Abstract
Bisphenol A (BPA) is a monomer used in manufacturing a wide range of chemical products, including epoxy resins and polycarbonate. BPA, an important endocrine disrupting chemical that exerts estrogen-like activities, is detectable at nanomolar levels in human serum worldwide. The pregnancy associated doses of 17β-estradiol (E2) plus tumor-necrosis factor-α (TNF-α) induce distorted maturation of human dendritic cells (DCs) that result in an increased capacity to induce T helper (Th) 2 responses. The current study demonstrated that the presence of BPA during DC maturation influences the function of human DCs, thereby polarizing the subsequent Th response. In the presence of TNF-α, BPA treatment enhanced the expression of CC chemokine ligand 1 (CCL1) in DCs. In addition, DCs exposed to BPA/TNF-α produced higher levels of IL-10 relative to those of IL-12p70 on CD40 ligation, and preferentially induced Th2 deviation. BPA exerts the same effect with E2 at the same dose (0.01–0.1 µΜ) with regard to DC-mediated Th2 polarization. These findings imply that DCs exposed to BPA will provide one of the initial signals driving the development and perpetuation of Th2-dominated immune response in allergic reactions.
Keywords: bisphenol A, dendritic cells, Th1/2, human
Introduction
There has recently been a significant increase in the prevalence of allergic diseases, including food allergies. Several factors characteristic of modern lifestyles that may originate from the chemical, physical, biological or psychosocial environment have been proposed to play a role in the development of allergies.1, 2 The hormone-like effects of environmental chemicals have been shown to interfere with reproduction and hormonal regulation in wildlife and humans.3, 4
Bisphenol A (BPA) is a widespread endocrine disrupting chemical that may have adverse effects on human health.5 It is a monomer of polycarbonate plastics used in many consumer products, including beverage bottles, canned food liners (50–100 nM) and epoxy dental sealants.6 Small amounts of BPA can migrate from polymers to food or water, especially when heated.7 Levels of BPA ranging from 1 to 20 nM are present in adult and fetal human plasma, urine and breast milk.8
BPA has weak estrogen activity approximately 1/1000- to 1/10 000-fold of 17β-estradiol (E2),9 and interacts with classical estrogen receptors alpha (ER-α) and beta (ER-β)10 or with non-classical G-protein coupled receptor 30 (GPR30).11 Although BPA induces target genes in both ER-dependent and ER-independent manners,12, 13 the mechanism by which BPA exerts its biological actions has not yet been fully elucidated.
BPA may be a potentially important modulator of immune responses.14, 15, 16 BPA was reported to enhance IL-4 production by T helper (Th) 2 cells in vitro while also enhancing the Th2 and Th1 immune responses, depending on the doses of BPA administered in vivo.17, 18 These experimental studies have indicated that BPA may help trigger allergic responses. However, the precise mechanism by which BPA regulates the Th responses has remained elusive.
Dendritic cells (DCs) play a key role in adaptive immunity, because they represent the most potent antigen-presenting cells and are able to activate naive T cells.19 Depending on the type of pathogen encountered and the profile of costimulatory and T-cell-polarizing molecules, DCs drive the development of Th1, Th2 or Th17 cells.20, 21, 22, 23 Several DC-derived molecules with Th-polarizing potential have been identified.24 IL-12 plays a central role in promoting the differentiation of naive CD4+ T cells into mature Th1 effector cells.25 In contrast, IL-10 has been suggested to downregulate the DC-derived IL-12 production, thus leading to a Th2 differentiation.26 Transforming growth factor-β in combination with IL-1β, IL-6 and IL-21 was identified to be a critical factor in human Th17 differentiation.27 However, during DC maturation, environmental substances such as pollen-associated phytoprostanes, diesel exhaust particles or formaldehyde have been demonstrated to have a great impact on their functions.28, 29, 30 The expression patterns of Th-polarizing molecules following maturation stimuli depend on the environment of the host individuals, thereby resulting in a specifically optimized pathogen–host balance which may occasionally result in allergic diseases.
Pregnancy-associated doses of E2 plus tumor-necrosis factor-α (TNF-α) induce distorted maturation of human DCs that result in an increased capacity to induce Th2 responses.31 Because BPA is an important endocrine-disrupting chemical that exerts estrogen-like activities, it is of interest to study whether BPA can directly modulate the DC function like estrogen. To date, the effect of BPA on human DCs and their downstream Th response is still not fully understood.
The present study shows that DCs exposed to BPA in combination with TNF-α enhance the CC chemokine ligand 1 (CCL1), a chemokine that is known to trigger chemotaxis of CCR8-expressing Th2 and a subset of T regulatory cells. In addition, they produce higher levels of IL-10 relative to those of IL-12p70 on CD40 ligation, and preferentially induce Th2 deviation.
Materials and methods
Antibodies and reagents
Recombinant human (rh) IL-4, rh granulocyte–macrophage colony-stimulating factor (GM-CSF) and rhTNF-α were purchased from Primmune (Osaka, Japan). Anti-CD3 (HIT3a) and anti-CD28 (CD28.2) were obtained from BD PharMingen (San Diego, CA, USA). Anti-CD14 and anti-CD45RO microbeads were from Miltenyi Biotec (Bergisch, Gladbach, Germany). CD40L-transfected L-fibroblast cells were kindly provided by Dr Y. J. Liu (M. D. Anderson Cancer Center, Houston, TX, USA).
Generation of immature DCs and their induction of maturation
Buffy coats of blood were obtained from healthy volunteers (Chinese PLA General Hospital, Beijing, China) and peripheral blood mononuclear cells were isolated by density gradient centrifugation. CD14+ monocytes were isolated from peripheral blood mononuclear cells by positive magnetic sorting using CD14 microbeads and cultured at 1.0×106 cells/ml in phenol red-free RPMI1640 supplemented with 5% dextran-coated charcoal-treated human serum in the presence of rhGM-CSF and rhIL-4 (50 ng/ml each). On days 2 and 3, the DC cultures received an additional dose of rhGM-CSF and rhIL-4 (50 ng/ml each). On day 6, non-adherent DCs were harvested and served as immature DCs. Further differentiation into mature DCs was induced by treatment for 48 h with the following factors alone or with a combination as indicated in the text: 25 µg/ml poly I∶C (Invivogen, San Diego, CA, USA), 1 µg/ml cholera toxin (Sigma, St Louis, MO, USA), E2 (Sigma), BPA (Sigma), 0.1 µΜ ICI 182,780 (ICI; Tocris Bioscience, Ellisville, MI, USA) and 20 ng/ml TNF-α.
Mixed leukocyte reaction and Th differentiation
Allogeneic CD4+CD45RO− (naive) Th cells were isolated from peripheral blood mononuclear cells by negative magnetic cell sorting using a CD4+ T Cell Isolation Kit II (Miltenyi Biotec) and CD45RO microbeads. Differentially conditioned DCs were irradiated (3000 cGy) and cultured with naive Th cells at a DC/T ratio of 1∶5. After 5 days of culture, the cells were pulsed with [3H]-thymidine (1 µCi/well) for 16 h and the proliferative responses were measured by [3H]-thymidine incorporation. For cytokine production, after 9 days of culture, Th cells were restimulated with plate-bound anti-CD3 monoclonal antibody (mAb, 10 µg/ml) and soluble anti-CD28 mAb (1 µg/ml). After 24 h, the levels of Th cytokines in the culture supernatants were evaluated with ELISA.25
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Genomic DNA was digested and removed using an RNase-Free DNase Kit (Qiagen). First strand cDNA was synthesized using oligo (dT)12–18 primer (Invitrogen, Carlsbad, CA, USA) and the Omniscript RT Kit (Qiagen). The synthesized cDNA was thermocycled for PCR amplification with 1 µΜ each primer and 1.5 U of Taq polymerase. The primers for amplification and the sizes of respective PCR products were: ER-α, 5′-AAGAGCTGCCAGGCCTGCC-3′ and 5′-TTGGCAGCTCTCATGTCTCC-3′ for 167 bp; ER-β, 5′-GCATGGAACATCTGCTCAAC-3′ and 5′-ACGCTTCAGCTTGTGACCTC-3′ for 228 bp. The GPR30 specific primers were designed according to the GPR30 sequence (NM_001505): GPR30, 5′-GAAGCGGCGAGTGAAAAT-3′ and 5′-TGGTTTGGGTTGGGTTTG-3′ for 366 bp. After an initial 10-min denaturation step at 95 °C, 40 cycles of PCR carried out on GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA) under the following conditions: 30 s denaturation at 95 °C, 1 min annealing at 60 °C for ER-α or 58 °C for ER-β and GPR30, 1 min extension at 72 °C, followed by a 5-min final extension at 72 °C. The primers for 21 different chemokines and PCR condition were previously described.31 As a positive control, β-actin cDNA was amplified by using specific primers as previously described.32
Real-time quantitative RT-PCR
The transcripts were quantified by real-time quantitative PCR using an ABI PRISM 7500 Sequence Detector (Applied Biosystems) with Applied Biosystems predesigned TaqMan Gene Expression Assays and reagents according to the manufacturer's instructions. The gene expression in the samples was normalized by comparing it with the glyceraldehyde-3-phosphate dehydrogenase mRNA expression using the ddCT method as previously described.33
Measurement of cytokines
The cytokine levels in the culture supernatants were evaluated with ELISA (human IL-5, IL-10, IL-12p70, IL-13, interferon (IFN)-γ and CCL1; R&D systems, Minneapolis, MN, USA).
Statistical analysis
The statistical significance of the differences between the groups of continuous variables was analyzed using the Mann-Whitney U test. A P value of <0.05 was considered to be statistically significant.
Results
Monocyte-derived dendritic cells (Mo-DCs) express E2-related receptors
Steroid hormone-reduced medium that was composed of dextran-coated charcoal-treated human serum in phenol red-free RPMI was used throughout the study to investigate the direct effect of BPA on the function of human Mo-DCs. The use of phenol red-free medium excludes the weak estrogen-like activity of phenol red.31 Mo-DCs were assessed for the presence of ER-α, ER-β or GPR30 mRNA using RT-PCR to determine the expression of E2-related receptors. Mo-DCs expressed mRNA for ER-α, ER-β and GPR30 (Figure 1), thus indicating that Mo-DCs may be directly subjected to regulation by BPA.
Figure 1.
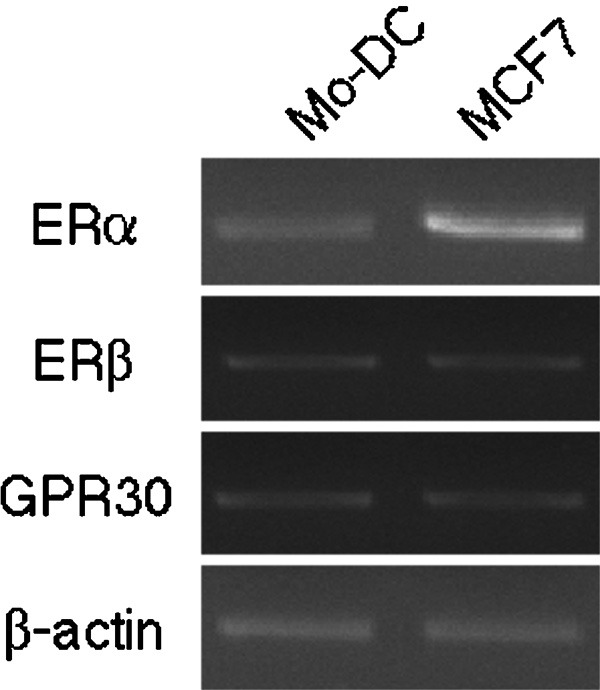
The expression of ER-α, ER-β and GPR30 mRNA in DCs. The expression of mRNA for ER-α, ER-β and GPR30 were analyzed by RT-PCR. cDNA from MCF-7 breast cancer cells were used as positive controls. β-actin was used as an internal positive control. The constitutively expressed β-actin is shown in the bottom panel. DC, dendritic cell; ER, estrogen receptor; GPR30, G-protein coupled receptor 30; Mo-DC, monocyte-derived dendritic cell; RT-PCR, reverse transcription-polymerase chain reaction.
BPA does not affect the maturation of DCs
Six days of culturing CD14+ monocytes with IL-4 and GM-CSF in the dextran-coated charcoal-treated human serum medium induced the cells to acquire a typical immature DC phenotype, that is, human leucocyte antigen-DR+, CD40+, CD80+, CD83low, CD86low and CD1ahigh (Figure 2a). The presence of BPA enhanced the expression of human leucocyte antigen-DR and CD1a in DCs. In contrast, the presence of ICI 182,780 (ICI: a specific antagonist for ERs) reduced the surface expression of these molecules (Figure 2a). However, BPA or BPA plus ICI had no effect on the surface expression of CD83 and CD86 in the presence of TNF-α, which is a maturation-inducing factor of DCs (data not shown). In addition, the allostimulatory capacity was not affected at all (Figure 2b).
Figure 2.
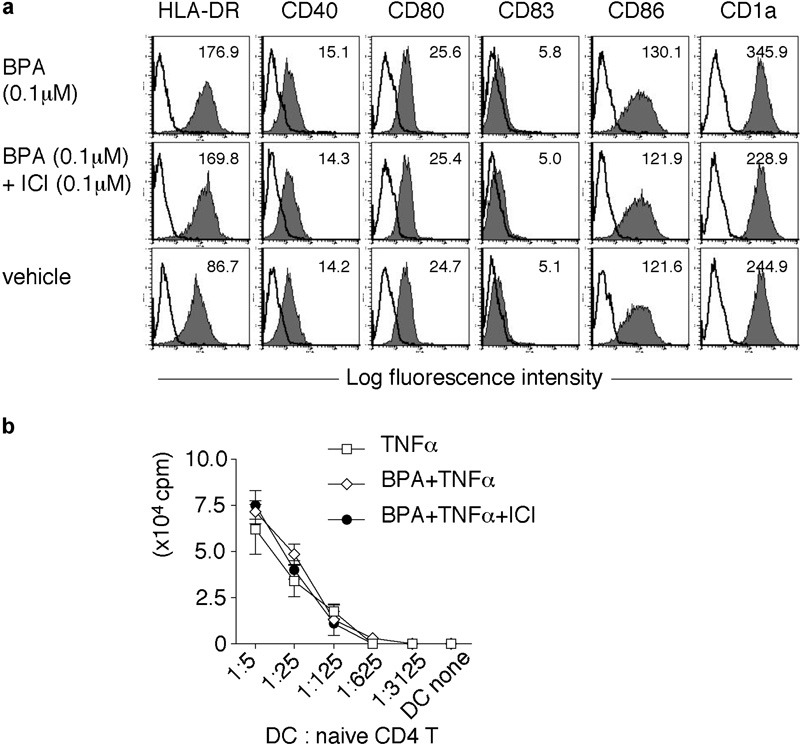
Characterization of BPA-exposed DCs. (a) The surface phenotype of BPA-treated immature DCs. DCs were treated for 24 h with BPA (0.1 µΜ: top panels), BPA (0.1 µΜ) plus ICI (0.1 µΜ: middle panels) or vehicle (1/1 000 000 vol ethanol: bottom panels) and were stained with mAbs against the indicated surface molecules (filled histogram) or with isotype control antibodies (open histogram). The MFI of each histogram is shown at the top of each panel. The data are representative of three separate experiments. (b) The allostimulatory activity of BPA/TNF-α-exposed DCs. DCs were treated for 24 h with vehicle, BPA (0.1 µΜ), or BPA (0.1 µΜ) plus ICI (0.1 µΜ) in the presence of TNF-α (20 ng/ml). Differentially conditioned DCs were cultured with allogeneic naive Th cells (5.0×104) for 5 days. The proliferative responses were assessed by [3H]-thymidine incorporation. The data represent the mean±SD of triplicate cultures. BPA, bisphenol A; DC, dendritic cell; HLA-DR, human leucocyte antigen-DR; ICI, ICI 182,780; mAb, monoclonal antibody; MFI, mean fluorescence intensity; Th, T helper; TNF, tumor-necrosis factor.
Enhanced CCL1 production by BPA/TNF-α DCs
The synthesis and release of chemokines and cytokines with important modulatory function on inflammation and T-cell differentiation is a major attribute of mature DCs. DCs were treated with vehicle (1/1 000 000 vol ethanol), BPA (0.1 µΜ), or BPA (0.1 µΜ) plus ICI (0.1 µΜ) in the presence of TNF-α (20 ng/ml) for 24 h and then they were extensively screened for 21 different chemokines by semiquantitative RT-PCR (CCL1/I-309, CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES, CCL17/TARC, CCL18/PARC, CCL19/ELC, CCL20/LARC, CCL21/SLC, CCL22/MDC, CCL25/TECK, CXCL8/IL-8, CXCL9/MIG, CXCL10/IP-10, CXCL11/I-TAC, CXCL12/SDF1, CXCL13/BLC, XCL1/lymphotactin, CX3CL1/fractalkine and CXCL16). The level of CCL1 mRNA specifically increased in response to BPA and the expression was then completely abrogated in the presence of ICI (data not shown). Next, the expression of CCL1 mRNA was investigated by real-time quantitative RT-PCR (Figure 3a and b). The CCL1 mRNA expression was robustly induced within 3 h of TNF-α stimulation alone and promptly disappeared. Although the presence of BPA on TNF-α stimulation showed similar patterns to TNF-α stimulation, the BPA enhanced the CCL1 mRNA expression and sustained the transcript abundance more. The expression level in 0.1 µΜ BPA/TNF-α DCs was the highest among the BPA concentrations tested (0.001, 0.01, 0.1 and 1 µΜ) and was twofold higher than that of TNF-α DCs (Figure 3a). An ELISA analysis confirmed that BPA (0.1 µΜ) enhanced the TNF-α-induced CCL1 production at the protein level (Figure 3c and d). The enhancing effect of BPA plus TNF-α on the release of CCL1 continued for 72 h whereas the release of CCL1 was limited to 48 h in stimulation with TNF-α alone (Figure 3c). The CCL1 production induced by TNF-α was enhanced in the presence of either BPA or E2, and that was completely abrogated by the addition of ICI (Figure 3c and d). The addition of ICI, BPA, or BPA plus ICI had little effect on CCL1 production in the absence of TNF-α (data not shown). These findings indicate that the enhanced CCL1 production induced by BPA is an estrogen-like activity in TNF-α-stimulated DCs. The TNF-α-induced CCL1 production was completely abrogated by the addition of ICI, even in the absence of BPA or E2 (Figure 3d). It is conceivable that ER activation can occur through pathways other than the classical ER activation pathway in TNF-α-stimulated DCs. Therefore, the marked inhibitory effect of ICI on TNF-α-induced CCL1 production may be due to the ICI-mediated inactivation of ER.
Figure 3.

Effect of BPA on the chemokine expression. (a,b) The kinetics of the CCL1 mRNA expression. The levels of CCL1 mRNA were determined by real-time PCR. The amounts of CCL1 mRNA are shown by arbitrary units relative to the amounts of glyceraldehyde-3-phosphate dehydrogenase mRNA. (a) DCs were treated with TNF-α plus BPA (0, 0.001, 0.01, 0.1 or 1 µM) for 0, 3, 6, 12, 24 and 48 h. (b) DCs were treated with vehicle, BPA (0.1 µM), or BPA (0.1 µM) plus ICI (0.1 µM) in the presence of TNF-α (20 ng/ml). (c) The kinetics of the CCL1 production evaluated with ELISA. DCs (5.0×104) were treated with vehicle, BPA (0.1 µM), or BPA (0.1 µM) plus ICI (0.1 µM) in the presence of TNF-α (20 ng/ml). The culture supernatants were collected at the indicated time points. (d) DCs (5.0×104) were treated with vehicle, BPA (0.1 µM), E2 (0.1 µM), ICI (0.1 µM), E2 (0.1 µM) plus ICI (0.1 µM), or BPA (0.1 µM) plus ICI (0.1 µM) in the presence of TNF-α (20 ng/ml) for 48 h. The data represent the mean±SD of triplicate cultures. *P<0.05. BPA, bisphenol A; CCL, CC chemokine ligand; DC, dendritic cell; E2, 17β-estradiol; ICI, ICI 182,780; PCR, polymerase chain reaction; TNF, tumor-necrosis factor.
Enhanced production of IL-10 relative to IL-12p70 by BPA/TNF-α DCs on CD40L ligation
The effect of the BPA pretreatment on IL-10 and the IL-12 production in DCs stimulated with CD40L-transfected L cells was examined. DCs were pretreated with vehicle, BPA (0.1 µΜ), or BPA (0.1 µΜ) plus ICI (0.1 µΜ) in the absence or presence of TNF-α (20 ng/ml). After the pretreatment (24 h), the differentially conditioned DCs were washed and then stimulated with CD40L-transfected L cells (48 h). This system mimics the CD40 engagement by CD40L on Th cells (Figure 4). The pattern of cytokines produced on CD40 ligation is an important determinant of Th1/2 differentiation. The presence of TNF-α in the pretreatment reduced the levels of IL-10 and IL-12 on CD40 ligation (Figure 4a and b). This finding is consistent with the reported observation that the pretreatment of DCs with maturation factors reduced the cytokine production on secondary stimulation.34, 35 The pretreatment with BPA alone significantly enhanced the production of both IL-10 and IL-12p70 (Figure 4a and b). In contrast, the pretreatment with BPA plus ICI inhibited the production of these cytokines. Although TNF-α-pretreated DCs showed the reduced production of both IL-10 and IL-12p70 on CD40 ligation, the pretreatment of BPA plus TNF-α revealed higher IL-10/IL-12p70 ratios than those of the DCs tested (Figure 4c).
Figure 4.
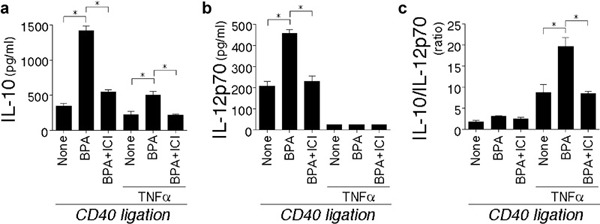
Effect of BPA treatment on DC function. DCs were cultured for 24 h with BPA (0.1 µM), BPA (0.1 µM) plus ICI (0.1 µM), or vehicle in the presence or absence of TNF-α (20 ng/ml). Subsequently, differentially conditioned DCs (5.0×104) were cocultured with CD40L-transfected L cells (1.0×104). (a) IL-10 and (b) IL-12p70 production of DCs cultured for 48 h with CD40L-transfected L cells. (c) The relative IL-10/IL-12p70 ratios in (a) and (b) are shown. The data are representative of three separate experiments from different individuals. The data represent the mean±SD of triplicate cultures. *P<0.05. BPA, bisphenol A; DC, dendritic cell; ICI, ICI 182,780; TNF, tumor-necrosis factor.
Enhanced ability of BPA/TNF-α DCs to induce the development of Th2 cells
After recognizing the antigenic peptides on DCs, naive T lymphocytes proliferate and differentiate into a variety of effector cells depending on the stimulatory conditions and cytokine milieu.24 Developing Th1 cells acquire the capacity to produce IFN-γ. Conversely, developing Th2 cells acquire the capacity to produce IL-4, IL-5 and IL-13. IL-12p70 released by mature DCs is the most important factor that drives the differentiation of naive T cells toward the Th1 phenotype. Given the increased production of IL-10 relative to IL-12p70 by BPA/TNF-α-matured DCs on CD40 ligation, the quality of primary T-cell response induced by DCs matured in the presence of BPA was examined. DCs were treated with vehicle, BPA, or BPA plus ICI (0.1 µΜ) in the presence of TNF-α (20 ng/ml) for 24 h and subsequently cultured with allogeneic naive Th cells. After 9 days of culture, the cytokine production (IFN-γ, IL-5, IL-10 and IL-13) of the Th cells was analyzed (Figures 5 and 6). The Th2 cytokine-producing capacities in differentiated Th cells gradually increased depending on the doses of BPA (0.001–0.1 µΜ) in the DC maturation cultures, except for the high dose of BPA (>1 µΜ Figure 5). Naive Th cells primed by BPA/TNF-α-matured DCs differentiated into Th2 cells with the characteristic of high IL-5/IFN-γ, IL-10/IFN-γ and IL-13/IFN-γ ratios (Figure 6e–g). However, the IFN-γ production did not change among the culture conditions tested (Figure 6a). The Th2 shift induced by the BPA/TNF-α-matured DCs was markedly abrogated when the DCs were matured in the presence of ICI (Figure 6b–g). The Th2-inducing capacities in BPA/TNF-α-matured DCs was similar to those of E2 (0.1 µΜ)/TNF-α-matured DCs.
Figure 5.
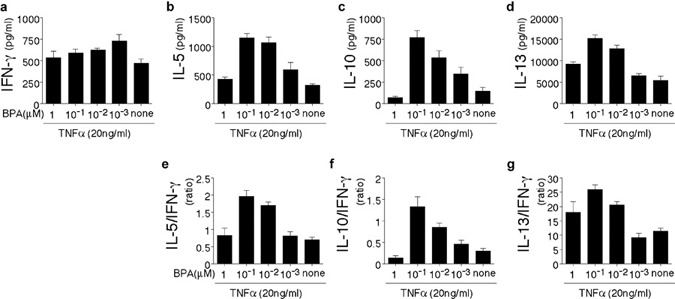
DCs matured in the presence of a high concentration of BPA (0.1 µM) enhance the development Th2 cells. DCs were treated for 24 h with the indicated doses of BPA in the presence of TNF-α (20 ng/ml). The differentially conditioned DCs were subsequently cultured with allogeneic naive Th cells for 9 days. The differentiated Th cells were restimulated with plate-bound anti-CD3 and soluble anti-CD28 mAbs for 24 h and the cytokine levels in the culture supernatants were measured with ELISA. (a–d) The absolute amounts of secreted cytokines (IFN-γ, IL-5, IL-10 and IL-13). (e–g) Relative IL-5/IFN-γ, IL-10/IFN-γ and IL-13/IFN-γ ratios are shown. The data are representative of three separate experiments from different individuals. The data represent the mean±SD of triplicate cultures. BPA, bisphenol A; DC, dendritic cell; IFN, interferon; mAb, monoclonal antibody; Th, T helper; TNF, tumor-necrosis factor.
Figure 6.
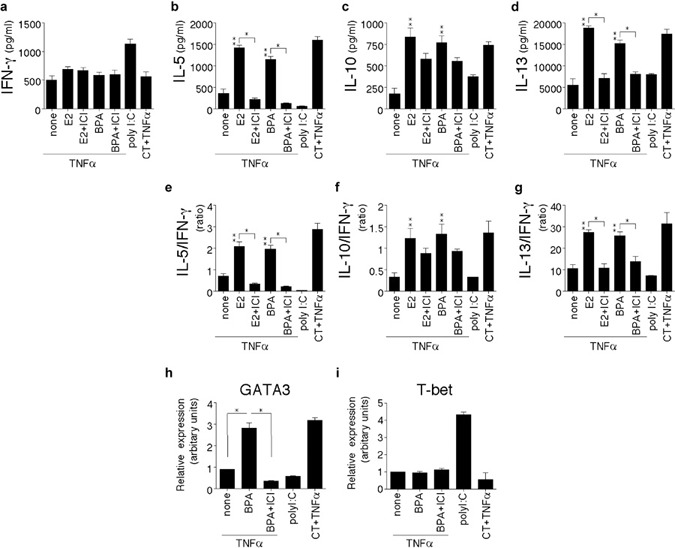
DCs matured in the presence of BPA show an intrinsic ability to induce the development Th2 cells. (a–g) DCs were treated with vehicle, E2 (0.1 µM), BPA (0.1 µM), BPA (0.1 µM) plus ICI (0.1 µM), or E2 (0.1 µM) plus ICI (0.1 µM) in the presence of TNF-α (20 ng/ml) for 24 h. The differentially conditioned DCs were subsequently cultured with allogeneic naive Th cells for 9 days. The differentiated Th cells were restimulated with plate-bound anti-CD3 and soluble anti-CD28 mAbs for 24 h and the cytokine levels in the culture supernatants were measured with ELISA. (a–d) The absolute amounts of secreted cytokines (IFN-γ, IL-5, IL-10 and IL-13). (e–g) Relative IL-5/IFN-γ, IL-10/IFN-γ and IL-13/IFN-γ ratios are shown. (h,i) The expression of transcriptional factors involved in Th1 and Th2 differentiation. DCs were treated with vehicle, BPA (0.1 µM), or BPA (0.1 µM) plus ICI (0.1 µM) in the presence of TNF-α (20 ng/ml) for 24 h. The differentially conditioned DCs were subsequently cultured with allogeneic naive Th cells for 7 days. GATA-3 and T-bet were quantitatively measured by real-time PCR in the Th cells. The amounts of mRNA were shown in arbitrary units relative to the amount of GAPDH mRNA. Data are representative of three separate experiments. The data represent the mean±SD of triplicate cultures. ** or *P<0.05 (**: E2 or BPA, P versus none control; a–i). Poly I∶C DCs (control for Th1 polarization) and CT/TNF-α DCs (control for Th2 polarization) served as a reference for the other culture conditions. BPA, bisphenol A; CT, cholera toxin; DC, dendritic cell; E2, 17β-estradiol; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ICI, ICI 182,780; IFN, interferon; mAb, monoclonal antibody; PCR, polymerase chain reaction; Th, T helper; TNF, tumor-necrosis factor.
The expression of the transcription factors GATA-3 and T-bet is critical for the differentiation of naive T cells into Th1 and Th2. The expression of GATA-3 and T-bet was examined by quantitative PCR in Th cells primed by BPA/TNF-α-DCs (Figure 6h and i). The GATA3 expression induced by TNF-α was enhanced in the presence of BPA, and that was completely abrogated by the addition of ICI. However, the T-bet expression did not change among the culture conditions tested. These findings suggest that the BPA elicits the generation of Th2 cells and this effect is mediated by DCs.
Discussion
This study, for the first time, provided evidence that BPA directly influences the function of human DCs, thereby influencing the subsequent Th response. BPA/TNF-α DCs revealed an improved ability to produce CCL1. In addition, BPA/TNF-α DCs increased the production of IL-10 relative to the IL-12p70 after CD40 ligation, while also inducing naive T-cell differentiation into the Th2 lineage. Moreover, the increased doses of BPA in the route of maturation did indeed further enhance Th2 differentiation.
The stimulatory effects of BPA on human DCs observed in this study may be at least in part explained by its estrogen-like action. Dominant Th2 differentiation is induced at a high E2 concentration (0.1 µΜ) typical during pregnancy.31 The relative binding affinity of BPA to ER-α or ER-β has known to be 1000–10 000 times lower than that of E2, indicating that BPA should activate these receptors only at higher doses.36 However, BPA exerts the same effect with E2 at the same dose (0.01–0.1 µΜ) with regard to DC-mediated Th2 differentiation (Figure 6). The precise mechanism by which BPA exposure resulted in the augmentation of Th2 differentiation to an extent equal to that of E2 exposure is unknown. The expression of multiple types of E2 receptor by DCs complicates the identification of the precise mechanism mediating the BPA effect on DC response. It is conceivable that: (i) BPA binds differently within the ligand-binding domain of ER-α or ER-β and recruits dissimilar coregulators;37 or (ii) BPA elicits rapid responses by binding to membrane-anchored ERs,38 an as-yet-unidentified non-classical membrane ER39 or GPR30.40 Although the effect of BPA on each E2 receptors is different from that of E2, the combined effect of BPA signals via each E2 receptor may be similar to that of E2 signals.
CCL1 production was induced by TNF-α stimulation alone and the CCL1-producing capacity was enhanced by either BPA or E2 (Figure 3d). In contrast, the addition of ICI completely abrogated the production of CCL1 even in the absence of BPA or E2 (Figure 3d). One potential explanation for this unexpected finding is that the non-classical ER activation pathway such as phosphatidylinositol 3-kinase and AKT may activate ER in the absence of BPA or E2 when DCs were stimulated with TNF-α.41 The ICI may have inhibited the hormone-independent activation of ER. As a result, the CCL1 production was therefore completely abrogated. In addition, BPA or E2 may elicit a non-genomic effect via cell surface E2 receptors linked to the signal transduction pathways, e.g., mitogen-activated protein kinase.42 The modulatory effects of BPA on this pathway may provide an additional mechanism by which BPA or E2 controls a variety of immune-related genes because TNF-α mediates its effect on the cytokine and chemokine gene expression by nuclear factor-κB activation. The cognate receptor and signal transduction pathways involved in these mechanisms are currently under investigation.
DCs maintain the cell surface expression of CCR8 in the presence of activation signals.43 In addition, CCL1 is a potent anti-apoptotic factor.44 The secretion of high amounts of CCL1 by BPA-exposed DCs might amplify certain immune responses by providing survival signals for Th cells and DCs and by recruiting more CCR8+ cells to sites of inflammation.
Naive Th cells primed by BPA/TNF-α-matured DCs differentiated into Th2 cells with characteristically high IL-5/IFN-γ, IL-10/IFN-γ and IL-13/IFN-γ ratios (Figures 5 and 6). However, the IFN-γ production was not affected at all, thus indicating that Th2 bias was induced by enhanced Th2 cytokine production (Figures 5 and 6). In the analysis of Th differentiation using a transwell system by which naive Th cells were separated from Th–DC coculture by porous membrane, the Th2 differentiation induced by BPA/TNF-α DCs was completely abrogated (data not shown). This finding indicates that the Th2 differentiation of naive Th cells in response to BPA/TNF-α DCs required Th–DC contact. It is conceivable that certain molecule(s) expressed on the surface of DCs by the stimulation with BPA may exclusively induce Th2 differentiation. The Th2-driving DCs express OX40L.35 The high expression of CD86 is also an additional factor that favors the induction of Th2.45 However, BPA/TNF-α-matured DCs did not reveal such characteristics (data not shown). The identification of the Th2 polarizing factors induced by BPA thus remains to be investigated in the future.
The Th cells differentiated by BPA/TNF-α-matured DCs did not produce IL-17 (data not shown). This finding is supported by the previous observation that other factors such as ligands for Toll-like receptors are required for DC-mediated differentiation of IL-17-producing Th cells.46, 47, 48 Whether Th polarization is also modified by BPA/Toll-like receptor-stimulated DCs remains to be determined.
ERs are not only involved in female reproduction, but also may be an important factor in regulating the normal and pathogenic immune response. The current data provide evidence for role of BPA in the decision-making process of DCs. DCs exposed to BPA will provide one of the initial signals driving the development and perpetuation of Th2-dominated immune response.
Acknowledgments
This work was supported in part by the Nursing Foundation for Science Development and Innovation 09KMM06 from Chinese PLA General Hospital, Grants-in-Aid 21791572, 21791473 and 20591190 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and research grants from the Kansai Medical University (Research grant C), the Osaka Cancer Research Foundation (2010), and the Princess Takamatsu Cancer Research Fund (09-24104).
References
- Ring J, Kramer U, Schafer T, Behrendt H. Why are allergies increasing. Curr Opin Immunol. 2001;13:701–708. doi: 10.1016/s0952-7915(01)00282-5. [DOI] [PubMed] [Google Scholar]
- McGeady SJ. Immunocompetence and allergy. Pediatrics. 2004;113:1107–1113. [PubMed] [Google Scholar]
- Safe SH. Environmental and dietary estrogens and human health: is there a problem. Environ Health Perspect. 1995;103:346–351. doi: 10.1289/ehp.95103346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelce WR, Wilson EM. Environmental antiandrogens: developmental effects, molecular mechanisms, and clinical implications. J Mol Med. 1997;75:198–207. doi: 10.1007/s001090050104. [DOI] [PubMed] [Google Scholar]
- Ohshima Y, Yamada A, Tokuriki S, Yasutomi M, Omata N, Mayumi M. Transmaternal exposure to bisphenol A modulates the development of oral tolerance. Pediatr Res. 2007;62:60–64. doi: 10.1203/PDR.0b013e3180674dae. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- Witorsch RJ. Low-dose in utero effects of xenoestrogens in mice and their relevance to humans: an analytical review of the literature. Food Chem Toxicol. 2002;40:905–912. doi: 10.1016/s0278-6915(02)00069-8. [DOI] [PubMed] [Google Scholar]
- Kurosawa T, Hiroi H, Tsutsumi O, Ishikawa T, Osuga Y, Fujiwara T, et al. The activity of bisphenol A depends on both the estrogen receptor subtype and the cell type. Endocr J. 2002;49:465–471. doi: 10.1507/endocrj.49.465. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Singleton DW, Feng Y, Chen Y, Busch SJ, Lee AV, Puga A, et al. Bisphenol-A and estradiol exert novel gene regulation in human MCF-7 derived breast cancer cells. Mol Cell Endocrinol. 2004;221:47–55. doi: 10.1016/j.mce.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Singleton DW, Feng Y, Yang J, Puga A, Lee AV, Khan SA. Gene expression profiling reveals novel regulation by bisphenol-A in estrogen receptor-alpha-positive human cells. Environ Res. 2006;100:86–92. doi: 10.1016/j.envres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Goto M, Takano-Ishikawa Y, Ono H, Yoshida M, Yamaki K, Shinmoto H. Orally administered bisphenol A disturbed antigen specific immunoresponses in the naive condition. Biosci Biotechnol Biochem. 2007;71:2136–2143. doi: 10.1271/bbb.70004. [DOI] [PubMed] [Google Scholar]
- Ohshima Y, Yamada A, Tokuriki S, Yasutomi M, Omata N, Mayumi M. Transmaternal exposure to bisphenol A modulates the development of oral tolerance. Pediatr Res. 2007;62:60–64. doi: 10.1203/PDR.0b013e3180674dae. [DOI] [PubMed] [Google Scholar]
- Yan H, Takamoto M, Sugane K. Exposure to Bisphenol A prenatally or in adulthood promotes TH2 cytokine production associated with reduction of CD4+CD25+ regulatory T cells. Environ Health Perspect. 2008;116:514–519. doi: 10.1289/ehp.10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Takamoto M, Sugane K. Bisphenol A promotes IL-4 production by Th2 cells. Int Arch Allergy Immunol. 2003;132:240–247. doi: 10.1159/000074305. [DOI] [PubMed] [Google Scholar]
- Yoshino S, Yamaki K, Yanagisawa R, Takano H, Hayashi H, Mori Y. Effects of bisphenol A on antigen-specific antibody production, proliferative responses of lymphoid cells, and TH1 and TH2 immune responses in mice. Br J Pharmacol. 2003;138:1271–1276. doi: 10.1038/sj.bjp.0705166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- Liu TY, Uemura Y, Suzuki M, Narita Y, Hirata S, Ohyama H, et al. Distinct subsets of human invariant NKT cells differentially regulate T helper responses via dendritic cells. Eur J Immunol. 2008;38:1012–1023. doi: 10.1002/eji.200737838. [DOI] [PubMed] [Google Scholar]
- Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. 1998;160:5936–5944. [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traidl-Hoffmann C, Mariani V, Hochrein H, Karg K, Wagner H, Ring J, et al. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J Exp Med. 2005;201:627–636. doi: 10.1084/jem.20041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani T, Nakagawa S, Kurosawa M, Mizuashi M, Ozawa M, Aiba S. Cellular basis of the role of diesel exhaust particles in inducing Th2-dominant response. J Immunol. 2005;174:2412–2419. doi: 10.4049/jimmunol.174.4.2412. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Ohtani T, Ito Y, Mizuashi M, Nakagawa S, Furukawa T, et al. Molecular events in human T cells treated with diesel exhaust particles or formaldehyde that underlie their diminished interferon-gamma and interleukin-10 production. Int Arch Allergy Immunol. 2009;148:239–250. doi: 10.1159/000161584. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Liu TY, Narita Y, Suzuki M, Matsushita S. 17 beta-estradiol (E2) plus tumor necrosis factor-alpha induces a distorted maturation of human monocytes-derived dendritic cells and promotes their capacity to initiate T-helper 2 responses. Hum Immunol. 2008;69:149–157. doi: 10.1016/j.humimm.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Liu TY, Narita Y, Suzuki M, Ohshima S, Muzukami S, et al. Identification of functional type 1 ryanodine receptors in human dendritic cells. Biochem Biophys Res Commun. 2007;362:510–515. doi: 10.1016/j.bbrc.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Suzuki M, Liu TY, Narita Y, Hirata S, Ohyama H, et al. Role of human non-invariant NKT lymphocytes in the maintenance of type 2 T helper environment during pregnancy. Int Immunol. 2008;20:405–412. doi: 10.1093/intimm/dxn001. [DOI] [PubMed] [Google Scholar]
- Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- de Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, Wierenga EA, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J Immunol. 2002;168:1704–1709. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Safe SH, Pallaroni L, Yoon K, Gaido K, Ross S, McDonnell D. Problems for risk assessment of endocrine-active estrogenic compounds. Environ Health Perspect. 2002;110:925–929. doi: 10.1289/ehp.02110s6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, Wozniak AL, Finnerty CC. Signaling from the membrane via membrane estrogen receptor-alpha: estrogens, xenoestrogens, and phytoestrogens. Steroids. 2005;70:364–371. doi: 10.1016/j.steroids.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Laribi O, Ropero AB, Fuentes E, Ripoll C, Soria B, et al. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect. 2005;113:969–977. doi: 10.1289/ehp.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Compbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptoralpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–1644. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- Gombert M, Dieu-Nosjean MC, Winterberg F, Bunemann E, Kubitza RC, Da Cunha L, et al. CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J Immunol. 2005;174:5082–5091. doi: 10.4049/jimmunol.174.8.5082. [DOI] [PubMed] [Google Scholar]
- Ruckes T, Saul D, van Snick J, Hermine O, Grassmann R. Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood. 2001;98:1150–1159. doi: 10.1182/blood.v98.4.1150. [DOI] [PubMed] [Google Scholar]
- Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- Roses RE, Xu S, Xu M, Koldovsky U, Koski G, Czerniecki BJ. Differential production of IL-23 and IL-12 by myeloid-derived dendritic cells in response to TLR agonists. J Immunol. 2008;181:5120–5127. doi: 10.4049/jimmunol.181.7.5120. [DOI] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura Y, Liu TY, Narita Y, Suzuki M, Nakatsuka R, Araki T, et al. Cytokine-dependent modification of IL-12p70 and IL-23 balance in dendritic cells by ligand activation of Valpha24 invariant NKT cells. J Immunol. 2009;183:201–208. doi: 10.4049/jimmunol.0900873. [DOI] [PubMed] [Google Scholar]


