Abstract
Autophagy is the major intracellular degradation system, by which cytoplasmic materials are delivered to and degraded in the lysosome. As a quality control mechanism for cytoplasmic proteins and organelles, autophagy plays important roles in a variety of human diseases, including neurodegenerative diseases, cancer, cardiovascular disease, diabetes and infectious and inflammatory diseases. The discovery of ATG genes and the dissection of the signaling pathways involved in regulating autophagy have greatly enriched our knowledge on the occurrence and development of this lysosomal degradation pathway. In addition to its role in degradation, autophagy may also promote a type of programmed cell death that is different from apoptosis, termed type II programmed cell death. Owing to the dual roles of autophagy in cell death and the specificity of diseases, the exact mechanisms of autophagy in various diseases require more investigation. The application of autophagy inhibitors and activators will help us understand the regulation of autophagy in human diseases, and provide insight into the use of autophagy-targeted drugs. In this review, we summarize the latest research on autophagy inhibitors and activators and discuss the possibility of their application in human disease therapy.
Keywords: autophagy, cancer, neurodegenerative diseases, PI3K inhibitor, cycloheximide, lysosomal lumen alkalizer, Rack1 protein, ER stress inducer, rapamycin, LiCl
Introduction
Cell growth and homeostasis are governed by tightly regulated biosynthetic and catabolic processes. The major cellular pathways for protein and organelle turnover are autophagy and proteasome-mediated degradation. Autophagy is a universal, dynamic process that takes place in all eukaryotic cells. There are three primary forms of autophagy: macroautophagy, microautophagy and chaperone-mediated autophagy1. Of these classifications, macroautophagy, herein referred to as autophagy, is the major form of autophagy and has been well characterized in recent years.
Increasing studies have shown that autophagy is involved in many human diseases (such as neurodegenerative diseases and cancer) and plays roles in their pathogenesis2,3. Uncovering the role of autophagy in these diseases is essential because it may present a novel therapeutic target. Unfortunately, available methods to monitor autophagy are limited. Enhancing or blocking autophagy by chemical drugs or genetic means will help us to reveal autophagic mechanisms implicated in these disease processes, and autophagy inhibitors or activators may become possible therapeutic strategies. Various compounds or strategies that have been utilized to induce or suppress autophagy in mammalian systems are summarized and discussed in this review.
Mechanisms and pharmacologic targeting of autophagy
Although autophagy and autophagy-related processes are dynamic, they can be broken down into several discrete steps for the purpose of discussion: (1) induction, (2) autophagosome formation, (3) autophagolysosome formation, and (4) delivery and degradation of the autophagic body1. Autophagy is induced as a response to both extracellular stress conditions (nutrient deprivation, hypoxia, and oxidative stress) and intracellular stress conditions (endoplasmic reticulum stress, accumulation of damaged organelles, and aggregation of proteins). The large number of stimuli able to trigger autophagy implies the involvement of multiple signaling pathways in autophagosome formation.
The autophagy-related genes and their products are named as ATG and Atg, respectively4. Once the phagophore has been formed, the membrane structure expands to sequester materials to form autophagosome; this process is mediated by two ubiquitin-like conjugation systems, the Atg12–Atg5 and Atg8 conjugation systems5. In fact, half of the ATG genes essential for autophagy are involved in these two conjugation systems, and they are well conserved among eukaryotes. In addition, Atg1 kinase and its regulators, the phosphoinositide 3-kinase (PI3K) complex, Atg9 and the Atg2–Atg18 complex, are all involved in autophagosome formation6. Of these Atgs, Beclin 1, the mammalian ortholog of yeast Atg6, interacts with class III PI3K (also named Vps34) and thus plays an important role in the initiation of autophagosome formation during autophagy7. Beclin 1 is the first autophagy-related tumor suppressor gene reported thus far, and it has been shown to stimulate autophagy and suppress tumorigenesis in cancer cells8. These specific Atgs may be potent autophagy-regulating targets for genetic intervention.
The central player in autophagic signaling complexes and pathways is the mammalian target of rapamycin (mTOR), which negatively regulates autophagy. mTOR forms two different multi-protein complexes, which are referred to as mTOR complex 1 (mTORC1) and mTORC2, which are largely defined by the presence of either raptor or rictor9. mTOR is highly conserved from yeast to mammals. As a central regulator of cell growth and metabolism, mTOR controls growth-related processes such as development, aging and the response to hypoxia10. Thus, inhibitors of mTOR may be useful for the treatment of human diseases such as neurodegenerative diseases and metabolic disorders. Apart from the classical mTOR pathway regulating mammalian autophagy, the Ca2+-calpain-Gsα and the cAMP-Epac-PLC-ε-IP3 pathways also play important roles in the regulation of autophagy in an mTOR-independent way11. Multiple unexplored drug targets may exist in these two mTOR-independent autophagic pathways.
With the development of intensive research on autophagy, it has been recognized that an increase in autophagosome number alone does not necessarily correlate with increased lautophagic activity or flux. Instead, the striking accumulation of autophagic vacuoles (AV) in cells likely reflects an imbalance between the rates of autophagic sequestration and completion of the degradative process. In other words, these cells can be thought of as undergoing “autophagic stress”12. Maintaining the function of the lysosome and/or promoting its fusion with autophagosomes are critical for the completion of autophagic flux. Histone deacetylase 6 (HDAC6), a microtubule-associated deacetylase, recruits misfolded proteins to dynein motors for transportation to aggresomes in an autophagy-dependent manner13. Mounting evidence suggests that although HDAC6 is not required for autophagy activation per se, it is critical for autophagosome-lysosome fusion14,15. Our recent findings showed that HDAC6 and dynein participated in the degradation of MPP+-induced misfolded α-synuclein aggregates by regulating the aggresome-autophagy pathway16,17.
Autophagy activators
Several recent articles address that autophagy upregulation may have therapeutic benefits in a range of diseases. New research related to autophagy activators has become a hot topic owing to their potential clinical value. The growing list of various compounds or strategies for inducing autophagy is shown below (Table 1).
Table 1. Autophagy activators.
| Name | Mechanism | Target point | Solubility | References |
|---|---|---|---|---|
| Earle's balanced salt solution (EBSS) | Starvation inducer | Autophagy induction | Water-soluble | 20,21,59 |
| Brefeldin A | ER stressing inducer | Autophagy induction | Water-insoluble | 28,60,61 |
| Thapsigargin | ER stressing inducer | Autophagy induction | Water-insoluble | 28,29,61 |
| Tunicamycin | ER stressing inducer | Autophagy induction | Water-insoluble | 28,29,60,61 |
| Rapamycin | mTOR inhibitor | mTOR-dependent signaling pathway | Water-insoluble | 32,33,34 |
| CCI-779 | mTOR inhibitor | mTOR-dependent signaling pathway | Water-insoluble | 37,39,62,63 |
| RAD001 | mTOR inhibitor | mTOR-dependent signaling pathway | Water-insoluble | 39,64,65,66 |
| AP23576 | mTOR inhibitor | mTOR-dependent signaling pathway | Water-insoluble | 39 |
| Small molecule enhancers rapamycin (SMER) | mTOR-independent activator | mTOR-independent signaling pathway | Water-insoluble | 40,41,42 |
| Trehalose | mTOR-independent activator | mTOR-independent signaling pathway | Water-soluble | 43,44,45 |
| Lithium chloride | IMPase inhibitor | mTOR-independent signaling pathway | Water-soluble | 47,48 |
| L-690,330 | IMPase inhibitor | mTOR-independent signaling pathway | Water-soluble | 49 |
| Carbamazepine | IMPase inhibitor | mTOR-independent signaling pathway | Water-insoluble | 50,67,68 |
| Valproic acid sodium salt | IMPase inhibitor | mTOR-independent signaling pathway | Water-soluble | 51,67 |
| N-Acetyl-D-sphingosine (C2-ceramide) | Class I PI3K inhibitor | mTOR-dependent signaling pathway | Water-insoluble | 54,69 |
| Penitrem A | Ca2+ channel blocker | mTOR-independent signaling pathway | Water-insoluble | 11,70 |
| Calpastatin | Calpain inhibitor | mTOR-independent signaling pathway | Water-soluble | 71 |
| Xestospongin B | IP3R blocker | mTOR-independent signaling pathway | Water-insoluble | 58,72,73,74 |
Starvation inducers
Physiologically, autophagy is induced by amino acid deprivation18. Electron microscopy studies have indicated that complete deprivation of serum and amino acids provides a useful model for the further study of cellular autophagy19. It is widely accepted that autophagy is induced in several cell types as a response to total nutrient and serum starvation by incubation in Earle's Balanced Salt Solution (EBSS) or DMEM without amino acids and serum20,21. Drosophila Rack1, a receptor of activated protein kinase C 1, increases 4.1- to 5.5-fold during nutrient deprivation in all three genotypes. Recently, it was reported that loss of Rack1 led to an attenuated autophagic response to starvation22. This starvation-induced protein potentially acts as a scaffold protein during autophagosome formation.
Endoplasmic reticulum stress inducers
Accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER) results in ER stress. Emerging data now indicate that ER stress is a potent inducer of autophagy, a process whereby eukaryotic cells recycle their macromolecules and organelles23,24,25. Sar1 and Rab1b are monomeric GTPases that control traffic from the ER to the Golgi, and there is evidence indicating that the activity of both proteins is also required for autophagosome formation26. ER stress enhances autophagy by negatively regulating the AKT/TSC/mTOR pathway27. ER stress inducers such as brefeldin A, thapsigargin and tunicamycin increase the formation of autophagic vesicles with the expression of Beclin and LC-3 (microtubule-associated protein1 light chain 3) II, two autophagic markers28,29. However, conflicting data have also been reported. For example, Gordon et al showed that autophagy is inhibited by thapsigargin, which releases Ca2+ from ER stores and thus increases intracytosolic Ca2+ levels30. Another study demonstrated that thapsigargin did not affect autophagosome formation but did lead to accumulation of mature autophagosomes by blocking autophagosome fusion with the endocytic system31. The opposite effects shown by these ER stress inducers may be caused by crosstalk between regulatory pathways of ER stress and autophagy.
Rapamycin and its derivatives
Rapamycin, also called sirolimus, is a natural product with potent antifungal and immunosuppressive activities. It forms a complex with the immunophilin FK506-binding protein 12 (FKBP12), which then stabilizes the raptor-mTOR association and inhibits the kinase activity of mTOR32. As an inhibitor of mTOR, rapamycin has been widely reported in the literature to induce autophagy both in vivo and in vitro33,34. In organisms from yeast to humans, TOR proteins control several cellular processes other than autophagy, including the repression of ribosome biogenesis and protein translation and transcriptional induction of compensatory metabolic pathways35,36. These effects may contribute to the complications observed with long-term rapamycin use, such as immunosuppression, which is not compatible with disease therapy. Temsirolimus (also known as CCI-779) is a water-soluble ester of rapamycin that decreased huntingtin aggregate formation in a mouse model of Huntington disease by inhibiting mTOR37. The results from a current clinical trial support the use of CCI-779 for mantle cell lymphoma intervention, likely in combination with other agents, such as antiangiogenic drugs or histone acetylase inhibitors38. Similar to CCI-779 (intravenous formulation), two other rapamycin analogs are RAD001 (or Everolimus, oral formulation) and AP23573 (intravenous formulation), both of which display a good safety profile with mild, dose-limiting toxicities compared with rapamycin39. These rapamycin derivatives are better therapeutic strategies against cancer.
Small molecule enhancers of rapamycin
Owing to the immunosuppressive side effects of rapamycin that preclude its use in therapy, a safer way of inducing autophagy urgently needs to be developed. Using a novel, two-step screening process, three small molecule enhancers of rapamycin (SMERs), SMERs 10, 18, and 28, were identified from 50 729 compounds screened in 200740. SMER10 is an aminopyrimidone, SMER18 is a vinylogous amide, and SMER28 is a bromo-substituted quinazoline. These SMERs have been confirmed to induce autophagy in mammalian cells, enhancing the clearance of autophagy substrates such as mutant huntingtin and A53T α-synuclein, which are associated with Huntington's disease and familial Parkinson's disease, respectively41. The SMERs did not affect the levels of various autophagic regulators, such as Beclin 1 (Atg6), Atg5, Atg7, and Atg12, nor did they enhance conjugation of Atg12 to Atg5, which is a critical step in autophagosome assembly preceding LC3 conjugation41. These SMERs induce mammalian autophagy in an mTOR-independent manner, appearing to act either independently or downstream of the target of rapamycin. However, a recent study shows that Atg5 plays an essential role in the degradation of Aβ and APP-CTF, which is induced by starvation or SMER28. In other words, SMER28 decreases Aβ and APP-CTF levels via an Atg5-dependent autophagic pathway42.
Trehalose
Trehalose was first extracted from rye ergot by Wiggers in 1832. Trehalose protects the integrity of cells against various environmental stresses such as heat, cold, desiccation, dehydration, and oxidation by preventing protein denaturation. Recently, a new role for trehalose as an mTOR-independent activator of autophagy was discovered43. Trehalose-induced autophagy enhanced the clearance of autophagy substrates such as mutant huntingtin and A30P and A53T α-synuclein43. Furthermore, as a natural hemolymph sugar of invertebrates, trehalose may be a safe strategy for the treatment of two other neurodegenerative diseases, AD44 and prion disease45. Notably, trehalose pre-treatment protected against pro-apoptotic insults by reducing mitochondrial load in addition to its autophagic induction role43,46. It is difficult to conclude that prolonged upregulation of autophagy would be beneficial for treatment without any risk. However, the dual protective properties of trehalose and its less toxic characteristics make it a unique candidate for developing therapeutic strategies of autophagy-targeted drugs.
IMPase inhibitors
In 2005, Prof Rubinsztein found that the lithium chloride (LiCl) used to treat bipolar disorders induced autophagy in an mTOR-independent manner. It was revealed that LiCl induced autophagy via inhibition of inositol monophosphatase (IMPase), leading to free inositol depletion and reduced inositol-1,4,5-triphosphate (IP3) levels47. This is the first report describing a pathway that regulates mammalian autophagy other than the canonical mTOR-dependent pathway. Autophagy induced by LiCl significantly promotes the degradation of mutant huntingtin and α-synuclein47. A recent study indicates that long-term oral lithium treatment attenuates p-tau-induced motor disturbances not only by inhibiting glycogen synthase kinase-3 but also by enhancing autophagy in tauopathy model mice48. L-690,330 is a bisphosphonate inhibitor of IMPase that mimics the effects of lithium49. It also facilitates the clearance of soluble EGFP-HDQ74 and mutant synucleins47. Furthermore, the mood stabilizing drugs carbamazepine50 and valproic acid51, which lower intracellular inositol levels, were also found to activate autophagy. These findings suggest that IMPase inhibitors may be a valuable strategy for the treatment of neurodegenerative diseases by upregulating autophagy.
Class I PI3K inhibitors
The PI3K-I/PKB pathway is involved in the negative regulation of autophagy52,53. N-Acetyl-D-sphingosine (C2-ceramide) is a cell-permeable and biologically active ceramide. It rescues the inhibition of the class I PI3K signaling pathway on autophagy by interfering with the IL-13-dependent activation of protein kinase B (PKB) and stimulation of the expression of Beclin 154. These data suggest a novel function for ceramide in autophagy upregulation. Recent studies show that CH513279955, GDC-098056, and GDC-094157 potently inhibit signal transduction downstream of both PI3K and mTOR. However, only the pro-apoptotic mechanisms of these three novel class I PI3K inhibitors have been discussed. Their roles in the regulation of autophagy are yet to be identified.
Other activators
Ca2+ is an important intracellular second messenger involved in regulating many cellular processes. Autophagy is inhibited by increasing intracytosolic Ca2+ in rat hepatocytes30. Penitrem A (an irreversible inhibitor of high conductance Ca2+-activated K+ channels) was shown to be a candidate autophagy activator by blocking Ca2+ channels11. The increase in intracytosolic Ca2+ activates a family of Ca2+-dependent cysteine proteases termed calpains, which inhibits autophagy by cleaving the α-subunit of heterotrimeric G-proteins (Gsα)11. Thus, the calpain inhibitor calpastatin may be a potential autophagy inducer. As mentioned above, the lower IP3 levels induced by lithium promote autophagic degradation of protein aggregates. Consistent with the effect of IP3 on autophagy, it is notable that the IP3 receptor (IP3R) inhibitor xestospongin B also acts as an mTOR-independent autophagy activator58.
Autophagy inhibitors
Autophagy could potentially be suppressed at any stage of autophagic flux. During the study of autophagy mechanisms, many chemical inhibitors have been identified and were used in various cell and animal models (Table 2). However, most chemical inhibitors of autophagy are not entirely specific, and it should be cautious to interpret the findings obtained with the use of these compounds, especially regarding their dose and incubation time.
Table 2. Autophagy inhibitors.
| Name | Mechanism | Target point | Solubility | References |
|---|---|---|---|---|
| 3-Methyladenine | PI 3-kinase inhibitor | Autophagosome formation | Water-soluble | 76,78,80 |
| Wortmaninn | PI 3-kinase inhibitor | Autophagosome formation | Water-insoluble | 80,102,103 |
| LY294002 | PI 3-kinase inhibitor | Autophagosome formation | Water-insoluble | 80,102,103 |
| Cycloheximide | protein synthesis inhibitor | Autophagosome formation | Water-insoluble | 85,86,87 |
| Bafilomycin A1 | Vacuolar-type H(+)-ATPase inhibitor | Autophagolysosome formation | Water-insoluble | 90,91 |
| Hydroxychloroquine | Lysosomal lumen alkalizer | Lysosome | Water-soluble | 95,104,105 |
| Lys05 | Lysosomal lumen alkalizer | Lysosome | Water-soluble | 97,106 |
| Leupeptin | Acid protease inhibitor | Lysosome | Water-soluble | 98,107 |
| E64d | Acid protease inhibitor | Lysosome | Water-insoluble | 100,108 |
| Pepstatin A | Acid protease inhibitor | Lysosome | Water-insoluble | 100 |
PI3K inhibitors
The activation of a population of PI3Ks located in a specific membrane domain may be responsible for autophagosome biogenesis. Several studies have demonstrated that PI3K inhibitors interfere with the formation of autophagosomes75,76,77. The PI3K inhibitor 3-methyladenine (3-MA) was the first identified and is the most widely used autophagy inhibitor78. In mammalian cells, there are three classes of PI3Ks. Class I PI3K is an inhibitor of autophagy79. Class II PI3K activity is thought to have no relevance to autophagic control. Class III PI3K, a functional ortholog of yeast Vps34, is an activator of autophagy and plays a crucial role in an early step of autophagosome formation in mammalian cells77. Subsequent studies confirmed that 3-MA, together with two other PI3K inhibitors, wortmannin and LY294002, suppresses autophagy via inhibition of class III PI3K80.
Previously, it was thought that the overall effect of these inhibitors was typically to block autophagy because the class III enzymes that are required to activate autophagy act downstream of the negative regulatory class I enzymes. However, a recent study presents a surprising finding that 3-MA has a dual role in autophagic regulation81. 3-MA promoted autophagic flux when administered under nutrient-rich conditions with a prolonged period of treatment, although it was still capable of suppressing starvation-induced autophagy. The inhibitory effects of wortmannin are the opposite of those of 3-MA: it has persistent effects on class III PI3K and transient effects on class I PI3K81. Data from this study also suggest that wortmannin is a more suitable autophagy inhibitor than 3-MA due to its persistent inhibition of class III PI3K activity. However, it is notable that wortmannin induces the formation of vacuoles that appear similar to autophagosomes, although they are swollen late endocytic compartments82. In addition, studies also have shown that LY294002 activated autophagy by inhibiting the class I PI3K signaling pathway83. LY294002 increased intracellular calcium, at least in part, by mobilizing intracellular calcium stores and inhibiting calcium transients84. Therefore, experiments where calcium is relevant should avoid using LY294002. Understanding the complex role of PI3K inhibitors in autophagy may help in choosing the proper inhibitor for a particular situation.
Cycloheximide
Cycloheximide is an inhibitor of protein biosynthesis in eukaryotic organisms and is produced by the bacterium Streptomyces griseus. It is a widely used method in biomedical research to inhibit protein synthesis that is inexpensive and fast acting. Studies have demonstrated that cycloheximide suppresses cellular autophagy induced by hyperosmotic sucrose or cadmium chloride in mouse pancreatic acinar cells85. An in vitro study has shown regression of autophagic vacuoles in seminal vesicle cells following cycloheximide treatment86. Cycloheximide has proven to be a fast and effective inhibitor of autophagic segregation and may inhibit segregational steps occurring prior to the actual formation of autolysosomes87. Although it is still currently used to inhibit the autophagy-lysosome pathway88, one should keep in mind that the inhibition of autophagic degradation and lysosomal enzyme delivery is rapidly reversed following the removal of cycloheximide89 and that the mechanism of cycloheximide action in short-term experiments remains poorly understood.
Vacuolar-type H (+)-ATPase inhibitors
Vacuolar-type H (+)-ATPases (V-ATPases) are found within the membranes of many organelles including lysosomes, endosomes, and secretory vesicles, where they play a variety of roles crucial for organelle function. Bafilomycin A1 is a specific inhibitor of V-ATPase in cells, and it inhibits the acidification of lysosomes and endosomes. As early as 1998, bafilomycin A1 was reported to prevent maturation of autophagic vacuoles by inhibiting the fusion between autophagosomes and lysosomes in the rat hepatoma cell line H-4-II-E90. Inhibition of autophagy by bafilomycin A1 decreased proliferation and induced apoptosis in colon cancer cells91. However, Prof Daniel revealed an apparently contradictory result that bafilomycin A1 did not block the fusion of autophagosomes with lysosomes92. Data from the relatively recent literature also show that bafilomycin A1 and rapamycin potentiate ethanol-increased LC3 lipidation, whereas wortmannin and a BECN1-specific shRNA inhibit ethanol-promoted LC3 lipidation93. Furthermore, concanamycin A, another selective V-ATPase inhibitor, also increased the accumulation of autophagosomes94.
Lysosomal lumen alkalizers
Lysosomal lumen alkalizers (chloroquine, hydroxychloroquine, NH4Cl, and neutral red) are used to block autophagic progress by impairing lysosomes. Of them, chloroquine and its analog hydroxychloroquine are the only clinically relevant autophagy inhibitors that are widely used as anti-malarial and anti-rheumatoid agents. It has been reported that chloroquine-mediated lysosomal dysfunction enhanced its anticancer effect95. A major concern with the use of hydroxychloroquine is that high micromolar concentrations, which are not consistently achieved in patients, are required to block autophagy in vitro.
Lys01 is a new, dimeric form of chloroquine that contains the spacer N,N-bis(2-aminoethyl)-methylamine as a connector between two chloroquine moieties. It is a 10-fold more potent autophagy inhibitor than hydroxychloroquine96. Compared with hydroxychloroquine, Lys05, a water-soluble salt of Lys01, more potently accumulates within and deacidifies the lysosome, resulting in impaired autophagy and tumor growth97. As a new lysosomal autophagy inhibitor, Lys05 has a better therapeutic index and has the potential to be developed further into a drug for autophagy-targeting therapy.
Acid protease inhibitors
The lysosome is the ultimate degradative autophagic compartment in the cell. Leupeptin is a naturally occurring protease inhibitor that inhibits cysteine, serine and threonine peptidases. It blocks autophagy at the step of degradation of the cytoplasm enclosed in lysosomes and causes the accumulation of autolysosomes and/or many cytoplasmic inclusions in the central vacuoles98. Cycloheximide administered simultaneously with leupeptin rapidly inhibited the formation of autophagic vacuoles and the sequestrations of both cytoplasmic and lysosomal enzymes99.
Lysosomal cathepsins, which are enclosed in lysosomes, help maintain the homeostasis of the cell's metabolism by participating in the degradation of autophagic bodies. Among the lysosomal hydrolases and proteases, cathepsins have an especially major role. E64d and pepstatin A are two autophagy inhibitors that function by suppressing lysosomal proteases. E64d is a membrane-permeable inhibitor of cathepsins B, H, and L, whereas pepstatin A is an inhibitor of cathepsins D and E. The lysosomal turnover of endogenous LC3-II could be investigated using E64d and pepstatin A. LC3-positive puncta, i.e., autophagolysosomes, highly accumulated in the presence of E64d and pepstatin A under starvation conditions100. It was recently reported that E64d plus pepstatin A increased free GFP fragments resulting from the degradation of GFP-LC3 within the autolysosome101. This observation is confusing because the level of free GFP fragments is thought to reflect autophagic flux. One possible reason for this is that under certain conditions (non-saturating concentrations) some lysosomal inhibitors only partially suppress cathepsin activity and may not completely suppress lysosomal degradation (or cleavage) of GFP-LC3.
Genetic intervention
The existence of autophagy inhibitors and activators greatly facilitates the investigation of autophagy and its therapeutic potential in human diseases. However, most chemical inhibitors of autophagy are not entirely specific; thus, genetic intervention is suggested as a preferred approach to block autophagy. The ATG genes are essential for autophagosome information109. The use of ATG gene deletions/inactivations or functional knockdown (eg, RNAi against the ATG genes) methods may produce a more specific manipulation of autophagy. A growing number of studies indicate that Atg-deficient cells and animals provide available experimental models for monitoring autophagy in different organisms. Furthermore, microRNAs may also be used for autophagy-related studies.
The tumor-suppressive miRNA miR-101 has been identified as a potent inhibitor of basal and rapamycin-induced autophagy110. Recently, miR-30a has been shown to be a potent autophagic inhibitor by downregulating Beclin 1 and ATG5 expression. In contrast, knockdown of miR-30a by antagomir-30a increases the expression of Beclin 1 and ATG5111. Although previous reports have shown that downregulation of ATG7, ATG5, or BECN1 by RNAi significantly decreases autophagy, it should be noted that autophagy may also occur in the absence of some of these key autophagic proteins. Recent evidence supports the idea that mammalian autophagy may occur through an Atg5/Atg7-independent pathway112. Furthermore, Beclin 1-independent autophagy was also found in dying cortical neurons113. These interesting data emphasize the limitations of Atg5/Atg7 and Beclin 1 as autophagic markers in some situations.
Therapeutic implications for autophagy regulators
Malfunctioning autophagy is observed in many human diseases including cancer, neurodegenerative diseases, cardiac and muscular diseases, infectious and inflammatory diseases, diabetes and obesity114.
However, the effect of autophagy on disease progression has not yet been discovered, and the identification and development of new drug targets is still a key focus. Further investigations are required to assess the clinical potential of autophagy activators and inhibitors in various diseases.
Autophagy is a universal, dynamic process that takes place in all eukaryotic cells and contributes to the turnover and rejuvenation of cellular components. It may also promote an autophagic death distinct from apoptosis, which is termed type II programmed cell death115. As a double-edged sword, autophagy plays a dual role in many diseases116. For example, autophagy acts both as a tumor suppressor and a protector of cancer cell survival in tumorigenesis117. A growing body of evidence demonstrates that cellular decisions toward autophagy depend on disease type, stage, microenvironment and drug treatment. Future work will be required to further investigate the mechanisms of autophagy underlying various diseases and to elucidate their exact roles in these diseases.
Many promising small molecules have been developed to regulate autophagy for therapeutic needs. Recently, a potent small molecule inhibitor of autophagy termed spautin-1 for specific and potent autophagy inhibitor-1 was discovered. Spautin-1 promotes the degradation of Vps34-PI3 kinase complexes by inhibiting two ubiquitin-specific peptidases, USP10 and USP13, that target the Beclin1 subunit of Vps34 complexes118. DEPTOR, an inhibitor of mTORC1 and mTORC2, accumulates upon glucose deprivation and mTOR inhibition and induces autophagy119. Similarly, the small-molecule inhibitor torin 1 was used to demonstrate that inhibition of mTOR kinase activity was a more potent inducer of autophagy than rapamycin120. These small molecule regulators of autophagy are more effective and likely to enhance the therapeutic arsenal against human diseases.
As mentioned above, many autophagy regulators are medicines that have been used in clinical therapy for years. Their new roles in autophagic regulation are surprising. FDA-approved antipsychotic drugs (fluspirilene, trifluoperazine, and pimozide) and FDA-approved drugs for cardiovascular indications (niguldipine, nicardipine and amiodarone) have been all described to promote autophagy11. The existence of autophagic regulators among FDA-approved drugs facilitates the investigation of the therapeutic potential of regulators of autophagy in vivo.
Novel regulators of autophagy with better therapeutic indexes are still needed. Because of their lower toxicity, traditional Chinese medicines should be considered for disease therapy by autophagic regulation. Recently, it was reported that the therapeutic effects of resveratrol121 and oridonin122 were both related to autophagy. Our studies also show that paeoniflorin, the principal bioactive component of Radix Paeoniae alba, potently protected PC12 cells against MPP+ or acidosis-induced injury by upregulating the autophagic pathway123.
The regulation of autophagy is complex and involves many signaling pathways. Thus, the safety and effectiveness of autophagy activators or inhibitors must be taken into account before clinical therapy development. A combination of mTOR and PI3 kinase inhibitors showed a synergistic antitumor effect124. Another study showed that the combination of the mTOR inhibitor rapamycin and the IMPase inhibitor lithium ameliorates toxicity of polyglutamine-expanded huntingtin125. These lines of evidence shed some light on the advantage of combination therapy and suggest that the combination therapy based on an mTOR inhibitor and an mTOR-independent activator deserves further investigation as a potential treatment.
Concluding remarks
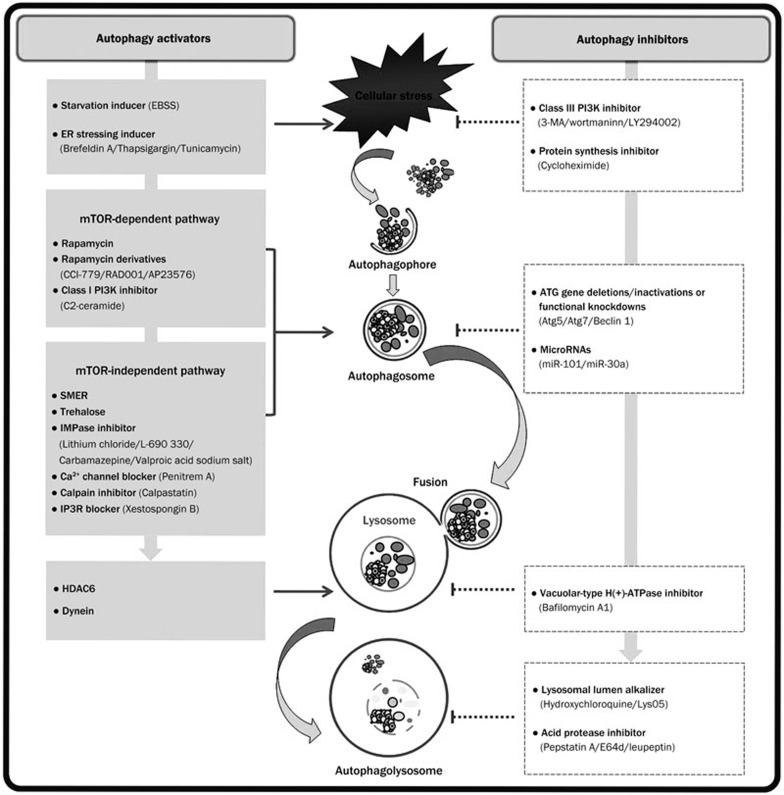
Autophagy is a process that involves the sequestration of intracellular components and their subsequent degradation in secondary lysosomes that is highly conserved from yeast to mammals. In the past several decades, the molecular mechanisms of autophagy and its role in human diseases have been well elucidated. However, there are still many questions that remain to be intensively investigated. Although autophagy activators and inhibitors have been widely used to monitor autophagy (Figure 1), the exact therapeutic doses of these autophagic regulators need to be optimized according to individual experimental conditions such as experimental purpose, cell type, culture characteristics, etc.
Figure 1.
Pharmacological targeting of control points in the autophagic pathway. EBSS, Earle's Balanced Salt Solution; ER, endoplasmic reticulum; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase; SMER, small molecule enhancers of rapamycin; IMPase, inositol monophosphatase; IP3R, inositol-1,4,5-triphosphate receptor; HDAC6, histone deacetylase 6; 3-MA, 3-methyladenine; ATG, autophagy-specific gene.
Discovering potential drug therapies that can be used to modulate autophagy is a major challenge that is likely to provide a huge therapeutic potential. Autophagy-targeted drugs should be selected based on the type and stage of the various diseases. Gene-targeting approaches may provide a novel therapeutic option for human diseases and deserve further exploration. Given the side effects caused by high drug dosage, the use of a combination therapy of autophagic regulators, rather than treatment with a single medicine, is strongly recommended whenever possible.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81171213), the Natural Science Foundation of Jiangsu Province of China (No BK2010228), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No 10KJB320016) and Plans for Graduate Research and Innovation in Colleges and Universities of Jiangsu Province (No CX10B-0492).
References
- Yang YP, Liang ZQ, Gu ZL, Qin ZH. Molecular mechanism and regulation of autophagy. Acta Pharmacol Sin. 2005;26:1421–34. doi: 10.1111/j.1745-7254.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. Autophagy: regulation and role in disease. Crit Rev Clin Lab Sci. 2009;46:210–40. doi: 10.1080/10408360903044068. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Cregg JM, Dunn WA, Jr, Emr SD, Sakai Y, Sandoval IV, et al. A unified nomenclature for yeast autophagy-related genes. Dev Cell. 2003;5:539–45. doi: 10.1016/s1534-5807(03)00296-x. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–6. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–32. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–5. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Weber JD, Gutmann DH. Deconvoluting mTOR biology. Cell Cycle. 2012;11:236–48. doi: 10.4161/cc.11.2.19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009;16:46–56. doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- Chu CT. Autophagic stress in neuronal injury and disease. J Neuropathol Exp Neurol. 2006;65:423–32. doi: 10.1097/01.jnen.0000229233.75253.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–38. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, et al. HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J. 2010;29:969–80. doi: 10.1038/emboj.2009.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Yao TP. Quality control autophagy: A joint effort of ubiquitin, protein deacetylase and actin cytoskeleton. Autophagy. 2010;6:555–7. doi: 10.4161/auto.6.4.11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Shi JJ, Yang YP, Li J, Zhang YL, Chen J, et al. HDAC6 regulates aggresome-autophagy degradation pathway of alpha-synuclein in response to MPP+-induced stress. J Neurochem. 2011;117:112–20. doi: 10.1111/j.1471-4159.2011.07180.x. [DOI] [PubMed] [Google Scholar]
- Cai ZL, Shi JJ, Yang YP, Cao BY, Wang F, Huang JZ, et al. MPP+ impairs autophagic clearance of alpha-synuclein by impairing the activity of dynein. Neuroreport. 2009;20:569–73. doi: 10.1097/WNR.0b013e32832986c4. [DOI] [PubMed] [Google Scholar]
- Seglen PO, Gordon PB. Amino acid control of autophagic sequestration and protein degradation in isolated rat hepatocytes. J Cell Biol. 1984;99:435–44. doi: 10.1083/jcb.99.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchener JS, Shelburne JD, Bradford WD, Hawkins HK. Cellular autophagocytosis induced by deprivation of serum and amino acids in HeLa cells. Am J Pathol. 1976;83:485–92. [PMC free article] [PubMed] [Google Scholar]
- Chan LL, Shen D, Wilkinson AR, Patton W, Lai N, Chan E, et al. A novel image-based cytometry method for autophagy detection in living cells. Autophagy. 2012;8:1371–82. doi: 10.4161/auto.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorov SO, Jardon MA, Piret JM, Blades MW, Turner RF. Raman microspectroscopy of live cells under autophagy-inducing conditions. Analyst. 2012;137:4662–8. doi: 10.1039/c2an35477b. [DOI] [PubMed] [Google Scholar]
- Erdi B, Nagy P, Zvara A, Varga A, Pircs K, Menesi D, et al. Loss of the starvation-induced gene Rack1 leads to glycogen deficiency and impaired autophagic responses in Drosophila. Autophagy. 2012;8:1124–35. doi: 10.4161/auto.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JJ, Li YB, Wang Y, Liu GD, Wang J, Zhu XO, et al. The role of autophagy in endoplasmic reticulum stress-induced pancreatic beta cell death. Autophagy. 2012;8:158–64. doi: 10.4161/auto.8.2.18807. [DOI] [PubMed] [Google Scholar]
- Kim DS, Li B, Rhew KY, Oh HW, Lim HD, Lee W, et al. The regulatory mechanism of 4-phenylbutyric acid against ER stress-induced autophagy in human gingival fibroblasts. Arch Pharm Res. 2012;35:1269–78. doi: 10.1007/s12272-012-0718-2. [DOI] [PubMed] [Google Scholar]
- Ciechomska IA, Gabrusiewicz K, Szczepankiewicz AA, Kaminska B.Endoplasmic reticulum stress triggers autophagy in malignant glioma cells undergoing cyclosporine A-induced cell death Oncogene 2012. May 14 Epub ahead of print. doi: 10.1038/onc.2012.174 [DOI] [PubMed]
- Zoppino FC, Militello RD, Slavin I, Alvarez C, Colombo MI. Autophagosome formation depends on the small GTPase Rab1 and functional ER exit sites. Traffic. 2010;11:1246–61. doi: 10.1111/j.1600-0854.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6:239–47. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- Kim DS, Kim JH, Lee GH, Kim HT, Lim JM, Chae SW, et al. p38 Mitogen-activated protein kinase is involved in endoplasmic reticulum stress-induced cell death and autophagy in human gingival fibroblasts. Biol Pharm Bull. 2010;33:545–9. doi: 10.1248/bpb.33.545. [DOI] [PubMed] [Google Scholar]
- Petrovski G, Das S, Juhasz B, Kertesz A, Tosaki A, Das DK. Cardioprotection by endoplasmic reticulum stress-induced autophagy. Antioxid Redox Signal. 2011;14:2191–200. doi: 10.1089/ars.2010.3486. [DOI] [PubMed] [Google Scholar]
- Gordon PB, Holen I, Fosse M, Rotnes JS, Seglen PO. Dependence of hepatocytic autophagy on intracellularly sequestered calcium. J Biol Chem. 1993;268:26107–12. [PubMed] [Google Scholar]
- Ganley IG, Wong PM, Gammoh N, Jiang X. Distinct autophagosomal-lysosomal fusion mechanism revealed by thapsigargin-induced autophagy arrest. Mol Cell. 2011;42:731–43. doi: 10.1016/j.molcel.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Tanemura M, Ohmura Y, Deguchi T, Machida T, Tsukamoto R, Wada H, et al. Rapamycin causes upregulation of autophagy and impairs islets function both in vitro and in vivo. Am J Transplant. 2012;12:102–14. doi: 10.1111/j.1600-6143.2011.03771.x. [DOI] [PubMed] [Google Scholar]
- Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82:381–8. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Hall MN. mTOR-what does it do. Transplant Proc. 2008;40:S5–8. doi: 10.1016/j.transproceed.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–95. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Galimberti S, Petrini M. Temsirolimus in the treatment of relapsed and/or refractory mantle cell lymphoma. Cancer Manag Res. 2010;2:181–9. doi: 10.2147/cmar.s7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–37. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- Floto RA, Sarkar S, Perlstein EO, Kampmann B, Schreiber SL, Rubinsztein DC. Small molecule enhancers of rapamycin-induced TOR inhibition promote autophagy, reduce toxicity in Huntington's disease models and enhance killing of mycobacteria by macrophages. Autophagy. 2007;3:620–2. doi: 10.4161/auto.4898. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL, et al. Small molecules enhance autophagy and reduce toxicity in Huntington's disease models. Nat Chem Biol. 2007;3:331–8. doi: 10.1038/nchembio883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Bustos V, Flajolet M, Greengard P. A small-molecule enhancer of autophagy decreases levels of Abeta and APP-CTF via Atg5-dependent autophagy pathway. FASEB J. 2011;25:1934–42. doi: 10.1096/fj.10-175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007;282:5641–52. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- Kruger U, Wang Y, Kumar S, Mandelkow EM. Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Neurobiol Aging. 2012;33:2291–305. doi: 10.1016/j.neurobiolaging.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Aguib Y, Heiseke A, Gilch S, Riemer C, Baier M, Schatzl HM, et al. Autophagy induction by trehalose counteracts cellular prion infection. Autophagy. 2009;5:361–9. doi: 10.4161/auto.5.3.7662. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Berger Z, Vacher C, O'Kane CJ, Rubinsztein DC. Rapamycin pre-treatment protects against apoptosis. Hum Mol Genet. 2006;15:1209–16. doi: 10.1093/hmg/ddl036. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–11. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Motoi Y, Ishiguro K, Kambe T, Matsumoto SE, Itaya M, et al. Long-term oral lithium treatment attenuates motor disturbance in tauopathy model mice: implications of autophagy promotion. Neurobiol Dis. 2012;46:101–8. doi: 10.1016/j.nbd.2011.12.050. [DOI] [PubMed] [Google Scholar]
- Atack JR, Cook SM, Watt AP, Fletcher SR, Ragan CI. In vitro and in vivo inhibition of inositol monophosphatase by the bisphosphonate L-690,330. J Neurochem. 1993;60:652–8. doi: 10.1111/j.1471-4159.1993.tb03197.x. [DOI] [PubMed] [Google Scholar]
- Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329:229–32. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- Fu J, Shao CJ, Chen FR, Ng HK, Chen ZP. Autophagy induced by valproic acid is associated with oxidative stress in glioma cell lines. Neuro Oncol. 2010;12:328–40. doi: 10.1093/neuonc/nop005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, et al. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–6. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275:992–8. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- Scarlatti F, Bauvy C, Ventruti A, Sala G, Cluzeaud F, Vandewalle A, et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem. 2004;279:18384–91. doi: 10.1074/jbc.M313561200. [DOI] [PubMed] [Google Scholar]
- Ohwada J, Ebiike H, Kawada H, Tsukazaki M, Nakamura M, Miyazaki T, et al. Discovery and biological activity of a novel class I PI3K inhibitor, CH5132799. Bioorg Med Chem Lett. 2011;21:1767–72. doi: 10.1016/j.bmcl.2011.01.065. [DOI] [PubMed] [Google Scholar]
- Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, et al. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011;10:2426–36. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- Wallin JJ, Guan J, Prior WW, Lee LB, Berry L, Belmont LD, et al. GDC-0941, a novel class I selective PI3K inhibitor, enhances the efficacy of docetaxel in human breast cancer models by increasing cell death in vitro and in vivo. Clin Cancer Res. 2012;18:3901–11. doi: 10.1158/1078-0432.CCR-11-2088. [DOI] [PubMed] [Google Scholar]
- Criollo A, Maiuri MC, Tasdemir E, Vitale I, Fiebig AA, Andrews D, et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14:1029–39. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- Martinet W, De Meyer GR, Herman AG, Kockx MM. Amino acid deprivation induces both apoptosis and autophagy in murine C2C12 muscle cells. Biotechnol Lett. 2005;27:1157–63. doi: 10.1007/s10529-005-0007-y. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Inagi R, Takano H, Sato S, Ingelfinger JR, Fujita T, et al. Endoplasmic reticulum stress induces autophagy in renal proximal tubular cells. Nephrol Dial Transplant. 2009;24:2665–72. doi: 10.1093/ndt/gfp215. [DOI] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, et al. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–10. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- Bray K, Mathew R, Lau A, Kamphorst JJ, Fan J, Chen J, et al. Autophagy suppresses RIP kinase-dependent necrosis enabling survival to mTOR inhibition. PLOS ONE. 2012;7:e41831. doi: 10.1371/journal.pone.0041831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies FM, Huebener J, Renna M, Bonin M, Riess O, Rubinsztein DC. Autophagy induction reduces mutant ataxin-3 levels and toxicity in a mouse model of spinocerebellar ataxia type 3. Brain. 2010;133:93–104. doi: 10.1093/brain/awp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Subhawong T, Albert JM, Kim KW, Geng L, Sekhar KR, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66:10040–7. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- Crazzolara R, Bradstock KF, Bendall LJ. RAD001 (Everolimus) induces autophagy in acute lymphoblastic leukemia. Autophagy. 2009;5:727–8. doi: 10.4161/auto.5.5.8507. [DOI] [PubMed] [Google Scholar]
- Lin CI, Whang EE, Donner DB, Du J, Lorch J, He F, et al. Autophagy induction with RAD001 enhances chemosensitivity and radiosensitivity through Met inhibition in papillary thyroid cancer. Mol Cancer Res. 2010;8:1217–26. doi: 10.1158/1541-7786.MCR-10-0162. [DOI] [PubMed] [Google Scholar]
- Xiong N, Jia M, Chen C, Xiong J, Zhang Z, Huang J, et al. Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y. Neuroscience. 2011;199:292–302. doi: 10.1016/j.neuroscience.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Kim H, Bernard ME, Flickinger J, Epperly MW, Wang H, Dixon TM, et al. The autophagy-inducing drug carbamazepine is a radiation protector and mitigator. Int J Radiat Biol. 2011;87:1052–60. doi: 10.3109/09553002.2011.587860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–70. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu J, Pan H, Hu P, Hao Y, Cai W, et al. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc Natl Acad Sci U S A. 2007;104:19023–8. doi: 10.1073/pnas.0709695104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrozo Z, Sanchez G, Torrealba N, Valenzuela R, Fernandez C, Hidalgo C, et al. Calpains and proteasomes mediate degradation of ryanodine receptors in a model of cardiac ischemic reperfusion. Biochim Biophys Acta. 2010;1802:356–62. doi: 10.1016/j.bbadis.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Decuypere JP, Welkenhuyzen K, Luyten T, Ponsaerts R, Dewaele M, Molgo J, et al. Ins(1,4,5)P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy. 2011;7:1472–89. doi: 10.4161/auto.7.12.17909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicencio JM, Ortiz C, Criollo A, Jones AW, Kepp O, Galluzzi L, et al. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 2009;16:1006–17. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- Criollo A, Vicencio JM, Tasdemir E, Maiuri MC, Lavandero S, Kroemer G. The inositol trisphosphate receptor in the control of autophagy. Autophagy. 2007;3:350–3. doi: 10.4161/auto.4077. [DOI] [PubMed] [Google Scholar]
- Hou H, Zhang Y, Huang Y, Yi Q, Lv L, Zhang T, et al. Inhibitors of phosphatidylinositol 3′-kinases promote mitotic cell death in HeLa cells. PLOS ONE. 2012;7:e35665. doi: 10.1371/journal.pone.0035665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castino R, Bellio N, Follo C, Murphy D, Isidoro C. Inhibition of PI3k class III — dependent autophagy prevents apoptosis and necrosis by oxidative stress in dopaminergic neuroblastoma cells. Toxicol Sci. 2010;117:152–62. doi: 10.1093/toxsci/kfq170. [DOI] [PubMed] [Google Scholar]
- Miller S, Tavshanjian B, Oleksy A, Perisic O, Houseman BT, Shokat KM, et al. Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps34. Science. 2010;327:1638–42. doi: 10.1126/science.1184429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1982;79:1889–92. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli AM, Chiarini F, Evangelisti C, Cappellini A, Buontempo F, Bressanin D, et al. Two hits are better than one: targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment. Oncotarget. 2012;3:371–94. doi: 10.18632/oncotarget.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuka C, Inoue Y, Matsuoka K, Moriyasu Y. 3-Methyladenine inhibits autophagy in tobacco culture cells under sucrose starvation conditions. Plant Cell Physiol. 2004;45:265–74. doi: 10.1093/pcp/pch031. [DOI] [PubMed] [Google Scholar]
- Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, et al. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285:10850–61. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright NA, Lindsay MR, Stewart A, Luzio JP. The relationship between lumenal and limiting membranes in swollen late endocytic compartments formed after wortmannin treatment or sucrose accumulation. Traffic. 2001;2:631–42. doi: 10.1034/j.1600-0854.2001.20906.x. [DOI] [PubMed] [Google Scholar]
- Xing C, Zhu B, Liu H, Yao H, Zhang L. Class I phosphatidylinositol 3-kinase inhibitor LY294002 activates autophagy and induces apoptosis through p53 pathway in gastric cancer cell line SGC7901. Acta Biochim Biophys Sin (Shanghai) 2008;40:194–201. doi: 10.1111/j.1745-7270.2008.00393.x. [DOI] [PubMed] [Google Scholar]
- Ethier MF, Madison JM. LY294002, but not wortmannin, increases intracellular calcium and inhibits calcium transients in bovine and human airway smooth muscle cells. Cell Calcium. 2002;32:31–8. doi: 10.1016/s0143-4160(02)00111-2. [DOI] [PubMed] [Google Scholar]
- Rez G, Kovacs J. Prevention by cycloheximide of cellular autophagy induced by hyperosmotic sucrose or cadmium chloride in mouse pancreatic acinar cells. Acta Biol Acad Sci Hung. 1973;24:201–5. [PubMed] [Google Scholar]
- Kovacs J. Regression of autophagic vacuoles in seminal vesicle cells following cycloheximide treatment. Exp Cell Res. 1983;144:231–4. doi: 10.1016/0014-4827(83)90460-3. [DOI] [PubMed] [Google Scholar]
- Oliva O, Rez G, Palfia Z, Fellinger E. Dynamics of vinblastine-induced autophagocytosis in murine pancreatic acinar cells: influence of cycloheximide post-treatments. Exp Mol Pathol. 1992;56:76–86. doi: 10.1016/0014-4800(92)90025-7. [DOI] [PubMed] [Google Scholar]
- Machiya Y, Hara S, Arawaka S, Fukushima S, Sato H, Sakamoto M, et al. Phosphorylated alpha-synuclein at Ser-129 is targeted to the proteasome pathway in a ubiquitin-independent manner. J Biol Chem. 2010;285:40732–44. doi: 10.1074/jbc.M110.141952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence BP, Brown WJ. Inhibition of protein synthesis separates autophagic sequestration from the delivery of lysosomal enzymes. J Cell Sci. 1993;105:473–80. doi: 10.1242/jcs.105.2.473. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- Wu YC, Wu WK, Li Y, Yu L, Li ZJ, Wong CC, et al. Inhibition of macroautophagy by bafilomycin A1 lowers proliferation and induces apoptosis in colon cancer cells. Biochem Biophys Res Commun. 2009;382:451–6. doi: 10.1016/j.bbrc.2009.03.051. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes. Autophagy. 2008;4:849–950. doi: 10.4161/auto.6845. [DOI] [PubMed] [Google Scholar]
- Chen G, Ke Z, Xu M, Liao M, Wang X, Qi Y, et al. Autophagy is a protective response to ethanol neurotoxicity. Autophagy. 2012;8:1577–89. doi: 10.4161/auto.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengjel J, Hoyer-Hansen M, Nielsen MO, Eisenberg T, Harder LM, Schandorff S, et al. Identification of autophagosome-associated proteins and regulators by quantitative proteomic analysis and genetic screens. Mol Cell Proteomics. 2012;11:M111.014035. doi: 10.1074/mcp.M111.014035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhaji-Trajkovic L, Arsikin K, Kravic-Stevovic T, Petricevic S, Tovilovic G, Pantovic A, et al. Chloroquine-mediated lysosomal dysfunction enhances the anticancer effect of nutrient deprivation. Pharm Res. 2012;29:2249–63. doi: 10.1007/s11095-012-0753-1. [DOI] [PubMed] [Google Scholar]
- Amaravadi RK, Winkler JD. Lys05: A new lysosomal autophagy inhibitor. Autophagy. 2012;8:1383–4. doi: 10.4161/auto.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee Q, Zhang Z, Samanta A, Levi SM, Ma XH, Piao S, et al. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc Natl Acad Sci U S A. 2012;109:8253–8. doi: 10.1073/pnas.1118193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyasu Y, Inoue Y. Use of protease inhibitors for detecting autophagy in plants. Methods Enzymol. 2008;451:557–80. doi: 10.1016/S0076-6879(08)03232-1. [DOI] [PubMed] [Google Scholar]
- Kominami E, Hashida S, Khairallah EA, Katunuma N. Sequestration of cytoplasmic enzymes in an autophagic vacuole-lysosomal system induced by injection of leupeptin. J Biol Chem. 1983;258:6093–100. [PubMed] [Google Scholar]
- Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, et al. Dissecting the dynamic turnover of GFP-LC3 in the autolysosome. Autophagy. 2011;7:188–204. doi: 10.4161/auto.7.2.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–6. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- Christian F, Anthony DF, Vadrevu S, Riddell T, Day JP, McLeod R, et al. p62 (SQSTM1) and cyclic AMP phosphodiesterase-4A4 (PDE4A4) locate to a novel, reversible protein aggregate with links to autophagy and proteasome degradation pathways. Cell Signal. 2010;22:1576–96. doi: 10.1016/j.cellsig.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Ramser B, Kokot A, Metze D, Weiss N, Luger TA, Bohm M. Hydroxychloroquine modulates metabolic activity and proliferation and induces autophagic cell death of human dermal fibroblasts. J Invest Dermatol. 2009;129:2419–26. doi: 10.1038/jid.2009.80. [DOI] [PubMed] [Google Scholar]
- Oikarinen A. Hydroxychloroquine induces autophagic cell death of human dermal fibroblasts: implications for treating fibrotic skin diseases. J Invest Dermatol. 2009;129:2333–5. doi: 10.1038/jid.2009.164. [DOI] [PubMed] [Google Scholar]
- Amaravadi RK, Winkler JD. Lys05: a new lysosomal autophagy inhibitor. Autophagy. 2012;8:1383–4. doi: 10.4161/auto.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson N, Jr, Dennis PA, Dunn WA. Metabolism of leupeptin and its effect on autophagy in the perfused rat liver. Acta Biol Med Ger. 1981;40:1531–8. [PubMed] [Google Scholar]
- Dong XX, Wang YR, Qin S, Liang ZQ, Liu BH, Qin ZH, et al. p53 Mediates autophagy activation and mitochondria dysfunction in kainic acid-induced excitotoxicity in primary striatal neurons. Neuroscience. 2012;207:52–64. doi: 10.1016/j.neuroscience.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Tanida I. Autophagy basics. Microbiol Immunol. 2011;55:1–11. doi: 10.1111/j.1348-0421.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- Frankel LB, Wen J, Lees M, Hoyer-Hansen M, Farkas T, Krogh A, et al. microRNA-101 is a potent inhibitor of autophagy. EMBO J. 2011;30:4628–41. doi: 10.1038/emboj.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yang L, Zhao M, Zhu S, Kang R, Vernon P, et al. Targeting microRNA-30a-mediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells. Leukemia. 2012;26:1752–60. doi: 10.1038/leu.2012.65. [DOI] [PubMed] [Google Scholar]
- Yuya N, Satoko A, Kenji F, Hirofumi Y, Takeshi M, Toku K, et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–8. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J. Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy. 2011;7:1115–31. doi: 10.4161/auto.7.10.16608. [DOI] [PubMed] [Google Scholar]
- Beau I, Mehrpour M, Codogno P. Autophagosomes and human diseases. Int J Biochem Cell Biol. 2011;43:460–4. doi: 10.1016/j.biocel.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Bursch W EA, Gerner C SR.(2004). Autophagocytosis and programmed cell death. In: Klionsky DJ, editor. Autophagy. Georgetown, TX: Landes Bioscience, 2004p287–303.
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–5. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel A, Zentgraf H, Buchler MW, Herr I. Autophagy-A double-edged sword in oncology. Int J Cancer. 2009;125:991–5. doi: 10.1002/ijc.24500. [DOI] [PubMed] [Google Scholar]
- Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–34. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xiong X, Sun Y. DEPTOR, an mTOR inhibitor, is a physiological substrate of SCF(betaTrCP) E3 ubiquitin ligase and regulates survival and autophagy. Mol Cell. 2011;44:304–16. doi: 10.1016/j.molcel.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–32. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci. 2011;1215:22–33. doi: 10.1111/j.1749-6632.2010.05843.x. [DOI] [PubMed] [Google Scholar]
- Li CY, Wang EQ, Cheng Y, Bao JK. Oridonin: An active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int J Biochem Cell Biol. 2011;43:701–4. doi: 10.1016/j.biocel.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Cao BY, Yang YP, Luo WF, Mao CJ, Han R, Sun X, et al. Paeoniflorin, a potent natural compound, protects PC12 cells from MPP+ and acidic damage via autophagic pathway. J Ethnopharmacol. 2010;131:122–9. doi: 10.1016/j.jep.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Yang S, Xiao X, Meng X, Leslie KK. A mechanism for synergy with combined mTOR and PI3 kinase inhibitors. PLOS One. 2011;6:e26343. doi: 10.1371/journal.pone.0026343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Krishna G, Imarisio S, Saiki S, O'Kane CJ, Rubinsztein DC. A rational mechanism for combination treatment of Huntington's disease using lithium and rapamycin. Hum Mol Genet. 2008;17:170–8. doi: 10.1093/hmg/ddm294. [DOI] [PubMed] [Google Scholar]