Abstract
Aim:
Recent studies have demonstrated that mesenchymal stem cells (MSCs) can differentiate into endothelial cells. The effect of shear stress on MSC differentiation is incompletely understood, and most studies have been based on two-dimensional systems. We used a model of tissue-engineered vascular grafts (TEVGs) to investigate the effects of shear stress on MSC differentiation.
Methods:
MSCs were isolated from canine bone marrow. The TEVG was constructed by seeding MSCs onto poly-ɛ-caprolactone and lactic acid (PCLA) scaffolds and subjecting them to shear stress provided by a pulsatile bioreactor for four days (two days at 1 dyne/cm2 to 15 dyne/cm2 and two days at 15 dyne/cm2).
Results:
Shear stress significantly increased the expression of endothelial cell markers, such as platelet-endothelial cell adhesion molecule-1 (PECAM-1), VE-cadherin, and CD34, at both the mRNA and protein levels as compared with static control cells. Protein levels of alpha-smooth muscle actin (α-SMA) and calponin were substantially reduced in shear stress-cultured cells. There was no significant change in the expression of α-SMA, smooth muscle myosin heavy chain (SMMHC) or calponin at the mRNA level.
Conclusion:
Shear stress upregulated the expression of endothelial cell-related markers and downregulated smooth muscle-related markers in canine MSCs. This study may serve as a basis for further investigation of the effects of shear stress on MSC differentiation in TEVGs.
Keywords: mesenchymal stem cells, shear stress, endothelial cells, differentiation, platelet-endothelial cell adhesion molecule-1, vWF, VE-cadherin, α-smooth muscle actin, calponin
Introduction
Vascular tissue engineering is a novel concept used to overcome the shortage of small-diameter vascular grafts. Traditional methods of vascular tissue engineering include sequential seeding of smooth muscle cells (SMCs) and endothelial cells (ECs) onto collagen1, biodegradable scaffolds2 or decelluarized matrices3. Procurement of SMCs and ECs involves sacrificing a blood vessel, and terminally differentiated vascular cells are prone to senescence4. Much attention has been focused on progenitor cells and stem cells, and they have been successfully used in cardiovascular tissue engineering. Kaushal et al5 successfully seeded endothelial progenitor cells (EPCs) onto a decellularized arterial matrix and transplanted it as a carotid graft. Sutherland et al6 successfully constructed heart valves using mesenchymal stem cells (MSCs) and biodegradable scaffolds. Among the stem cells used in cardiovascular tissue engineering, MSCs seem to be an ideal cell source because they can differentiate into SMCs and ECs in vitro7, 8 and in vivo6, 9 and can be expanded by several million-fold in culture. MSCs harvested from patients' bone marrow do not have the problem of immune rejection, unlike embryonic stem cells. In fact, many studies have indicated that MSCs themselves could be the cell source for cardiovascular tissue engineering6, 10, 11, 12.
Biochemical and biomechanical factors can have large effects on MSC differentiation. MSCs will differentiate into ECs when cultured in 2% fetal calf serum (FCS) and 50 ng/mL vascular endothelial growth factor (VEGF)7. Wang et al13 demonstrated endothelial differentiation in a mesenchymal progenitor cell line, CH3H/10T1/2, resulting from exposure to shear. Most studies investigating the effects of shear stress on the differentiation of stem cells have used planar model systems13, 14. It is necessary to investigate whether MSCs seeded onto tubular scaffolds can differentiate into ECs after exposure to shear stress conferred by a pulsatile flow apparatus in order to construct vascular grafts with MSCs as the cell source.
We attempted to investigate the response of MSCs to shear stress in a model of tissue-engineered vascular grafts (TEVGs). MSCs were isolated from canine bone marrow, expanded in vitro, and seeded onto scaffolds comprising a copolymer of ɛ-caprolactone and lactic acid (PCLA). MSC-seeded scaffolds were loaded into a pulsatile bioreactor, and shear stress was increased from 1 dyne/cm2 to 15 dyne/cm2 for two days and then to 15 dyne/cm2 for another two days. Cellular responses were characterized by assays of cellular morphology, flow cytometry, and gene expression.
Materials and methods
All procedures were carried out according to the Regulations for the Administration of Affairs Concerning Experimental Animals of the Republic of China.
Cell source
Bone marrow aspirates were obtained from the iliac crest of mongrel dogs. MSCs were isolated and expanded in culture as described by Pittenger et al15. MSCs at passage 2 or passage 3 were used for the experiments.
Biodegradable scaffolds
PCLA was synthesized by ring-opening polymerization. The copolymer was a polyester with a molar composition of ɛ-caprolactone and lactic acid at a ratio of 79:21. Using this copolymer, we fabricated a tubular scaffold that was 4 mm in diameter, 6 cm in length, and 0.7 mm in thickness by electrospinning. Pore sizes in the scaffold varied from approximately 10 μm to 30 μm, and the corresponding porosities were 75%–80%. Scaffolds were sterilized by γ-irradiation before cell seeding.
Cell seeding and shear stress experiments
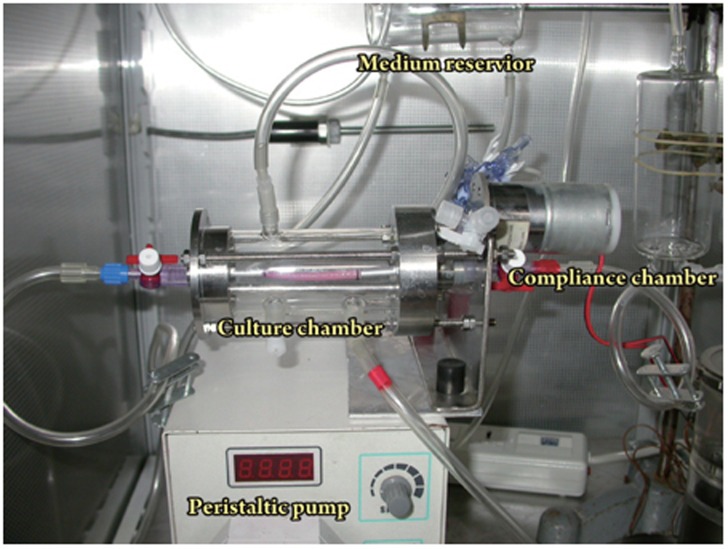
Tubular scaffolds were seeded with MSCs at passage 2 or passage 3 at a concentration of 3×106 cell/mL. They were then mounted onto a low-speed motor at a constant rate of 6 rotations per hour and incubated at 37 °C for 3 h. MSC-seeded scaffolds were installed horizontally in a pulsatile flow bioreactor (Figure 1), which consisted of a peristaltic pump, a glass culture reservoir with inlets and outlets for medium circulation and air exchange, a compliance chamber, and a culture chamber. The glass reservoir upstream of the peristaltic pump provided fluid for circulation and a cap for gasification with 5% CO2/95% air. The flow rate was measured downstream of the tube using a T106 small blood flow meter (Transsonic Ithaca, NY, USA). The perfusion pressure was 40−50 mmHg. Mean shear stress (τmean) was calculated as follows:
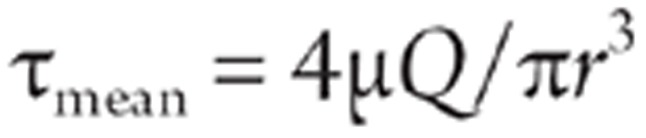 |
where μ is the viscosity of the culture medium. In this study, μ was 1 cP. Q is the flow rate of the bioreactor, which ranged from 0 to 1000 ml/min, and r is the radius of the scaffolds16. Direct exposure of MSCs seeded onto the tubular scaffolds to shear stress of a physiological level (15 dyne/cm2) would cause the cells to detach from the scaffolds. Kaushal5 et al reported that gradually increasing the shear stress from low (1 dyne/cm2) to high (25 dyne/cm2) over two days resulted in high retention of the cells on the scaffolds. In this study, shear stress was initiated at 1 dyne/cm2 and gradually increased from 1 dyne/cm2 to 15 dyne/cm2 over a two-day period. Scaffolds were then subjected to 15 dyne/cm2 for another two days. MSCs cultured statically in culture dishes were used as the control group.
Figure 1.
Overview of the whole bioreactor. (1) Culture chamber, (2) Peristaltic pump; (3) Compliance chamber, and (4) Culture reservoir.
Flow cytometry
Cultured cells were detached with 0.25% trypsin–0.02% ethylenediamine tetraacetic acid (EDTA) and incubated with monoclonal antibodies against CD34 or CD44 followed by Texas Red dye-conjugated anti-mouse IgG antibody or fluorescein isothiocyanate (FITC)-conjugated anti-rat IgG antibody. Background fluorescence was obtained from negative control cells stained with the secondary antibody and subtracted from the mean fluorescence of the specific staining patterns. Quantitative analysis was done using a FACScalibur flow cytometer.
Real-time reverse transcriptase polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted and reverse-transcribed into cDNA. Real-time quantitative RT-PCR primers targeting canine CD31, VE-cadherin, smooth muscle (SM) α-actin, smooth muscle myosin heavy chain (SMMHC), and calponin were designed by Primer Premier 5 software (sequences are listed in Table 1). The SYBR Green I assay and Line-gene Real-time RT-PCR detection system were used for detecting real-time quantitative PCR products from 50 ng of reverse-transcribed cDNA. The Ct value is the cycle number at which fluorescence reaches a threshold. The ΔCt is determined by subtracting the Ct of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control from the Ct of the target gene [ΔCt=Ct (target)–Ct (GAPDH)]. The relative value of target genes to the endogenous reference is described as the fold of GAPDH=2−ΔCt.
Table 1. Oligonucleotide primers used for gene expression analysis by real-time PCR.
| Gene | Primer sequences, 5′–3′ | Accession number | Size of amplified fragment, bp |
|---|---|---|---|
| CD31 | Fwd: TGGTCAGTGAAGTTCTGCGTGT Rev: ACCGAGCACAGAAGCTCAATG | XM_848326.1 | 114 |
| VE-cadherin | Fwd: TCTTCCGAATAACCAAGCAA Rev: TGTGGGGTACCCACTAGAAT | XM_546894.2 | 119 |
| α-SMA | Fwd: CGCCACATCTCAACTCTGAA Rev: GCTGAAGCCTGTTCTTGGTC | XM_857817 | 190 |
| SMMHC | Fwd: TGCGTGACATCAAGGAGAAG Rev: TGCTGTTGTAGGTGGTCTCG | XM_534781 | 228 |
| GAPDH | Fwd: AACATCATCCCTGCTTCCAC Rev: GACCACCTGGTCCTCAGTGT | NM_001003142.1 | 234 |
VE–cadherin, vascular endothelial cadherin; α-SMA, α-smooth muscle actin; SMMHC, smooth muscle myosin heavy chain; GAPDH, glyceralde-hyde-3-phosphate dehydrogenase; Fwd, forward; Rev, reverse.
Western blotting
Western blotting was used to evaluate expression of alpha smooth muscle actin (α-SMA) and calponin after 4 days of shear stress. Cultured cells were detached from scaffolds or culture dishes with 0.25% trypsin and 0.02% EDTA. They were homogenized in lysis buffer, and 30 μg of each sample was separated on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel. After protein transfer to nitrocellulose membrane, the immunoreactivity of α-SMA and calponin was detected by enhanced chemiluminescence.
Scanning electron microscopy (SEM)
Samples were subjected to SEM to study the gross morphology and orientation of the cells. Each scaffold was imaged at 5 kV on a Hitachi S-4800 field emission scanning electron microscope.
Statistical analysis Results are mean±standard deviation (SD) from at least three experiments. The statistical significance of the differences was evaluated by Student's t-test. P<0.05 was considered significant.
Results
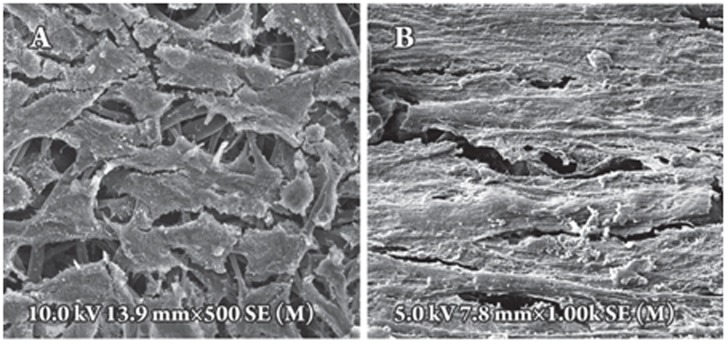
Effect of shear stress on cell morphology of MSCs Shear stress had a distinct effect on the morphology of MSCs. The appearance of cells changed from a fibroblast-like appearance (Figure 2A) to a more fusiform shape (Figure 2B) after exposure to shear stress for four days. Under static culture conditions, MSCs were randomly distributed with little alignment (Figure 2A). After four days of shear stress, cells were elongated and aligned in the direction of flow (Figure 2B).
Figure 2.
Effect of shear stress on the morphology and alignment of cells. SEM images of MSCs cultured under static conditions with the MSCs oriented randomly (A), or of MSCs exposed to shear stress for four days in which the MSCs are elongated and aligned parallel to the direction of flow (B).
Flow cytometry analysis
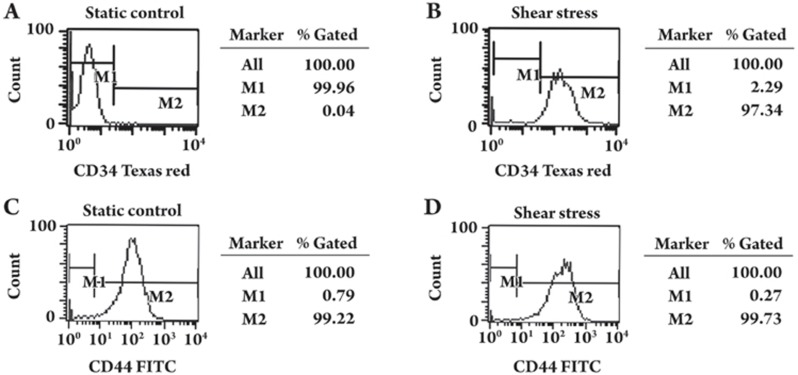
The cellular protein level of CD34 and CD44 was determined by flow cytometry. Statically cultured MSCs were 99.96% CD34-negative (Figure 3A) and 99.22% CD44-positive (Figure 3C). After being subjected to shear stress for four days, 97.34% of cells were CD34-positive (Figure 3B), and no significant change was found in the expression of CD44 (99.73% were positive after exposure to shear stress) (Figure 3D).
Figure 3.
Effect of shear stress on the expression of CD34 and CD44 by flow cytometric analysis. MSCs were cultured under static conditions (A, C) or exposed to shear stress (B, D) for four days (two days at 1 dyne/cm2 to 15 dyne/cm2 and two days at 15 dyne/cm2). MSCs were labeled with fluorescent antibodies to CD34 or CD44, and relative protein levels were determined by flow cytometry. M1: negative; M2: positive.
Gene expression profiles by real-time RT-PCR
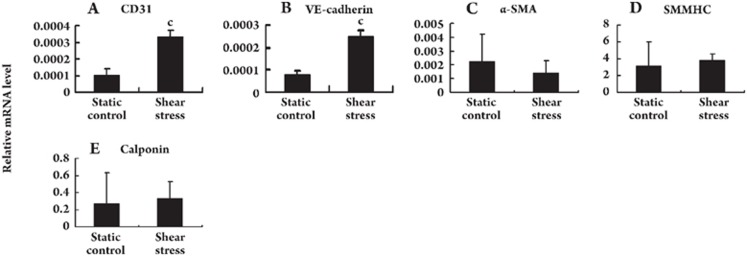
Gene expression in MSCs was examined by real-time RT-PCR. Shear stress markedly increased the mRNA levels of the EC markers CD31 (Figure 4A) and VE-cadherin (Figure 4B). The mRNA levels of the SM cell markers α-SMA (Figure 4C), SMMHC (Figure 4D) and calponin (Figure 4E) did not show a significant change.
Figure 4.
Effect of shear stress on the mRNA levels of various cell lineage markers. MSCs were cultured under static conditions or exposed to shear stress for four days (two days at 1 dyne/cm2 to 15 dyne/cm2 and two days at 15 dyne/cm2), and total RNA was extracted. Relative mRNA expression levels of CD31 (A), VE-cadherin (B), α-SMA (C), SMMHC (D), and calponin (E) were determined by real-time quantitative PCR. Values of mRNA amounts were normalized to GAPDH expression. Error bars represent SD. cP<0.01 vs static control.
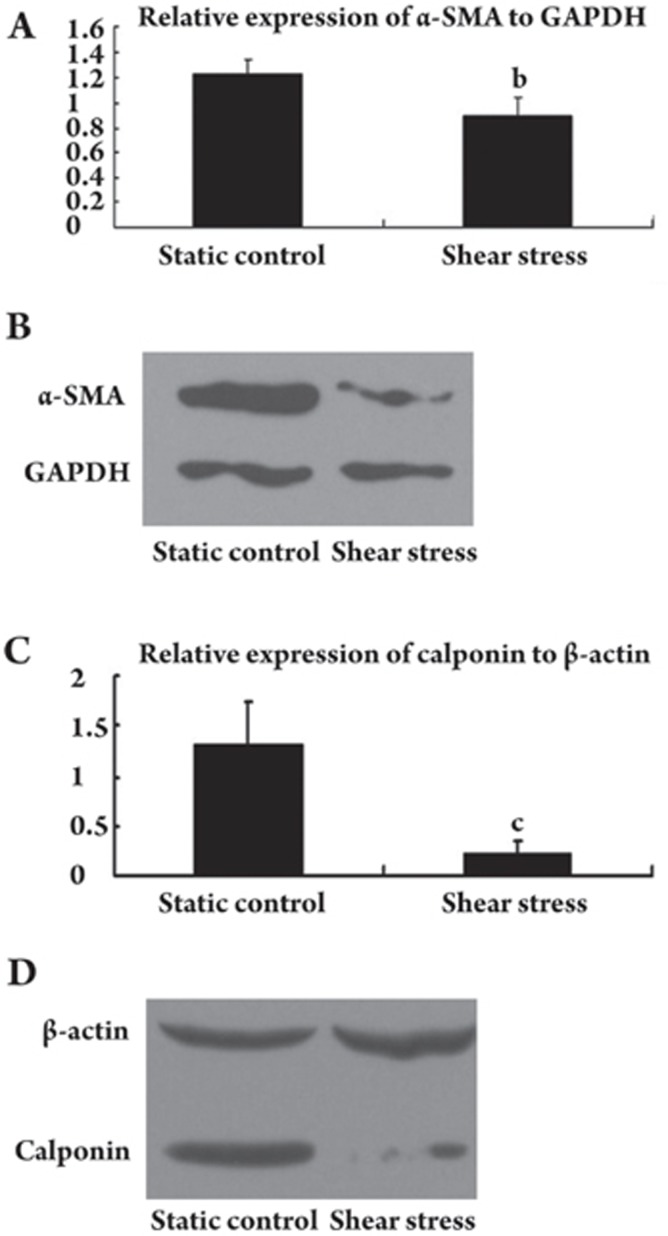
Expression of SMC markers in MSCs by Western blotting
The cellular protein levels of α-SMA and calponin were determined by Western blotting. Protein levels of α-SMA and calponin substantially decreased in the cells cultured under shear stress compared with cells cultured statically. The relative expression of α-SMA compared with GAPDH was 1.23±0.12 and 0.89±0.15 for statically cultured and shear stress-cultured MSCs, respectively (n=3, t=3.194, P=0.033; Figure 5A, 5B). The relative expression of calponin compared with β-actin was 1.32±0.42 and 0.22±0.12 for the statically cultured and shear stress-cultured MSCs, respectively (n=3, t=4.319, P=0.12; Figure 5C, 5D).
Figure 5.
Effect of shear stress on the expression of α-SMA and calponin in MSCs at the protein level. (A and B) MSCs cultured statically or exposed to shear stress (two days at 1 dyne/cm2 to 15 dyne/cm2 and two days at 15 dyne/cm2). Relative α-SMA protein expression levels were determined by Western blot analysis. Values of protein amounts were normalized to GAPDH expression. Error bars represent SD. bP<0.05 vs Static control. (C and D) Relative calponin protein expression levels determined by Western blot analysis. Values of protein amounts were normalized to β-actin expression. Error bars represent SD. cP<0.01 vs Static control.
Discussion
The current study demonstrated that shear stress changed the morphology and alignment of canine MSCs and induced the expression of EC-specific markers, such as CD31, VE-cadherin, and von Willebrand factor (vWF), at both the mRNA and protein levels in TEVGs. Shear stress induced CD34 expression, which is a surface maker of hematopoietic stem cells (HSCs) and EPCs, at the protein level. Shear stress also reduced the expression of the smooth muscle cell markers SMA and calponin at the protein level. A significant change in the expression of SMA and calponin was not detected at the mRNA level.
MSCs are bone marrow-derived cells with the potential to differentiate along several mesenchymal lineages, including differentiation to adipocytes, osteoblasts and chondrocytes15. Recent studies revealed that MSCs can differentiate into ECs and vascular SMCs in vivo6, 17, 18 or in vitro after chemical stimulation7, 19. Besides biochemical stimuli, biomechanical forces also have important roles in MSC differentiation. Fluid shear stress is a frictional force caused by blood flow, which acts parallel to the longitudinal axis of the vessel wall. Studies have suggested that laminar shear stress is responsible for arteriogenesis20, the migration of ECs21, the pathogenesis of atherosclerosis22 and EC differentiation23. Recent studies have shown that shear stress can induce stem cells (including embryonic stem cells14 and MSCs13) or SMCs24 to differentiate into ECs. In order to use MSCs as cell source in the tissue engineering of vascular grafts, it would be necessary to investigate whether MSCs can differentiate along the endothelial lineage after exposure to shear stress.
MSCs changed their morphology in response to shear stress. Cells were elongated and oriented in the direction of flow after exposure to shear stress for four days. Morphology and orientation were similar to those of the endothelium in the vasculature.
There are no specific surface markers for MSCs, and it is recognized that MSCs are positive for SH2, SH3, CD29, CD44, CD71, and CD106 and are negative for CD14, CD34, and CD4515. Flow cytometry demonstrated that cultured cells were 99.2% CD44-positive, whereas 99.96% were CD34-negative; ie, cultured cells were MSCs. After exposure to shear stress for four days (two days at 1 dyne/cm2 to 15 dyne/cm2 and two days at 15 dyne/cm2), 97.3% of the cells were CD34-positive, whereas there was no significant change in CD44 expression (99.2% and 99.7% in static and shear stressed cultures, respectively). CD34 is a 115-kDa transmembrane glycoprotein that is present on hematopoietic stem cells, EPCs25, and ECs26. A recent study revealed that CD34+ cells can differentiate into ECs under stimulation with VEGF and fibronectin27. CD34 expression may therefore indicate a transition from stem cells or progenitor cells to ECs.
Platelet-endothelial cell adhesion molecule-1 (PECAM-1; CD31), VE-cadherin and vWF are believed to be the most reliable markers for ECs and are often used to validate endothelial differentiation. Shear stress significantly increased the mRNA levels of CD31 and VE-cadherin. We also found vWF expression in the MSCs after exposure to shear stress (data not shown). This result was consistent with the findings from other studies that examined the effect of shear stress on cell differentiation13, 14, 23, 24, 28. The present study suggests that shear stress induces differentiation along an endothelial lineage in canine MSCs. O'Cearbhaill et al29 did not observe vWF expression on the protein level under static or mechanically stimulated conditions, nor did they observe a significant change in vWF at the mRNA level. The combined effect of radial stress and hoop stress used in the experiment may have surpassed the effect of shear stress in cell differentiation, because cyclic stress promotes the expression of smooth muscle-like properties10, 30, 31.
Because MSCs can also differentiate along SMC lines, we hypothesized that differentiation along the endothelial line might accompany shear stress-induced negative regulation of the expression of SMC-related markers, such as SM α-actin and calponin. A significant downregulation of SM α-actin and calponin were exhibited at the protein level when MSCs were cultured under shear stress. However, a significant change in the expression of SM α-actin and calponin at the mRNA level was not detected. Wang et al13 demonstrated that downregulation of transforming growth factor-beta (TGF-β), platelet-derived growth factor-B (PDGF-B) and the platelet-derived growth factor receptor (PDGFR) are associated with SMC differentiation. We suggest that the reduction in the expression of SMC-related markers occurred at the protein level but not at the level of mRNA, and was regulated by SMC differentiation-related growth factors, such as TGF-β and PDGF-B.
Cells that are seeded onto a compliant tube and then subjected to pulsatile flow will experience shear stress and circumferential strain. In the study by Huang et al32, embryonic stem cells differentiated along endothelial cell and smooth muscle cell lines on the luminal surface and in the interstices, respectively. In this study, MSCs seeded onto PCLA scaffolds were cultured in a pulsatile flow bioreactor. After 4 days of culture in the bioreactor, most of the cells were on the luminal surface and exhibited an endothelial phenotype. This could be the combinatorial result of shear stress and circumferential strain, in which the shear stress played the major role in this study.
Traditionally, tissue-engineered vascular grafts are formed by sequentially seeding SMCs and ECs onto a biodegradable2 or decellularized matrix8. This is a more complicated procedure and is more prone to contamination than a single-seeding process. Because MSCs can be induced to differentiate into ECs under shear stress, there is no need to seed ECs, which means less work and less expense. In vitro shear stress preconditioning can serve two purposes in TEVGs: (i) to promote adhesion of seeded cells onto scaffolds, preventing cells from dislodging after exposure to high stress in vivo5, 33, and (ii) to induce seeded MSCs to differentiate into ECs, which will provide an antithrombogenic surface for the grafts.
In summary, our study demonstrated that a physiological level of shear stress upregulated the expression of several EC surface markers at the mRNA and protein levels and downregulated the expression of SMC surface markers at the protein level in canine MSCs in a model of vascular tissue engineering. This study may serve as a basis for further investigation of the effect of shear stress on the differentiation of MSCs in TEVGs.
Author contribution
Jian-de DONG, Yong-quan GU, Chun-min LI, Jian ZHANG designed the research; Jian-de DONG, Chun-min LI, Shu-wen ZHANG performed the research; Chun-ren WANG, Zeng-guo FENG, Rong-xin QIU contributed new analytical tools and reagents; Bing CHEN, Jian-xin LI, Zhong-gao WANG analyzed the data; Jian-de DONG wrote the paper.
Acknowledgments
We are grateful to Dr Ye-qing CUI, Dr Cui-fei YE, and Dr Xue-jing SUN for their technical assistance and advice. We also thank David CUSHLEY for his critical editing of the paper (International Science Editing Compuscript Ltd, Co Clare, Republic of Ireland). This study was funded by the National High Technology Research and Development Program (“863” program) of China (No 2006AA02A134).
References
- Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Ito A, Arinobe M, Murase Y, Iwata Y, Narita Y, et al. Effective cell-seeding technique using magnetite nanoparticles and magnetic force onto decellularized blood vessels for vascular tissue engineering. J Biosci Bioeng. 2007;103:472–8. doi: 10.1263/jbb.103.472. [DOI] [PubMed] [Google Scholar]
- McKee JA, Banik SS, Boyer MJ, Hamad NM, Lawson JH, Niklason LE, et al. Human arteries engineered in vitro. EMBO Rep. 2003;4:633–8. doi: 10.1038/sj.embor.embor847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–40. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland FW, Perry TE, Yu Y, Sherwood MC, Rabkin E, Masuda Y, et al. From stem cells to viable autologous semilunar heart valve. Circulation. 2005;111:2783–91. doi: 10.1161/CIRCULATIONAHA.104.498378. [DOI] [PubMed] [Google Scholar]
- Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–84. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- Cho SW, Lim SH, Kim IK, Hong YS, Kim SS, Yoo KJ, et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506–15. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura G, Miyagawa-Tomita S, Shin'oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108:1729–34. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- Engelmayr GC, Jr, Sales VL, Mayer JE, Jr, Sacks MS. Cyclic flexure and laminar flow synergistically accelerate mesenchymal stem cell-mediated engineered tissue formation: implications for engineered heart valve tissues. Biomaterials. 2006;27:6083–95. doi: 10.1016/j.biomaterials.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Hashi CK, Zhu Y, Yang GY, Young WL, Hsiao BS, Wang K, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A. 2007;104:11915–20. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerstrup SP, Kadner A, Melnitchouk S, Trojan A, Eid K, Tracy J, et al. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation. 2002;106 12 Suppl 1:I143–50. [PubMed] [Google Scholar]
- Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, et al. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817–23. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H1915–24. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Cheng CP, Parker D, Taylor CA. Quantification of wall shear stress in large blood vessels using Lagrangian interpolation functions with cine phase-contrast magnetic resonance imaging. Ann Biomed Eng. 2002;30:1020–32. doi: 10.1114/1.1511239. [DOI] [PubMed] [Google Scholar]
- Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–6. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–8. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin (Shanghai) 2008;40:681–92. [PubMed] [Google Scholar]
- Urbich C, Dernbach E, Reissner A, Vasa M, Zeiher AM, Dimmeler S. Shear stress-induced endothelial cell migration involves integrin signaling via the fibronectin receptor subunits alpha(5) and beta(1) Arterioscler Thromb Vasc Biol. 2002;22:69–75. doi: 10.1161/hq0102.101518. [DOI] [PubMed] [Google Scholar]
- Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Takahashi T, Asahara T, Ohura N, Sokabe T, Kamiya A, et al. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol. 2003;95:2081–8. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- Wang H, Yan S, Chai H, Riha GM, Li M, Yao Q, et al. Shear stress induces endothelial transdifferentiation from mouse smooth muscle cells. Biochem Biophys Res Commun. 2006;346:860–5. doi: 10.1016/j.bbrc.2006.05.196. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Muller AM, Hermanns MI, Skrzynski C, Nesslinger M, Muller KM, Kirkpatrick CJ. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002;72:221–9. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- Wijelath ES, Rahman S, Murray J, Patel Y, Savidge G, Sobel M. Fibronectin promotes VEGF-induced CD34 cell differentiation into endothelial cells. J Vasc Surg. 2004;39:655–60. doi: 10.1016/j.jvs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Wu CC, Chao YC, Chen CN, Chien S, Chen YC, Chien CC, et al. Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J Biomech. 2008;41:813–21. doi: 10.1016/j.jbiomech.2007.11.008. [DOI] [PubMed] [Google Scholar]
- O'Cearbhaill ED, Punchard MA, Murphy M, Barry FP, McHugh PE, Barron V. Response of mesenchymal stem cells to the biomechanical environment of the endothelium on a flexible tubular silicone substrate. Biomaterials;2008;29:1610–9. doi: 10.1016/j.biomaterials.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Yasu T, Ueba H, Sata M, Hashimoto S, Kuroki M, et al. Mechanical stress promotes the expression of smooth muscle-like properties in marrow stromal cells. Exp Hematol. 2004;32:1238–45. doi: 10.1016/j.exphem.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359–68. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- Huang H, Nakayama Y, Qin K, Yamamoto K, Ando J, Yamashita J, et al. Differentiation from embryonic stem cells to vascular wall cells under in vitro pulsatile flow loading. J Artif Organs. 2005;8:110–8. doi: 10.1007/s10047-005-0291-2. [DOI] [PubMed] [Google Scholar]
- Zhang ZX, Xi TF, Wang YJ, Chen XS, Zhang J, Wang CR, et al. In vitro study of endothelial cells lining vascular grafts grown within the recipient's peritoneal cavity. Tissue Eng Part A. 2008;14:1109–20. doi: 10.1089/ten.tea.2007.0219. [DOI] [PubMed] [Google Scholar]