Abstract
Aim:
The purpose of this work was to search for potential drugs with potent antitussive and expectorant activities as well as a low toxicity, but without addictive properties. Cholic acid-verticinone ester (CA-Ver) was synthesized based on the clearly elucidated antitussive and expectorant activities of verticinone in bulbs of Fritillaria and different bile acids in Snake Bile. In our previous study, CA-Ver showed a much more potent activity than codeine phosphate. This study was carried out to investigate the central antitussive mechanism and the addictive evaluation of CA-Ver.
Methods:
Testing on a capsaicin-induced cough model of mice pretreated with naloxone, a non-selective opioid receptor antagonist, was performed for the observation of CA-Ver's central antitussive mechanism. We then took naloxone-induced withdrawal tests of mice for the judgment of CA-Ver's addiction. Lastly, we determined the opioid dependence of CA-Ver in the guinea pig ileum.
Results:
The test on the capsaicin-induced cough model showed that naloxone could block the antitussive effect of CA-Ver, suggesting the antitussive mechanism of CA-Ver was related to the central opioid receptors. The naloxone-urged withdrawal tests of the mice showed that CA-Ver was not addictive, and the test of the opioid dependence in the guinea pig ileum showed that CA-Ver had no withdrawal response.
Conclusion:
These findings suggested that CA-Ver deserved attention for its potent antitussive effects related to the central opioid receptors, but without addiction, and had a good development perspective.
Keywords: cholic acid-verticinone ester, addictive evaluation, opioid receptors, agonist action, naloxone, ileum, opioid dependence, addiction
Introduction
Hubeibeimu, listed in Pharmacopoeia of the People's Republic of China (Chp)2000 and Chp2005, is the dried bulb of Fritillaria hupehensis Hsiao et KC Hsia. Within the realm of Chinese medicine, Hubeibeimu has been claimed to resolve phlegm, stop cough, and clear away heat, and dispel accumulation; it is widely used in Traditional Chinese Medicine. Recent pharmacological studies demonstrated that the total and single alkaloids (eg, verticinone) of this medicine had antitussive activity1. Verticinone is the main alkaloid of F hupehensis Hsiao et KC Hsia, comprising about 0.3% of the crude herb. Verticine, the other main alkaloid of F hupehensis Hsiao et KC Hsia, is the alcohol derivative of verticinone. Verticine is easily oxidized to verticinone. Together, the two make up over 80% of the total alkaloids. Shedan is a valuable Chinese crude drug. Systematic chemical and pharmacological research studies have confirmed that its main components are taurocholic acid and various kinds of free cholic acids2, 3, 4.
Enlightened with the “combination principle” in drug discovery, we synthesized cholic acid-verticinone ester (CA-Ver), a novel derivative of verticinone and cholic acid. The pharmacodynamic studies on the antitussive and expectorant activities of CA-Ver were then screened with different animal models. CA-Ver showed a much more potent antitussive activity than codeine phosphate. A further acute toxicity study showed that the LD50 value of CA-Ver exceeded 3.5 g·kg−1 by intraperitoneal injection in mice. CA-Ver showed a potential future in the development of a new antitussive drug.
In a previous study5, we assayed the influences of CA-Ver on five kinds of monoamine neurotransmitters in mouse brain, using reverse-phase HPLC method with a fluorescent light detector6, 7 and with codeine and dextromethorphan as positive drugs8. The results indicated that the antitussive mechanism of dextromethorphan concerned the monoamine neurotransmitters 5-HT. Further, dextromethorphan could increase the amount of 5-HT in the animal's brain to produce antitussive effects, while the antitussive effects of CA-Ver had no relationship with serotonergic mechanisms, just as codeine. This study was carried out to investigate the central antitussive mechanism of CA-Ver. Codeine, a centrally acting, narcotic antitussive drug, is one of the most commonly used potent antitussive agents; however, addiction to codeine limits the clinical usefulness of this agent9. In order to determine whether CA-Ver is addictive like codeine, naloxone-urged withdrawal tests of mice were performed for the observation of addiction to CA-Ver. We then undertook a method for the quantitative determination of opioid dependence in the guinea pig ileum in vitro to confirm an absence of addiction to CA-Ver10, 11.
Materials and methods
Preparation of CA-Ver
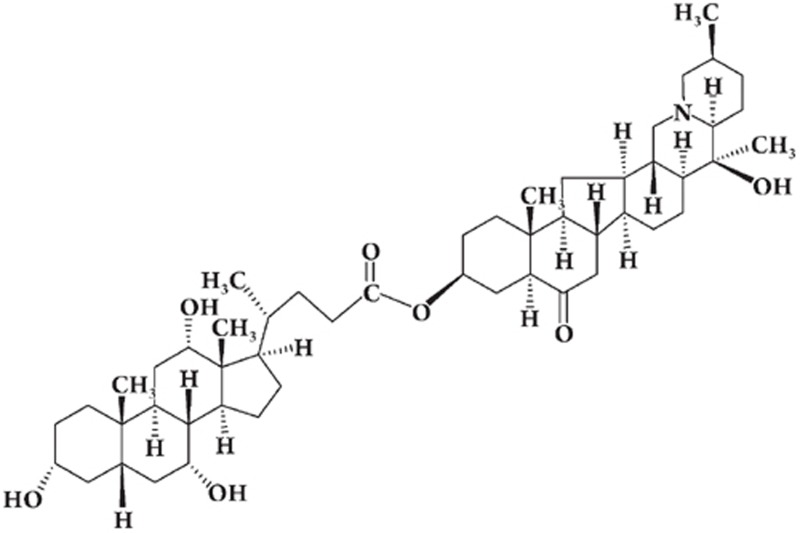
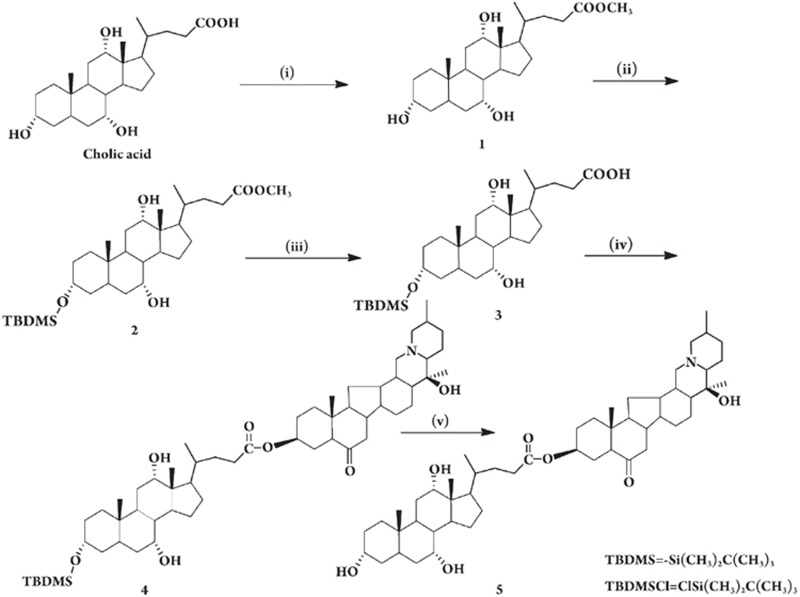
CA-Ver (Figure 1) was synthesized from cholic acid and verticinone via esterification, using dicyclohexylcarbodiimide (DCC) as the acid-activating agent and leading to the linkage of the carboxylic acid group on the C24 position of cholic acid, with the hydroxyl group on the C3 position of verticinone. The synthesis of CA-Ver is illustrated in Figure 2 (five steps, 33% overall yield). The reaction of cholic acid with absolute MeOH followed by esterification resulted in the formation of methyl 3α,7α,12α-trihydroxy-5β-cholan-24-oate. Then, the reaction of 1 with tert-butyl dimethyl chlorosilane (TBDMSiCl)12 via ether bond afforded the intermediate 2. Hydrolysis of the methyl ester groups of 2 with 5% sodium hydroxide aqueous solution produced 3. Then treatment of 3 with verticinone in CH2Cl2, followed by esterification, gave the compound 5α,14α-cevanin-6-O-20β-hydroxy-3β-yl-3α-tert-butyldimethylsilyloxy-7α,12α-dihydroxy-5β-cholan-24-oate, 4. In this reaction, 4-dimethylaminopyridine (DMAP) was used as a catalyst and N,N′-dicyclohexylcarbodiimide (DCC) as a dehydration agent13. Finally, the deprotection reaction of 4 in 5% HF14 aqueous solution gave the desired 5α,14α-cevanin-6-O-20β-hydroxy-3β-yl-3α,7α,12α-trihydroxy-5β-cholan-24-oate, 5. The chemoselective esterification reaction of 3 with verticinone in CH2Cl2 provided 4 in 58.9% yield.
Figure 1.
Structure of CA-Ver. (5α,14α-cevanin-6-O-20β-hydroxy-3β-yl-3α,7α,12α-trihydroxy-5β-cholan-24-oate)
Figure 2.
Reagents and conditions. (i) MeOH/concentrated sulfuric acid, room temperature (rt) 1 day; (ii) TBDMSCl/imidazole/THF, low temperature, 48 h; (iii) 1: 10% NaOH/THF, rt, 12 h; 2: H2O/H+, rt, 30 min; (iv) verticinone/DCC/DMAP/CH2Cl2, rt, 48 h. (v) 5% HF(aq)/THF, rt, 24 h.
Chemicals
The CA-Ver was synthesized by us (the purity of CA-Ver was determined by HPLC to be ≥98.0%). The codeine phosphate and morphine hydrochloride were purchased from the Qinghai Pharmaceutical Factory (Qinghai, China). The naloxone hydrochloride injection was purchased from the Chengdu Beite Pharmaceutical Factory (Chengdu, China). Acetylcholine chloride (99%) was obtained from Sigma-Aldrich. Verticinone was isolated from F hupehensis Hsiao et KC Hsia, which is commercially available from the Hubei Institute of Chinese Materia Medica (Wuhan, China) and was identified by Prof De-tai PENG (Lichuan Institute of Chinese Materia Medica, Lichuan, Hubei province, China). A voucher specimen (F030823) was deposited at the Faculty of Pharmaceutical Science, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). The capsaicin was purchased from the Fluka reagent company (EC No 2069698, Purity ≥97.0% by HPLC).
Animals
Kunming mice of both sexes (Experimental Animal Center, Tongji Medical College, Huazhong University of Science and Technology, China), weighing about 30−40 g, were used for the test on capsaicin-induced cough model, and mice weighing about 13−17 g were used for the naloxone-urged withdrawal test. Male Dunkin-Hartley guinea pigs (300−350 g, Experimental Animal Center, Tongji Medical College, Huazhong University of Science and Technology, China) were killed by CO2 inhalation. All mice and guinea pigs had free access to food and water, and were maintained in a designated animal room, at 24±1 °C and with a 12-h light-dark cycle. This study was carried out in accordance with the Declaration of Helsinki and/or the “Regulation for the Administration of Affairs Concerning Experimental Animals” (State Council of China, 1988). Minimum numbers of animals were used to result in meaningful interpretation of data, and animal discomfort was kept to a minimum.
Antitussive assay
The cough reflex was induced as previously described by Kamei15, 16. Briefly, the unanesthetized animals were placed individually in a transparent tailor-made bottle (dimensions: 10 cm×5 cm×5 cm) and exposed to a nebulized solution of capsaicin (100 μmol/L) for 5 min using a body plethysmograph. The same nebulizer was used throughout the experiment. Capsaicin was dissolved in 10% ethanol and 10% Tween 80 saline solution at a concentration of 0.15 mg/mL, and the mixture was diluted with saline. The coughs produced during a 5 min exposure period were counted. The animals were selected by the number of coughs 72 h before the test. Animals with more than 30 or fewer than 8 coughs in a 5 min period were not used for the studies. After 72 h of recovery, the selected mice were randomly divided into several groups, with at least ten animals in each group, for different treatments. The CA-Ver and codeine phosphate were suspended in 0.5% carboxyl methylcellulose solution and were administered orally. The mice were exposed to capsaicin for 5 min beginning 30 min before the administration of antitussive agents to determine the frequency of the control coughs. The animals were again exposed to capsaicin aerosol for 60 min after the administration of the antitussive agents. The number of coughs produced after the drug administration (Ct) was compared with the number of control coughs (Cc). The antitussive effect was expressed as the percentage of inhibition of the number of control coughs [(Cc–Ct)/Cc]×100%.
The naloxone-urged withdrawal tests of mice
We used naloxone-urged withdrawal tests of mice for the judgment of addiction to the CA-Ver17, 18. In this study, we assayed the addiction to CA-Ver using codeine phosphate and morphine hydrochloride as the positive drugs. All drugs were mixed with quantitative forage powders, and then the mixtures were pressed into newly made animal feeds. The production of animal feed mixed with codeine is described as an example of the process. First, 1600 mg codeine phosphate was ground to a powder and mixed with 800 g of forage powder by the method of the isometric increasing principle. Then, we added 800 mL of distilled water to the mixture. Finally, we passed the farina through a 5 mL syringe to produce a strip, which was dried, first at room temperature for 24 h, then at 50 °C for 24 h. Other animal feeds mixed with different drugs were made with the same procedure as described for codeine phosphate, but with different dosages. Mice were divided into five groups of ten animals. Group 1 animals (Control) were treated with the animal feeds without drug for 15 days. Group 2 was treated with codeine phosphate as the positive drug. Group 3 was treated with morphine hydrochloride and Group 4 was treated with verticinone. Group 5 was treated with CA-Ver. Each mouse's body weight was measured at 9:00 am daily. Each group was fed with forage plus drugs for 15 days. Each mouse's body weight was recorded at 12:00 am on the 16th day. Immediately thereafter, naloxone (4 mg/kg) was injected (ip) and the mouse was put on a 30 cm diameter frustum at a height of 35 cm for observation. During the 30 min observation period, the mouse was watched continuously by a trained observer and the frequency of jumps was recorded. At 30 min, the mouse was once again weighed, fed with forage without drugs, and then weighed every 30 min, until the mouse's body weight recovered.
The test of opioid dependence in the guinea pig ileum in vitro
Guinea pigs were deprived of food for 24 h and killed by CO2 inhalation. Pieces of ileum were immediately removed from a point within 10 cm from the ileo-caecal junction. These pieces were dissected and placed in Krebs-Henseleit solution (mmol/L): NaCl, 112.08; KCl, 5.90; CaCl2, 1.97; MgCl2, 1.18; NaH2PO4, 1.22; NaHCO3, 25.00; and glucose, 11.49. The ileum was placed under 1 g of tension in a 5 mL organ bath containing the nutrient solution, at 37 °C, with continuous bubbling of 95% O2 and 5% CO2. After 60 min equilibration in Krebs-Henseleit solution, the contraction tension of the ileum was recorded by a multi-channel physiological signal-gathering processing system (RM-6280C, Chengdu Instrument Factory, Chengdu, China). At the start of each experiment, a maximum response to acetylcholine (3 μmol/L) in each tissue was obtained to check its stability. Dependence, as indicated by a strong contraction of the ileum when challenged with naloxone, was produced by incubating the ileum from a native guinea pig with opioid at 37 °C for 4 h10, 11. The response of the ileum to naloxone was time-dependent and directly related to the codeine phosphate concentration in the incubation fluid (10−1 000 nmol/L) and to the challenge dose of naloxone (10−1 000 nmol/L)19. The guinea pig ileum was incubated with drugs at 37 °C for 4 h. The drug concentration in the incubation fluid was 3 μmol/L, and the challenge dose of naloxone was 3 μmol/L. The withdrawal responses were expressed as the ratio to the acetylcholine (3 μmol/L) responses. The mean amplitude of the stimulated contraction by naloxone was about 50% of the maximal response to acetylcholine (3 μmol/L). The dependence of CA-Ver was determined by comparing it with the effects of codeine phosphate in the ileum.
Statistical analysis
In all studies, the values were expressed as means±SEM, of n observations. The results were analyzed by one-way analysis of variance followed by a Bonferroni post-hoc test for multiple comparisons. P<0.05 was considered significant.
Results
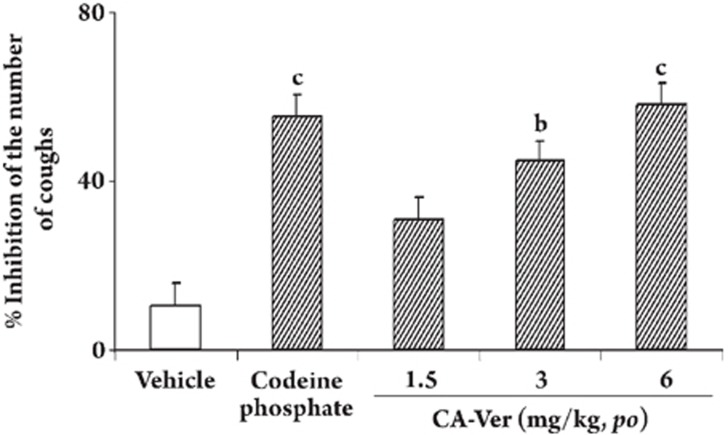
Antitussive effect of CA-Ver on capsaicin-induced coughs CA-Ver, at doses of 1.5, 3, and 6 mg/kg, po, dose-dependently inhibited the number of capsaicin-induced coughs when the antitussive effect was examined 60 min after administration (Figure 3).
Figure 3.
Dose-response data for the antitussive effect induced by CA-Ver in mice. The antitussive effects were assessed 60 min after the administration of each drug. Each point or column represents the mean±SEM for ten mice in each group. bP<0.05, cP<0.01 vs the value for the respective vehicle-treated group (open column). The dose of codeine phosphate was 30 mg/kg. CA-Ver and codeine phosphate were suspended in a 0.5% carboxyl methylcellulose solution. Vehicle: 0.5% carboxyl methylcellulose solution.
Effects of naloxone on the antitussive effect of CA-Ver
The antitussive effect of CA-Ver (6 mg/kg, po) was abolished by pretreatment with naloxone (4 mg/kg, ip). As shown in Figure 4, the pretreatment with naloxone, a non-selective opioid receptor antagonist, significantly (P<0.05) reduced the antitussive effect of codeine phosphate, a centrally acting, narcotic antitussive drug (with saline, 62.18%±8.14%, n=10; with naloxone, 35.03%±10.93%, n=10). The antitussive effect of CA-Ver was also significantly (P<0.05) antagonized by naloxone (with saline, 60.15%±10.54%, n=10; with naloxone, 35.60%±9.68%, n=10) (Figure 4).
Figure 4.
Effects of naloxone on the antitussive effect of codeine phosphate and CA-Ver in mice. Naloxone (4 mg/kg) was injected (ip) 45 min after the administration of codeine phosphate and CA-Ver. The antitussive effects of codeine phosphate (30 mg/kg) and CA-Ver (6 mg/kg) were assessed 60 min after oral administration. Each column represents the mean±SEM for ten mice in each group. bP<0.05 vs the respective saline-treated group (open column).
Addictive evaluation of CA-Ver in mice
The number of jumps and the percentage of weight loss in the mice in the CA-Ver group were not significantly different from the negative control group, but were both less than the two positive control groups (P<0.01). The percentage of jumping animals in the CA-Ver group was similar to the negative control group, but lower than the positive control groups. The drug dosages administered to the mice were based upon the median lethal doses (LD50) of the different drugs. The dose of codeine phosphate and morphine was 400 mg·kg−1·d−1 and 200 mg·kg−1·d−1, respectively. However, the dose of verticinone was only 6 mg·kg−1·d−1 and CA-Ver was 200 mg·kg−1·d−1. As shown in Table 1, the naloxone urged withdrawal tests of mice showed that CA-Ver was not addictive.
Table 1. Number of naloxone-urged jumps and the weight decrease ratio of mice. n=10. Mean±SEM. cP<0.01 vs vehicle; eP<0.05 vs codeine phosphate group; iP<0.01 vs morphine hydrochloride group; Vehicle: purified water.
| Group | Dose/mg·kg−1·d−1 | Number of jumps | Rate of jumping animals/% | Weight decrease ratio/% |
|---|---|---|---|---|
| Vehicle | – | 0.50±0.71 | 40 | 3.38±2.04 |
| Codeine phosphate | 400 | 6.20±6.06c | 80 | 5.93±0.57c |
| Morphine hydrochloride | 200 | 8.40±6.79c | 100 | 6.55±1.54c |
| Verticinone | 6 | 1.30±3.09ei | 40 | 3.08±3.49ei |
| CA-Ver | 200 | 0.10±0.32ei | 10 | 4.58±1.30ei |
Dependence evaluation of CA-Ver in the guinea pig ileum in vitro
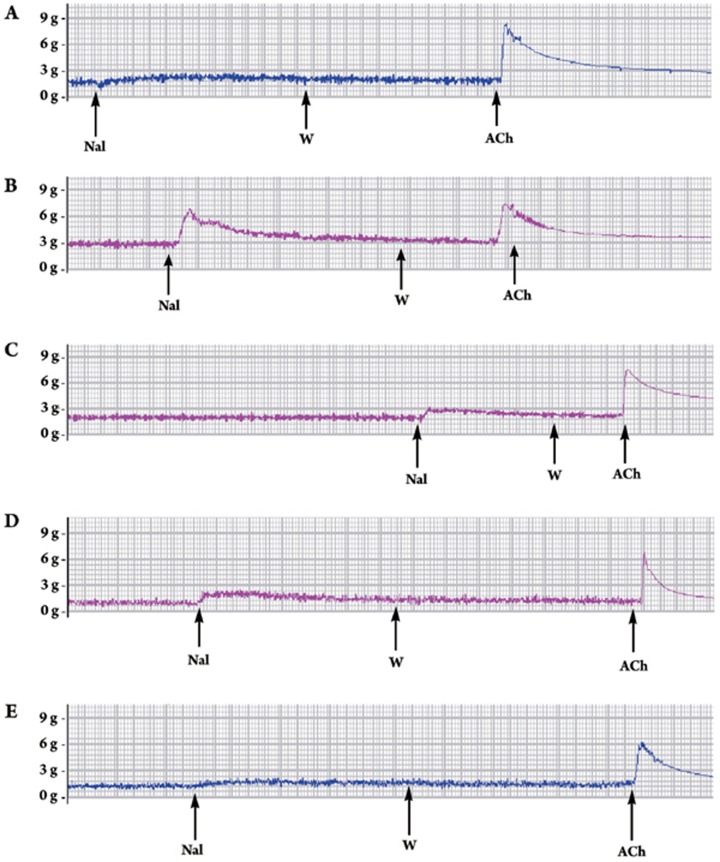
Segments of the ileum taken from the same native guinea pig, were each incubated in Krebs solution containing a different drug (codeine phosphate 3 μmol/L, verticinone 3 μmol/L, cholic acid 3 μmol/L, and CA-Ver 3 μmol/L) at 37 °C for 4 h. The withdrawal contracture was elicited by a challenge with naloxone (3 μmol/L). As shown in Figure 5 , in the ileum incubated in Krebs solution with codeine phosphate for 4 h, the addition of naloxone induced a withdrawal contracture. As shown in Table 2, the codeine phosphate group showed a significant difference from negative control group (P<0.01). But the CA-Ver group showed no significant difference from the negative control group. Taken together with the naloxone-urged withdrawal tests of mice, our results indicated that CA-Ver induced no addiction.
Figure 5.
Naloxone (Nal, 3 μmol/L)-induced contraction in the guinea pig ileum incubated with different drugs at 37 °C for 4 h. (A) Vehicle. (B) Codeine phosphate (3 μmol/L). (C) Cholic acid (3 μmol/L). (D) Verticinone (3 μmol/L). (E) CA-Ver (3 μmol/L). W: Wash. ACh: Acetylcholine 3 μmol/L. Withdrawal responses were expressed as the ratio of the ACh 3 μmol/L responses. Vehicle: purified water. Other drugs were dissolved in purified water.
Table 2. Naloxone (3 μmol/L)-precipitated withdrawal responses of the guinea pig ileum incubated with different drugs at 37 °C for 4 h. Withdrawal responses %=withdrawl contracture/acetylcholine contracture×100%. n=10 guinea pigs. Mean±SEM. cP<0.01 vs vehicle; fP<0.01 vs codeine phosphate group. Vehicle: purified water. All other drugs were dissolved in purified water.
| Group | Dose/μmol·L−1 | Withdrawal responses/% |
|---|---|---|
| Vehicle | – | 4.7±4.6 |
| Codeine phosphate | 3 | 50.4±17.1c |
| Verticinone | 3 | 3.2±4.1f |
| Cholic acid | 3 | 5.6±3.5f |
| CA-Ver | 3 | 1.9±2.9f |
Discussion
Cough remains among the most common complaints for which patients seek medical attention. Presently available therapies to treat cough are limited due to a lack of effective medications. Moreover, most existing antitussive drugs can bring inevitable or intolerable side effects; codeine or dextromethorphan, for example, have limited efficacy in chronic cough at the recommended doses, and unacceptable side effects at higher doses20, 21, 22. The most pressing and unmet current need is for safe and more effective cough suppression23. To search for potential drugs with potent antitussive and expectorant activities as well as low toxicity but no addiction, CA-Ver was synthesized based on the clearly elucidated antitussive, expectorant, and antiasthmatic activities of verticinone in bulbs of Fritillaria and different bile acids in Snake Bile.
Capsaicin, the active ingredient of red pepper, is a commonly used cough stimulant in the study of cough reactivity and for the evaluation of antitussive agents24, 25. It has been shown that capsaicin induces cough mainly by stimulating C-fiber endings and also by stimulating some rapidly adapting receptors with myelinated fibers26. Different studies27, 28 have demonstrated that capsaicin is safe to use and produces a dose-dependent and reproducible cough response in a variety of conditions. Therefore, testing on a capsaicin-induced cough model in mice pretreated with naloxone, a non-selective opioid receptor antagonist, was done to investigate CA-Ver's central antitussive mechanism. The study demonstrated that po administration of CA-Ver produced a dose-dependent antitussive effect in mice. The antitussive effect of CA-Ver was significantly reduced by pretreatment with naloxone. In a parallel study, codeine phosphate, one of the most commonly used potent antitussive agents29, was used as the positive control. The antitussive effect of codeine phosphate, a centrally acting, narcotic antitussive drug, was also significantly reduced by pretreatment with naloxone. These results indicate that the naloxone-sensitive opioid receptor plays an important role in mediating the antitussive effect of CA-Ver. In our previous study, we had elucidated that the antitussive mechanism of the total alkaloids from F hupehensis Hsiao et KC Hsia was central through the experimental cough model elicited in pentobarbital-anesthetized cats by electrical stimulation of the superior laryngeal nerve, which had been used to determine the site of the antitussive drugs30. Moreover, we had assayed the influences that CA-Ver had on the five kinds of monoamine neurotransmitters in mouse brain by the reverse-phase HPLC method with a fluorescent light detector, using codeine and dextromethorphan as the positive drugs. All the results indicated that the antitussive mechanism of CA-Ver was related to central opioid receptors and had no relationship with serotonergic mechanisms, just as with codeine.
However, most centrally acting antitussive drugs can lead to the development of tolerance and physical and mental dependence, characteristics that limit the clinical usefulness of these agents. Codeine, a centrally acting (μ2-opioid receptor agonist), narcotic antitussive drug, was one of the most commonly used potent antitussive agents; however, the addictive nature of codeine limits its clinical usefulness9. A physical dependence constitutes an abnormal physiological state produced by the repeated administration of a drug that leads to the appearance of specific symptoms reflective of autonomic, somatic, and behavioral hyperactivity when the drug administration is reduced or discontinued. The overall intensity of the physical dependence is gauged by the severity of the withdrawal syndrome. In order to determine whether CA-Ver is addictive like codeine, we conducted naloxone-urged withdrawal tests of mice. The results indicated that CA-Ver was not addictive.
The isolated guinea pig ileum has been proved to be a model for the study of opioid dependence19, as it contains populations of functional μ- and κ-opioid receptors31, 32. A contractile response was elicited when naloxone was administered to the naive ileum incubated with opioids. These withdrawal responses have pharmacological characteristics similar to the withdrawal signs in the intact animal. So the naloxone-precipitated withdrawal contracture in the ileum has been used as an indication of opioid dependence, since their actions in the enteric nervous system are thought to mimic those in the central nervous system. In the present study, when the ileum was incubated in Krebs solution containing CA-Ver for 4 h, the addition of naloxone did not induce a withdrawal contracture. However, a withdrawal contracture was induced by the addition of naloxone to the ileum treated with codeine phosphate. Taken together with the naloxone-urged withdrawal tests of mice, our results indicated that CA-Ver induced no addiction.
Our present study indicated that the potent antitussive effect of CA-Ver was through the opioid receptor. In both the intact mouse model and the isolated guinea pig ileum, CA-Ver showed no addictive properties. However, opioids such as morphine and codeine also work through the opioid receptor, and the addiction to opioids limits the clinical usefulness of these agents. Do our results contradict these facts? Recent investigations have demonstrated that an antinociceptive response can be mediated at both supraspinal and spinal sites by μ-, δ-, and κ-opioid receptors33. Therefore, not only the μ-opioid receptor but also the δ- and κ-opioid receptors may play an important role in the antitussive effects of opioids. According to reports in the literature, the antitussive effects of opioids are mediated predominantly by μ- and κ-opioid receptors, and some literature indicated that the μ2-, rather than the μ1-, opioid receptors were involved in μ-opioid receptor-mediated antitussive effects. However, the addiction of opioids is mediated predominantly by the μ-opioid receptors, not the κ-opioid receptors. Therefore, codeine (a μ2-opioid receptor agonist) is a narcotic antitussive drug. However, it is not certain whether CA-Ver is a selective κ-opioid receptor agonist or both a κ-opioid receptor agonist and a δ-opioid receptor agonist. The lack of addiction to CA-Ver suggests that the potent antitussive effect of CA-Ver is possibly through the κ-opioid receptor.
Due to the potent antitussive activity but low toxicity of CA-Ver, we investigated its addictive characteristics. This study allowed us to conclude that CA-Ver deserved attention for its potent antitussive effects related to the central opioid receptors without addiction, and that it had a good potential for development as a new antitussive drug.
Author contribution
Jiu-liang ZHANG designed research; Hui WANG and Chang CHEN performed research; Hui-fang PI, Han-li RUAN, Peng ZHANG and Ji-zhou WU contributed analytical tools and analytical support; Jiu-liang ZHANG wrote the paper.
Acknowledgments
This project was supported by the Fund of the Key Project of the Education Ministry, China, 2006, and the Fund of the International Cooperation Project of Science and Technology, the Ministry of Science and Technology, China (China and Korea, No 2006DFA33210). The present study has applied for a patent in China (application No 200810048722.9).
The authors are grateful to the Huazhong University of Science and Technology Analytical and Testing Center for analytical support.
References
- Xiao PG.A review on the study of Hubeibeimu Zhongguo Zhong Yao Za Zhi 200227726–8.Chinese. [PubMed] [Google Scholar]
- Mei HS, Shi CZ, Wang KP, Xiang Y, Peng H.The comparative study of pharmacology on sodium taurocholate and sodium taurochenodeoxycholate Zhongguo Sheng Hua Yao Wu Za Zhi 199920198–200.Chinese. [Google Scholar]
- Fang HF, Shi CZ.Preliminary studies on synthesis and pharmacological effects of taurobitocholic acid Zhongguo Yi Yao Gong Ye Za Zhi 199930540–3.Chinese. [Google Scholar]
- Li S, Shi CZ.Synthesis of sodium taurine-conjugated bile salts and their comparison in antitussive and expectorant effects Acta Univ Med Tongji 199928114–6.Chinese. [Google Scholar]
- Chen X.Researches on the antitussive mechanism of total alkaloids, single alkaloids and their derivatives of Hubeibeimu [Master Dissertation] Wuhan: Tongji Medical College, Huazhong University of Science and Technology; 2008. Chinese. [Google Scholar]
- Wang CY, Pang ZG, Wang BQ, Zhang P.Determination of the content of monoaminergic transmitters in SD rat brain by fluorometry Fenxi Huaxue 199927101–3.Chinese. [Google Scholar]
- Lu YX, Cui J, Ling XY, Lu ZY.The content determination of the five transmitters in rat brain by RP-HPLC method with fluorescence detector Pharm J Chin PLA 200319262–3.Chinese. [Google Scholar]
- Kamei J. Role of opioidergic and serotonergic mechanisms in cough and antitussives. Pulm Pharmacol. 1996;9:349–56. doi: 10.1006/pulp.1996.0046. [DOI] [PubMed] [Google Scholar]
- Sullivan ME, Hall SR, Milne B, Jhamandas K. Suppression of acute and chronic opioid withdrawal by a selective soluble guanylyl cyclase inhibitor. Brain Res. 2000;859:45–56. doi: 10.1016/s0006-8993(99)02481-6. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Li JL, Li N, Tian CL, Zhang KG. Effect of tetrandrine on morphine dependence in isolated guinea pig ileum. Acta Pharmacol Sin. 1999;20:1000–4. [PubMed] [Google Scholar]
- Matsumoto K, Takayama H, Ishikawa H, Aimi N, Ponglux D, Watanabe K, et al. Partial agonistic effect of 9-hydroxycorynantheidine on μ-opioid receptor in the guinea-pig ileum. Life Sci. 2006;78:2265–71. doi: 10.1016/j.lfs.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Corey EJ, Venkateswarlu A. Protection of hydroxyl groups as tert-butyldimethylsilyl derivatives. J Am Chem Soc. 1972;94:6190–1. [Google Scholar]
- Sellergren B, Wieschemeyer J, Boos KS, Seidel D. Imprinted polymers for selective adsorption of cholesterol from gastrointestinal fluids. Chem Mater. 1998;10:4037–46. [Google Scholar]
- Neton RF, Reynolds DP. An excellent reagent for the removal of the t-butyldimethylsilyl protecting group. Tetrahedron Lett. 1979;20:3981–2. [Google Scholar]
- Kamei J, Iwamoto Y, Kawashima N, Suzuki T, Nagase H, Misawa M, et al. Possible involvement of μ2-mediated mechanisms in μ-mediated antitussive activity in the mouse. Neurosci Lett. 1993;149:169–72. doi: 10.1016/0304-3940(93)90763-b. [DOI] [PubMed] [Google Scholar]
- Kamei J, Iwamoto Y, Suzuki T, Misawa M, Nagase H, Kasuya Y. The role of the μ2-opioid receptor in the antitussive effect of morphine in μ1-opioid receptor-deficient CXBK mice. Eur J Pharmacol. 1993;240:99–101. doi: 10.1016/0014-2999(93)90553-t. [DOI] [PubMed] [Google Scholar]
- Xu SY, Bian RL, Chen X.Methodology in Phamacological Experiments 2nd ed.Beijing: People's Medical Publishing House; 1991. p707–8.Chinese. [Google Scholar]
- Xu Y, Xie JM, Han J, Xu WF, Yu CX.A stable model of naloxone-induced withdrawal jumps in morphine-dependent experimental mice J Fujian Med Univ 200539307–9.Chinese. [Google Scholar]
- Chen JQ, Jin WQ, Chi ZQ. Opioid dependence and tolerance in the guinea-pig ileum in vitro. Acta Pharmacol Sin. 1990;11:488–91. [PubMed] [Google Scholar]
- Chung KF. Chronic cough: future directions in chronic cough: mechanisms and antitussives. Chron Respir Dis. 2007;4:159–65. doi: 10.1177/1479972307077894. [DOI] [PubMed] [Google Scholar]
- Chung KF. Drugs to suppress cough. Expert Opin Investig Drugs. 2005;14:19–27. doi: 10.1517/13543784.14.1.19. [DOI] [PubMed] [Google Scholar]
- Chung KF. Effective antitussives for the cough patient: an unmet need. Pulm Pharmacol Ther. 2007;20:438–51. doi: 10.1016/j.pupt.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV. Potential new cough therapies. Pulm Pharmacol Ther. 2004;17:459–62. doi: 10.1016/j.pupt.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Morice AH. Inhalation cough challenge in the investigation of cough reflex and antitussives. Pulm Pharmacol. 1996;9:281–4. doi: 10.1006/pulp.1996.0036. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Kamio Y, Myou S, Hashimoto T. Effect of oral mexiletine on the cough response to capsaicin and tartaric acid. Thorax. 2000;55:126–8. doi: 10.1136/thorax.55.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudry NB, Fuller RW, Anderson N, Karlsson JA. Separation of cough and reflex bronchochonstriction by inhaled local anaesthetics. Eur Respir J. 1990;3:579–83. [PubMed] [Google Scholar]
- Choudry NB, Fuller RW. Sensitivity of the cough reflex in patients with chronic cough. Eur Respir J. 1992;5:296–300. [PubMed] [Google Scholar]
- Midgren B, Hansson L, Karlsson JA, Simonsson BG, Persson CGA. Capsaicin-induced cough in humans. Am Rev Respir Dis. 1992;146:347–51. doi: 10.1164/ajrccm/146.2.347. [DOI] [PubMed] [Google Scholar]
- Adcock JJ, Schneider C, Smith TW. Effects of codeine, morphine and a novel opioid pentapeptide BW443C, on cough, nociception and ventilation in the unanaesthetized guinea pig. Br J Pharmacol. 1988;93:93–100. doi: 10.1111/j.1476-5381.1988.tb11409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey DM, Morris K, Gestreau C, Li Z, Lindsey B, Shannon R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. J Physiol. 2001;534:565–81. doi: 10.1111/j.1469-7793.2001.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–9. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Goldstein A. Demonstration of a specific dynorphin receptor in guinea pig ileum myenteric plexus. Nature. 1981;291:591–3. doi: 10.1038/291591a0. [DOI] [PubMed] [Google Scholar]
- Heyman JS, Mulvaney SA, Mosberg HI, Porreca F. Opioid δ receptor involvement in supraspinal and spinal antinociception in mice. Brain Res. 1987;420:100–8. doi: 10.1016/0006-8993(87)90244-7. [DOI] [PubMed] [Google Scholar]