Abstract
Aim:
To study the inducing effect of bicyclol on heat shock protein 27 (HSP27) and its role on anti-apoptosis in HepG2 cells intoxicated with D-galactosamine (D-GaIN).
Methods:
HepG2 cells were treated with various concentrations of bicyclol and then subjected to D-GaIN intoxication. Apoptosis was assayed by hoechst 33258 staining and flow cytometry analysis. HSP27, cytochrome c, apoptosis inducing factor (AIF) and c-Jun N-terminal kinase (JNK) were assayed by Western blot. Heat shock factor 1 (HSF1) was determined by electrophoretic mobility shift assay and the interactions of HSP27 with cytochrome c and AIF were detected by co-immunoprecipitation.
Results:
The results showed that bicyclol induced HSP27 protein and mRNA expression in HepG2 cells in both time- and dose-dependent manners (the maximal response: 1.23 fold increase at 100 μmol/L). Bicyclol treatment stimulated HSF1 activation and increased the HSF1-HSE binding activity (the maximal response: 2.1 fold increase at 100 μmol/L). This inducing effect of bicyclol on HSP27 and HSF1 was markedly blocked by quercetin. Pretreatment of the cells with bicyclol markedly attenuated D-GaIN-induced apoptosis and the release of cytochrome c and AIF from mitochondria. The induced HSP27 by bicyclol suppressed the activity of caspase-3 and the phosphorylation of JNK caused by D-GaIN in HepG2 cells. All the above effect of bicyclol against D-GaIN-induced hepatocytes apoptosis were significantly reversed by quercetin.
Conclusion:
HSP27 is involved in the anti-hepatocytes apoptosis of bicyclol, and this effect of bicyclol-induced HSP27 is mainly through inhibition of mitochondria and JNK apoptotic pathways.
Keywords: bicyclol, heat shock protein, mitochondria, c-Jun N-terminal kinase, apoptosis, HepG2 cells, cytochrome c, D-galactosamine
Introduction
Bicyclol is a novel anti-hepatitis drug which has been wildly used to treat chronic viral hepatitis in China since 2004. Its chemical name is 4,4′-dimethoxy-5,6,5′,6′-bis(methylene-dioxy)-2-hydroxy-methyl-2′-methoxycarbonyl biphenyl. Pharmacologically, bicyclol protects against chemical-induced liver injury in mice and rats1, 2, 3, inhibits hepatitis virus replication in duck viral hepatitis and in a HepG2.2.15 cell line4, 5 and anti-liver fibrosis in rat carbon tetrachloride model6. Bicyclol exerts most of its effects by multiple pathways such as eliminating free radical, maintenance of mitochondrial glutathione redox status, anti-inflammation and inhibiting fibrogenesis7.
Heat shock proteins (HSPs) are a family of constitutive and inducible expressed gene products that collectively function to maintain cellular protein conformation during stressful conditions. The synthesis of HSPs, which allows cells to adapt to gradual changes in their environment and to survive in otherwise lethal conditions, can be induced by a variety of mild stressors including exposure to oxidants, heat, hypoxia, and low pH, all of which can affect protein conformation8. It was reported that overexpression of HSPs suppressed apoptosis of hepatocytes in vitro and in vivo9, 10. In addition, induction of small HSPs such as HSP27 has been shown to exert cytoprotective effects against various stresses in many tissues including the liver11.
Hepatocytes apoptosis plays an important role in pathogenesis of liver disease including viral hepatitis12. D-galactosamine (D-GaIN) induces hepatocyte cell death in vivo13 and in vitro14, which is a suitable experimental model based on its capacity to reduce the intracellular pool of uridine monophosphate in hepatocytes, thus inhibiting the synthesis of RNA and proteins. Our recent study demonstrated that bicyclol significantly induced the hepatic heat shock protein 27 and 70 in normal mice, and the protective action of bicyclol against acetaminophen hepatotoxicity15 and concanavalin A (Con A)16 induced liver injury also attributed to its induction of hepatic HSPs. However, the molecular mechanism against hepatocyte injury is still not thoroughly elucidated. In this study, HepG2 cells were used to explore the mechanisms of bicyclol-induced HSP27 in the protection against D-GaIN-induced apoptosis of hepatocyte.
Materials and methods
Cell culture and treatment
Human hepatoma HepG2 cells were cultured in minimal essential medium with Earle's salts supplemented with 10% (v/v) heat-inactivated fetal bovine serum and incubated in a humidified incubator with 5% CO2/95% air at 37 °C.
In the time-course study of the inducing effect of bicyclol on HSP27, a dose of bicyclol 50 μmol/L was used. Three different dosages of bicyclol 25 μmol/L, 50 μmol/L, and 100 μmol/L were added to the cells for dose-effect relationship study, each with 20 μmol/L of quercetin (Sigma,USA) simultaneously. To investigate the protective effects of bicyclol and its active mechanism on HepG2 cells challenged by D-GaIN (Sigma, USA), the cells were pre-treated with bicyclol 100 μmol/L and quercetin 20 μmol/L for 2 h, and the cells were then stimulated with D-GaIN 50 mmol/L, and harvested 8 h later.
Hoechst 33258 staining and flow cytometry analysis
HepG2 cells were grown in 24 microwell plates and treated as described above. To observe cells undergoing apoptosis, Hoechst 33258 staining was performed according to the kit instruction (Beyotime Institute Biotechnology, China). The cells were counted and examined by fluorescence microscopy at 480 nm (Eclipse TE300, Nikon, Japan). At minimum, 500 cells were counted from more than 5 random microscopic fields by two observers (Darzynkiewicz et al, 1992). For the flow cytometry study, HepG2 cells were cultured in 25 cm2 culture flask and treated as described in materials and methods. Cells were washed in a balanced salt solution, resuspended in 70% ethanol and stored at −20 °C for analysis. One hour before flow cytometry analysis, the fixed cells were washed twice and incubated for 30 min at room temperature in Hank's balanced salt solution in order to allow the release of apoptotic cells characterized with DNA of low molecular weight. Cells were resuspended in PBS, and then incubated in the presence of propidium iodide (PI) and DNase-free RNase A for 20 min at room temperature. The samples were analyzed using an EPICS Profile II flow cytometer (Coulter) equipped with an argon laser working at 15 mW. EPICS Profile Software (Coulter) was run for data acquisition and MultiCycle AV (Phoenix Flow System, San Diego, CA) for DNA ploidy. A minimum of 105 cells within the gated region was analyzed17.
Caspase-3 activity determination
Caspase-3 activity was determined according to the manufacturer's protocol (Sigma). In brief, HepG2 cell lysates were prepared by lysis buffer (25 mmol/L HEPES, pH 7.4, 2.5 mmol/L CHAPS, 2.5 mmol/L dithiothreitol). Caspase-3 activity was determined by monitoring proteolysis of the colorimetric substrates. Ac-DEVD-p-nitroaniline was used as colorimetric p-nitroaniline linked substrate. The whole-cell lysate was added to a buffer containing 200 μmol/L substrates. After 1.5 h of incubation, the cleavage of the peptide by the caspase was quantified spectrophotometrically at 405 nm in a 96 well plate. The unit of the optical density was converted to nmols of p-nitroaniline using a standard curve generated with free p-nitroaniline.
Reverse transcription-polymerase chain reaction (RT-PCR) assay
Total RNA was isolated from HepG2 cells using Trizol reagent (Invitrogen CA, USA) following the manufacturer's protocol. RT-PCR was performed using One-Step RT-PCR Kit (Promega, WI, USA). The reaction mixture contains AMV/Tfl reaction buffer 10 μL, dNTP 0.2 mmol/L, 1 μmol/L of each primer, 1 mmol/L MgSO4, 0.1 u/μL AMV reverse transcriptase and Tfl DNA polymerase, 2 μg RNA template. The reaction was heated at 45 °C for 45 min for reverse transcription, and 94 °C for 2 min for AMV RT inactivation and RNA/cDNA/primer denaturation for 40 cycles. Denaturation, annealing and extension steps for detecting HSP27 transcripts were 94 °C for 30 s, 55 °C for 1 min, and 68 °C for 2 min, respectively. The final extension was at 68 °C for 7 min. The following primers used in the PCR reactions were synthesized by Shanghai Sangon Biological Engineering Technology & Services Company (Shanghai, China): HSP27-forward 5′-CCCACCCTCTATCACGGCTAC-3′ and reverse 5′-GGGCTCAACTCTGGCTATCTC-3′, which leads to a 426-bp product. Amplified products were separated on a 1% agarose gel in TBE buffer (45 mmol/L Tris borate, 1 mmol/L EDTA). RT-PCR bands were photographed with a Kodak Gel Logic 100 Imaging System (Life Technologies, Inc, Eastman Kodak Co, New Haven, CT) and the density of the bands was determined using Gel-Pro Analyzer 4.0 software.
Western blot analysis
HepG2 cells were lysed in nondenaturing lysis buffer (Applygen Technologies Inc Beijing, China). 30 μg of sample proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in a 10% polyacrylamide gel, and transferred to a polyvinylidene difluoride (PVDF) membrane. Membranes were blocked in 5% skim milk-TBS-T (20 mmol/L Tris-HCl, pH 7.5, 500 mmol/L NaCl. 0.1% Tween 20) at 4 °C overnight. Blots were probed with antibodies against HSP27, cytochrome c, apoptosis inducing factor (AIF) (Santa Cruz, CA, USA), apoptosis protease activating factor-1 (Apaf-1), (p)JNK (Cell Signaling, MA, USA) in 5% skim milk-TBS-T for 2 h at room temperature, and then incubated with the horseradish peroxidase-conjugated secondary antibody in skim milk-TBST for 2 h at room temperature. The blot was developed with LAS3000 chemiluminescence system (Fujifilm, Tokyo, Japan) and the density of the bands was determined using Gel-Pro Analyzer 4.0 software.
Electrophoretic mobility shift assay (EMSA)
HepG2 cells nuclear extracts for EMSA were prepared using nuclear-cytosol extraction kit (Applygen Technologies Inc). Annealed double-stranded heat shock element (HSE) oligonucleotides (5′-AGA CGC GAA ACT GCT GGA AGA TTC CGT GCC-3′) labeled with biotin were synthesized by IDT (NJ, USA). EMSA Kit (Pierce, IL, USA) was used to perform the reaction. The binding reaction (20 μL in total) consists of 10 μg of protein extracts, 20 fmol of biotin labeled DNA, 2.5% glycerol, 5 mmol/L MgCl2, 50 ng/μL Poly (dI dC), and incubated for 20 min at room temperature. DNA-protein complexes were resolved by electrophoresis on a 6% polyacrylamide gel at 4 °C in 0.5×TBE buffer (45 mmol/L Tris borate, 1 mmol/L EDTA), and transferred to a nylon membrane. Then the membrane was detected with the enhanced LAS3000 chemiluminescence system.
Co-immunoprecipitation (Co-IP)
HepG2 cells were lysed in nondenaturing lysis buffer (Applygen Technologies Inc) The Co-IP assay was performed following the protocol of Co-IP kit (Pierce, IL, USA). Briefly, 50 μg of the purified HSP27 antibody was immobilized in 100 μL, 50% antibody coupling gel. Protein extracts 300 μg were incubated with gentle end-over-end mixing for 2 h at room temperature. Immunoprecipitated complexes were eluted thrice with 50 μL elution buffer, boiled and separated by SDS-PAGE, transferred to a PVDF membrane, incubated with cytochrome c, AIF or Apapf-1 antibodies, and detected with the enhanced LAS3000 chemiluminescence system.
Statistical analysis
Data were expressed as means±SD. Changes in different assays were analyzed by ANOVA followed by Tukey–Kramer test as the post hoc test. P<0.05 was considered to be significant statistically.
Results
Bicyclol induced expression of HSP27 in HepG2 cells
Since the induction of HSP is a universal stress response against various insults and it has been widely shown to have an anti-apoptosis effect, the expression of HSP27, one of the major members in HSPs family, was first detected by western blot in this experiment. Bicyclol 50 μmol/L induced HSP27 expression in HepG2 cells in a time-dependent manner. The maximum of the induction was at 6 h (0.93-fold increase, P<0.05), after which the induction gradually declined to the control level at 24 h (Figure 1A).
Figure 1.
Bicyclol induced the expression of HSP27 and activation of HSF1 in HepG2 cells. HepG2 cells were treated with bicyclol 25, 50, and 100 μmol/L alone or co-treated with quercetin 20 μmol/L. Cells were collected 6 h later. HSP27 level was measured by Western blot and RT-PCR. HSF1 was measured by Western blot and EMSA. Data were described as Mean±SD by three separate experiments. (A) Time-course of HSP27 induction by bicyclol. bP<0.05, cP<0.01 vs 0 h control cells. (B) Dose-effect relationship of bicyclol in inducing HSP27 in HepG2 cells. bP<0.05, cP<0.01 vs control cells, eP<0.05 vs cells treated with bicyclol 100 μmol/L. (C, D) Bicyclol induced activation of HSF1 in HepG2 cells. bP<0.05, cP<0.01 vs control cells, eP<0.05, fP<0.01 vs cells treated with bicyclol 100 μmol/L.
The dose-effect relationship of the inductive effect of HSP27 by bicyclol is shown in Figure 1B. Bicyclol induced both protein and mRNA levels of HSP27 expression in a dose-dependent manner. The inducing effect of HSP27 by bicyclol 100 μmol/L was more potent than those by bicyclol 50 μmol/L and 25 μmol/L (25 μmol/L: 0.37-fold increase; 50 μmol/L: 0.58-fold increase; 100 μmol/L: 1.23-fold increase, P<0.01).
Quercetin is a flavanoid and a known inhibitor of HSP synthesis which functions by inhibiting heat shock factor 1 (HSF1). Co-treatment of quercetin 20 μmol/L significantly inhibited bicyclol-induced HSP27 expression in protein (0.82-fold decrease, P<0.05) and mRNA level (0.65-fold decrease, P<0.05), suggesting that the inductive effect of bicyclol on HSP27 expression occurred at mRNA level.
Heat shock gene expression is mainly regulated at the transcriptional level by HSF1. The results of the effect of bicyclol on the activation of HSF1 indicated that bicyclol treatment resulted in decreased level of HSF1 in cytosol (bicyclol treatment: 50 μmol/L: 86.9% decrease, P<0.01; 100 μmol/L: 87.9% decrease, P<0.01) and concomitantly, increased level of HSF1 in nucleus (p-HSF1) (bicyclol treatment: 50 μmol/L: 2.3-fold increase, P<0.05; 100 μmol/L: 4.1-fold increase, P<0.01). EMSA assay showed that bicyclol was able to enhance HSF1-HSE binding activity in a concentration-dependent manner (bicyclol treatment: 25 μmol/L: 1.7-fold increase, P<0.05; 50 μmol/L: 1.9-fold increase, P<0.05; 100 μmol/L: 2.1-fold increase, P<0.01), and co-treatment of quercetin attenuated these effects of bicyclol (Figure 1C, 1D). The results indicated that bicyclol activated HSF1, which in turn induced HSP27 gene expression.
HSP27 induced by bicyclol attenuated apoptosis of HepG2 cells intoxicated with D-GaIN
As shown in Figure 2A, the majority of HepG2 cells in control group had uniformly stained nuclei after staining with the membrane-permeable DNA-binding dye Hoechst 33258. Exposure of HepG2 cells to 50 mmol/L of D-GaIN for 8 h resulted in nuclei fragmentation as indicated in condensed chromatin and bright staining in morphology of HepG2 cells under fluorescent microscope, indicating apoptosis. The treatment of 100 μmol/L bicyclol attenuated apoptosis by 57.6% (P<0.01) as expressed in decrease of nuclei fragmentation of HepG2 cells induced by D-GaIN. When HSP27 synthesis was inhibited by 20 μmol/L quercetin, the protective effect of bicyclol against nuclei fragmentation was abrogated. The apoptosis percentage increased by 1.2-fold (P<0.05), suggesting that HSP27 was involved in the attenuating effect of bicyclol on nuclei fragmentation.
Figure 2.
Bicyclol-induced HSP27 inhibited apoptosis of HepG2 cells intoxicated with D-GaIN. The cells were pre-treated with bicyclol 100 μmol/L alone or co-treated with quercetin 20 μmol/L for 2 h simultaneously. The cells were then stimulated with D-GaIN 50 mmol/L, and harvested 8 h later. (A) Hoechst 33258 staining of DNA in HepG2 cells. (B) Flow cytometry analysis of DNA in HepG2 cells. cP<0.01 vs D-GaIN treated cells; eP<0.05 vs bicyclol+D-GaIN treated cells. Each bar is the mean±SD of five separate experiments.
The flow cytometry analysis showed that 50 mmol/L of D-GaIN induced significant hypodiploid DNA peak before the narrow peak of diploid DNA, which indicated DNA degradation and the undergoing apoptosis of HepG2 cells. The treatment of bicyclol 100 μmol/L inhibited D-GaIN-induced hypodiploid DNA peak development by 61.5% (P<0.01). This effect of bicyclol was also attenuated by co-addition of 20 μmol/L quercetin, indicating that HSP27 participated in the protective effect of bicyclol on hepatocytes apoptosis (Figure 2B).
Bicyclol-induced HSP27 inhibited caspase-3 activation in HepG2 cells intoxicated with D-GaIN
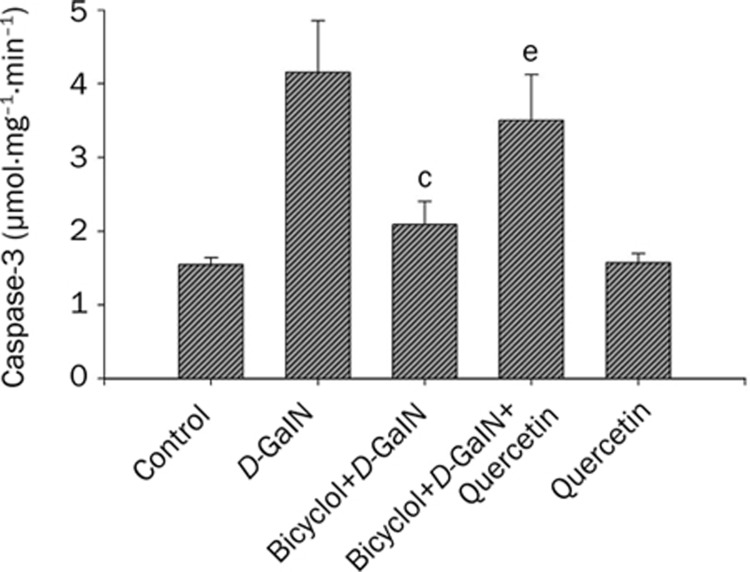
Caspase-3 has been identified as a key mediator of apoptosis of mammalian cells. The results of caspase-3 colorimetric assay indicated that cultivation of HepG2 cells with 50 mmol/L D-GaIN for 8 h caused a significant increase in caspase-3 activity in the cells. Pre-incubation of HepG2 cells with 100 μmol/L bicyclol significantly inhibited D-GaIN-induced activation of caspase-3 by 35.3% (P<0.01). Similarly, 20 μmol/L quercetin attenuated this effect of bicyclol as shown in 30.5% increase of caspase-3 activity (P<0.05) (Figure 3). The results suggested that HSP27 might be involved in the inhibitory effect of bicyclol on caspase-3 activity.
Figure 3.
Inhibitory effect of bicyclol-induced HSP27 on caspase-3 activation in HepG2 cells intoxicated with D-GaIN. The procedure of cell treatment was same as Figure 2. Data were described with Mean±SD by five separate experiments. cP<0.01 vs D-GaIN treated cells; eP<0.05 vs bicyclol+D-GaIN treated cells.
Bicyclol induced HSP27 inhibited the release of cytochrome c and AIF from mitochondria in HepG2 cells intoxicated with D-GaIN
The releases of cytochrome c and AIF from mitochondria play a crucial role in apoptosis. The present results showed that the amount of cytochrome c and AIF released from mitochondria to cytosol significantly increased, accordingly, the levels of cytochrome c and AIF in mitochondria decreased significantly in HepG2 cells intoxicated with D-GaIN. The ratio of cytochrome c and AIF in cytosol/mitochondria increased 8.5-foldand 4.2-fold, respectively. Pre-treatment of bicyclol 100 μmol/L inhibited the release of cytochrome c and AIF from mitochondria to cytosol. The decrease of cytochrome c was 6.3-fold (P<0.01) and AIF decreased 3.8-fold (P<0.01). These effects of bicyclol were attenuated by simultaneously addition of quercetin as shown in 3.85-fold increase of release of cytochrome c (P<0.01) and 3.5-fold of AIF P<0.01) (Figure 4), indicating the role of HSP27 in the protection against D-GaIN induced hepatocytes apoptosis.
Figure 4.
Bicyclol-induced HSP27 inhibited the releases of cytochrome c and AIF from mitochondria in HepG2 cells intoxicated with D-GaIN. The treatment of cells was described above. Cytochrome c and AIF was measured by Western blot. A representative from three results is shown. cP<0.01 vs D-GaIN treated cells; fP<0.01 vs bicyclol+D-GaIN treated cells.
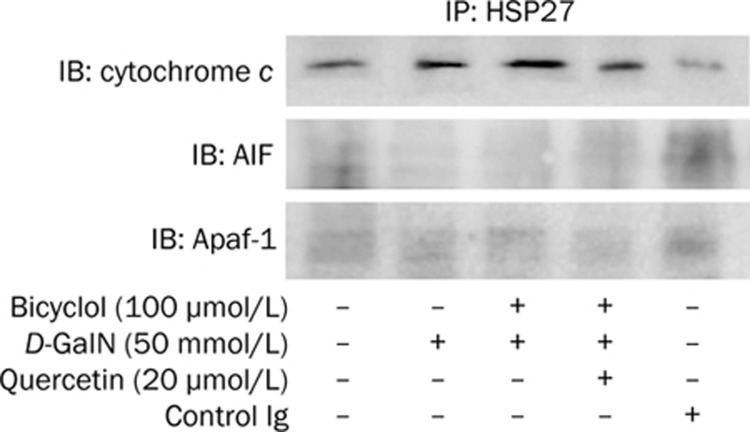
Bicyclol induced HSP27 interacts with cytochrome c, AIF, and Apaf-1 in HepG2 cells intoxicated with D-GaIN
To further study the effect of HSP27 on cytochrome c, AIF and Apaf-1, a set of co-immunoprecipitation experiments were performed with cytochrome c, AIF, and Apaf-1 antibodies in HepG2 cell intoxicated with D-GaIN. As shown in Figure 5, the interaction of HSP27 with cytochrome c was quite distinct, however, the interactions of HSP27 with AIF and Apaf-1were very weak. Blocking of HSP27 with quercetin reduced the association of HSP27 with cytochrome c (Figure 5), suggesting that bicyclol-induced HSP27 directly binds to the pro-apoptosis protein and inhibited the activity of the protein, thereby suppressed hepatocytes apoptosis.
Figure 5.
Interactions of bicyclol-induced HSP27 with cytochrome c, AIF, and Apaf-1 in HepG2 cell intoxicated with D-GaIN. Cells were pre-treated with bicyclol 100 μmol/L, and then they were subjected to D-GaIN. Co-immunoprecipitation assays were performed with cytochrome c, AIF, and Apaf-1 antibodies. A representative of three experiments is shown.
HSP27 induced by bicyclol inhibited JNK activation in HepG2 cells intoxicated with D-GaIN
JNK pathway is also involved in hepatocyte apoptosis. The protective action of HSP27 induced by bicyclol on the activation of JNK pathway was further studied. As shown in Figure 6, 50 mmol/L D-GaIN caused JNK phosphorylation. The pretreatment of 100 μmol/L of bicyclol suppressed JNK phosphorylation by 42% (P<0.05). Co-treatment of quercetin at 20 μmol/L significantly reversed the inhibitory effect of bicyclol on JNK phosphorylation (P<0.05), suggesting that bicyclol induced-HSP27 was involved in the inhibition of JNK.
Figure 6.
Inhibition of JNK activation by bicyclol-induced HSP27 in HepG2 cell intoxicated with D-GaIN. The treatment of cells was described above. JNK was measured by Western blot. bP<0.05 vs D-GaIN treated cells; eP<0.05 vs bicyclol+D-GaIN treated cells. Similar results were obtained in three separate experiments.
Discussion
In the present study, bicyclol was further confirmed to induce the expression of HSP27 in protein and mRNA levels in HepG2 cells. HSP synthesis is tightly regulated at the transcriptional level by HSF1. The activation of HSF1 is associated with its oligomerization, nuclear localization, and acquisition of binding activity to HSE in the heat shock gene promoter to exert the transcriptional activation18, 19. The results of western blot and gel mobility shift assay indicated that bicyclol treatment led to HSF1 translocation to nucleus, phosphorylation and therefore produced an HSE sequence-specific complex in HepG2 cells, which resulted in a marked up-regulation of HSE-binding activity, indicating that bicyclol activates HSF1, which in turn induces HSP27 gene expression.
It was reported that apoptosis is a key pathologic change in liver disease, including viral hepatitis20, liver ischemia21, chemical1 and drug-induced2 liver injury as well as fatty liver disease22. The results of our study indicated that in associated with the induction of heat shock response by bicyclol, HepG2 cells acquired high resistance to apoptosis caused by D-GaIN insult, which expressed in a decrease of percentage of hepatocytes apoptosis and caspase-3 activity. Recent paper pointed out that mitochondrial dysfunction is the commitment step in hepatocyte apoptosis23. Cell death signals induce the release of cytochrome c from the mitochondria, which then binds to Apaf-1, facilitating the formation of the apoptosome. Apoptosome formation results in the processing and activation of caspase-9, which triggers the caspase pathway by activating the downstream caspase-324. AIF is another mitochondrial intermembrane protein released upon apoptotic stimulus, which translocates to the nucleus and triggers caspase-independent nuclear changes upon activation of the intrinsic pathway25. The highly conserved HSPs provide protection to injured cells. Overexpression of HSP27 increases the resistance of cells to various apoptotic stimuli26. One mechanism by which HSP27 could interfere with apoptosis is by directly binding to cytosolic cytochrome c and sequestering it from Apaf-127. The present paper demonstrated that pretreatment of bicyclol inhibited the release of cytochrome c and AIF from mitochondria caused by D-GaIN, and enhanced the association of HSP27 with cytochrome c. It might be possible that overexpressed bicyclol-induced HSP27 may inhibit the apoptotic pathways. So, quercetin, an inhibitor of HSPs biosynthesis, was therefore chosen as a tool in the present study. Quercetin has been shown to block binding of activated HSF1 to its cognate DNA sequence, reduce the activation of the HSR, and decrease HSP expression28, 29. The present results also demonstrated that quercetin at 20 mol/L has no protective effect on hepatocyte apoptosis challenged by D-GaIN. Moreover, quercetin alone also does not affect cytochrome c, AIF and JNK expression. Therefore, the only effect of quercetin at 20 is to inhibit the synthesis HSPs. The present results showed that blocking HSP27 biosynthesis with quercetin markedly attenuated the effects of bicyclol on cytochrome c and AIF release from mitochondria as well as apoptosis of hepatocytes. A question arises as to why the interaction of HSP27 induced by bicyclol with cytochrome c was significant, whereas the interactions with AIF and Apaf-1 were very weak. The cause of different effects of bicyclol on the interaction with AIF and Apaf-1 may be interpreted by other reports that Apaf-1 and AIF mainly interact with HSP70 to antagonize AIF-dependent apoptosis30 and prevent the recruitment of caspases to the apoptosome complex and thereby to block the apoptotic signaling relay. In the present paper, HSP27 was shown to have no interactions with AIF and Apaf-1. However, western blot data indicated that bicyclol can also inhibit the release of AIF from mitochondria to cytosol. Therefore, there might be other proteins such as HSP70 that were involved in the inhibition of bicyclol on AIF and Apaf-1 in HepG2 cells intoxicated by D-GaIN.
Caspase-3 can be activated by caspase-9, which is cleaved and activated by the release of cytochrome c from mitochondria to cytosol. Experimental depletion of HSP27 suggests that HSP27 mainly functions as an inhibitor of caspase activation. Knock-down of HSP27 by small interfering RNAs induces apoptosis through caspase-3 activation31. This phenomenon can be explained by the ability of HSP27 to prevent the formation of the apoptosome and the subsequent activation of caspases32. In agreement with these reports, the present paper provides evidence that HSP27 induced by bicyclol inhibited the activity of caspase-3 in HepG2 cells intoxicated with D-GaIN. It still needs further investigation for the inhibition of caspase-3 by HSP27 due to its effect on apoptosome formation and/or directly on caspase-3.
Mitochondrial outer membrane permeabilization can activate intracellular stress kinases, such as JNK, which is the most important kinase that determines the cell fate to death or survival33. Mice lacking either JNK1 or JNK2 are highly resistant to Con A-induced liver failure and show considerably lower amounts of apoptotic and necrotic hepatocyte death34. Recent studies further proposed that down-regulation of JNK activity is a mechanism of HSP27 mediated protection. Overexpression of HSP27 in stress can definitely play an inhibitory role in JNK signal transduction pathway, and thus blocks cell apoptosis induced by JNK signal transduction pathway35. In our study, the phosphorylation of JNK was also inhibited by pretreatment of bicyclol in HepG2 cells intoxicated by D-GaIN, which was attenuated by simultaneous administration of quercetin, inhibitor of HSPs synthesis. The suppression of JNK signaling by HSP27 induced bicyclol is also involved in anti-apoptosis process of hepatocytes.
Taken together, the present study revealed that bicyclol at non-toxic concentration elicits a significant induction of HSP27 response that is capable of protection against hepatocytes apoptosis mainly through inhibition of mitochondrial and JNK apoptotic pathways in HepG2 cells-intoxicated with D-GaIN.
Author contribution
Geng-tao LIU designed research; Xiu-qi BAO performed research, analyzed data, and wrote the paper.
Acknowledgments
This work was supported by grants from the Chinese Ministry of Science and Technology (96-901-01-45) and from the Chinese Medical Board in New York (93-582).
References
- Zhao DM, Liu GT. Protective effect of bicyclol on concanavalin A induced liver nuclei DNA injury in mice. Natl Med J China. 2001;81:844–8. [PubMed] [Google Scholar]
- Li Y, Dai GW, Li Y, Liu GT. Effect of bicyclol on acetaminophen-induced hepatotoxicity: energetic metabolism and mitochondrial injury in acetaminophen-intoxicated mice. Yao Xue Xue Bao. 2001;36:723–6. [PubMed] [Google Scholar]
- Liu GT, Li Y, Wei HL, Zhang H, Xu JY, Yu LH. Mechanism of protective action of bicyclol against CCl4-induced liver injury in mice. Liver Int. 2005;25:872–9. doi: 10.1111/j.1478-3231.2005.01103.x. [DOI] [PubMed] [Google Scholar]
- Liu GT. The anti-virus and hepatoprotective effect of bicyclol and its mechanism of action. Chin J New Drugs. 2001;10:325–7. [Google Scholar]
- Yao GB, Ji YY, Wang QH, Zhou XQ, Xu DZ, Chen XY, et al. A randomized double-blind controlled trial of bicyclol in treatment of chronic hepatitis B. Chin J New Drugs Clin Rem. 2002;21:457–62. [Google Scholar]
- Li Y, Li Y, Liu GT. Protective effects of bicyclol on liver fibrosis induced by carbon tetrachloride. Natl Med J China. 2004;84:2096–101. [PubMed] [Google Scholar]
- Liu GT. Bicyclol: a novel drug for treating chronic viral hepatitis B and C. Med Chem. 2009;5:29–43. doi: 10.2174/157340609787049316. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–10. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Park KJ, Gaynor RB, Kwak YT. Heat shock protein 27 association with the I-kappa B kinase complex regulates tumor necrosis factor alpha-induced NF-kappa B activation. J Biol Chem. 2003;278:35272–8. doi: 10.1074/jbc.M305095200. [DOI] [PubMed] [Google Scholar]
- Sumioka I, Matsura T, Kai M, Yamada K. Potential roles of hepatic heat shock protein 25 and 70i in protection of mice against acetaminophen-induced liver injury. Life Sci. 2004;74:2551–61. doi: 10.1016/j.lfs.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Zborek A, Małusecka E, Krzyowska-Gruca S, Wysocka A, Krawczyk Z. Immunohistochemical studies on the expression pattern of molecular chaperones HSC70 and HSP25 and cell cycle-related proteins cyclin D1 and PCNA in rat liver after thioacetamide intoxication. Histochem Cell Biol. 2002;118:311–9. doi: 10.1007/s00418-002-0456-5. [DOI] [PubMed] [Google Scholar]
- Fischer R, Baumert T, Blum HE. Hepatitis C virus infection and apoptosis. World J Gastroenterol. 2007;13:4865–72. doi: 10.3748/wjg.v13.i36.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntane J, Montero JL, Lozano JM, Miranda-Vizuete A, de la Mata M, Mino G. TNF-alpha but not Il-1alpha are correlated with PGE1-dependent protection against acute D-galactosamine-induced liver injury. Can J Gastroenterol. 2000;14:175–80. doi: 10.1155/2000/416705. [DOI] [PubMed] [Google Scholar]
- Fouad D, Siendones E, Costán G, Muntané J. Role of NF-kappaB activation and nitric oxide expression during PGE protection against d-galactosamine-induced cell death in cultured rat hepatocytes. Liver Int. 2004;24:227–36. doi: 10.1111/j.1478-3231.2004.00913.x. [DOI] [PubMed] [Google Scholar]
- Bao XQ, Liu GT. Bicyclol: a novel antihepatitis drug with hepatic heat shock protein 27/70-inducing activity and cytoprotective effects in mice. Cell Stress Chaperones. 2008;13:347–55. doi: 10.1007/s12192-008-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao XQ, Liu GT. Induction of heat shock protein 27 and 70 overexpression by bicyclol attenuates concanavalin A-induced liver injury through suppression of NF-{kappa}B in mice. Mol Pharmacol. 2009;75:1180–8. doi: 10.1124/mol.108.053280. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz MA, Lassota P, et al. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Santoro MG. Heat shock factors and the control of the stress response. Biochem Pharmacol. 2000;59:55–63. doi: 10.1016/s0006-2952(99)00299-3. [DOI] [PubMed] [Google Scholar]
- Han SI, Oh SY, Woo SH, Kim KH, Kim JH, Kim HD, et al. Implication of a small GTPase Rac1 in the activation of c-Jun N-terminal kinase and heat shock factor in response to heat shock. J Biol Chem. 2001;276:1889–95. doi: 10.1074/jbc.M006042200. [DOI] [PubMed] [Google Scholar]
- Wang WH, Hullinger RL, Andrisani OM. Hepatitis B virus X protein via the p38MAPK pathway induces E2F1 release and ATR kinase activation mediating p53 apoptosis. J Biol Chem. 2008;283:25455–67. doi: 10.1074/jbc.M801934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursio R, Colosetti P, Auberger P, Gugenheim J. Liver apoptosis following normothermic ischemia-reperfusion: in vivo evaluation of caspase activity by FLIVO assay in rats. Transplant Proc. 2008;40:2038–41. doi: 10.1016/j.transproceed.2008.05.039. [DOI] [PubMed] [Google Scholar]
- Tessari P, Coracina A, Cosma A, Tiengo A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2009;19:291–302. doi: 10.1016/j.numecd.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths. Hepatology. 2006;43:S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- Wright KM, Vaughn AE, Deshmukh M. Apoptosome dependent caspase-3 activation pathway is non-redundant and necessary for apoptosis in sympathetic neurons. Cell Death Differ. 2007;14:625–33. doi: 10.1038/sj.cdd.4402024. [DOI] [PubMed] [Google Scholar]
- Joza N, Susin SA, Daugas E, Stanford W, Cho SK, Li CY, et al. Essential role of the mitochondrial apoptosis inducing factor in programmed cell death. Nature. 2001;410:549–54. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274:18947–56. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–52. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Jakubowicz-Gil J, Rzymowska J, Paduch R, Gawron A. The effect of quercetin on the expression of heat shock proteins and apoptosis induction in monkey kidney cell line GMK. Folia Histochem Cytobio. 2002;40:137–8. [PubMed] [Google Scholar]
- Masuda Y, Sumita S, Fujimura N, Namiki A. Geranylgeranylacetone attenuates septic diaphragm dysfunction by induction of heat shock protein 70. Crit Care Med. 2003;31:2585–91. doi: 10.1097/01.CCM.0000094230.44674.D8. [DOI] [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–43. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- Rocchi P, Jugpal P, So A, Sinneman S, Ettinger S, Fazli L, et al. Small interference RNA targeting heat-shock protein 27 inhibits the growth of prostatic cell lines and induces apoptosis via caspase-3 activation in vitro. BJU Int. 2006;98:1082–9. doi: 10.1111/j.1464-410X.2006.06425.x. [DOI] [PubMed] [Google Scholar]
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999;13:2061–70. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- Singh R, Czaja MJ. Regulation of hepatocyte apoptosis by oxidative stress. J Gastroenterol Hepatol. 2007;22 Suppl 1:S45–48. doi: 10.1111/j.1440-1746.2006.04646.x. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNF-α-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Nakagomi S, Suzuki Y, Namikawa K, Kiryu-Seo S, Kiyama H. Expression of the activating transcription factor 3 prevents c-Jun N-terminal kinase-induced neuronal death by promoting heat shock protein 27 expression and Akt activation. J Neurosci. 2003;23:5187–96. doi: 10.1523/JNEUROSCI.23-12-05187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]