Abstract
Chronic stress has deleterious effects on immune function, which can lead to adverse health outcomes. However, studies investigating the impact of stress reduction interventions on immunity in clinical research have yielded divergent results, potentially stemming from differences in study design and genetic heterogeneity, among other clinical research challenges. To test the hypothesis that reducing glucocorticoid levels enhances certain immune functions, we administered influenza vaccine once (prime) or twice (boost) to mice housed in either standard control caging or environmental enrichment (EE) caging. We have shown that this approach reduces mouse corticosterone production. Compared with controls, EE mice had significantly lower levels of fecal corticosterone metabolites (FCMs) and increased splenic B and T lymphocyte numbers. Corticosterone levels were negatively associated with the numbers of CD19+ (r2 = 0.43, p = 0.0017), CD4+ (r2 = 0.28, p = 0.0154) and CD8+ cells (r2 = 0.20, p = 0.0503). Vaccinated mice showed nonsignificant differences in immunoglobulin G (IgG) titer between caging groups, although EE mice tended to exhibit larger increases in titer from prime to boost than controls; the interaction between the caging group (control versus EE) and vaccine group (prime versus boost) showed a strong statistical trend (cage-group*vaccine-group, F = 4.27, p = 0.0555), suggesting that there may be distinct effects of EE caging on primary versus secondary IgG vaccine responses. Vaccine-stimulated splenocytes from boosted EE mice had a significantly greater frequency of interleukin 5 (IL-5)-secreting cells than boosted controls (mean difference 7.7, IL-5 spot-forming units/106 splenocytes, 95% confidence interval 0.24–135.1, p = 0.0493) and showed a greater increase in the frequency of IL-5–secreting cells from prime to boost. Our results suggest that corticosterone reduction via EE caging was associated with enhanced secondary vaccine responses, but had little effect on primary responses in mice. These findings help identify differences in primary and secondary vaccine responses in relationship to stress mediators that may be relevant in clinical studies.
INTRODUCTION
Stress can be defined as a relationship between a person and his or her environment that is appraised as being taxing or exceeding the ability to cope (1). The perception of a stressor activates a cascade of physiological responses in the body including the sympathetic-adrenal-medullary and hypothalamic–pituitary–adrenal (HPA) axes, culminating in systemic release of catecholamines and glucocorticoids, respectively. Cells of the immune system express receptors for these stress mediators and are susceptible to paracrine immunomodulation as well as signaling via direct innervation of lymphoid organs (2).
Accumulating evidence suggests that acute stressors (those lasting minutes to hours) can augment immune responses. Studies in rodents and humans have demonstrated that acute stress near the time of antigen exposure can amplify delayed-type hypersensitivity (DTH) reactions, increase natural killer cell lytic activity and enhance antibody production in response to vaccination (3–5). In contrast, chronic stressors (those lasting weeks to years) are associated with dysregulation of the HPA axis, impaired innate and adaptive immune responses, and adverse health outcomes. For example, chronically stressed rodents exhibit increased lymphocyte apoptosis, suppressed DTH responses and accelerated tumor growth (5–7). Human studies have demonstrated that chronically stressed populations exhibit a variety of immune impairments, such as diminished wound healing and increased susceptibility to upper respiratory infection (8–10). Vaccination has become an important model for studying the impact of stress on immune function. Studies have shown that chronically stressed individuals mount weaker responses to influenza, hepatitis B and pneumococcal vaccines, compared with matched control subjects, and repeated activation of the stress response and exposure to stress mediators are thought to be related to the observed immune dysfunction (11–13). Interestingly, these studies, as well as others, have reported a negative association between psychological stress level and performance in a variety of tests of immune function (14,15).
Although prior data reporting an association between stress and immune responses to vaccination in humans have come from observational studies, several studies have reported the effects of stress reduction interventions on vaccine responses. A key intervention that has been tested is mindfulness-based stress reduction (MBSR), a standardized meditation-based intervention that has become widely used in hospitals, schools and community settings internationally. MBSR has been shown to reduce anxiety and depression, improve mood and may influence HPA axis activity (16–21). Given the negative relationship between chronic psychological stress and indices of immune function, several groups have hypothesized that stress reduction interventions may enhance responses to an immune challenge, in part, due to reduced exposure to neuroendocrine stress mediators. To date, reports from three randomized controlled trials have been published in which vaccination has been used to compare antigen-specific immune responses in MBSR-trained subjects and controls (22–24). Of the three studies, the first reported that MBSR-trained subjects had greater antigen-specific antibody titers, the second reported no difference between MBSR-trained subjects and controls and the third reported that MBSR-trained subjects had significantly lower antibody titers than controls. These contradictory findings may stem from difference in study design such as choice of immunogen, sampling time points, subject age range, how the intervention was delivered or statistical power. Additionally, inherent challenges of human research, such as genetic heterogeneity, unforeseen life events and variability in previous exposure to the immunogen, may add to the complexity of identifying relationships between stress reduction and vaccine responses.
In previous work, we described a standardized approach to environmental enrichment (EE) in which we housed BALB/c mice, a mouse strain known to be relatively anxious, in caging containing a targeted collection of enrichments, to reduce corticosterone production (25). EE cages are larger and contain shredded nesting material, a cardboard nest box and a polycarbonate tube, which allow the mice to create a customized habitat including the construction of a three- dimensional nest. Mice housed in standard cages did not have access to any enrichment materials. We showed that EE-caged mice exhibited significant reductions in corticosterone excretion and increased body mass when compared with mice housed in standard control caging, although no differences in elevated-plus-maze behavior were observed. Additionally, we found that EE-caged mice had greater spleen mass than controls, which led us to hypothesize that EE-caged mice may have altered spleen composition and enhanced response to an immune challenge and that these results may be related to reduced glucocorticoid signaling.
Given the incongruent findings in human studies of MBSR and vaccine responses, we reasoned that conducting a rodent vaccination study, focused on modeling reduced HPA axis activity, would provide insights into the relationship between glucocorticoid signaling and immune function. Such a study design would also be useful for shaping future immunologically driven studies of mind-body interventions. In the current study, we investigated whether control- and EE-caged mice exhibited differences in spleen composition and/or humoral and cellular immune responses to influenza vaccination. Through studying genetically identical inbred mice, with a known immunogen exposure history, and housing the animals in a controlled environment, we were able to circumvent some of the human research challenges described above.
MATERIALS AND METHODS
Animals
Six-week-old male BALB/c mice were purchased from The Jackson Laboratory and housed in the Laboratory Animal Resource Center at San Francisco General Hospital. Cages were kept in a temperature-controlled room (22°C) with a light–dark 12:12 cycle (lights on 0600–1800 h). All cages were provided with water and PicoLab Rodent Diet 20 (LabDiet, PMI Nutrition International [see http://www.labdiet.com/ [LabDiet, St. Louis, MO, USA]) ad libitum. All studies were approved by the University of California San Francisco Institutional Animal Care and Use Committee and were conducted in accordance with national guidelines of humane laboratory animal care.
Caging and Enrichment
Mice were randomly assigned to control or EE caging (n = 20 per group). For both groups, each cage housed five animals (four cages per group). All caging, bedding and enrichment items were autoclaved before use or sterilized with Coverage disinfectant spray (STERIS Corporation, Mentor, OH). Standard wire-bar lids for food and water and filter-top bonnets were used for all cages. Cages for the control group (189 × 297 × 128 mm; 484 cm2 surface area; Allentown Inc., Allentown, NJ, USA) contained standard Paperchip bedding (Shepherd Specialty Papers, http://www.ssponline.com/index.htm) and lacked enhancements. Cages for the EE group were larger (257 × 483 × 152 mm; 980 cm2 surface area) and contained standard bedding, one paper nest box (Bio-Serv, Frenchtown, NJ, USA), one red polycarbonate mouse tunnel (Bio-Serv) and 236 cm3 (1 cup) of compressed Enviro-dri Eco-bedding shredded paper strips (FiberCore, Cleveland, OH, USA). Once weekly, cages and washable enrichment items were cleaned, and bedding, Enviro-dri strips and nest boxes were replaced.
Influenza Vaccination Timeline
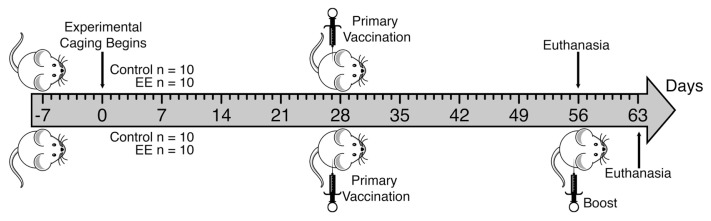
Figure 1 summarizes the cage enrichment and vaccination timeline carried out in these studies. After arrival from the vendor, all mice were group-housed (five animals per cage) in standard control caging for a period of 14 d (d –14 to 0) to allow them to acclimatize to the Laboratory Animal Resource Center husbandry environment. Body mass and fecal corticosterone measurements began 1 wk before the onset of experimental caging (d –7 onward). After acclimatization, animals were placed in their respective caging conditions and remained there for an additional period of either 56 or 63 d. Animals were weighed during weekly cage cleaning. Additionally, fecal samples were taken from each animal at regular intervals and kept at −20°C for batched fecal corticosterone metabolite analysis (see below). Inactivated Fluarix trivalent influenza vaccine (GlaxoSmithKline, Brentford, Middlesex, UK) was diluted in sterile phosphate-buffered saline (PBS). The vaccine contained equal quantities of hemagglutinin (HA) from the following strains: A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2), B/Brisbane/60/2008. Mice were placed under light isoflurane anesthesia and injected intramuscularly in the right hindlimb with dilute vaccine (1 μg HA in 50 μL) or vehicle (50 μL PBS). Mice were injected with vaccine once (prime, n = 5 per caging group) or twice (boost, n = 5 per caging group) or were injected with PBS alone (naive, n = 10 per caging group). Primed mice were vaccinated after 4 wks of experimental caging and euthanized 4 wks after injection. Boosted mice received the first injection 4 wks after the onset of experimental caging and the second injection 4 wks after the first injection, and were euthanized 1 wk after boost.
Figure 1.
Caging enrichment and influenza vaccination timeline. After arriving at the husbandry facility, 6-wk-old male BALB/c mice were housed in standard control caging for 14 d (day −14 to 0) for acclimatization. At d 0, mice were placed, five per cage, into either standard control caging (n = 20) or enriched EE caging (n = 20) for either 56 or 63 d. At d 28, all mice were injected intramuscularly with trivalent influenza vaccine (1 μg HA in 50 μL PBS) or vehicle (50 μL PBS). At 28 d after primary vaccination, half of the animals were euthanized for blood and tissue collection and half were given a second intramuscular vaccination (boost). Remaining animals were euthanized for blood and tissue collection 7 d after boost.
Fecal Corticosterone Metabolites
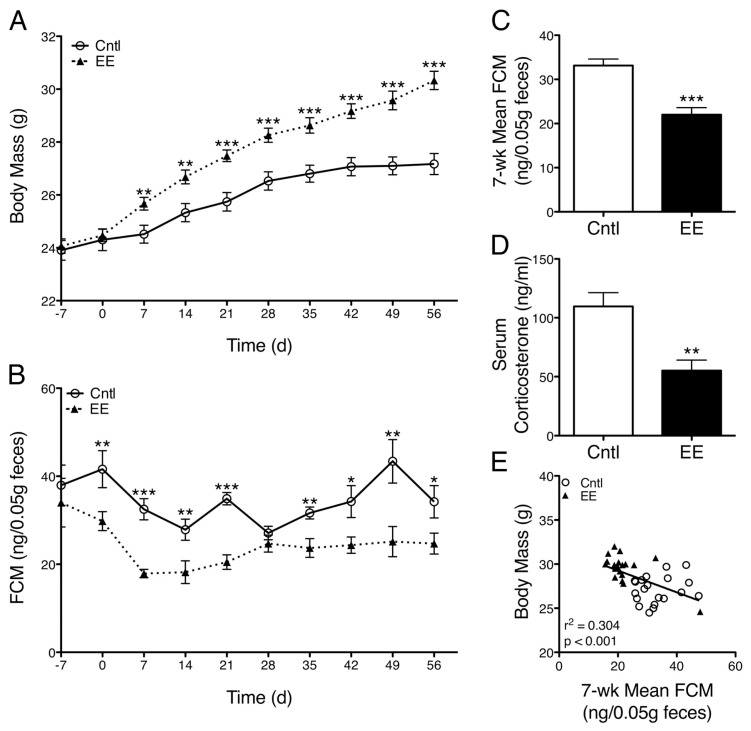
During cage changing, animals were temporarily placed in individual containers, and fecal pellets produced at this time were collected and stored at −20°C. Weekly samples were taken from each animal at the same time of day for 7 wks. Glucocorticoid metabolite quantification in fecal samples has been extensively validated for laboratory mice (26–29). Collected samples were analyzed for immunoreactive fecal corticosterone metabolite (FCMs) by using a 5α-pregnane-3β,11β,21-triol-20-one enzyme immunoassay (EIA) as described previously (27). Samples were run in one batch to enhance data measure consistency. The intraassay coefficient of variation was 8.8%. Area under the curve was determined by using the trapezoidal rule. The 7-wk mean FCM was calculated by averaging the weekly FCM data for each animal beginning at the first measurement after the onset of experimental caging (7 d) and ending at the last time point in which FCM data were collected for all animals in all caging conditions (49 d). Group means (Figure 2B) were determined by averaging the 7-wk mean FCM values for all animals in each caging condition, whereas regression analyses used 7-wk mean FCM data from each individual animal.
Figure 2.
EE-caged mice have greater body mass and reduced corticosterone concentration. (A) EE-caged mice exhibited greater body mass than control-caged mice over the course of the study (caging, p = 0.0037; time*caging, p < 0.0001). (B) Longitudinal FCM levels, a noninvasive measure highly correlated with serum corticosterone concentration, were consistently lower in EE-caged mice compared with controls (caging, p < 0.0001; area under curve, p < 0.0001). (C) Mean FCM concentration, averaged over the course of the experiment, was also significantly lower in EE mice compared with control-caged mice (p < 0.0001). (D) Serum corticosterone concentration measured at the time of euthanasia was 43% lower in EE mice than control mice (p = 0.0029). (E) A negative relationship was observed between body mass and mean FCM concentration across caging groups (r2 = 0.30, p = 0.0002). Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Euthanasia
At the experimental endpoint, d 56 or 63, cardiac blood was collected while the animals were under deep isoflurane anesthesia, and animals were then euthanized via cardiac puncture one at a time. Depth and duration of anesthesia were similar for all animals, and sample collection was done within 2 h of lights on in the husbandry facility.
Flow Cytometry
Spleens were taken and cells were mechanically dissociated. Cell number was counted using standard methods after red blood cell lysis (eBioscience, San Diego, CA, USA). Splenocytes were plated at 1.0 × 106 cells per well in a V-bottom 96-well plate. Cells were stained with phycoerythrin (PE)- conjugated anti-Thy1.2, Alexa 700– conjugated anti-CD4, phycoerythrin-Texas Red (PE-Tx)-conjugated anti-CD8, and allophycocyanin-Cy7 (APC-Cy7)-conjugated anti-CD19. Dead cells were labeled and excluded from analyses by using Alexa 430 amine-reactive live/dead dye (BD Biosciences [San Jose, CA, USA] and Molecular Probes/Life Technologies [Carlsbad, CA, USA]). After staining, cells were washed with fluorescence-activated cell sorter buffer (PBS + 0.5% bovine serum albumin + 2 mmol/L ethylenediaminetetraacetic acid [EDTA]), fixed with 2% paraformaldehyde in PBS, and collected on an LSR II (BD Biosciences). Anti-rat immunoglobulin G (IgG)-coated beads were stained with each fluorochrome-conjugated rat antibody separately and used for software-based compensation. All samples were analyzed on a custom four-laser LSR II (BD Biosciences) equipped with a 50 mW blue (488 nmol/L), 50 mW violet (406 nmol/L), 40 mW red (640 nmol/L) and 150 mW green (532 nmol/L) laser.
Gating Strategy
Flow cytometry analyses were carried out using FlowJo software, version 9.5 (TreeStar, Ashland, OR, USA). Cell doublets were excluded by plotting forward scatter height and area parameters. A lymphocyte gate was created using side scatter area and forward scatter height area parameters. Live cells were selected as those not stained positively by Alexa 430 amine-reactive live/dead dye. B lymphocytes were identified as Thy1.2−CD19+ cells. CD4+ T lymphocytes were identified as CD19−Thy1.2+CD4+CD8− cells. CD8+ T lymphocytes were identified as CD19−Thy1.2+CD4−CD8+ cells. CD4−CD8−T lymphocytes were identified as CD19−Thy1.2+CD4−CD8− cells.
IgG Titer
Vaccine-specific IgG levels were assessed by using an enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well Immulon-2 High Binding flat-bottom microtiter plates (Thermo Scientific [Thermo Fisher Scientific Inc., Waltham, MA, USA]) were coated with 100 μL diluted Fluarix vaccine (1 μg/mL HA protein) and incubated overnight at 4°C. Animal sera were diluted with blocking buffer (0.5% gelatin, 0.15% Tween-20, 1% bovine serum albumin in PBS) and incubated for 1 h at 37°C. Plates were washed and blocked. Serum samples were twofold serially diluted starting from 1:200, plated in duplicate and incubated for 1.5 h at 37°C. Plates were washed and then incubated with horseradish peroxidase–conjugated anti-mouse IgG (Abcam, Cambridge, UK) for 45 min at 37°C. Plates were washed and tetramethylbenzidine (TMB) substrate (Invitrogen/Life Technologies) was added, followed by 2 mol/L H2SO4 stop solution. A SPECTRAmax absorbance microplate reader (Molecular Devices/Life Technologies) was used to read each plate at optical densities of 450 and 650 nm. The 650-nm values were subtracted from the 450-nm values to account for plate background. Serum from one vaccinated mouse was added to each plate in triplicate and used as an interplate normalization control (coefficient of variation: interassay 7.2%, intraassay 6.7%). Negative control wells were not coated with vaccine and incubated with serum samples or coated with vaccine and incubated with blocking buffer only. Optical density (OD) values from negative control wells were subtracted from serum sample OD values as a background correction. IgG titer was set as the greatest dilution of serum generating an OD value greater than twice the vaccine-uncoated negative control OD value.
ELISPOT Assay
Interleukin (IL)-5 production was measured with a mouse IL-5 ELISPOTplus kit (Mabtech, Cincinnati, OH, USA) according to the manufacturer’s instructions. Briefly, splenocytes were plated in 96-well ELISPOT polyvinylidene fluoride plates (EMD Millipore, Billerica, MA, USA) at 1 × 106 cells per well. Wells were treated with Fluarix vaccine (3 μg/mL), concanavalin A (2 μg/mL) or PBS in duplicate. Plates were then incubated for 24 h at 37°C, washed with PBS and developed per the manufacturer’s instructions. Spots were counted by using AID EliSpot Reader and Software (Autoimmun Diagnostika, Straßberg, Germany). To correct for background signal, twice the number of spots counted in PBS-treated negative control wells was subtracted from each sample spot number.
Serum Corticosterone
Whole blood was collected via cardiac puncture at the study endpoint within 2 h of lights on, kept at 4°C overnight until coagulated and centrifuged for 10 min at 17,970 × g. Supernatants were frozen at −20°C. ELISA kits were used as instructed by the manufacturer to measure serum concentrations of corticosterone (IBL America, Minneapolis, MN, USA). Samples were assayed in one batch (intraassay coefficient of variation was ≤8.3%).
Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM). Analyses were performed by using Prism software v5.0a (GraphPad Software Inc., La Jolla, CA, USA) or SAS PROC GLM 9.2. Unpaired t tests were used to compare control-caged animals with EE-caged animals or vaccine-primed animals with vaccine-boosted animals (Figures 2A–D, 3A–D, and 4A, B, D). Two-way repeated-measures analysis of variance (ANOVA) was used to compare increases in body mass between caging groups over time (Figure 2A) and FCM level over time (Figure 2B). Relationships between corticosterone concentration and body mass (Figure 2E), lymphocyte number (Figures 3E–J and 4F, G), IL-5 production (Figure 4C) and spleen mass (Figure 4E) were measured by using a linear regression followed by two-tailed statistical comparison of regression coefficients. Interaction between cage group and vaccine group (Figure 4A, B) was determined by using a 2 × 2 ANOVA. Partial η2, the proportion of residual variation explained by a given effect, was used for effect size in ANOVA (Figure 4A, B). In all cases, p < 0.05 was considered statistically significant.
Figure 3.
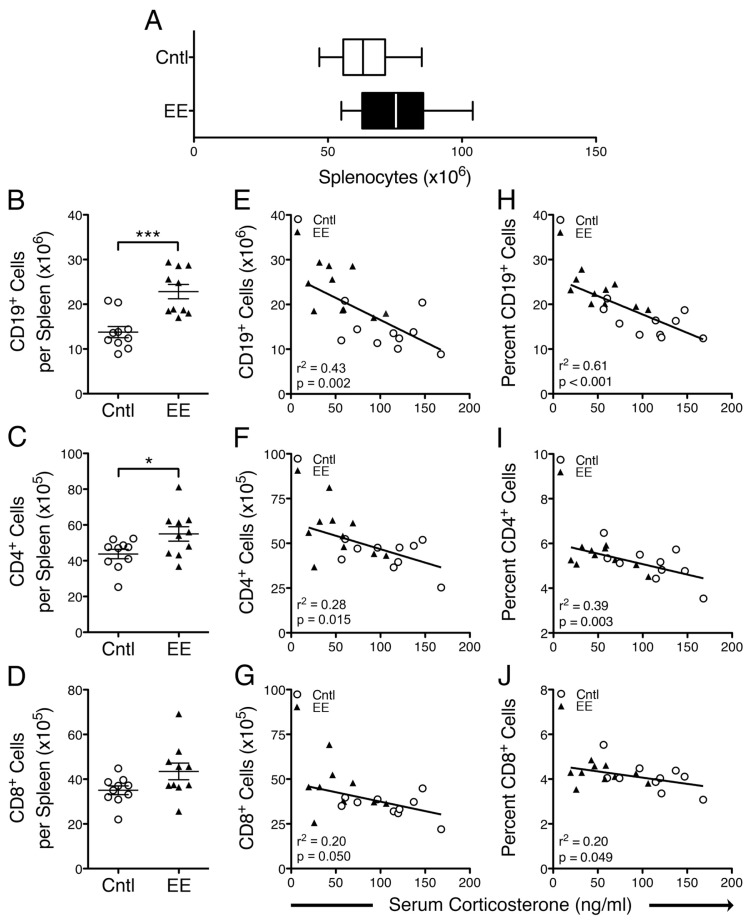
Splenic B and T lymphocyte numbers are greater in naive EE-caged mice than naive controls and negatively associated with corticosterone concentration. (A) Naive (PBS-injected) EE mice exhibited a nonsignificant trend toward a greater number of splenocytes than naive controls (p = 0.0588). (B–D) The absolute number of splenic CD19+, CD4+ and CD8+ cells was greater in naive EE mice than naive controls (CD19+, p = 0.0003; CD4+, p = 0.0321; CD8+, p = 0.0605). (E–G) Negative relationships were observed between the absolute number of splenic CD19+, CD4+ and CD8+ cells and serum corticosterone concentration (CD19+, r2 = 0.43, p = 0.0017; CD4+, r2 = 0.28, p = 0.0154; CD8+, r2 = 0.20, p = 0.0503). (H–J) Similarly, the proportion (% of total splenocytes) of CD19+, CD4+, and CD8+ cells exhibited a significant negative relationship with serum corticosterone concentration (CD19+, r2 = 0.61, p < 0.0001; CD4+, r2 = 0.39, p = 0.0034; CD8+, r2 = 0.20, p = 0.0492). Data are mean ± SEM. *p < 0.05, ***p < 0.001.
Figure 4.
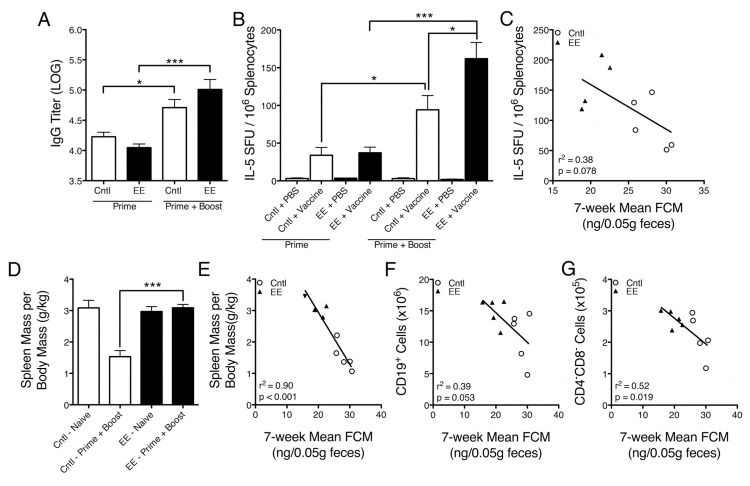
Effects of EE caging on primary and secondary vaccine responses and spleen composition. (A) Influenza vaccine–specific IgG titers were measured in serum from mice vaccinated once (prime) or twice (boost) using an ELISA. In primed groups, EE mice had slightly lower IgG titers than control-caged mice (p = 0.0943), whereas in boosted groups, EE mice had slightly higher IgG titers than controls (p = 0.1950), although neither difference was statistically significant. Control mice exhibited an increase in IgG titer, from prime to boost, of 11.4%, whereas EE mice exhibited an increase of 23.8% (control, p = 0.0138; EE, p = 0.0006). (B) 1 × 106 splenocytes were stimulated with either PBS or vaccine for 24 h in an IL-5 ELISPOT assay. Primed animals showed no difference between caging groups, whereas splenocytes from boosted EE mice that were stimulated with vaccine had a significantly greater frequency of IL-5–secreting cells than boosted controls (p = 0.0493). The increase in frequency of IL-5–secreting cells from prime to boost was larger in EE mice (334.2%) than controls (177.9%) (control, p = 0.0230; EE, p = 0.0005). (C) A nonsignificant negative trend was observed between IL-5 production and mean FCM concentration (r2 = 0. 38, p = 0.0781). (D) Spleen mass per unit body mass was found to be significantly lower in boosted control-caged mice than boosted EE-caged mice (p = 0.0001). (E) A negative relationship was observed between mean FCM concentration and spleen mass per unit body mass in vaccine-boosted animals (r2 = 0.90, p < 0.0001). (F, G) Negative relationships were also observed between mean FCM concentration and CD19+ B cell number and CD4−CD8− lymphocyte number (CD19+, r2 = 0.39, p = 0.0533; CD4−CD8−, r2 = 0.52, p = 0.0190). Data are mean ± SEM. *p < 0.05, ***p < 0.001.
RESULTS
Body Mass and Corticosterone Concentration
Mice housed in EE caging gained significantly greater body mass than control-housed animals within 1 wk of the onset of experimental caging (7 d, difference in means [Md] 1.15 g, 95% confidence interval [CI] 0.32–1.99, p = 0.0082) and significantly greater body mass over the course of the study (caging, p = 0.0037; time*caging, p < 0.0001; Figure 2A). These results replicate our previous findings (25). To monitor stress hormone production noninvasively and longitudinally, we measured FCM concentrations from each animal weekly (a measure highly correlated with plasma corticosterone concentration) (29). EE-caged animals had significantly lower FCM concentrations than control-caged animals over the course of the experiment (caging, p < 0.0001; area under curve [trapezoidal rule], Md −425.3 ng/0.05 g feces, CI −247.2 to −603.4, p < 0.0001; Figure 2B) and a significantly lower 7-wk mean FCM concentration (Md −11.1 ng/0.05 g feces, CI −6.7 to −15.5, p < 0.0001; Figure 2C). Interestingly, in the first week of experimental caging (d 0–7) both caging groups exhibited a reduction in FCM level, which may have been related to latent acclimatization or habituation to regular handling. Serum corticosterone concentrations were also measured at the time of euthanasia and, similarly, were 43% lower in EE-caged animals compared with controls (Md −43.1 ng/mL, CI −15.7 to −70.5, p = 0.0029; Figure 2D). In regression analyses, we found a negative relationship between body mass and mean FCM concentration (r2 = 0.30, p = 0.0002; Figure 2E), a finding in line with previous reports of the relationship between dominance hierarchy and corticosterone production (30).
Spleen Immunophenotype of Naive Mice
Consistent with our previous findings of increased spleen mass in EE mice, we found that PBS-injected (naive), EE-caged animals exhibited a trend toward greater numbers of splenocytes after erythrolysis compared with PBS-injected controls (Md 11.7 × 106 cells, CI −0.49 × 106 to 24.0 × 106, p = 0.0588, Cohen’s d = 0.90; Figure 3A). By using multicolor flow cytometry, we enumerated splenic Thy1.2+, CD4+, CD8+ and CD19+ cells to identify potential shifts in the absolute number and proportion of these cell populations between caging groups (see Materials and Methods). We found that EE-caged mice had a markedly greater number of CD19+ B lymphocytes per spleen compared with control-caged mice (Md 9.0 × 106 CD19 cells per spleen, CI 4.8 × 106 to 13.3 × 106, p = 0.0003; Figure 3B). We also found a trend toward greater numbers of Thy1.2+ T lymphocytes in spleens from EE-caged mice (Md 20.0 × 105 CD3+ cells per spleen, CI −0.06 × 105 to 40.0 × 105, p = 0.0506; data not shown). Among the T cells, we found that EE-caged mice also had greater numbers of CD4+ and CD8+ T-cell subsets than controls, although the differences in CD8+ T cell numbers were not statistically significant (Md 11.2 × 105 CD4+ cells per spleen, CI 1.1 × 105 to 21.4 × 105, p = 0.0321; Figure 3C; Md 8.4 × 105 CD8+ cells per spleen, CI −0.4 × 105 to 17.25 × 105, p = 0.0605; Figure 3D).
To determine whether serum corticosterone concentration was related to the differences observed in the number of splenic immune cell subpopulations, we applied regression analyses focused on absolute cell number per spleen (Figure 3E–G) and the proportion of each subpopulation per spleen (Figure 3H–J). We found a statistically significant negative relationship between the absolute number of CD19+ B lymphocytes and serum corticosterone concentration (r2 = 0.43, p = 0.0017; Figure 3E). CD4+ and CD8+ T lymphocyte counts also displayed a negative relationship with corticosterone concentration (CD4+, r2 = 0.28, p = 0.0154, Figure 3F; CD8+, r2 = 0.20, p = 0.0503, Figure 3G). Similarly, the proportion of CD19+, CD4+ and CD8+ cells, as a percentage of total splenocytes, exhibited a statistically significant negative relationship with corticosterone concentration (CD19+, r2 = 0.61, p < 0.0001, Figure 3H; CD4+, r2 = 0.39, p = 0.0034, Figure 3I; CD8+, r2 = 0.20, p = 0.0492, Figure 3J). These results demonstrate that in naive mice, decreased glucocorticoid levels were associated with greater spleen cellularity, a pronounced increase in the number of CD19+ B lymphocytes and a modest increase in CD4+ and CD8+ T lymphocyte numbers per spleen.
Vaccine-Specific IgG Titer
To test the hypothesis that EE caging and related corticosterone reduction may enhance vaccine responses, we assessed humoral and cellular antigen-specific immunity after vaccination. We measured influenza vaccine–specific IgG titers in serum from control- and EE-caged mice that were injected with influenza vaccine intramuscularly either once (prime) or twice (boost). Naive mice injected with PBS served as negative controls and exhibited uniform influenza vaccine–specific IgG titers near or below the detection threshold (data not shown). For vaccine-primed groups, EE-caged mice had lower IgG titers than control-caged mice, whereas for boosted groups, EE-caged mice had higher IgG titers than control-caged mice, although neither difference was found to be statistically significant (prime, Md −0.18 log IgG titer, CI 0.04 to −0.40, p = 0.0943, Cohen’s d = 1.20; boost, Md 0.30 log IgG titer, CI −0.20 to 0.79, p = 0.1950, Cohen’s d = 0.89; Figure 4A). Within caging groups, we found that control-caged animals exhibited an increase in IgG titer, from prime to boost, of 11.4%, whereas EE-caged mice exhibited an increase of 23.8% (control, Md 0.4816 log IgG titer, CI 0.13–0.84, p = 0.0138; EE, Md 0.96 log IgG titer, CI 0.56–1.37, p = 0.0006; Figure 4A). There was a strong statistical trend toward an interaction between caging group (control versus EE) and vaccine group (prime versus boost), suggesting there may be distinct effects of enriched caging on primary versus secondary IgG response to vaccination (cage-group*vaccine-group, F = 4.27, p = 0.0555, partial η2 = 0.21).
Vaccine-Specific IL-5 Responses
BALB/c mice display a genetic bias toward T helper type 2 (TH2)-mediated immune responses and have been reported to produce greater quantities of IgG antibodies in response to vaccination when compared with other strains such as C57BL/6 (31,32). We investigated differences between caging groups in splenocyte production of IL-5, a TH2 cytokine known to promote B lymphocyte growth, differentiation and increased IgG secretion (33). Splenocytes were stimulated with either PBS or vaccine for 24 h in an IL-5 ELISPOT assay. We found that in primed animals, IL-5 responses to vaccine stimulation were similar in control-and EE-caged groups (Figure 4B). However, for vaccine-boosted animals, a significantly greater frequency of splenocytes from EE-caged mice produced IL-5 after vaccine stimulation when compared with control-caged mice (Md 67.7 IL-5 spot-forming units (SFU)/106 splenocytes, CI 0.24–135.1, p = 0.0493; Figure 4B). The increased frequency in IL-5–secreting cells from prime to boost was larger in magnitude in EE-caged mice (334.2%) compared with control-caged mice (177.9%) (control, Md 60.3 IL-5 SFU/106 splenocytes, CI 10.7–109.9, p = 0.0230; EE, 6 splenocytes, CI Md 124.7 IL-5 SFU/10 75.8–173.6, p = 0.0005; Figure 4B). We observed a statistically significant interaction between caging group and vaccine group, suggesting there may be distinct effects of corticosterone reduction caging on primary versus secondary cellular immune responses to vaccination (cage-group*vaccine-group, F = 4.60, p = 0.0487, partial η2 = 0.23). Given these results, we applied regression analyses to investigate the relationship between the frequency of IL-5–secreting splenocytes in boosted mice and the 7-wk mean FCM concentration. We observed a trend suggesting a negative relationship between mean FCM concentration and the frequency of IL-5–secreting cells (r2 = 0.38, p = 0.0781; Figure 4C). Together, these results suggest that the caging environment influenced secondary, but not primary, cell-mediated immune responses upon vaccine stimulation.
Spleen Composition of Vaccine-Boosted Mice
Our observations of caging-group effects on functional immunity led us to further investigate splenic differences in vaccine-boosted animals. To account for variance in body mass within cages and across groups, we quantified spleen mass as a function of body mass (g/kg). We found that boosted EE-caged mice had a significantly greater spleen mass per unit body mass than boosted control-caged mice (Md 1.55 g/kg, CI 1.05–2.06, p = 0.0001; Figure 4D). Notably, regression analysis demonstrated a strong negative relationship between the 7-wk mean FCM concentration and spleen mass per unit body mass in vaccine-boosted animals (r2 = 0.90, p < 0.0001; Figure 4E). On the basis of the large increase we observed in CD19+ B lymphocyte numbers in naive EE-caged mice, we anticipated that the FCM-associated differences in spleen mass in boosted mice might be partially attributed to differences in B-cell number. To investigate this relationship further, we looked at regressions of mean FCM concentration and absolute numbers of splenocyte subpopulations generated from our flow cytometry–based phenotype analysis of vaccine-boosted animals. We observed negative relationships between mean FCM concentration and CD19+ B-cell number and, unexpectedly, CD4−CD8− T lymphocyte number (CD19+, r2 = 0.39, p = 0.0533; CD4−CD8−, r2 = 0.52, p = 0.0190; Figure 4F–G). These results suggest that, among vaccine-boosted animals, spleen mass is influenced by caging condition and mean glucocorticoid exposure and that distinct subpopulations of splenocytes, including CD19+ B cells and CD4−CD8− T cells, appear to be more influenced by glucocorticoid exposure.
DISCUSSION
In the current work, we demonstrate that our standardized approach of EE housing reduces corticosterone levels in mice and modulates splenic immune cell composition and influenza vaccine responses. Naive EE-caged mice had a significantly greater number and proportion of splenic B and T lymphocytes than controls, and we observed a negative relationship between corticosterone concentration and B and T lymphocyte number and proportion. The difference in primary influenza vaccine–specific IgG titers between caging groups was not statistically significant; however, EE-caged mice tended to exhibit a greater increase in titer from prime to boost than controls, although this finding did not quite reach the level of statistical significance (p = 0.0555). Vaccine-stimulated splenocytes from boosted EE-caged mice had a significantly greater frequency of IL-5–secreting cells than boosted controls, whereas there was little difference between groups in the frequency of IL-5–secreting cells in primed mice. Lastly, we found that, in boosted mice, spleen mass per unit body mass was significantly lower in control-caged mice compared with EE-caged mice and the number of splenic B lymphocytes in both caging groups was negatively correlated with the average corticosterone level.
In previous studies, by using the same enriched environment parameters and mouse strain used here, we found that EE-caged mice displayed no significant differences in elevated-plus-maze behavior (25). We have since repeated this experiment by using an elevated-zero-maze and again found no significant behavioral differences between EE- and control-caged animals (data not shown), although a marked reduction of corticosterone production in EE-caged mice remained reproducible. We speculate that the lack of behavioral differences may be related to strain, such as BALB/c mice being relatively less exploratory than other strains, and the sensitivity of the behavioral tests we have used to detect subtle behavioral changes that may result from caging enrichment. As a product of these findings, we have determined that further behavioral testing needs to be done to validate this approach to caging enrichment as a stress reduction model. In this article, we have referred to the enhanced caging as “enriched environment” instead of the former label “Calm” caging.
Other studies have demonstrated that enriched environment has effects on immune function such as increased natural killer activity, elevated tumor resistance and increased antibody production after vaccination (34–36). A key detail of the enrichment protocols in these studies is that they included access to a running wheel. In previous work, we have shown that, compared with EE caging alone, EE caging with the addition of a running wheel enhances the observed effects on spleen mass and corticosterone reduction. A limitation of the current study is that we did not have resources to include separate EE and control caging conditions with and without exercise wheels and hence cannot distinguish the effect of exercise from that of other elements of the EE caging. In contrast to our data, the above studies, and others, also reported that mice housed in enriched conditions had similar or increased concentration of plasma corticosterone compared with control-caged mice (37,38). These disparate findings may be related to differences in the approach to EE. Some enrichment protocols call for frequent rotation of enrichment items into and out of the cage, which increases the level of enrichment novelty but may also be a source of mild stress. In our studies, the enrichment items and locations within the cage were kept consistent throughout the experiment so as to minimize cage disruption and its potential effect as a mild stressor. Additionally, genetic differences among the strains of mice used in EE studies may, in part, contribute to the divergent results. The BALB/c strain used in our studies is relatively anxious, with higher concentrations of basal serum corticosterone and lower rates of corticosterone catabolism, which may amplify the effects of cage enrichment on reduction of corticosterone production (39,40). In contrast, the above studies that found increased corticosterone concentrations in EE-caged mice used C57BL/6 and C3H/eB strains. It will be interesting to use other strains of laboratory mice in our standardized approach to EE, both to determine whether it is an effective tool for corticosterone reduction across genetic strains and to address the high degree of variability that exists in immune phenotype across strains (41).
Previous studies in rodents have shown that restraint stress or treatment with exogenous corticosterone is negatively associated with spleen mass and splenic lymphocyte number (42). Similarly, adrenalectomized mice have greater numbers of splenic IgG-secreting cells compared with sham surgery controls, although a statistically significant correlation between corticosterone and IgG-secreting cell number was not observed as it was in our data (43). In a study investigating how glucocorticoids affect B lymphocyte populations, it was found that chronic corticosterone exposure causes depletion of developing B-lineage cells in the bone marrow by reducing the number of cycling precursor cells and inducing apoptosis (44). Data from our enriched environment approach (a mild intervention relative to restraint, corticosterone treatment or adrenalectomy) are consistent with the above findings. Our results also suggest a relationship between corticosterone and the immune system in which small, physiologically relevant decrements in corticosterone production are associated with substantial increases in splenic B and, to a lesser extent, T lymphocyte numbers. Though further work is needed to establish the immunologic and clinical significance of having a greater number of naive B lymphocytes in the spleen, we speculate that, under conditions of infection or vaccination, there may be an advantage to having a larger pool of B lymphocytes available such that a greater diversity of antigen-specific antibodies can be generated. This might be of particular importance for infectious diseases in which the pathogen undergoes rapid mutations such as influenza. Further, B cells are also capable of functioning as antigen-presenting cells, and an increased number of B cells in the spleen, and possibly other lymphoid tissues, could result in enhanced antigen presentation (45,46).
Both human and animal studies have demonstrated the suppressive effects of chronic stress on humoral immunity. For example, a study of caregivers of spouses with dementia demonstrated that the percentage of caregivers who mounted a clinically protective IgG antibody response after influenza vaccination was significantly lower than in vaccinated nonstressed controls (47). In related work, restraint stress altered the kinetics of the antibody response and delayed class switching from IgM to IgG and IgA isotypes in mice exposed to influenza virus (48). Repeated activation of the stress response and exposure to stress mediators are believed to be related to the immune modulation observed in these studies. Given these data, we hypothesized that mice with reduced corticosterone, a principal stress hormone, would exhibit enhanced humoral responses after vaccination. However, influenza vaccine–specific antibody titers in EE-caged mice were not significantly different from controls. Inspection of the data revealed a notable pattern, however, in that EE mice had lower IgG titers than control-caged mice after primary vaccination, but tended to have higher titers than controls after vaccine boost. Further, EE animals tended to have a greater increase in titer from prime to boost than did control mice. This interaction between caging group and number of vaccine administrations was nearly statistically significant and is consistent with another study in which keyhole limpet hemocyanin was administered once or twice to male mice housed from 1 per cage up to 12 per cage (49). It was observed that increased housing density was positively associated with corticosterone production. Whereas singly housed mice receiving a booster immunization had significantly greater IgM and IgG titers than group-housed mice, there was no effect of caging density on antibody production in mice that only received a primary immunization with keyhole limpet hemocyanin. Notably, the authors report administering 5–150 μg of keyhole limpet hemocyanin antigen per vaccination, whereas we administered 1 μg HA antigen in our studies. In follow-up work, it will be important to determine the degree to which the quantity of immunogen has effects on the interaction between stress level and humoral immune responses.
Cellular immune function is modified by stress and stress hormone signaling. For example, restraint stress has been shown to suppress IL-2 production 14 d after influenza viral infection and also to attenuate delayed-type hypersensitivity reactions (5,48). However, no investigations have been made into the effects of enriched environment and reduced glucocorticoid production on cell-mediated responses after vaccination. Because BALB/c mice display a genetic bias toward TH2-predominant immune responses, we investigated differences between caging groups in splenocyte production of IL-5, a TH2 cytokine known to promote B lymphocyte growth, differentiation and increased IgG secretion (33). We anticipated that splenocytes from both primed and boosted EE-caged mice would exhibit enhanced cellular immune responses when exposed to vaccine in vitro. While we observed that vaccine-stimulated splenocytes from boosted EE mice had a significantly greater frequency of IL-5–secreting cells than boosted control-caged mice, we found no differences in the frequency of IL-5–secreting cells between vaccine-primed EE and control animals. Similar to the IgG titer data, we found that EE mice displayed a greater increase in IL-5–secreting cells from prime to boost than did controls, and this result was supported by a statistically significant interaction between the caging group and number of vaccine administrations. Together with the humoral immune data, these findings support the argument that enriched environment and associated changes in corticosterone may preferentially affect secondary humoral and cellular immune responses and have less of an impact on primary responses. Additionally, while these preliminary studies were mainly focused on adaptive immunity, glucocorticoid signaling is also known to influence important innate cell types such as macrophages and dendritic cells, which are important for efficacious vaccine responses. In future studies, we intend to assess cell type–specific functional effects across innate and adaptive arms of the immune system.
Analysis of the spleen data revealed that spleen mass per unit body mass was significantly lower in boosted control mice than boosted EE mice. We also found that in boosted animals, glucocorticoid concentration was negatively associated with spleen mass and the absolute number of splenic CD19+ B cells and CD4−CD8− T cells. These findings are consistent with our observations related to glucocorticoid levels and spleen composition in naive animals as well as work by others reporting the effects of corticosterone signaling on B lymphocyte populations (42–44).
It is well established that both glucocorticoids and autonomic nervous system mediators, such as epinephrine and nor-epinephrine, are important for regulating immune cell distribution and function. Our approach in the current work was to minimize animal handling and stressful procedures such as blood collection to prevent regular activation of the acute stress response. We were able to collect detailed longitudinal data on glucocorticoid production by measuring fecal corticosterone metabolites noninvasively. However, one disadvantage of this approach was that we were not able to measure plasma or urinary catecholamines.
Lastly, it is important to note that the relationship between stress and HPA axis activity is complex and remains an area of ongoing investigation. For example, in some scenarios of chronic stress, such as posttraumatic stress disorder, humans and rodent models can display increased or decreased glucocorticoid production, findings that are currently poorly understood (50,51). In the present work, although we found consistent relationships between corticosterone and a number of immunologic outcomes, the complexity of the stress-HPA axis relationship should be carefully considered when relating our results to stress physiology.
CONCLUSION
Overall, the current data highlight key issues that should be carefully addressed in future human studies investigating the relationship between psychological stress, glucocorticoid signaling and vaccine responses. In particular, it may be important to use an experimental design capable of testing the differential effects of stress mediators (that is, glucocorticoids and catecholamines) on primary versus secondary immune responses. Furthermore, assessment of both humoral and cell-mediated immune responses in this context will provide a more thorough characterization of the impact of behavioral interventions on immune function and health.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. Springer; New York: 1984. [Google Scholar]
- 2.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–51. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 3.Edwards KM, et al. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav Immun. 2006;20:159–68. doi: 10.1016/j.bbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Schedlowski M, et al. Changes of natural killer cells during acute psychological stress. J Clin Immunol. 1993;13:119–26. doi: 10.1007/BF00919268. [DOI] [PubMed] [Google Scholar]
- 5.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 6.Yin D, Tuthill D, Mufson RA, Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J Exp Med. 2000;191:1423–8. doi: 10.1084/jem.191.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaker PH, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 8.Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–6. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–12. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S, Doyle WJ, Skoner DP. Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosom Med. 1999;61:175–80. doi: 10.1097/00006842-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher S, Phillips AC, Drayson MT, Carroll D. Parental caregivers of children with developmental disabilities mount a poor antibody response to pneumococcal vaccination. Brain Behav Immun. 2009;23:338–46. doi: 10.1016/j.bbi.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Vedhara K, et al. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet. 1999;353:627–31. doi: 10.1016/S0140-6736(98)06098-X. [DOI] [PubMed] [Google Scholar]
- 13.Glaser R, et al. Stress-induced modulation of the immune response to recombinant hepatitis B vaccine. Psychosom Med. 1992;54:22–9. doi: 10.1097/00006842-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen AF, Zachariae R, Bovbjerg DH. Psychological stress and antibody response to influenza vaccination: a meta-analysis. Brain Behav Immun. 2009;23:427–33. doi: 10.1016/j.bbi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038–49. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Jain S, et al. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Ann Behav Med. 2007;33:11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- 18.Kabat-Zinn J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936–43. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- 19.Miller JJ, Fletcher K, Kabat-Zinn J. Three-year follow-up and clinical implications of a mindfulness meditation-based stress reduction intervention in the treatment of anxiety disorders. Gen Hosp Psychiatry. 1995;17:192–200. doi: 10.1016/0163-8343(95)00025-m. [DOI] [PubMed] [Google Scholar]
- 20.Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait-list controlled clinical trial: the effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom Med. 2000;62:613–22. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Witek-Janusek L, et al. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969–81. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayney MS, et al. Age and psychological influences on immune responses to trivalent inactivated influenza vaccine in the meditation or exercise for preventing acute respiratory infection (MEPARI) trial. Hum Vaccin Immunother. 2014;10:83–91. doi: 10.4161/hv.26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson RJ, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65:564–70. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 24.Moynihan JA, et al. Mindfulness-based stress reduction for older adults: effects on executive function, frontal alpha asymmetry and immune function. Neuropsychobiology. 2013;68:34–43. doi: 10.1159/000350949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurfein BT, et al. The calm mouse: an animal model of stress reduction. Mol Med. 2012;18:606–17. doi: 10.2119/molmed.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palme R, Touma C, Arias N, Dominchin MF, Lepschy M. Steroid extraction: get the best out of faecal samples. Wiener Tiera?rztliche Monatsschrift. 2013;100:238–46. [Google Scholar]
- 27.Touma C, Sachser N, Mostl E, Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol. 2003;130:267–78. doi: 10.1016/s0016-6480(02)00620-2. [DOI] [PubMed] [Google Scholar]
- 28.Touma C, Palme R. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann N Y Acad Sci. 2005;1046:54–74. doi: 10.1196/annals.1343.006. [DOI] [PubMed] [Google Scholar]
- 29.Touma C, Palme R, Sachser N. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav. 2004;45:10–22. doi: 10.1016/j.yhbeh.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Louch CD, Higginbotham M. The relation between social rank and plasma corticosterone levels in mice. Gen Comp Endocrinol. 1967;8:441–4. doi: 10.1016/s0016-6480(67)80006-6. [DOI] [PubMed] [Google Scholar]
- 31.Gessner A, Blum H, Rollinghoff M. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993;189:419–35. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- 32.Locksley RM, Heinzel FP, Sadick MD, Holaday BJ, Gardner KD., Jr Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann Inst Pasteur Immunol. 1987;138:744–49. doi: 10.1016/s0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- 33.Moon BG, Takaki S, Miyake K, Takatsu K. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. J Immunol. 2004;172:6020–9. doi: 10.4049/jimmunol.172.10.6020. [DOI] [PubMed] [Google Scholar]
- 34.Cao L, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benaroya-Milshtein N, et al. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur J Neurosci. 2004;20:1341–7. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- 36.Benaroya-Milshtein N, et al. Environmental enrichment augments the efficacy of idiotype vaccination for B-cell lymphoma. J Immunother. 2007;30:517–22. doi: 10.1097/CJI.0b013e31804efc5e. [DOI] [PubMed] [Google Scholar]
- 37.Marashi V, Barnekow A, Ossendorf E, Sachser N. Effects of different forms of environmental enrichment on behavioral, endocrinological, and immunological parameters in male mice. Horm Behav. 2003;43:281–92. doi: 10.1016/s0018-506x(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 38.Marashi V, Barnekow A, Sachser N. Effects of environmental enrichment on males of a docile inbred strain of mice. Physiol Behav. 2004;82:765–76. doi: 10.1016/j.physbeh.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl) 1993;111:323–31. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- 40.Shanks N, Griffiths J, Zalcman S, Zacharko RM, Anisman H. Mouse strain differences in plasma corticosterone following uncontrollable footshock. Pharmacol Biochem Behav. 1990;36:515–9. doi: 10.1016/0091-3057(90)90249-h. [DOI] [PubMed] [Google Scholar]
- 41.Petkova SB, et al. Genetic influence on immune phenotype revealed strain-specific variations in peripheral blood lineages. Physiol Genomics. 2008;34:304–14. doi: 10.1152/physiolgenomics.00185.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pruett SB, Fan R, Myers LP, Wu WJ, Collier S. Quantitative analysis of the neuroendocrine-immune axis: linear modeling of the effects of exogenous corticosterone and restraint stress on lymphocyte subpopulations in the spleen and thymus in female B6C3F1 mice. Brain Behav Immun. 2000;14:270–87. doi: 10.1006/brbi.2000.0605. [DOI] [PubMed] [Google Scholar]
- 43.del Rey A, Besedovsky H, Sorkin E. Endogenous blood levels of corticosterone control the immunologic cell mass and B cell activity in mice. J Immunol. 1984;133:572–5. [PubMed] [Google Scholar]
- 44.Garvy BA, King LE, Telford WG, Morford LA, Fraker PJ. Chronic elevation of plasma corticosterone causes reductions in the number of cycling cells of the B lineage in murine bone marrow and induces apoptosis. Immunology. 1993;80:587–92. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong G, Reis e Sousa C, Germain RN. Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility complex class II complexes after soluble protein exposure in vivo or in vitro. J Exp Med. 1997;186:673–82. doi: 10.1084/jem.186.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Townsend SE, Goodnow CC. Abortive proliferation of rare T cells induced by direct or indirect antigen presentation by rare B cells in vivo. J Exp Med. 1998;187:1611–21. doi: 10.1084/jem.187.10.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93:3043–7. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng N, et al. The effect of restraint stress on the kinetics, magnitude, and isotype of the humoral immune response to influenza virus infection. Brain Behav Immun. 1991;5:370–82. doi: 10.1016/0889-1591(91)90032-6. [DOI] [PubMed] [Google Scholar]
- 49.Karp JD, Moynihan JA, Ader R. Effects of differential housing on the primary and secondary antibody responses of male C57BL/6 and BALB/c mice. Brain Behav Immun. 1993;7:326–33. doi: 10.1006/brbi.1993.1032. [DOI] [PubMed] [Google Scholar]
- 50.Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–92. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 51.Daskalakis NP, Yehuda R, Diamond DM. Animal models in translational studies of PTSD. Psychoneuroendocrinology. 2013;38:1895–911. doi: 10.1016/j.psyneuen.2013.06.006. [DOI] [PubMed] [Google Scholar]