Abstract
Objective:
The purpose of this study was to examine the effectiveness of a patient–provider educational intervention in reducing at-risk drinking among older adults.
Method:
This was a cluster-randomized controlled trial of 31 primary care providers and their patients ages 60 years and older at a community-based practice with seven clinics. Recruitment occurred from July 2005 to August 2007. Eligibility was determined by telephone and a baseline mailed survey. A total of 1,186 at-risk drinkers were identified by the Comorbidity Alcohol Risk Evaluation Tool. Follow-up patient surveys were administered at 3, 6, and 12 months after baseline. Study physicians and their patients were randomly assigned to usual care (n = 640 patients) versus the Project SHARE (Senior Health and Alcohol Risk Education) intervention (n = 546 patients), which included personalized reports, educational materials, drinking diaries, physician advice during office visits, and telephone counseling delivered by a health educator. Main outcomes were alcohol consumption, at-risk drinking (overall and by type), alcohol discussions with physicians, health care utilization, and screening and intervention costs.
Results:
At 12 months, the intervention was significantly associated with an increase in alcohol-related discussions with physicians (23% vs. 13%; p ≤ .01) and reductions in at-risk drinking (56% vs. 67%; p ≤ .01), alcohol consumption (-2.19 drinks per week; p ≤ .01), physician visits (-1.14 visits; p = .03), emergency department visits (16% vs. 25%; p ≤ .01), and nonprofessional caregiving visits (12% vs. 17%; p ≤ .01). Average variable costs per patient were $31 for screening and $79 for intervention.
Conclusions:
The intervention reduced alcohol consumption and at-risk drinking among older adults. Effects were sustained over a year and may have been associated with lower health care utilization, offsetting screening and intervention costs.
Fifty-three percent of adults ages 65–74 years and 40% of adults ages 75 years and older report consuming alcohol (Schiller et al., 2012). Although drinking declines with advancing age (Moore et al., 2005), older adults who consume alcohol have increased risks associated with drinking compared with younger adults because of increased morbidity and medication use as well as age-related physiological changes that increase the effects of a given amount of alcohol (Linnoila et al., 1980; Moore et al., 1999a, 1999b, 2006, 2007; Vestal et al., 1977).
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines recommended drinking limits for adults age 65 years and older as no more than seven drinks per week and no more than three drinks per occasion (NIAAA, 1995). Lower limits or abstinence are recommended for those who take medications that interact with alcohol or have a health condition exacerbated by alcohol. The prevalence of older adults who exceed these recommended drinking limits or who have other reasons to limit drinking (e.g., at-risk drinking) has been estimated to be 18% of men and 5% of women (Moore et al., 2006), and at-risk drinking has been associated with increased mortality rates among older men (Moore et al., 2006). Because the older adult population continues to grow, so that approximately 20% of the population will be age 65 years or older by 2030, the number of older at-risk drinkers is also likely to increase (Balsa et al., 2008; United States Census Bureau, 2012).
Project SHARE (Senior Health and Alcohol Risk Education) is a cluster-randomized trial of an educational intervention to reduce at-risk drinking in primary care and to assess the costs of screening and intervention as well as whether the intervention reduced health care utilization. Older at-risk drinkers were identified using the Comorbidity Alcohol Risk Evaluation Tool (CARET), which assesses drinking risks by collecting information on amount of alcohol use, comorbidities, symptoms, and medication use (Barnes et al., 2010; Fink et al., 2002b; Moore et al., 2000, 2002). Compared with usual care, the intervention was hypothesized to reduce at-risk drinking, quantity and frequency of alcohol use, and use of medical care, especially hospitalizations and emergency department visits. We also measured costs of the screening and intervention.
Randomized controlled trials of brief alcohol interventions in primary care settings have previously been shown to reduce alcohol use (Kaner et al., 2009). However, just three studies have focused on older adults (Fink et al., 2005; Fleming et al., 1999; Moore et al., 2011). All three of the studies among older adults were modestly successful in reducing alcohol use and/or at-risk drinking, although each used an approach that differed from Project SHARE (see the Discussion for details). In addition, none of the earlier studies addressed the effect of the intervention on health care utilization or assessed the costs of screening and intervention. Our study, therefore, complements and extends the existing literature on the effectiveness of primary care–based brief alcohol intervention among the growing population of elderly at-risk drinkers.
Method
Trial design
The study was a two-arm, cluster-randomized controlled trial with outcomes at the individual patient level measured over a 12-month follow-up period. Randomization at the level of participating physicians was used so that physicians would not see patients in both the intervention and control arms of the study, thereby avoiding contamination effects. The study was approved by the Office of Human Research Protection Program at the University of California at Los Angeles (UCLA).
Setting and participating physicians
The study was conducted at Sansum Clinic, a community-based group practice with seven clinics in and around Santa Barbara, CA. Primary care physicians (both internists and family physicians) were recruited in 2005 by the Sansum Medical Director (Kurt Ransohoff) and invited to attend a 60-minute informational meeting. Forty-three physicians were initially approached, and 29 agreed to participate. Physicians who did not attend the informational meeting met individually with one of the study investigators (Alison A. Moore). Before randomization, all participating physicians completed written informed consent and baseline surveys including questions on age, gender, and specialty type. During the course of the study, three physicians left the group (one left before patient recruitment began), and three physicians joined the group and the study. Thus, over the course of the study, 31 physicians had at least one patient in the study. The mean age of the 31 physicians who had participating patients was 48.3 years, and they included 20 men and 11 women, 17 internists, and 14 family physicians.
Cluster randomization and blinding
We aimed to match each participating physician to another with the same specialty and clinic site. Physicians from each of the matched pairs were randomly assigned by a statistician who drew random numbers from a uniform [0,1] distribution for the pair; the physician having the lower number was assigned to the intervention group. The patient’s treatment assignment was then based on the random assignment of the patient’s primary care physician. Among the 31 physicians who ever participated from the seven clinics, 14 were randomized to the control group and 17 to the intervention group (the two physicians who left after the study began were in the intervention group and were replaced by the next two physicians joining the group, who took over their patient panels; the third physician who joined the group during the course of the study was randomly assigned to the intervention group). Research assistants, blinded to treatment allocation, entered all data collected.
Patient participants
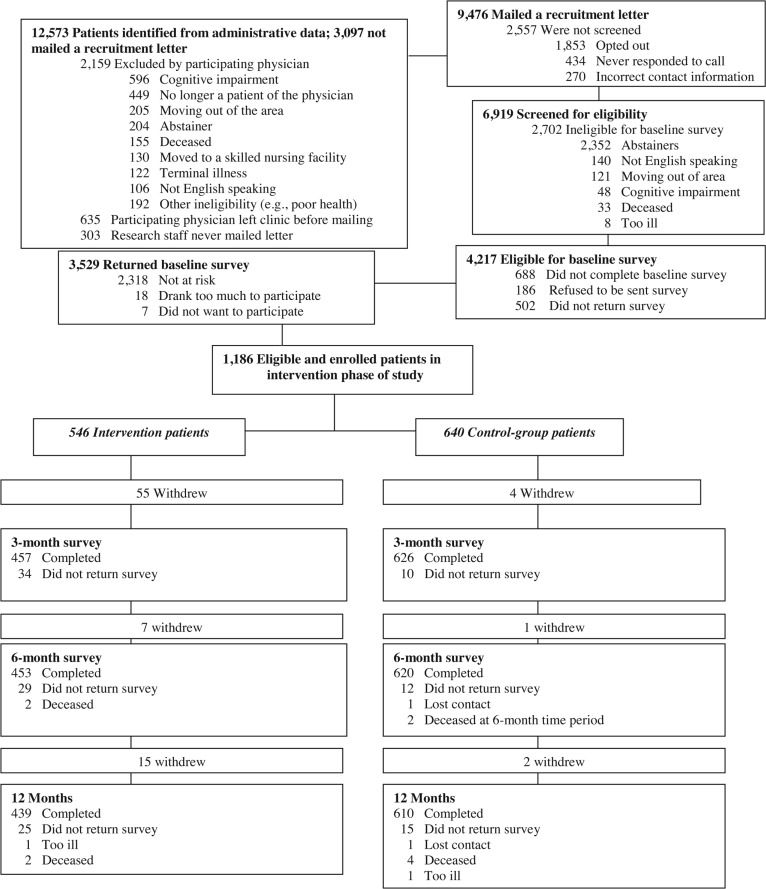
Patient recruitment took place from July 2005 to August 2007. First, administrative data were used to identify all patients of participating physicians who were age 60 years and older (n = 12,573). Then, participating physicians were asked to review the identified patients and exclude those who they knew had severe cognitive impairment, were terminally ill or deceased, were residing in a skilled nursing facility, were moving out of the area in the next year, did not speak English, were no longer a patient of the physician, or did not drink alcohol; patients could also be removed for any other reason the physician thought would exclude them from participating in the study (e.g., other health reasons, too frail) (n excluded = 2,159) (Figure 1). Patients of physicians who had left the practice were also removed from the list of those eligible (n = 635). Because of competing time demands unrelated to the study or its outcomes, study staff never mailed recruitment letters to 303 patients.
Figure 1.
Participant flow in Project SHARE (Senior Health and Alcohol Risk Education)
Pre-screening at-risk drinkers
The remaining patients (n = 9,476) were mailed a letter inviting them to participate in the study. Unless the patient called a toll-free number to opt out of the study, research staff called patients within a week of the mailing to further explain the study. Among those who expressed interest, the staff ascertained the following eligibility criteria: whether the individual had consumed at least one alcoholic beverage in the past 3 months, was at least 60 years old, was planning to remain in the area for the next 12 months, spoke English, and was physically and cognitively well enough to participate. Of the 6,919 persons who were reached via telephone, 4,217 met all eligibility criteria and were invited to participate; 3,529 returned a baseline survey.
Identification of at-risk drinkers
Eligible, interested patients were mailed an information sheet (required by the UCLA Institutional Review Board in lieu of standard informed consent forms when surveys are not conducted in person) and a baseline survey that included the CARET, an updated and revised version of the Alcohol-Related Problems Survey (Barnes et al., 2010; Grimshaw et al., 2001; Fink et al., 2002a, 2002b; Moore et al., 2000, 2002), which identifies older adults at risk for harm from their alcohol consumption for any of the following seven reasons: (a) exceeding a particular quantity and frequency of alcohol use, (b) heavy episodic drinking (four or more drinks at least once a week), (c) driving within 2 hours of having had three or more drinks, (d) concern from another person about the patient’s drinking, (e) alcohol use with comorbidities (e.g., gout, depression, ulcer disease), (f) alcohol use with symptoms (e.g., nausea, falls, insomnia), or (g) alcohol use with medications (e.g., antidepressants, sedatives). Examples of at-risk drinkers include people who drink (a) four drinks (or more) per occasion at least weekly, (b) two drinks per day and take a sedative three to four times a week, or (c) two drinks at least twice a week and have memory problems. Table 1 provides a summary of the CARET risk classification scoring rules.
Table 1.
Comorbidity Alcohol Risk Evaluation Tool
| Item | Amount of drinking considered at risk |
| Alcohol use and behaviors in the last 12 months | |
| a. Number of drinks and frequency of drinking | a. ≥5/day at any frequency, 4/day at least 2 times/month, 3/day at least 4 times/week |
| b. Four or more drinks on one occasion (heavy episodic drinking) | b. At least 1 time/week |
| c. Driving within 2 hours of drinking three or more drinks | c. Any frequency |
| d. Someone concerned about participant’s alcohol use | d. Any amount |
| e. Someone concerned about participant’s alcohol use more than 12 months ago | e. ≥4/day at any frequency, 2–3/day at least 4 times/week |
| Alcohol use and medications taken at least 3–4 times per week currently | |
| a. Medications that may cause bleeding, dizziness, sedation | a. ≥4/day at any frequency, 2–3/day at least 4 times/week |
| b. Medications used for gastroesophageal reflux, ulcer disease, depression | b. ≥4/day at any frequency, 2–3/day at least 4 times/week |
| c. Medications for hypertension | c. ≥5/day at any frequency, 4/day at least 2 times/week, 3/day at least 4 times/week |
| Alcohol use and comorbidities in the past 12 months | |
| a. Liver disease, pancreatitis | a. Any amount |
| b. Gout, depression | b. ≥4/day at any frequency, 3/day at least 2 times/week, 2/day at least 4 times/week |
| c. High blood pressure, diabetes | c. 5/day at any frequency, 4/day at least 2 times/month |
| d. Sometimes have problems with sleeping, falling | d. 3/day at least 4 times/week |
| e. Memory problems, heartburn, stomach pain, nausea, vomiting, or feel sad/blue | e. ≥5/day at any frequency, 4/day at least 2 times/month, 3/day at least 2 times/week |
| f. Often have problems with sleeping, falling, memory, heartburn, stomach pain, nausea, vomiting, or feel sad/blue | f. ≥4/day at any frequency, 2–3/day at least 2 times/week |
Among the 3,529 patients who returned a survey, 2,318 were not at-risk drinkers, 18 reported drinking seven or more drinks daily and were excluded after the research staff notified the patient’s physician, and 7 were at risk but refused to participate further. Of the remaining 1,186 patients who were at-risk drinkers and therefore eligible to participate further, 546 were patients of intervention group physicians and 640 were patients of control group physicians.
Measures and outcomes
Patient participants were mailed surveys at baseline and at 3, 6, and 12 months. The measures collected for the current analyses were the CARET instrument (baseline, 6 months, and 12 months); sociodemographic characteristics (baseline); a question on when the individual had discussions related to alcohol use with his or her physician (baseline); and self-reported health care utilization, including past-year visits to physicians, hospitals, emergency departments, skilled nursing home stays, and other nonprofessional caregiver assistance (baseline, 3 months, 6 months, 12 months).
The primary outcomes were whether the patient continued to be an at-risk drinker at 6 and 12 months. Secondary outcomes included whether the patient was at risk for each of the seven specific reasons (e.g., alcohol quantity and frequency alone) previously described, usual number of drinks per week, whether the patient had alcohol-related discussions with the physician in the past 12 months, and health care utilization. Usual number of drinks per week at 6 and 12 months was constructed by combining responses to the following questions included in the CARET: (a) “During the past six (twelve) months, how often did you have a drink containing alcohol, such as beer, wine, liquor, sherry or liqueurs?” (b) “During the past six (twelve) months, on days that you drank alcohol, how many drinks did you usually have?” Alcohol-related discussions with the physician were ascertained at the 12-month follow-up, from the following question: “When did a doctor last discuss your alcohol use with you?” If patients reported at least one discussion within the past year, this was coded as yes. Health care utilization was ascertained by responses to the 3-, 6-, and 12-month surveys via cumulative measures of the number of physician visits and whether the patient had any hospital stay, had any emergency department visit, had any home health visit, or received any assistance from a nonprofessional caregiver (e.g., family member or friend) during the year after completing the baseline survey.
Control group
Patients of control group physicians completed the baseline and 3-, 6-, and 12-month surveys. They received care as usual, which could have included alcohol counseling.
Intervention group
All patient participants of physicians in the intervention group were mailed a personalized patient report; an educational booklet on alcohol and aging; a drinking diary to track alcohol consumption; and, depending on the individual patient’s reported alcohol-associated risks (as identified on the CARET), up to 13 “tip sheets” (e.g., on drinking sensibly, sleep, preventing falls and fractures, gout, etc.). The patient report was generated using software used to score the CARET and included specific reasons for the “at-risk drinking” classification (e.g., the individual’s use of alcohol in combination with benzodiazepines and sedating antihistamines) and potential harms that could result (e.g., sedation and impaired coordination). New patient reports were generated and mailed to the patients after completion of the 6-month CARET survey.
After patient participants of physicians in the intervention group completed the CARET at baseline and 6 months, provider reports similar to the patient reports were generated. Immediately before each regularly scheduled appointment of an intervention patient, all available provider reports for that patient were placed on the front of the medical record. Intervention physicians were asked to review and use the information in the provider reports to discuss the patient’s drinking and associated risks during the appointment and advise the patient to reduce alcohol use if the patient was still an at-risk drinker.
Via telephone, a health educator contacted intervention patients three times: (a) 2 weeks after sending the baseline patient report, (b) 3 months after sending the baseline patient report, and (c) 2 weeks after sending the patient’s 6-month patient report. During these calls, the health educator answered questions about the written materials and engaged in the following five steps: (a) assessment and direct feedback, (b) negotiation and goal setting, (c) behavioral modification techniques, (d) self-help-directed bibliotherapy, and (e) follow-up and reinforcement. Among intervention patients, 88.1%, 72.9%, and 85.5% received the baseline, 3-month, and 6-month health educator calls, respectively.
Statistical analysis
Frequencies for baseline risk categories and sociodemographic characteristics are reported, and chi-square tests and t tests were used for differences between the two treatment groups. Intent-to-treat analysis was used to examine differences in outcomes between patients assigned to the intervention versus control groups. The intervention was randomized at the physician level, and some baseline differences were found in patient characteristics. In addition, as noted by Fink et al. (2005), regression adjustment generally increases the efficiency of estimated treatment effects. For these reasons, all comparisons used multiple linear (for continuous outcomes) or logistic (for dichotomous outcomes) regression to adjust for the patient’s baseline categories of drinking risk, baseline sociodemographic characteristics (age group, race, Latino ethnicity, sex, education, marital status, annual household income, and home ownership), and time between the baseline and follow-up surveys. Models for usual number of drinks per week, alcohol discussions, and health care utilization were adjusted for the baseline value of the dependent variable. Models for alcohol discussions with physicians additionally adjusted for physician characteristics (age, sex, and specialty) and the number of physician visits during the previous year and its square. All regressions included random physician intercepts to account for within-physician correlation of the error terms. For dichotomous outcomes, we report the mean predicted probabilities of dichotomous outcomes with the intervention indicator set equal to 1 versus 0, holding all other covariate values constant at their original values.
Response rates at 12 months were 80.4% among the 546 intervention patients and 95.3% among the 640 control patients (p ≤ .01 for the difference). To address the possibility of bias attributable to higher attrition rates among intervention patients, we performed “worst case” sensitivity analyses by assuming that 100% of study dropouts remained at-risk drinkers.
Missing data for predictors were multiply imputed using 10 data sets. Multiple imputation (Rubin, 1987) involves three steps. The first step in multiple imputation is to run an imputation model to “fill in” values for missing data in the original data set. This procedure is carried out multiple times (in our case, 10 times) to create a series of imputed data sets. The second step involves the analysis model that is then estimated separately with each of the imputed data sets, in our case creating 10 separate sets of regression estimates. The third and final step is to combine these different sets of estimates into a single set of parameter estimates. The advantage of multiple imputation is that (unlike single imputation) it accounts for uncertainty in the imputation by incorporating the variability between imputed data sets in the variance estimates.
Measurement of screening and intervention costs
To measure screening costs, we first estimated the time spent by the data coordinators to conduct the phone screeners (including multiple call attempts), compile and mail the baseline survey packet, enter the CARET data, and generate the baseline patient reports. These time estimates were multiplied by the data coordinator’s hourly compensation rate (wages plus benefits). We also included the cost of postage, envelopes, self-addressed stamped envelopes, and copies of the baseline CARET and instruction sheets. The total costs of screening the entire population were then divided by the number of “positive” screens to calculate the average cost of identifying an at-risk drinker.
The CARET screening tool and software for generating reports are publicly available at no cost; therefore, the fixed costs associated with the intervention primarily result from the time spent by the physician instructor and intervention physicians who participated in the group educational training activity. Primary care physician time was costed out at $200 per hour, to be consistent with the hourly equivalent of Medicare rates for routine office visits. (All monetary amounts are in U.S. dollars.) The total variable costs associated with the intervention fell into four major categories: supply costs, physician time, data coordinator time, and health educator time. The sum of these costs was then divided by the total number of patients who received the intervention in order to estimate the mean incremental cost of adding another patient to the existing intervention. In all cases, the base year used for prices was 2007.
Supply costs included printing and/or copying costs for the CARET instrument, patient and provider reports, tip sheets, drinking diaries, cover sheets, and call logs; the cost of postage, envelopes, and self-addressed stamped envelopes; and cell phone costs. Physician time talking to intervention patients about their at-risk drinking was tracked using physician visit logs. The intervention-related activities of the data coordinators consisted of compiling and mailing packets including the CARET instrument; entering CARET data, and generating and mailing patient reports; and printing provider reports and attaching them to patient charts before visits. Health educator time was tracked using the call logs and included unsuccessful call attempts. We excluded costs resulting from the research rather than intervention per se (e.g., the costs associated with the portions of the survey that were needed for the evaluation but not the intervention). We used hourly compensation (actual wages plus fringe benefits) to cost out the time spent on the Project SHARE intervention by the data coordinators and health educators.
Results
Baseline characteristics of patient study sample
The average age of patient participants was 71 years, and most were male, non-Latino White, highly educated, and married, with a roughly equal age distribution across the five groups (60–64, 65–69, 70–74, 75–79, and ≥80 years) (Table 2). Compared with control group patients, intervention group patients were significantly more likely to be female; have lower income; and be divorced, separated, or widowed.
Table 2.
Baseline patient characteristics
| Characteristic | Total (N = 1,186) | Intervention (n = 546) | Usual care (n = 640) | p |
| Age in years, M (SD) | 70.95 (7.27) | 71.37 (7.39) | 70.58 (7.15) | .06 |
| Age group in years, n (%) | .08 | |||
| 60–64 | 256 (21.59) | 107 (20) | 149 (23) | |
| 65–69 | 337 (28.41) | 160 (29) | 177 (28) | |
| 70–74 | 226 (19.06) | 92 (17) | 134 (21) | |
| 75–79 | 192 (16.19) | 100 (18) | 92 (14) | |
| ≥80 | 175 (14.76) | 87 (16) | 88 (14) | |
| Race, n (%) | .34 | |||
| White | 1,142 (97.27) | 524 (97) | 618 (98) | |
| Black | 4 (<1) | 2 (<1) | 2 (<1) | |
| Asian/Pacific Islander | 11 (<1) | 8 (1) | 3 (<1) | |
| American Indian | 17 (1.45) | 7 (1) | 10 (2) | |
| Ethnicity, n (%) | .50 | |||
| Latino | 69 (5.87) | 29 (5.4) | 40 (6.3) | |
| Non-Latino | 1,106 (94.13) | 511 (94.6) | 595 (93.7) | |
| Sex, n (%) | .03 | |||
| Male | 779 (65.68) | 341 (62) | 438 (68) | |
| Female | 407 (34.32) | 205 (38) | 202 (32) | |
| Education, n (%) | .28 | |||
| Less than high school | 37 (3.16) | 17 (3) | 20 (3) | |
| High school graduate | 123 (10.49) | 62 (12) | 61 (10) | |
| Some college | 316 (26.96) | 155 (29) | 161 (25) | |
| College graduate | 291 (24.83) | 134 (25) | 157 (25) | |
| Graduate degree | 405 (34.56) | 169 (31) | 236 (37) | |
| Marital status, n (%) | .01 | |||
| Married | 895 (76.24) | 390 (72) | 505 (80) | |
| Widowed | 134 (11.41) | 73 (14) | 61 (10) | |
| Divorced or separated | 118 (10.05) | 66 (12) | 52 (8) | |
| Never married | 27 (2.30) | 11 (2) | 16 (3) | |
| Annual income,a n (%) | .02 | |||
| <$40,000 | 221 (19.23) | 122 (23) | 99 (16) | |
| $40,000 to <$80,000 | 387 (33.68) | 186 (35) | 201 (32) | |
| $80,000 to <$100,000 | 185 (16.10) | 74 (14) | 111 (18) | |
| $100,000 to <$200,000 | 242 (21.06) | 102 (19) | 140 (23) | |
| ≥$200,000 | 114 (9.92) | 48 (9) | 66 (11) | |
| Owns home, n (%) | .77 | |||
| Yes | 1,042 (88.31) | 482 (89) | 560 (88) | |
| No. of drinks per week, M (SD) | 13.6 (8.0) | 13.3 (7.9) | 13.9 (8.0) | .17 |
| No. of risks, range: 0–7, M (SD) | 2.62 (1.83) | 2.55 (1.81) | 2.68 (1.84) | .20 |
| Type of risk categoryb | ||||
| Alcohol amount | 426 (36) | 183 (34) | 243 (38) | .11 |
| Alcohol and symptoms | 541 (46) | 243 (45) | 298 (47) | .48 |
| Alcohol and comorbidities | 467 (39) | 205 (38) | 262 (41) | .23 |
| Alcohol and medication | 720 (61) | 332 (61) | 388 (61) | .95 |
| Heavy episodic drinking | 232 (20) | 101 (19) | 131 (20) | .39 |
| Driving after drinking | 375 (32) | 167 (31) | 208 (33) | .48 |
| Anyone concerned about drinking | 348 (29) | 160 (29) | 180 (29) | .98 |
Notes: No. = number.
In U.S. dollars;
percentages may add up to more than 100% if individuals have multiple risk factors.
On average, patient participants reported drinking almost 14 drinks per week in the past 12 months (Table 2). Of the seven possible types of risks defining at-risk drinking, patients had an average of 2.6 risks; 45% had just one risk; and 15%, 13%, 8%, 10%, 7%, and 5% had two, three, four, five, six, or seven risks, respectively. There were no significant differences between intervention and control groups in these percentages or in the prevalence of each risk category. Most of the patients were at risk because of their alcohol use combined with select medications (61%), with select symptoms (46%), and/or with comorbidities (39%).
Impact of intervention on probability of at-risk drinking
The percentage of at-risk drinkers declined in both groups at both 6 and 12 months, with significantly greater declines observed in the intervention group compared with the control group for overall at-risk drinking and in all categories of at-risk drinking except driving after drinking and someone expressing concern about one’s drinking (Table 3). Overall, predicted rates of at-risk drinking at 6 months were 60% for intervention patients versus 72% among the controls (p ≤ .01). At 12 months, rates of at-risk drinking were lower for both groups (56% among intervention patients vs. 67% among controls), and the difference remained statistically significant.
Table 3.
Impact of Project SHARE intervention on probability of at-risk drinking
| Outcome | Intervention % | Control % | Difference % | p |
| At-risk drinking at 6 months | 60 | 72 | -12 | ≤.01 |
| Alcohol amount | 20 | 29 | -09 | ≤.01 |
| Alcohol and symptoms | 23 | 35 | -12 | ≤.01 |
| Alcohol and comorbidities | 23 | 29 | -06 | ≤.01 |
| Alcohol and medication | 38 | 49 | -11 | ≤.01 |
| Heavy episodic drinking | 10 | 18 | -08 | ≤.01 |
| Driving after drinking | 14 | 17 | -03 | .27 |
| Anyone concerned about drinking | 25 | 23 | 02 | .39 |
| At-risk drinking at 12 months | 56 | 67 | -11 | ≤.01 |
| Alcohol amount | 18 | 27 | -09 | ≤.01 |
| Alcohol and symptoms | 22 | 32 | -10 | ≤.01 |
| Alcohol and comorbidities | 21 | 27 | -06 | .03 |
| Alcohol and medication | 36 | 46 | -10 | ≤.01 |
| Heavy episodic drinking | 10 | 16 | -06 | ≤.01 |
| Driving after drinking | 11 | 16 | -05 | .06 |
| Anyone concerned about drinking | 23 | 21 | 02 | .45 |
Notes: n = 1,073 for 6-month outcomes and n = 1,049 for 12-month outcomes. Numbers shown are predicted probabilities derived from logistic regressions with random provider effects, adjusting for baseline risk reasons (alcohol behaviors, alcohol with symptoms or comorbidities, alcohol with medications); number of months between the baseline and follow-up survey; and indicators for age group, race/ethnicity, gender, education category, marital status, income category, and home ownership. Multiple imputation methods (10 data sets) were used to impute missing data for covariates. Project SHARE = Senior Health and Alcohol Risk Education.
Intervention effects were smaller in magnitude and less significant in sensitivity analyses using a “worst-case scenario” in which all dropouts were assumed to remain at risk and retained in the analytic sample. At 6 months, 67% of intervention patients versus 72% of usual care patients were predicted to remain at risk (p = .05 for the difference). By 12 months, the difference between groups was still 5 percentage points, but the estimated effect was no longer statistically significant (64% vs. 69%, p = .12).
Impact of intervention on usual number of drinks per week, alcohol-related discussions with physician, and health care utilization
The effects of the intervention on usual number of drinks per week reported by patients were significant at both 6 and 12 months (-2.42 and -2.19, respectively, p ≤ .01) (Table 4). Intervention group patients were also significantly more likely to have discussed their alcohol use with a physician during the 12-month intervention window compared with control group patients (23% vs. 13%, p ≤ .01). Compared with control group patients, intervention group patients were predicted to use 1.14 fewer physician visits during the follow-up year (p = .03), were less likely to have had any emergency department visit in the past year (16% vs. 25%, p ≤ .01), and were less likely to have received assistance from a nonprofessional caregiver (12% vs. 17%, p ≤ .01).
Table 4.
Impact of Project SHARE intervention on alcohol consumption, alcohol-related discussions with primary care physician, and health care utilization
| Outcome | Intervention | Control | Difference | p |
| Alcohol consumption at 6 and 12 months | ||||
| Drinks per week (6 months) | 9.82 | 12.24 | -2.42 | ≤.01 |
| Drinks per week (12 months) | 9.45 | 11.64 | -2.19 | ≤.01 |
| Alcohol-related discussions at 12 months | ||||
| Discussed alcohol with primary care physician in past year | 23% | 13% | 10% | ≤.01 |
| Health care utilization at 12 months | ||||
| Number of physician visits in past year† | 7.93 | 9.07 | -1.14 | .03 |
| Had any hospitalization in past year | 13% | 16% | -3% | .09 |
| Had any emergency department visit in past year | 16% | 25% | -9% | ≤.01 |
| Had any skilled nurse home visit in past year | 2% | 3% | -1% | .47 |
| Had any assistance from nonprofessional caregiver in past year | 12% | 17% | -5% | ≤.01 |
Notes: n = 1,073 for 6-month outcomes and n = 1,049 for 12-month outcomes. Estimates shown are probabilities from logistic regressions (for dichotomous outcomes) or changes in expected value from linear regressions (for continuous outcomes). All regressions included random provider effects and adjusted for the baseline value of the dependent variable; baseline risk reasons (alcohol behaviors, alcohol with symptoms or comorbidities, alcohol with medications); number of months between the baseline and follow-up survey; and indicators for age group, race/ethnicity, gender, education category, marital status, income category, and home ownership. In addition, the regression for alcohol-related discussions controlled for the (centered) number of physician visits and its square as well as the physician’s specialty, age, and gender. Multiple imputation methods (N = 10 data sets) were used to impute missing data for covariates. Project SHARE = Senior Health and Alcohol Risk Education.
Includes primary care and specialty physicians, other mental health professionals, and other primary care providers (nurse practitioners and physician’s assistants).
Costs of screening and intervention
The average cost to identify one older patient engaging in at-risk drinking was $31 using the Project SHARE screening methods. Total variable intervention costs included $6,980 in supplies, $5,268 in data coordinator time, $23,660 in health educator time, and $7,237 in physician time (representing 2,171 total minutes or about 36 hours at $200 per hour). Average variable intervention costs were $79 per intervention patient. In addition, the one-time total fixed cost of physician training was estimated to be $3,600 for the 17 physicians assigned to the intervention group.
Discussion
We found that an educational intervention was successful at reducing at-risk drinking among older adults and that this effect persisted over 12 months. The intervention was associated with significant reductions in all categories of at-risk drinking except for driving after drinking and having others express concern about one’s drinking (in the latter case, perhaps because family members of intervention patients became more aware of their at-risk drinking as a result of the intervention). The intervention also increased self-reported rates of alcohol-related discussions between study patients and their physicians and reduced the patients’ reported consumption of alcohol and health care utilization.
The data from this trial compare favorably with those from the three other published trials in primary care aimed at reducing some aspect of alcohol consumption among older adults (Fink et al., 2002a; Fleming et al., 1999; Moore et al., 2011). Project GOAL (Guiding Older Adult Lifestyles) was a randomized trial testing the efficacy of brief physician advice and follow-up nurse calls in reducing alcohol use in problem drinkers ages 65 and older (Fleming et al., 1999). Project GOAL found significant reductions in 7-day alcohol use, episodes of heavy episodic drinking, and frequency of “high-quantity” drinking. However, Project GOAL had a smaller sample size (N = 158) than Project SHARE and did not examine at-risk drinking because of harmful interactions between alcohol use and prescription medications. Fink et al. (2005) classified respondents in three sites as nonhazardous, hazardous, and harmful drinkers at baseline. At Site 1, reports describing patients’ drinking risks were sent to both patients and their physicians; at Site 2, reports were sent only to patients; and at Site 3, no reports were sent. All patients receiving reports had reduced drinking risks 12 months later, and those in Site 1 had reduced amount of drinking compared with Site 3. However, more than half of the participants in the Fink et al. (2005) study were nonhazardous drinkers at baseline, and unmeasured site heterogeneity may have confounded the associations between the treatment arm and patient outcomes.
Participants in the Healthy Living as You Age (HLAYA) study were those identified as at-risk drinkers at baseline using the CARET (Moore et al., 2011). All participants in the intervention arm received baseline personalized reports and physician feedback as well as health educator telephone calls at 2, 4, and 8 weeks. This “front-loading” of the intervention contrasts with the approach used by Project SHARE, in which the intervention included health educator calls over a longer period (baseline, 3 months, and 6 months), and the intervention physicians were asked to discuss the reports with their study patients throughout the entire year whenever the patients came in for a visit. Three months after baseline, the HLAYA study observed reduced rates of at-risk drinking, amount of drinking, and heavy episodic drinking in the intervention arm compared with the control arm; however, by 12 months, only the difference in number of drinks remained significant. Both the HLAYA and Project SHARE interventions initially reduced rates of at-risk drinking by about 12 percentage points, yet the Project SHARE intervention effects were more persistent, suggesting that a low-intensity but sustained intervention may be a useful approach for older at-risk drinkers. Sustained reductions in at-risk drinking may reduce negative health-related events such as hospitalization, injury, and development of new comorbidities.
The Project SHARE intervention may have reduced health care costs, as intervention patients reported lower use of a variety of health care services; in many cases, the differences were statistically significant. Most notably, the probability of having an emergency department visit in the postbaseline year is 9 percentage points lower among the intervention patients. The Project SHARE intervention itself required significant resources, primarily the “opportunity cost” of health educator and physician time; adding another patient to the intervention was calculated to require $110 in screening plus intervention costs. However, during the same period, the median charge for outpatient emergency department visits was $1,233 (Caldwell et al., 2013), suggesting that the estimated reduction in emergency department visits alone might cover screening and intervention costs. Although a full analysis of cost-effectiveness is outside the scope of the current study, reductions in health care utilization resulting from the Project SHARE intervention improve its benefits relative to its costs and should be explored further. Our findings should be interpreted in light of several study limitations. The Project SHARE study enrolled relatively few physicians in a small number of clinics and enrolled primarily White, well-educated patients in a relatively affluent area. Therefore, our conclusions may not generalize to other settings and patient populations, although our study patients are typical of older Americans who consume alcohol (Fink et al., 2005; Fleming et al., 1999) and those who participate in brief alcohol interventions in community-based primary care (Falk et al., 2006; Grant, 1997; Moore et al., 1999b).
The outcomes were based on patient self-report, which could lead to underreporting of at-risk drinking. Nonetheless, patient-reported alcohol use is thought to be generally reliable and valid (Del Boca and Darkes, 2003), and, anecdotally, the Project SHARE health educators did not experience reluctance on the part of the intervention patients to acknowledge alcohol consumption. The use of a mail rather than phone survey or in-person interview is likely to have reduced the potential for social desirability bias even further.
Self-reported health care utilization is also subject to recall bias (Bhandari and Wagner, 2006). However, major events such as hospitalizations and emergency department visits are likely to be remembered with greater accuracy than routine physician visits (Bhandari and Wagner, 2006), especially when the measure is qualitative (whether the patient had any hospitalization or emergency department visit) rather than an exact count.
As is common with brief alcohol interventions (Kaner et al., 2009), the intervention group experienced a significantly higher dropout rate than the control group (20% vs. 5% by 12 months). Sensitivity analyses suggested that even in the worst-case scenario, the Project SHARE intervention reduced at-risk drinking by 5 percentage points, although this differential was only statistically significant at 6 months and no longer significant by 12 months.
Average screening costs will depend heavily on the proportion of the screened population that is at risk and the method of screening. The average cost to identify a single at-risk drinker will be lower if fewer people need to be screened to find an at-risk drinker, or it might be reduced if screening were built into routine practice.
Because of the difficulties with estimating the dollar value of the time of retired individuals, our estimated intervention costs excluded the opportunity costs of the time the intervention subjects spent on alcohol-related discussions with their physicians or the health educators (an average of 27 minutes per subject over the duration of the intervention).
Finally, we did not include overhead in calculating the costs associated with data coordinator and health educator time because overhead costs will vary substantially across institutions. In our case, there was excess capacity in terms of the pre-existing infrastructure; therefore, no new office space or other overhead costs were needed to extend the existing activities of these staff members to include the Project SHARE intervention. This caveat should be kept in mind when generalizing our cost estimates to other settings where new staff may need to be hired and additional overhead costs may be incurred.
Although the Project SHARE intervention shows promise for being an effective as well as potentially budget-neutral intervention, its effects were relatively modest in magnitude; in “worst-case” sensitivity analyses, they were nonsignificant at 12 months. Future work should examine ways to bolster the effectiveness of the intervention. Patient retention in the intervention might be enhanced through greater physician engagement with the patient. Adding periodic “booster” calls from the health educators after 6 months could help sustain long-term effects. Adherence among physicians might be enhanced through financial incentives to compensate them for discussing potential alcohol-related problems with their patients. A more intensive intervention would likely lead to better outcomes, although the additional resources used would also increase intervention costs. However, some of these discussions might be reimbursable through third-party payers, thus improving the potential for sustainability of the intervention. For example, Medicare covers annual screening for alcohol misuse (excluding dependence). For patients who screen positive for alcohol misuse, Medicare then covers up to four brief, face-to-face behavioral counseling interventions per year provided by primary care providers.
In summary, Project SHARE is one of the few trials to specifically target older adults in primary care settings who are at-risk drinkers and is the first study using a comprehensive definition of at-risk drinking and focusing on older adults already engaging in at-risk drinking to show sustained reductions in at-risk drinking and amount of drinking. Project SHARE is also the first of these studies to report improved rates of alcohol-related discussions with physicians and reduced use of emergency department visits among intervention recipients. The study’s intervention may be an effective approach to increase alcohol-related discussions and reduce at-risk alcohol use and health care utilization.
Acknowledgments
Author contributions: Dr. Ettner had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Ettner, Xu, Tseng, Duru, Moore. Acquisition of data: Ettner, Ang, Tallen, Mirkin, Ransohoff. Analysis and interpretation of data: Ettner, Duru, Xu, Tseng, Ang. Drafting of the manuscript: Ettner. Critical revision of the manuscript for important intellectual content: Ettner, Xu, Duru, Tseng, Barnes, Ransohoff, Moore. Statistical analysis: Ettner, Xu, Tseng, Ang. Obtained funding: Ettner. Administrative, technical, or material support: Ettner, Ang, Tallen, Mirkin, Xu, Tseng. Study supervision: Ettner, Tallen, Mirkin, Ransohoff.
Conflict of interest: All authors have declared no potential conflicts of interest.
Role of sponsor: The sponsor provided only financial support for the study and had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the study; or in the preparation, review, or approval of the manuscript.
Additional contributions: The authors thank the following: the leadership, research staff, and information technology staff of Sansum Clinic, in particular, Mr. Paul Jaconette, Ms. Chris McNamara, Ms. Linda Chapman, and Mr. Tom Colbert; Ms. Deborah Marshall and the UCLA research staff; the Project SHARE health educators; and the members of our Research Advisory Board. Finally, we are indebted to the Sansum Clinic patients and physicians who participated in Project SHARE, without whom the study could not have been conducted.
Footnotes
This project was funded by National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant 1RO1AA013990 (Principal investigator: Susan L. Ettner). Alison A. Moore’s time was additionally supported by NIAAA Grants R01 AA15957 and K24 AA15957 (Principal investigator: Alison A. Moore). O. Kenrik Duru’s time was supported by National Institute on Aging Grants 5P30AG021684-12 and 5K08AG033630-05. The authors thank the following: the leadership, research staff, and information technology staff of Sansum Clinic, in particular Mr. Paul Jaconette, Ms. Chris McNamara, Ms. Linda Chapman, and Mr. Tom Colbert; Ms. Deborah Marshall and the University of California at Los Angeles research staff; the Project SHARE (Senior Health and Alcohol Risk Education) health educators; and the members of our research advisory board. Finally, we are indebted to the Sansum Clinic patients and physicians who participated in Project SHARE, without whom the study could not have been conducted. This study is registered as a clinical trial with ClinicalTrials.gov Identifier: NCT00107640.
References
- Balsa AI, Homer JF, Fleming MF, French MT. Alcohol consumption and health among elders. The Gerontologist. 2008;48:622–636. doi: 10.1093/geront/48.5.622. [DOI] [PubMed] [Google Scholar]
- Barnes AJ, Moore AA, Xu H, Ang A, Tallen L, Mirkin M, Ettner SL. Prevalence and correlates of at-risk drinking among older adults: The Project SHARE study. Journal of General Internal Medicine. 2010;25:840–846. doi: 10.1007/s11606-010-1341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari A, Wagner T. Self-reported utilization of health care services: Improving measurement and accuracy. Medical Care Research and Review. 2006;63:217–235. doi: 10.1177/1077558705285298. [DOI] [PubMed] [Google Scholar]
- Caldwell N, Srebotnjak T, Wang T, Hsia R. “How much will I get charged for this?” Patient charges for top ten diagnoses in the emergency department. PLoS ONE. 2013;8(2):e55491. doi: 10.1371/journal.pone.0055491. Retrieved from doi:10.1371/journal.pone.0055491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction, 98, Supplement. 2003;2:1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhöfel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Research & Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Fink A, Elliott MN, Tsai M, Beck JC. An evaluation of an intervention to assist primary care physicians in screening and educating older patients who use alcohol. Journal of the American Geriatrics Society. 2005;53:1937–1943. doi: 10.1111/j.1532-5415.2005.00476.x. [DOI] [PubMed] [Google Scholar]
- Fink A, Morton SC, Beck JC, Hays RD, Spritzer K, Oishi S, Moore AA. The alcohol-related problems survey: Identifying hazardous and harmful drinking in older primary care patients. Journal of the American Geriatrics Society. 2002;50:1717–1722. doi: 10.1046/j.1532-5415.2002.50467.x. [DOI] [PubMed] [Google Scholar]
- Fink A, Tsai MC, Hays RD, Moore AA, Morton SC, Spritzer K, Beck JC. Comparing the alcohol-related problems survey (ARPS) to traditional alcohol screening measures in elderly outpatients. Archives of Gerontology and Geriatrics. 2002;34:55–78. doi: 10.1016/s0167-4943(01)00198-4. [DOI] [PubMed] [Google Scholar]
- Fleming MF, Manwell LB, Barry KL, Adams W, Stauffacher EA. Brief physician advice for alcohol problems in older adults: A randomized community-based trial. Journal of Family Practice. 1999;48:378–384. [PubMed] [Google Scholar]
- Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: Results of the National Longitudinal Alcohol Epidemiologic Survey. Journal of Studies on Alcohol. 1997;58:464–473. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, O’Brien MA. Changing provider behavior: An overview of systematic reviews of interventions. Medical Care, 39, Supplement. 2001;2:II-2–II-45. [PubMed] [Google Scholar]
- Kaner EFS, Dickinson HO, Beyer F, Pienaar E, Schlesinger C, Campbell F, Heather N. The effectiveness of brief alcohol interventions in primary care settings: A systematic review. Drug and Alcohol Review. 2009;28:301–323. doi: 10.1111/j.1465-3362.2009.00071.x. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Erwin CW, Ramm D, Cleveland WP. Effects of age and alcohol on psychomotor performance of men. Journal of Studies on Alcohol. 1980;41:488–495. doi: 10.15288/jsa.1980.41.488. [DOI] [PubMed] [Google Scholar]
- Moore AA, Beck JC, Babor TF, Hays RD, Reuben DB. Beyond alcoholism: Identifying older, at-risk drinkers in primary care. Journal of Studies on Alcohol. 2002;63:316–324. doi: 10.15288/jsa.2002.63.316. [DOI] [PubMed] [Google Scholar]
- Moore AA, Blow FC, Hoffing M, Welgreen S, Davis JW, Lin JC, Barry KL. Primary care-based intervention to reduce at-risk drinking in older adults: A randomized controlled trial. Addiction. 2011;106:111–120. doi: 10.1111/j.1360-0443.2010.03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AA, Giuli L, Gould R, Hu P, Zhou K, Reuben D, Karlamangla A. Alcohol use, comorbidity, and mortality. Journal of the American Geriatrics Society. 2006;54:757–762. doi: 10.1111/j.1532-5415.2006.00728.x. [DOI] [PubMed] [Google Scholar]
- Moore AA, Gould R, Reuben DB, Greendale GA, Carter MK, Zhou K, Karlamangla A. Longitudinal patterns and predictors of alcohol consumption in the United States. American Journal of Public Health. 2005;95:458–464. doi: 10.2105/AJPH.2003.019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AA, Hays RD, Greendale GA, Damesyn M, Reuben DB. Drinking habits among older persons: Findings from the NHANES I Epidemiologic Followup Study (1982-84). National Health and Nutrition Examination Survey. Journal of the American Geriatrics Society. 1999a;47:412–416. doi: 10.1111/j.1532-5415.1999.tb07232.x. [DOI] [PubMed] [Google Scholar]
- Moore AA, Hays RD, Reuben DB, Beck JC. Using a criterion standard to validate the Alcohol-Related Problems Survey (ARPS): A screening measure to identify harmful and hazardous drinking in older persons. Aging (Milano) 2000;12:221–227. doi: 10.1007/BF03339839. [DOI] [PubMed] [Google Scholar]
- Moore AA, Morton SC, Beck JC, Hays RD, Oishi SM, Partridge JM, Fink A. A new paradigm for alcohol use in older persons. Medical Care. 1999b;37:165–179. doi: 10.1097/00005650-199902000-00007. [DOI] [PubMed] [Google Scholar]
- Moore AA, Whiteman EJ, Ward KT. Risks of combined alcohol/medication use in older adults. American Journal of Geriatric Pharmacotherapy. 2007;5:64–74. doi: 10.1016/j.amjopharm.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. 1995. The physicians’ guide to helping patients with alcohol problems (National Institutes of Health Publication No. 95–3769). Rockville, MD: Author.
- Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: J. Wiley & Sons; 1987. [Google Scholar]
- Schiller JS, Lucas JW, Peregoy JA. 2012. Summary health statistics for U.S. adults: National Health Interview Survey, 2011 (DHHS Publication No. PHS 2013–1584). Vital and Health Statistics, Series 10, Number 256. Hyattsville, MD: U.S. Government Printing Office. Retrieved from http://www.cdc.gov/nchs/data/series/sr_10/sr10_256.pdf.
- United States Census Bureau, Population Division. 2012. Table 4. Percent Distribution of the Projected Population by Selected Age Groups and Sex for the United States: 2015 to 2060 (NP2012–T3)
- Vestal RE, McGuire EA, Tobin JD, Andres R, Norris AH, Mezey E. Aging and ethanol metabolism. Clinical Pharmacology and Therapeutics. 1977;21:343–354. doi: 10.1002/cpt1977213343. [DOI] [PubMed] [Google Scholar]