using a novel functional reporter gene high-throughput screening assay. Peptides. 2007;28:928–34. doi: 10.1016/j.peptides.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Durocher Y, Perret S, Thibaudeau E, Gaumond MH, Kamen A, Stocco R, et al. A reporter gene assay for high-throughput screening of G-protein-coupled receptors stably or transiently expressed in HEK293 EBNA cells grown in suspension culture. Anal Biochem. 2000;284:316–26. doi: 10.1006/abio.2000.4698. [DOI] [PubMed] [Google Scholar]
- Kunapuli P, Ransom R. Development of an intact cell reporter gene beta-lactamase assay for G protein-coupled receptors for high-throughput screening. Anal Biochem. 2003;314:16–29. doi: 10.1016/s0003-2697(02)00587-0. [DOI] [PubMed] [Google Scholar]
- Bai G, Yang Y, Shi Q, Liu Z, Zhang Q, Zhu YY. Identification of higenamine in Radix Aconiti Lateralis Preparata as a beta2-adrenergic receptor agonist. Acta Pharmacol Sin. 2008;29:1187–94. doi: 10.1111/j.1745-7254.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- Forsberg K, Karlsson JA, Theodorsson E, Lundberg JM, Persson CG. Cough and bronchoconstriction mediated by capsaicin-sensitive sensory neurons in the guinea-pig. Pulm Pharmacol. 1988;1:33–9. doi: 10.1016/0952-0600(88)90008-7. [DOI] [PubMed] [Google Scholar]
- Chu X, Xu Z, Wu D, Zhao A, Zhou M, Qiu M, et al. In vitro and in vivo evaluation of the anti-asthmatic activities of fractions from Pheretima. J Ethnopharmacol. 2007;111:490–5. doi: 10.1016/j.jep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Hashiba E, Sato T, Hirota K, Hashimoto Y, Matsuki A. The relaxant effect of propofol on guinea pig tracheal muscle is independent of airway epithelial function and β-adrenoceptor activity. Anesth Analg. 1999;89:191–6. doi: 10.1097/00000539-199907000-00034. [DOI] [PubMed] [Google Scholar]
- Vogel HG, Vogel WH.Drug discovery and evaluation: pharmacological assaysSpringer-Verlag: Berlin Heidelberg; 1997.
- Lin FY, Chen YH, Chen YL, Wu TC, Li CY, Chen JW, et al. Ginkgo biloba extract inhibits endotoxin-induced human aortic smooth muscle cell proliferation via suppression of toll-like receptor 4 expression and NADPH oxidase activation. J Agric Food Chem. 2007;55:1977–84. doi: 10.1021/jf062945r. [DOI] [PubMed] [Google Scholar]
- Takei H, Hirabuki M, Yoshizaki F. Analysis of synephrine in the peel of citrus fruit, immature citrus fruit and decoctions of Chinese medicinal prescriptions containing these crude drugs by capillary electrophoresis. Anal Sci. 1999;15:1017–20. [Google Scholar]
- Fugh-Bergman A, Myers A. Citrus aurantium, an ingredient of dietary supplements marketed for weight loss: current status of clinical and basic research. Exp Biol Med (Maywood) 2004;229:698–704. doi: 10.1177/153537020422900802. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV. Chronic cough due to asthma: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:75S–9S. doi: 10.1378/chest.129.1_suppl.75S. [DOI] [PubMed] [Google Scholar]
- Trinchant JC, Boscari A, Spennato G, Van de Sype G, Le Rudulier D. Proline betaine accumulation and metabolism in alfalfa plants under sodium chloride stress. Exploring its compartmentalization in nodules. Plant Physiol. 2004;135:1583–94. doi: 10.1104/pp.103.037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana T, Mathews AA, Vijetha P. Phytochemical and pharmacological review of some Indian Capparis species. Phcog Rev. 2008;2 4 Suppl:36–45. [Google Scholar]
- Barnes PJ. Effect of nedocromil sodium on airway sensory nerves. J Allergy Clin Immunol. 1993;92:182–6. doi: 10.1016/0091-6749(93)90103-m. [DOI] [PubMed] [Google Scholar]