Abstract
Aim:
Flt4 plays a key role in promoting tumor metastasis by stimulating solid tumor lymphangiogenesis. In this study, mouse Flt4 (mFlt4) was displayed on T4 phage in order to explore the feasibility of breaking immune tolerance to “self-antigens” and to evaluate the phage's antitumor activity.
Methods:
A T4 phage nanometer particle expressing mFlt4 on the surface was constructed for evaluation as a recombinant vaccine. The presence of the mFlt4 gene in the T4-mFlt4 recombinant vaccine was verified by PCR and Western blot analysis. The immunotherapeutic potential of T4-mFlt4 was tested in mice injected with Lewis lung carcinoma (LLC) cells. Anti-Flt4 antibody producing B cells were detected by ELISPOT. The effects of T4-mFlt4 on lymphatic metastasis and lymphangiogenesis were investigated in a mouse antimetastasis assay and by Flt4 and CD105 immunohistochemistry.
Results:
The T4-mFlt4 recombinant vaccine demonstrated antitumor activity and elicited autoantibodies against mFlt4. Mice carrying LLC-derived tumors exhibited prolonged survival when given the vaccine compared with control-treated animals. The vaccine also inhibited lymphangiogenesis and tumor metastasis in the mouse models. However, T4-mFlt4 was not observed to inhibit tumor growth.
Conclusion:
The T4-mFlt4 recombinant vaccine induced protective antitumor immunity and antimetastasis against LLC. Induction of an autoimmune response directed against tumor progression merits further study as a new strategy for immunotherapy in cancer.
Keywords: Flt4, self-immunization, cancer therapy, T4 bacteriophage
Introduction
Vascular endothelial growth factor receptors (VEGFRs) play a significant role in the generation and development of tumors, especially in lung cancer, hepatoma1, breast cancer2 and colon carcinoma3. The expression of VEGF-C is higher in carcinoma tissue samples than in para-tumoral tissue samples. Fms-like tyrosine kinase 4 (Flt4), also called VEGFR3, is a receptor for VEGF-C and D. Flt4 was believed to be critical for the development of the embryonic vascular system but postnatally restricted to the endothelial cells of lymphatic vessels and specialized fenestrated capillaries4. Recent studies have indicated that Flt4, which has been proposed as a marker for lymphatic endothelial cells, is also expressed in a variety of human malignancies. It is the only regulatory factor reported to regulate production of embryonic lymphatic vessels, promote functional maturation of individual lymph vessels, revoke and aggravate growth of blood and lymph vessels, increase vasopermeability, and support the migration of endothelial cells and the capillary network. Transgenic expression of an Flt4-specific mutant of VEGF-C in mouse skin resulted in increased growth of dermal lymphatic but not vascular endothelium5, 6, 7. The VEGF-C/Flt4 axis exerts different biological effects on cancer cells to cause tumor progression. Flt4 in colon cancer has been associated with poorer survival, suggesting an axis between VEGF-C and Flt4 in colorectal cancer8. Similarly, suppression of VEGF-C and D levels in tumor-bearing mice using neutralizing antibodies or a soluble form of Flt4 resulted primarily in antilymphangiogenic rather than anti-angiogenic effects7, 9, 10, 11. Expression of Flt4 has also been reported to be significantly correlated with the different stages of cervical carcinogenesis12. These observations indicate that Flt4 may represent a good therapeutic target for inhibiting tumor metastasis and prolonging survival.
Here we describe a novel strategy for achieving a protective immune response with an mFlt4-based T4 phage recombinant vaccine. Phage display is a powerful technique for identifying peptides or proteins that have desirable binding properties. First, the T4 phage display system is capable of displaying multiple copies of two different peptide/proteins and creating the “high concentration/density hot spot” of expressed protein to achieve its Kd and simulate a relevant protein-protein interaction13. Second, the exogenous or syngeneic protein/peptide can be fused to both the N- and the C-termini of T4 SOC and HOC proteins at higher copy number than other filamentous phage systems. The foreign proteins can be displayed both on T4 capsids and on polyheads (long planar tubules of the major capsid protein). Third, secretion of the fusion protein does not occur14, 15, 16, 17. Moreover, T4 phage particles possess high antigenicity without adjuvant13, 18. Finally, scientists have already conducted T4 phage safety testing of oral administration in humans and found it to be safe and accompanied by non-pathogenic side effects due to infection of enteric E coli19.
Direct and indirect evidence indicates that breaking immune tolerance against VEGFR activity associated with angiogenesis or lymphogenesis inhibited the growth and persistence of solid tumors. In this study, we used mFlt4 constructed on T4 phage to display VEGFR activity as an autologous vaccine. Thus, use of a T4 phage display system to construct a recombinant vaccine based on Flt4 can produce high antigenicity to induce immune responses in animal models and exert inhibitory effects on lymphogenesis.
Take together, the results from this study illustrate that with respect to cancer anti-lymphogenesis therapy, mFlt4-based active specific immunotherapy can be an alternative or complementary approach for the treatment of tumors, provided that induction of an immune response against self Flt4 is feasible20. Breaking immune tolerance by self Flt4 against autologous angiogenic endothelial cells, assisted by the T4 phage display system, indicates an attempt at cancer therapy with active immunity. In this experiment, we constructed mouse self-Flt4 (mFlt4) recombinant T4 phage (T4-mFlt4) as a new vaccine to test this concept along with blank T4 phage without the inserted protein and SM buffer prepared as controls.
Materials and methods
mFlt4 gene source
For this study, plasmid pD-mFLT4 was provided by Expression Biosciences LLC (Somerset, NJ, USA). It was previously constructed by reverse transcription from mouse embryo RNA and contains a mVEGFR-3/Flt-4 gene DNA sequence that spans the whole region of the cytomembrane outside portion, is 2226 nucleotide in length and encodes 742 amino acids (for the detailed sequence see mFlt4 cDNA in GenBank)
T4 phage display system T4-S-GPDS
The T4 bacteriophage nanoparticle surface gene-protein display system (T4-S-GPDS) has been developed and used as a nanoparticle phage vector by using capsid surface Soc and Hoc bipartite expression and display. In this study, the T4 phage display system includes phage T4 capsid surface display vector T4-Z, integration plasmid vector pE-SII, and pRH21. Phage vector T4-Z was generated by recombination of the Soc gene-deleted plasmid with phage T4 vector eG326 by Dr Ren21. Host bacteria E coli HB101 (sup°), CR63 (sup D), DH5α, BL21 (DE3), and wild-type T4+D phage were stored in Expression Biosciences LLC laboratory (Somerset, NJ, USA).
PCR techniques
For mFlt4-encoding cDNA generation in this study, PCR was conducted with pD-mFlt4 DNA as template and a pair of synthesized oligonucleotides as primer:
S-mFLT5: 5′ -GGGGCCCATGGATGGAGGATTCATATGTGATTG -3′ (NcoI site)
S-mFLT3: 5′-CCCCCGTTTAAACCCTCCATGCTGCCTTTATCTTCAGAGCCTTCCAC-3′ (PmeI site)
The PCR cycles were programmed as follows: one cycle of 94 °C for 3 min, 52 °C for 1 min and 72 °C for 2.5 min; 30 cycles of 94 °C for 1 min, 52 °C for 1 min and 72 °C for 2.5 min; one end cycle of 94 °C for 1 min, 52 °C for 1 min and 72 °C for 5 min.
Integration plasmid construction for fusion of mFlt4 cDNA to the T4 Soc gene
The PCR product representing full-length mFlt-4 cDNA and the T4-phage integration plasmid pRH were cut with NcoI and PmeI and then ligated with T4 DNA ligase to insert the mFlt-4 cDNA at the Soc gene N-terminus. The ligated plasmid was transformed into competent E coli HB101 cells to produce the recombinant plasmid pRH-mFlt4, which was further used for cross-integration with phage vector T4-Z1.
Construction of recombinant bacteriophage T4-mFlt4 nano-particle as a vaccine
The full-length mFlt4 cDNA was inserted into the T4-phage genome by plasmid- phage cross homologous recombination. First, the recombinant integration plasmid pRH-mFlt4 was transformed into E coli HB101 cells. Second, the cells were infected with expression phage T4-Z1 at a multiplicity of infection (MOI) of 0.5 to allow integration of the mFlt4 cDNA into the phage T4-Z1 genome and leading to pRH-mFlt4 protein display on the Soc site of the phage T4-Z1 capsid surface as the mFlt4-Soc fusion antigenic protein. The desired recombinant phage T4-mFlt4 strain was identified by lysozyme-dependent growth and absence of the Soc proteins on polyacrylamide gel electrophoresis (PAGE).
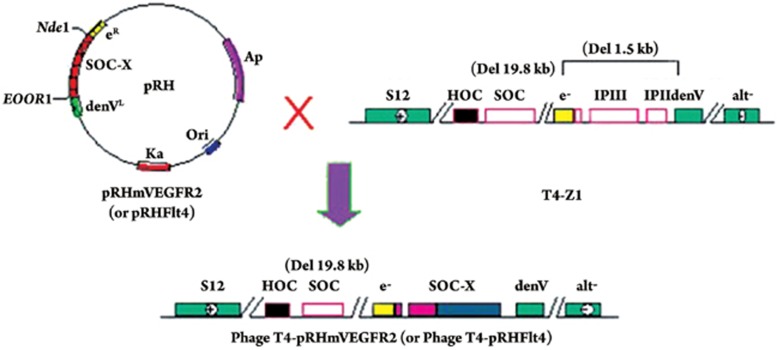
The recombinant phage T4-mFlt4 particles were directly used in animal tests as an immunotherapy vaccine. Because the T4 phage particle size is 110 nanometer long by 41 nanometer wide, it can be referred to as a nanometer particle. The recombinant T4 phage nano-particle vaccine generation strategy is illustrated in Figure 1.
Figure 1.
Strategy for constructing recombinant vaccine phage T4-mFlt4 from integration plasmid pRH-mFlt4 and phage expression vector T4-Z1. The pRH-derived plasmid contains a partially deleted T4 phage lysozyme gene eR and endonuclease gene denVL, which allow homologous recombination at both ends of the SOC gene with the T4-Z phage vector, on which another part of gene e− and denV were deleted. The recombination produced an entire gene e− and incorporated any target foreign gene X into the phage T4 genome as an SOC fusion gene.
Proliferation of phage T4-mFlt4
In a large flask, E coli BL21 (DE3) was incubated in LB medium to OD600 around 0.3, infected with phage T4-mFlt4 at MOI=0.9–1.0, incubated at 37 °C for 5 h when cells started to lyse. Cells were collected by centrifugation at 4 000×g for 5 min, and the phage pellet was suspended with SM buffer. Following the addition of about 100 μL CHCl3, the suspension was incubated with lysozyme for 30 min at 37 °C and with DNA enzyme for 10 min at 37 °C. The lysate was subjected to centrifugation at 12 000×g for 40 min and the supernatant was collected. The phage concentration ranged from 1011–1012 pfu/mL and contained around 10 mg/mL mFlt4 protein. The roughly purified T4 phage nanoparticles were subjected to centrifugation at 12 000×g for 40 min again and then used as vaccine in a series of animal tests. SM Buffer (SM) and the blank T4 phage were used in control animals.
Immunogenicity analysis by Western blotting
The immunogenicity of the recombinant phage T4-mFlt4 nanoparticles was analyzed by PAGE and Western blotting. The recombinant T4-mFlt4 phage sample PAGE gel was blotted on a nitrocellulose membrane and then probed with rabbit anti-mFlt4 serum as the primary antibody and goat anti-rabbit immunoglobulin G conjugated with HRP as the secondary antibody. Reactive protein bands were visualized by an ECL chemiluminescece detection kit (Amersham Biosciences Inc).
Animals
Female C57BL/6 inbred mice weighing 19 to 23 g were obtained from the Model Animal Research Center of Nanjing University, Nanjing, China. Mice were kept under specific pathogen-free (SPF) conditions and obtained food and water ad libitum. The protocols of animal use complied with the Principles of Laboratory Animal Care (NIH Publication 85–23, revised 1995).
Immunotherapy and tumor models
C57BL/6 mice (n=8) aged 6 weeks were injected subcutaneously once weekly for 4 consecutive weeks with various doses (1×1010–1×1013 pfu/mL, 100 μL per mouse) of T4-mFlt4 recombinant vaccine. Two control groups with 8 mice per group were treated with subcutaneous injection of SM buffer or blank T4-phage in SM. On the 7th day after 4 weeks of administration, the mice were injected subcutaneously with 1×106 Lewis lung carcinoma cells (LLC). LLC cells are a VEGFR-positive tumor cell strain and are dependent on the VEGFR pathway. LLC cells have been an important tumor model for metastatic, angiogenesis studies and neoadjuvant chemotherapy22, 23, 24, 25. Folkman's group demonstrated that removal of subcutaneously implanted LLC cells increased metastatic growth15. Tumor volume was determined by the following formula: tumor volume (mm3)=1/2×A (mm)×B2 (mm2), where ‘A' denotes the largest dimension of the tumor and ‘B' represents the smallest dimension.
Antimetastatic effect assay
To explore whether the autologous vaccine can have protective effects on widespread metastasis, mice were immunized with the T4-mFlt4 vaccine for three consecutive weeks or with SM as a control. One day before the fourth immunization, the mice were challenged with 1×106 LLC cells in the foot pad. Three weeks after malignant cell inoculation, tumors were surgically removed. Fifteen days after surgery, mice were sacrificed and the spontaneous lung metastases were measured by weighing the lungs of the mice.
Enzyme-linked immunospot assay
The enzyme-linked immunospot (ELISPOT) assay for the enumeration of the anti-Flt4 antibody-producing cells has been described13. Briefly, polyvinylidene difluoride (PVDF)-bottomed 96-well Filtration Plates (Millipore, Bedford, MA) were coated with 150 μL/mL T4-mFlt4 recombinant vaccine at 4°C overnight and then incubated at 37 °C for 1 h. Mononuclear cells prepared from spleen were enumerated from 1×106/well and diluted 1:3 and then incubated on the plates at 37 °C for 5 h. Immunoglobulin G (IgG) bound to the membrane was revealed as spots with alkaline phosphatase-conjugated antimouse IgG antibodies. Mononuclear cells from mice injected with SM buffer were used as negative controls.
Immunohistochemistry
Mouse tumors were washed once in PBS and then fixed in 10% formalin. Tumor tissues were then processed by paraffin embedding. Semiserial sections were cut 4 μm apart from the paraffin-embedded blocks. The sections of tumors from the vaccinated mouse group and the control group were assessed by immunohistochemistry using rabbit-anti-human CD105 and Flt4 monoclonal antibodies (Boster, Wuhan, China) as described26. Tissue sections (4 μm thick) were placed onto treated slides (Vectabond, Vector Laboratories, Burlingame, CA). Sections then were heat fixed, deparaffinized and rehydrated through graded alcohols (100%, 95%, 80%, 50%) to distilled water, boiled in citrate buffer at high temperature for antigen retrieval, and treated with 3% hydrogen peroxide to block endogenous peroxidase activity. The slides were incubated with a protein-blocking agent (SA1020, Boster, Wuhan, China) prior to the application of the primary antibody and then incubated with the primary antibody at 4 °C overnight. The tissues were then incubated with the secondary biotinylated anti-species antibody and labeled using a modification of the avidin–biotin complex immunoperoxidase staining procedure according to the Ultra Sensitive™ SP kit manual. Counterstaining was done with Harris hematoxylin. All reagents were supplied by Boster-Bio Co, Wuhan, China. Microvessel counts (MVCs) and lymphatic microvessel counts (LMVCs) were assessed according to Weidner27. The hot spots were selected under a microscope (×100), and individual counts were made under ×400 field (NIKON YS100 microscope, 0.155 mm2 per field). The average counts from five fields were recorded. Any single highlighted endothelial cell or endothelial cell cluster clearly separated from adjacent microvessels and distinct clusters of brown-staining endothelial cells were counted as separate microvessels.
Statistics
Values were expressed as mean±SEM, and statistical significance was analyzed by Student's t-test. All probability (P) values were based on two-tailed tests. Results were considered statistically significant when P<0.05. All statistical tests used Graph Pad Prism version 5.01 software.
Results
Generation of a T4 phage platform immunotherapy vaccine
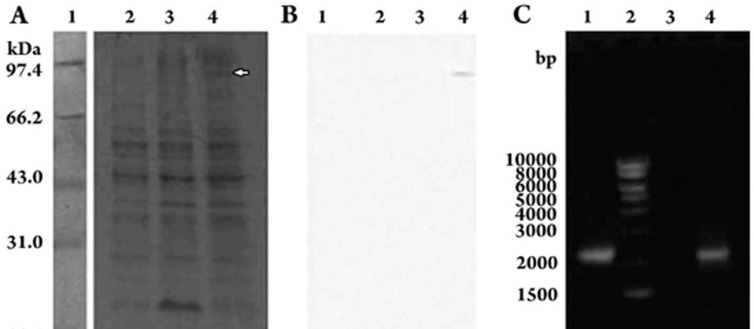
A T4 phage immunotherapy vaccine was constructed following the strategy shown in Figure 1 using the T4 bacteriophage nanoparticle surface gene-protein display system (T4-S-GPDS). First, the 2 226-bp mFlt4 cDNA gene was inserted into the integration plasmid pRH-mFlt4. Second, through plasmid-phage cross homologous recombination, the mFlt4 gene was successfully inserted into the phage vector (T4-Z1) genome to encode a 742 amino acid protein that covered the Flt4 outside-cytomembrane portion and was expressed and displayed on the phage particle surface. We named the recombinant vaccine phage strain T4-mFlt4. The phage could grow on lysozyme-absent medium. T4-mFlt4 lacked a 9.4-kb Soc protein band and instead expressed an approximately 90-kDa Soc-VEGFR target fusion protein band, as illustrated in Figure 2A, 2B. The recombinant phage T4-mFlt4 particles were propagated in E coli HB101, roughly purified by differential low (5 000×g) and high (12 000×g) speed centrifugation from the lysate, suspended in SM, and then directly used to immunize mice.
Figure 2.
Verification of mFlt4 expression. (A) Coomassie blue stained SDS-PAGE gel. Lane 1, protein mass markers, with the Mw shown in kDa; Lane 2, Phage vector T4-Z1 as negative control; Lane 3, Phage from E coli HB101 carrying the non-integration plasmid pD-Flt4 and infected by vector phage T4-Z1, also as negative control; Lane 4, Phage T4-mFlt4; the target mFlt4 band is indicated by an arrow. All lanes of phage samples were not highly purified. (B) Western blotting of gel-A transferred to a nitrocellulose membrane and probed with rabbit serum against mFlt4. The lanes are the same as in A. (C) PCR of vaccine phage T4-mFlt4, with the vaccine phage nano-particle DNA as template, Lane 1, PCR positive band from plasmid pD-mFlt4 DNA as template; Lane 2, DNA marker; the length in base pairs is shown; Lane 3, PCR from phage vector T4-Z1 genome DNA as a vacant control; Lane 4, PCR positive band from phage T4-mFlt4 genome DNA as template.
T4-mFlt4 recombinant vaccine gene expression
From the recombinant phage T4-mFlt4 vaccine particles, the whole phage genome DNA was extracted and used as a PCR template to amplify the mFlt4 cDNA, as shown in Figure 2C. The results confirmed that the heterologous mFlt4 cDNA gene was inserted into the genome of the vaccine phage T4- mFlt4 particles.
Expression and display of protein mFlt4 on the T4 phage external surface
The recombinant phage T4-mFlt4 nanoparticles were analyzed by PAGE and Western blot, revealing a T4 phage-expressed Soc-mFlt4 fusion protein band of ∼90 kDa (Soc 9.4 kDa+mFlt4 ∼81 kDa). As a comparison, the non-integration plasmid pD-mFlt4 carrying E coli cells infected by phage T4-Z1 was unable to express the target mFlt4 band. The resulting negative bands are also illustrated on the same gel in Figure 2A. Thus, the Soc-mFlt4 fusion protein expressed from recombinant T4-mFlt4 phage was successfully immunologically identified (Figure 2B).
Exploration of protective immune effects
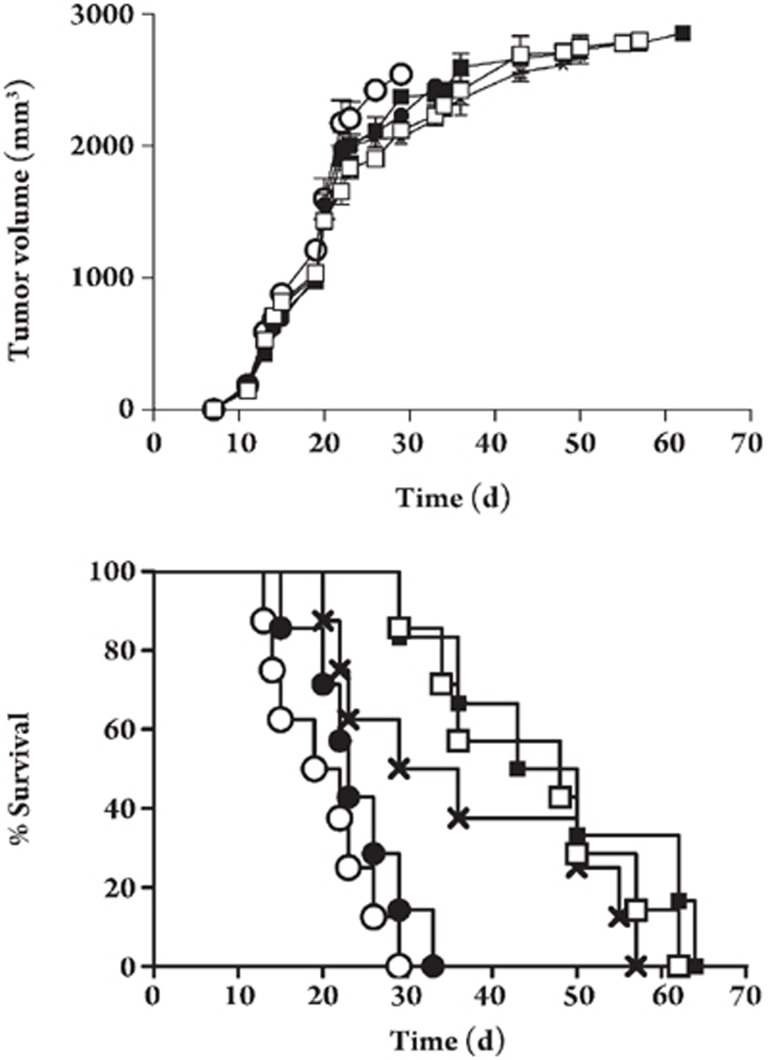
To explore the protective effects of the T4-mFlt4 phage, mice were immunized once weekly continuously for 4 weeks with T4-mFlt4 recombinant vaccine (1×1010–1×1013 pfu/mL; 100 μL per mouse) or the blank T4 phage in SM (blank T4 phage group) or SM alone. Mice were then challenged with LLC cells (1×106) at day 7 after the fourth immunization. The effects of this treatment are described in Figure 3A, 3B. Tumors grew progressively in the mice from groups that were injected with blank T4 phage and SM. In mice from the groups immunized with T4-mFlt4 recombinant vaccine, protective effects against tumor growth were not apparent. No statistical difference in tumor growth was observed between the T4-mFlt4 treated and non-treated groups (P>0.05). On day 29, the mice of the SM group died, and the tumor volumes of the control groups were 2543 mm3 and 2232±44.6 mm3 for the SM and blank T4 phage groups, respectively. On the same day, among the T4-mFlt4 treated groups, the tumor volumes of the groups with T4-mFlt4 doses of 1×1010, 1×1012, and 1×1013 pfu/mL were, respectively, 2062±129 mm3, 2271±130 mm3, and 2119±174 mm3. The inhibitory effects of T4-mFlt4 on tumor progression were not dose-dependent and the high dose groups (1×1012–1×1013 pfu/mL; 100 μL per mouse) showed no significant protective effects on tumor growth (Figure 3A).
Figure 3.
Induction of therapeutic antitumor immunity. Mice (8 per group) were immunized once weekly for 4 weeks with T4-mFlt4 (1×1010–1×1013 pfu/mL; 100 μL per mouse) (groups are presented as ×, ▪, and □, respectively), the blank T4 phage in SM (blank T4 phage group) (○), or SM alone (○), starting at day 7 after 1×106 Lewis lung carcinoma cells (LLC) were introduced subcutaneously into mice. Results are expressed as means±SEM. No significant differences were found between groups with respect to tumor size (P>0.05) (A). However, the survival length in tumor-bearing mice was extended in the T4-mFlt4 groups compared with the untreated groups (B).
Although four immunizations with T4-mFlt4 (1×1012 pfu/mL; 100 μL per mouse) led to no amelioration of tumor volume, the survival length was extended compared with untreated mice (n=8). Survival length was 64 days for the treated group compared with 29 and 33 days in the control groups. On day 62 the survival rate was 25% in the experimental groups, whereas for the blank T4 phage control group the survival rate was 12.5% at 29 days (Figure 3B, data not shown).
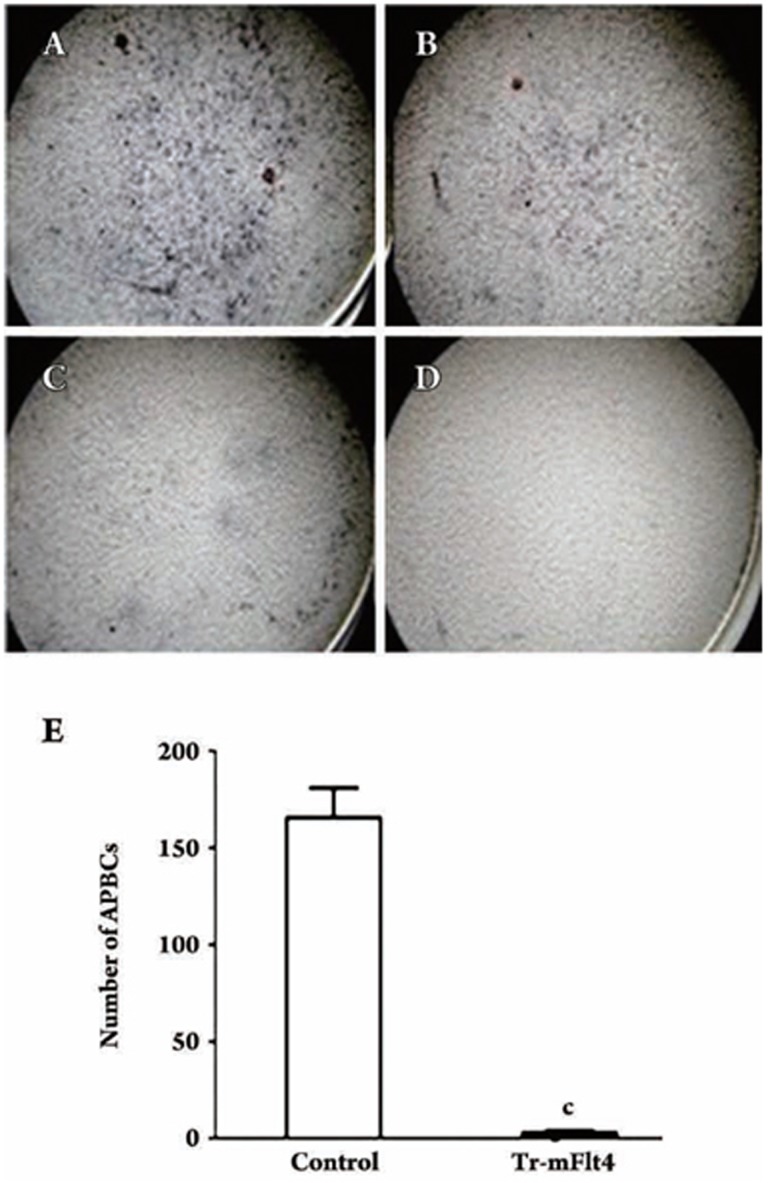
ELISPOT tested effects of T4-mFlt4 immunization generates specific B cells
We wondered whether immunization with T4-mFlt4 could induce antibodies in specific B cells (APBCs). The results indicated that splenic mononuclear cells from mice immunized with T4-mFlt4 recombinant vaccine could generate specific anti-mFlt4 antibody- producing B cells. In contrast, no anti-mFlt4 APBCs recovered from mice in the blank T4 phage control group (Figure 4). Individual counts of APBCs were made microscopically under a 40×field (NIKON YS100 microscope, 0.155 mm2 per field) and showed that the number of APBs in the T4-mFlt4 treated group was 165.7±15.3 per 106 splenic mononuclear cells, whereas no APBCs were detected from the control group splenic mononuclear cells (Figure 4A–4E).
Figure 4.
Characterization of the autoantibodies. Mice (8 per group) were treated with 100 μL 1×1012 pfu/mL T4-mFlt4 once a week for 4 weeks, and then splenic mononuclear cells were prepared 1 week after the fourth immunization. The splenic mononuclear cells were diluted 1:3 from 1×106 cells/mL. The high concentration (A) was 1×106; the middle concentration (B) was 3.5×105 and the low concentration (C) was 1×105 spleen cells. The splenic mononuclear cells from mice treated with SM were used as control (D). The numbers of anti-mFlt4 antibody-producing B lymphocytes in spleens of mice immunized with T4-mFlt4 or SM were counted in an ELISPOT assay. Data presented are means±SEM (E); original magnifications, ×40. cP<0.01 vs control group.
Antimetastatic effects assay
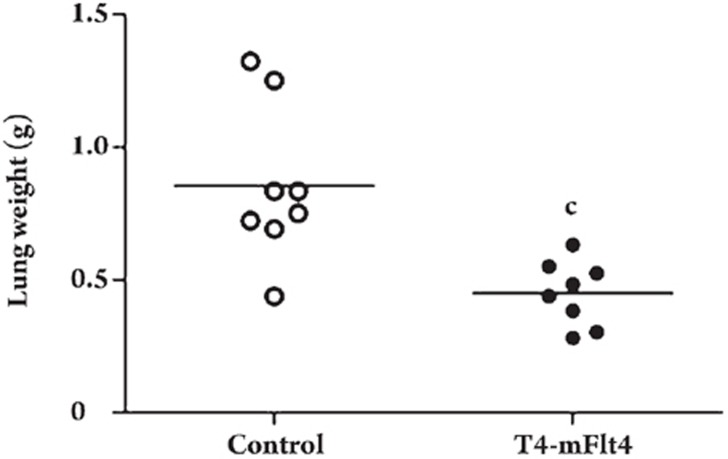
To test whether the T4-mFlt4 recombinant vaccine can induce widespread antimetastatic effects, mice were immunized and then sacrified 15 days after surgery. The group treated with T4-Flt4 vaccine had reduced lung metastasis (0.45 g±0.12 g, P=0.002), whereas no apparent effects on antimetastasis were seen with the control group (0.84 g±0.29 g) (Figure 5).
Figure 5.
Antimetastatic effect of T4-mFlt4 vaccine. Mice were immunized with T4-mFlt4 for three consecutive weeks and SM was used as control. One day before the fourth immunization, the mice were challenged with 1×106LLC cells in the foot pad. Three weeks after malignant cell inoculation, tumors were surgically removed. Fifteen days after surgery, mice were sacrificed and the spontaneous lung metastases quantified by weighing the lungs. In the T4-mFlt4 group, the lung weights were significantly reduced (P<0.01) compared with those of the corresponding control group. cP<0.01 vs control group.
Lymphogenesis and angiogenesis inhibition effects
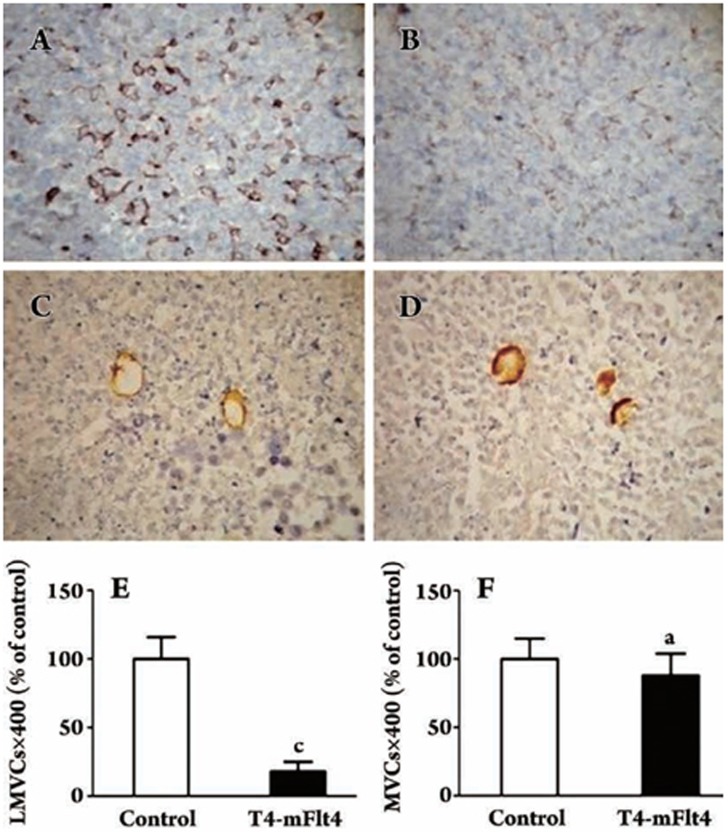
Histological examination of the tumor paraffin sections stained with hematoxylin and eosin showed that the tumor sections from the T4-mFlt4 vaccine group and the control group (SM alone) were stained with the endothelial-specific antibodies Flt4 (Boster, Wuhan, China) and CD105 (Boster, Wuhan, China) to identify tumor lymphogenesis and angiogenesis, respectively. Microvessels and lymphatic microvessels were represented by brown capillaries or small clusters (Figure 6). The mean lymphatic microvessel counts (LMVCs) were reduced in tumors after treatment (Figure 6A, 6B). The mean Flt4 positive vessel count in LLC was significantly higher than that in the control group (SM alone) (P<0.05) (Figure 6C, 6D). The average vascular microvessel counts (MVCs) in lungs, however, were not significantly different between the groups. These results suggest that T4-mFlt4 has little effect on angiogenesis (P>0.05) but has a significant inhibitory effect on lymphatic vessel generation (P<0.01).
Figure 6.
Immunohistochemistry of specific endothelial cell antibody (CD105) and Flt4 of tumor tissues. Lymphatic microvessel counts (LMVCs) and vascular microvessel counts (MVCs) of tumors from mice treated with the T4-mFlt4 vaccine were enumerated compared with the control group (SM alone). LMVCs of Flt4 in five randomly selected fields were reduced significantly in tumors from T4-mFlt4-treated animals (A, B), whereas MVCs did not differ (C, D). LMVCs ×400 of Flt4 is shown in 6E; MVCs×400 of CD105 is shown in 6F. aP>0.05, cP<0.01 vs control group.
Discussion
Phage display is a powerful technique for identifying peptides or proteins that have desirable biological and pharmaceutical properties. The T4 phage display system makes it possible to construct macromolecular complexes that display hundreds of identical copies of a designated protein with antigenic, antibody/scFv or enzymatic activities on a single macromolecular particle. The T4 phage display system has numerous applications, including vaccine development. Assembling massively multimeric copies of rare, expensive, unstable or enzymatic activities onto a T4 macromolecular platform can lead to generation of biopharmaceutical reagents and drugs. Protein secretion-related toxicity can be avoided by the T4 phage lytic process, and protein folding takes place in a fundamental intracellular environment to prevent the secretion of fusion protein. One of the most valuable and attractive features of the T4 phage display system over other phages is the ability to express large proteins (>700 amino acids) in an ordered array on the T4 phage surface.
However, this system is also associated with several limitations. For one, the fused target protein displayed copy number decreases as the insert becomes longer. Ren et al showed that the target protein copy number was inversely proportional to the exogenous insert's length21. Second, it is not known whether in large animals and humans a recombinant T4 phage particle carrying a large fragment with biological activity would lose its insert from the capsid surface. Third, in the case of T4-recombinant phage used in domestic animals and humans, it is unclear whether the T4 phage particle would evade and overcome the reticuloendothelial system and intestine-liver barrier to enter the target organs or circulatory system. These questions need to be investigated in small animal models. The method of administration (oral versus injection) also merits further study.
In this study, we tested whether the self mFlt4 based on a T4 phage recombinant vaccine was able to stimulate self immune responses and induce specific antibodies. Previous studies suggested that immunity of angiogenic vessels was difficult to acquire by a vaccine based on autologous or syngeneic molecules due to immune tolerance acquired during early development of the immune system and that only a xenogeneic protein could provoke the immune response. However, more current studies demonstrated the feasibility of circumventing tolerance of self proteins. Self EGFR protein extracellular domains produced by plasmid DNA encoding the murine EGFR-ECD could offer active antimetastatic immunotherapy to Lewis lung carcinoma28. Recently, a preclinical study of a DNA vaccine based on self EGFR-ECD has been published, demonstrating the validity of this active immunotherapy29. Our experimental results are consistent with these findings.
More importantly, in this study the recombinant T4-mFlt4 vaccine exhibited incomparable advantages, ie, T4 phage particles possess high antigenicity without any adjuvant added. This characteristic is attributed to the T4 phage coat major scaffold protein gp23 and surface accessory HOC protein's higher antigenicity resulting in enhanced immune response in animals. Furthermore, the self protein with T4 display system makes it easier to elicit immune responses in vivo. In comparison, proteins generated from a plasmid usually require adjuvant like FA to boost immune responses. Unfortunately, the inflammatory reaction produced by FA could damage the self immune system. An incomplete immune system cannot defend tumor progression when challenged with tumor cells; thus, we still need more evidence to evaluate the real effects of this vaccine prior to its use in clinical research.
Lewis lung carcinoma is a VEGFR-positive tumor, and the results presented here demonstrated that the phage vaccine could induce protective immunity against tumor cell metastasis and prolong the survival of tumor-bearing animals. In our experiments, the T4 phage functioned as a vector carrying the Flt4 gene and encoding Flt4 protein, as confirmed by PCR and Western blot. Flt4 immunity arose from the natural conformation of Flt4 outside-cytomembrane epitopes. The T4 phage surface gene-protein display system (T4-S-GPDS) has been proven to elicit a strong immune response by many studies.
In order to explore the efficacy of the T4-mFlt4 recombinant vaccine, we injected mice once a week for four weeks and then implanted LLC cells. Our in vivo and in vitro results indicate that the T4-mFlt4 recombinant vaccine was profoundly effective at preventing lymphatic vessel generation and tumor metastasis, but had little effect on tumor growth. It is possible that VEGFR3 overexpression by experimental tumors did not enhance tumor growth but selectively promoted tumor metastasis30, 31. Although VEGFR3 suppression successfully blocked lymphatic hyperplasia and limited the delivery of tumor cells into the lymph node, it was unable to prevent the growth of tumor cells already seeded in the lymph node. In contrast, VEGFR2 suppression could reduce tumor growth in both primary and secondary sites32. The interaction of VEGF-C with VEGFR3 in leukemia cells promotes cell survival and proliferation, as shown by Dias et al in two cell lines and in five cases of VEGFR3 primary leukemia33. Therefore, it seems that this treatment is unable to prevent tumor growth.
We also used ELISPOT to determine whether the specific anti-Flt4 antibody can be secreted by B lymphatic cells. Although the specific anti-Flt4 antibody did not inhibit tumor growth, it did restrict tumor metastasis. The mechanism of the T4-mFlt4 recombinant vaccine may include the humoral immune response and the cellular immune response. The cellular immune response plays a key role in generating effective anti-metastatic immune responses. However, further investigation is required to understand the mechanism of this phage recombinant vaccine.
In conclusion, we demonstrated that the T4-mFlt4 vaccine could inhibit tumor metastasis and prolong the survival of experimental animals. It is also a potent lymphogenesis inhibitor through its interference with VEGFC/Flt4 signal transduction. Such dual activity of the T4-mFlt4 recombinant vaccine, as opposed to the relatively single effect of angiogenesis inhibitors, can combine lymphogenesis with angiogenesis inhibitors for use in cancer therapy. Therefore, the immunogens expressed and displayed by T4-S-GPDS might be a novel anti-tumor metastatic agent in the future.
Author contribution
Shun-xiang REN and Feng YU designed research; Shun-xiang REN, Zhao-jun REN, Min-yi ZHAO, Shu-guang ZUO, and Feng YU performed research; Zhao-jun REN and Xiao-bin WANG contributed new analytical tools and reagents; Shun-xiang REN analyzed data; Shun-xiang REN, Zhao-jun REN, and Feng YU wrote the paper.
Acknowledgments
We are grateful to Prof Jia-hua CHEN and Qing-long GUO of China Pharmaceutical University for their assistance in this study.
References
- Clarijs R, Schalkwijk L, Hofmann UB, Ruiter DJ, de Waal RM. Induction of vascular endothelial growth factor receptor-3 expression on tumor microvasculature as a new progression marker in human cutaneous melanoma. Cancer Res. 2002;62:7059–65. [PubMed] [Google Scholar]
- Gunningham SP, Currie MJ, Han C. The short form of the alternatively spliced flt-4 but not its ligand vascular endothelial growth factor C is related to lymph node metastasis in human breast cancers. Clin Cancer Res. 2000;6:4278–86. [PubMed] [Google Scholar]
- Valtola R, Salven P, Heikkila P. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol. 1999;154:1381–90. doi: 10.1016/S0002-9440(10)65392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen TA, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, et al. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14:2087–96. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–5. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet. 2000;25:153–9. doi: 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA. 2001;98:12677–82. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte D, Thomas A, Ali N, Carlson N, Younes M. Expression of the vascular endothelial growth factor receptor-3 (VEGFR-3) and its ligand VEGF-C in human colorectal adenocarcinoma. Anticancer Res. 2002;22:1463–6. [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–91. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. Natl Cancer Inst. 2002;94:819–25. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- Van PO, Steele D, Lowe DG, Baithun S, Beasley N, Thiele W, et al. Expression of vascular endothelial growth factor (VEGF)-C and VEGF-D, and their receptor VEGFR-3, during different stages of cervical carcinogenesis. J Pathol. 2003;201:544–54. doi: 10.1002/path.1467. [DOI] [PubMed] [Google Scholar]
- Wu JM, Tu CC, Yu XL, Zhang ML, Zhang NZ, Zhao MY, et al. Bacteriophage T4 nanoparticle capsid surface soc and hoc bipartite display with enhanced classical swine fever virus immunogenicity: a powerful immunological approach. J Virol Methods. 2007;139:50–60. doi: 10.1016/j.jviromet.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Black LW, Showe MK, Steven AC.Morphogenesis of the T4 head. In: Karam JD, editor. Molecular biology of bacteriophage T4 Washington (DC): ASM Press 1994. p218–58.
- Yanagide M. Molecular organization of the shell of the T even bacteriophage head. II Arrangement of subunits in the head shells of giant phages. J Mol Biol. 1977;109:515–37. doi: 10.1016/s0022-2836(77)80089-2. [DOI] [PubMed] [Google Scholar]
- Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–21. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- Ishii T, Yanagida M. The two dispensable structural proteins (Soc and Hoc) of the T4 phage capcid; their purification and properties, isolation and characterization of the defective mutants, and their binding with the defective heads in vitro. J Mol Biol. 1977;109:487–514. doi: 10.1016/s0022-2836(77)80088-0. [DOI] [PubMed] [Google Scholar]
- Fokine A, Chipman PR, Leiman PG, Mesyanzhinov VV, Rao VB, Rossmann MG. Molecular architecture of the prolate head of bacteriophage T4. Proc Natl Acad Sci USA. 2004;101:6003–8. doi: 10.1073/pnas.0400444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZJ, Tian CJ, Zhao MY, Xin AG, Nie WX, Ling SR, et al. Orally delivered foot-and-mouth disease whole envelop vaccine displayed on T4 bacteriophage surface: 100% protection from potency challenge in mice. Vaccine. 2008;26:1471–81. doi: 10.1016/j.vaccine.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Bruttin A, Brussow H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrobial Agents Chemother. 2005;49:2874–8. doi: 10.1128/AAC.49.7.2874-2878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZJ, Lewis GK, Wingfield PT, Locke EG, Steven AC, Black LW. Phage display of intact domains at high copy number: a system based on SOC, the small outer capsid protein of bacteriophage T4. Protein Sci. 1996;5:1833–43. doi: 10.1002/pro.5560050909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly M, Holmgren L, Shing Y. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–25. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Fichtner I, Tanneberger S. Preoperative (neoadjuvant) chemotherapy in the murine Lewis lung carcinoma and possible implications for clinical use. Anticancer Res. 1987;7:227–33. [PubMed] [Google Scholar]
- Himmele JC, Rabenhorst B, Werner D. Inhibition of Lewis lung tumor growth and metastases by Ehrlich ascites tumor growth in the same host. J Cancer Res Clin Oncol. 1986;111:160–5. doi: 10.1007/BF00400757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik E, Segal S, Feldman M. Control of lung metastases progression in mice: role of growth kinetic of 3LL Lewis lung carcinoma and host immunity reactivity. J Natl Cancer Inst. 1980;65:1257–64. [PubMed] [Google Scholar]
- Lu N, Gao Y, Guo QL. Wogonin suppresses tumor growth in vivo and VEGF-induced angiogenesis through inhibiting tyrosine phosphorylation of VEGFR2. Life Sci. 2008;82:956–63. doi: 10.1016/j.lfs.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treatment. 1995;36:169–80. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- Ramirez BS, Pestana ES, Hidalgo GG, Hernandez GT, Rodriguez PR, Ullrich A, et al. Active antimetastatic immunotherapy in Lewis lung carcinoma with self EGFR extracellular domain protein in VSSP adjuvant. Int J Cancer. 2006;119:2190–9. doi: 10.1002/ijc.22085. [DOI] [PubMed] [Google Scholar]
- Hu B, Wei Y, Tian L, Zhao X, Lu Y, Wu Y, et al. Active antitumor immunity elicited by vaccine based on recombinant form of epidermal growth factor receptor. J Immunother. 2005;28:236–44. doi: 10.1097/01.cji.0000161394.11831.3f. [DOI] [PubMed] [Google Scholar]
- Su JL, Yang PC, Shih JY, Yang CY, Wei LH, Hsieh CY, et al. The VEGF-C/VEGFR-3 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9:209–23. doi: 10.1016/j.ccr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–8. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065–75. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- Dias S, Choy M, Alitalo K, Rafii S. Vascular endothelial growth factor (VEGF)-C signaling through VEGFR3 mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood. 2002;99:2179–84. doi: 10.1182/blood.v99.6.2179. [DOI] [PubMed] [Google Scholar]