Abstract
Cell-based immunotherapy for lymphoid malignancies has gained increasing attention as patients develop resistance to conventional treatments. γδ T cells, which have major histocompatibility complex (MHC)-unrestricted lytic activity, have become a promising candidate population for adoptive cell transfer therapy. We previously established a stable condition for expanding γδ T cells by using anti-γδ T-cell receptor (TCR) antibody. In this study, we found that adoptive transfer of the expanded γδ T cells to Daudi lymphoma-bearing nude mice significantly prolonged the survival time of the mice and improved their living status. We further investigated the characteristics of these antibody-expanded γδ T cells compared to the more commonly used phosphoantigen-expanded γδ T cells and evaluated the feasibility of employing them in the treatment of lymphoid malignancies. Slow but sustained proliferation of human peripheral blood γδ T cells was observed upon stimulation with anti-γδ TCR antibody. Compared to phosphoantigen-stimulated γδ T cells, the antibody-expanded cells manifested similar functional phenotypes and cytotoxic activity towards lymphoma cell lines. It is noteworthy that the anti-γδ TCR antibody could expand both the Vδ1 and Vδ2 subsets of γδ T cells. The in vitro-expanded Vδ1 T cells displayed comparable tumour cell-killing activity to Vδ2 T cells. Importantly, owing to higher C–C chemokine receptor 4 (CCR4) and CCR8 expression, the Vδ1 T cells were more prone to infiltrate CCL17- or CCL22-expressing lymphomas than the Vδ2 T cells. Characterizing the peripheral blood γδ T cells from lymphoma patients further confirmed that the anti-γδ TCR antibody-expanded γδ T cells could be a more efficacious choice for the treatment of lymphoid malignancies than phosphoantigen-expanded γδ T cells.
Keywords: adoptive cell therapy, anti-γδ TCR antibody, γδ T cells, lymphoid malignancies, Vδ1 subset
Introduction
Conventional therapeutic strategies, including chemotherapy and radiotherapy, effectively suppress lymphoid malignancies during the initial treatments. However, the majority of patients become resistant to these therapies. Therefore, other therapeutic strategies, such as cell-based immunotherapy, are attracting increasing attention.1 Innate immune cells are promising candidates for tumour immunotherapy because they play a critical role in tumour immunosurveillance.2
γδ T cells, which function in both innate and acquired immunity, are considered to be potent candidates for immunotherapy.3, 4 Unlike conventional αβ T cells, γδ T cells can be activated and expanded by several phosphorylated non-peptide antigens (phosphoantigens), including isopentenyl pyrophosphate, bromohydrin pyrophosphate, (E)-4-hydroxy-3-methyl-but-2-enyl-pyrophosphate and aminobisphosphonates.5 Phosphoantigen-activated γδ T cells have already been tested in several clinical trials. Considerable anti-tumour effects were often obtained without any significant toxicity. In some clinical trials, the γδ T-cell population was increased through in vivo γδ T-cell expansion upon phosphoantigen administration. Studies in patients with solid tumours have demonstrated that treatment with bromohydrin pyrophosphate (IPH1101) in combination with low-dose interleukin 2 (IL-2) is safe and well-tolerated and induces potent γδ T lymphocyte expansion in patients.6 In another clinical trial, in patients with advanced breast cancer treated with zoledronate and IL-2, a statistically significant correlation between clinical outcome and peripheral Vγ9Vδ2 T cell numbers emerged.7 In addition, the direct transfer of in vitro-expanded γδ T cells has been used in some clinical trials and was proven to be safe.8, 9, 10 In a trial of adoptive transfer of in vitro-expanded γδ T cells to non-small cell lung cancer patients, immunomonitoring data showed that the number of peripheral γδ T cells gradually increased with increasing numbers of infusions.10 Potential anti-tumour effects have been reported in some patients upon γδ T-cell transfer,8, 9, 10 even the complete remission of lung metastasis appeared following adoptive immunotherapy with activated autologous γδ T cells in a patient with renal cell carcinoma.11 However, the number of participants in these studies was low, and the anti-tumour effect remains to be confirmed in further studies using larger numbers of participants.
It should be noted that phosphoantigens can only activate and expand the Vδ2 subset, while the Vδ1 subset, another major population of circulating human γδ T cells, cannot be expanded. It is generally accepted that Vδ2 T cells, which circulate in the peripheral blood, have the capacity to kill lymphoma and myeloma cells, while Vδ1 T cells, which are mainly located in the mucosa-associated lymphoid tissue, possess the function of defending against epithelial cancers.12, 13 However, several studies have suggested that Vδ1 T cells also play a role in defending against lymphoid malignancies. Vδ1 T cells have been frequently found in lymphoma tissues and have demonstrated cytotoxicity against stress-associated antigens, such as major histocompatibility complex class I-related chain molecules A and B (MICA/B) and unique long 16-binding proteins (ULBPs)-expressing tumour cells.14, 15
Herein, we have used an anti-γδ T-cell receptor (TCR) antibody (Ab) to expand γδ T cells. As previously reported, expansion of only the Vδ2 subset occurred upon stimulation with phosphoantigens. In contrast, the anti-γδ TCR Ab caused expansion of both the Vδ1 and Vδ2 subsets of γδ T cells. Notably, the expanded Vδ1 T cells manifested an enhanced migration tendency towards several lymphoid malignancies compared to Vδ2 T cells. Our results indicate a non-redundant role of Vδ1 T cells in anti-lymphoma immunotherapy. As a consequence, Ab-expanded γδ T cells may have an intrinsic advantage over phosphoantigen expanded γδ T cells in the treatment of certain lymphoid malignancies.
Materials and methods
Cell lines
The human tumour cell lines Daudi (Burkitt's lymphoma cell line), Ramos (RA.1, Burkitt's lymphoma cell line), HuT-78 (cutaneous T lymphoma cell line), Jurkat (T cell lymphoma cell line), K562 (chronic myelogenous leukaemia cell line) and RPMI 8226 (myeloma cell line) were obtained from the Cell Culture Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China). The L-428 (human Hodgkin's lymphoma cell line) was kindly provided by Professor Xiangmin Tong (Department of Hematology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China). All of the tumour cell lines were maintained in RPMI 1640 medium (Gibco, Gaithersburgh, MD, USA) supplemented with 10% foetal calf serum (FCS; Gibco).
Mice
Athymic BALB/c nu/nu mice (female, 4–6 week old) were purchased from the Laboratory Animal Center of the Chinese National Institute for the Control of Pharmaceutical and Biological Products. Mice were maintained in specific pathogen-free conditions at the Animal Maintenance Facility of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. All animal experiments were carried out in compliance with the animal ethics regulations of Chinese Academy of Medical Sciences.
Patients
Twelve lymphoma patients who had been diagnosed with lymphoma at Peking Union Medical College Hospital were enrolled. Clinical information, including age, gender, diagnosis, Ann Arbor stage at presentation and International Prognostic Index, was collected and listed in Table 1. The study was approved by the ethical committee of Peking Union Medical College Hospital. Informed consent was obtained from every subject.
Table 1. Clinical characteristics of 12 patients with lymphoma.
| Patient no. | Age | Gender | Diagnosis | Tumor stage | International Prognostic Index |
|---|---|---|---|---|---|
| 1 | 72 | Female | Non-Hodgkin's lymphoma(small lymphocytic) | IV | 2 |
| 2 | 71 | Female | Non-Hodgkin's lymphoma(diffuse large cell) | IB | 1 |
| 3 | 41 | Female | Non-Hodgkin's lymphoma (follicular) | IIIB | 2 |
| 4 | 70 | Male | Non-Hodgkin's lymphoma (mucosa lymphoid tissue associated) | IIA | 1 |
| 5 | 56 | Male | Stomach Hodgkin's lymphoma (diffuse large B cells), lugano | IIE | 1 |
| 6 | 41 | Male | Interfollicular Hodgkin's lymphoma | IIIB | 3 |
| 7 | 41 | Female | Non-Hodgkin's lymphoma(B cells) | IIE | 0 |
| 8 | 58 | Female | Non-Hodgkin's lymphoma | IVA | 1 |
| 9 | 58 | Female | Non-Hodgkin's lymphoma(B cells) | IVB | 4 |
| 10 | 63 | Male | Non-Hodgkin's lymphoma(diffuse large B cells) | IIIB | 3 |
| 11 | 71 | Male | Non-Hodgkin's lymphoma(small B cells) | IIA | 1 |
| 12 | 19 | Male | Non-Hodgkin's lymphoma | IVB | 3 |
Expansion of γδ T cells in vitro
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (TBD, Tianjin, China) centrifugation. The expansion of γδ T cells was performed as previously described.16 Briefly, 24-well plates were coated with 500 µl purified anti-γδ TCR Ab (IMMU510, 1 µg/ml; Immunotech, Beckman Coulter, Fullerton, CA, USA) at 37 °C for 2 h. Then PBMCs were added to the Ab-coated wells and cultured in RPMI 1640 medium supplemented with 10% FCS and 200 IU/ml recombinant human IL-2 (Beijing Read United Cross Pharmaceutical Co., Ltd, Beijing, China). The culture medium was replaced every other day. Alternatively, PBMCs were stimulated with 10 µM pamidronate (PAM) disodium (Aredia, Novartis, Switzerland) and cultured in the same culture medium described above. The trypan blue exclusion method was employed to assess cell viability.
Adoptive immunotherapy in an animal model
To test the in vivo anti-tumour effect of human γδ T cells, nude mice were divided into three groups and injected intravenously (i.v.) with Daudi cells (2×106 cells per mouse). Three days later, mice were injected intravenously with γδ T cells (2.5×107) plus rhIL-2 (1×104 IU), rhIL-2 (1×104 IU) or phosphate-buffered saline (PBS) (n=8 for each group). IL-2 was given on the day of cell transfer, and administration was continued twice daily for 4 days. Body weight and survival rate were measured every other day. After 48 days of treatment, two mice from each group were killed. Blood films and bone marrow smears were prepared, fixed using absolute methanol, stained with May–Grunwald–Giemsa stain and evaluated by light microscopy for the presence of lymphoma cells. A manual differential count was performed for each blood film by counting 200 white blood cells per film. Lymphoma cells which have a large size, irregular nucleus shape and dark-purple cytoplasm, were included in the differential counts. A differential count was also performed by counting 1000 nucleated cells from each bone marrow smears.
Chemotaxis assays
Chemotaxis assays were performed using 5-µm pore polycarbonate filters in 24-well transwell chambers (Costar; Corning, Lowell, MA, USA). Briefly, RPMI 1640 (600 µl) containing 0.5% bovine serum albumin and rhCCL17 (1 µg/ml; R&D System, Minneapolis, MN, USA) or rhCCL22 (1 µg/ml; R&D System) was added to the bottom chamber of the transwell. Human γδ T cells (1×106) suspended in 200 µl RPMI 1640 containing 0.5% bovine serum albumin were placed in the upper wells. In some experiments, γδ T cells were pre-incubated with anti-C–C chemokine receptor (CCR4) Ab (1 µg/1×106 cells; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-CCR8 Ab (1 µg/1×106 cells; Santa Cruz Biotechnology) for 1 h at room temperature before being placed in the upper wells. The chambers were incubated at 37 °C, 5% CO2 for 4 h. At the end of the incubation, the cells that migrated into the lower chambers and those left in the upper chambers were collected separately, stained with PE-anti-CD3 and FITC-anti-TCR Vδ1 or FITC-anti-TCR Vδ2, and analysed by flow cytometry using a FACScan flow cytometer (BD, San Jose, CA, USA). The chemotaxis index was calculated as the ratio of cells migrating towards the chemokine and untreated cells randomly migrating across the membrane.
Flow cytometry
Cells were washed with PBS containing 1% bovine serum albumin and incubated with surface-staining antibodies. After incubation at 4 °C for 20 min, cells were washed and resuspended in 300 µl PBS containing 1% formaldehyde. For intracellular staining, cells were incubated with 20 ng/ml phorbol 12-myristate-13-acetate (PMA; Sigma, St Louis, MO, USA), 0.5 µg/ml ionomycin (Sigma) and 3 µg/ml Brefeldin A (eBioscience, San Diego, CA, USA). After 6 h, the cells were collected and stained for surface markers. Next, cells were fixed, permeabilized, incubated with intracellular antibodies and measured by flow cytometry using a FACScan flow cytometer. Analysis of the FACS data was performed using FlowJo software (Version 5.7.2; Tree Star, Inc., Ashland, OR, USA). The following antibodies, purchased from Immunotech, were used for staining: FITC-conjugated anti-γδ TCR (IMMU510), PE-conjugated anti-γδ TCR (IMMU510), PE-conjugated anti-αβ TCR (BMA031), FITC-conjugated anti-TCR Vδ2 (IMMU389), PE-conjugated anti-CD3 (IM1282U) and the respective isotype control monoclonal antibodies. PE-conjugated anti-interferon (IFN)-γ (4S.B3) and FITC-conjugated anti-granzyme B (GB11) were purchased from BD Pharmingen (San Diego, CA, USA). PE-conjugated anti-CD6 (BL-CD6), tumour-necrosis factor (TNF)-α (MAb11), CD27 (O323), CD107a (H4A3), TNF-related apoptosis-inducing ligand (TRAIL) (RIK-2), APC-conjugated CD45RA (HI100), NKG2D (1D11), programmed-death receptor 1 (PD1) (12.2H7) and PerCP-cy5.5-conjugated CCR4 (TG6/CCR4) were purchased from BioLegend (San Diego, CA, USA). FITC-conjugated TCR Vδ1 (TS8.2) was purchased from Pierce (Rockford, IL, USA). Purified anti-CCR4 and anti-CCR8 antibodies were products of Santa Cruz Biotechnology.
Cell sorting
After 2 weeks in culture, γδ T cells were collected and labelled with FITC-conjugated anti-TCR Vδ1 or FITC-conjugated anti-TCR Vδ2 antibodies and anti-FITC MicroBeads (Miltenyi, Bergisch Gladbach, Germany). Subsequent positive selection was carried out using MS columns (Miltenyi) according to the manufacturer's protocol. Isolated γδ T cells were cultured in RPMI 1640 medium supplemented with 10% FCS and 200 IU/ml rhIL-2. After 72 h of rest, cells were collected and used for cytotoxicity assays.
Cytotoxicity assays
The CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI, USA), based on the colorimetric detection of the released lactate dehydrogenase, was used. Tumour cells used as target cells were seeded into a 96-round well plate (5×104 cells per well). Expanded γδ T cells served as effector cells were directly added to individual wells at different effector/target (E/T) ratios. The plate was incubated at 37 °C for 4 h, the supernatants were collected and the lactate dehydrogenase activity was detected. Controls for spontaneous lactate dehydrogenase release from effector and target cells, the target maximum release, as well as the culture medium background were assayed simultaneously. The cytotoxicity was calculated as follows:
 |
Reverse transcription-polymerase chain reaction (PCR)
Total RNA was extracted from distinct tumour cell lines with the RNeasy Mini Kit (QIAGEN, Hilden, Germany). cDNA was synthesized using oligo-dT as primers and the Moloney murine leukaemia virus reverse transcriptase (Promega) in the reverse transcription reaction, and sequences were amplified by polymerase chain reaction using the following primers.
β-ACTIN: forward: 5′-ACTGTGCCCATCTACGAGGG-3′
reverse: 5′-GTGGTGGTGAAGCTGTAGCC-3′
CCL17: forward: 5′-CCTGGTCACCCTCCTCCTG-3′
reverse: 5′-GGTACCACGTCTTCAGCTTTCT-3′
CCL22: forward: 5′-GTTGTCCTCGTCCTCCTTGC-3′
reverse: 5′-GGAGTCTGAGGTCCAGTAGAAGTG-3′.
Real-time PCR was performed in an Applied Biosystems 7500 Fast Real Time PCR System to quantify the levels of CCL17 and CCL22 mRNA. The amplification reaction was performed in a volume of 20 µl, containing oligonucleotide primers (5 µM each) and SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), which contained Taq DNA Polymerase, the reaction buffer, dNTP and the double-stranded DNA-specific fluorescent dye SYBR Green. The amplification reaction was carried out using the following two-step procedure: denaturation at 95 °C for 10 min and 40 cycles with denaturation at 95 °C for 15 s, and annealing and elongation at 60 °C for 1 min. The fluorescent signal from the samples was measured at the end of the elongation step. The results were analysed using Sequence Detection Software (version 1.2; Applied Biosystems) and reported as the relative ratios to the expression levels in Jurkat cells.
Enzyme-linked immunosorbent assay (ELISA)
Tumour cells (5×105) in 500 µl RPMI 1640 supplemented with 10% FCS were seeded in 24-well plates and cultured for 48 h. Next, the cell culture supernatants from each well were collected to measure chemokine concentration. Quantikine Human TARC/CCL17 ELISA Kits and Quantikine Human MDC/CCL22 ELISA Kits (R&D Systems) were used to quantify thymus and activation-regulated chemokine (TARC/CCL17) and macrophage-derived chemokine (MDC/CCL22) levels in tumour cell culture supernatants. The assay procedure was performed according to the manufacturer's instructions. The optical density in each well was determined within 30 min using a microplate reader set at the spectrum of 450 nm with λ correction at 570 nm.
Statistical analysis
Data are presented as mean±s.d. The comparisons of quantitative data between two groups were performed by Student's t-test. Repeated-measures analysis of variance followed by least significant difference was used to compare the mouse weights. The log-rank test was used in Kaplan–Meier survival analysis. A P value of less than 0.05 was considered statistically significant.
Results
Anti-lymphoma effect of Ab-expanded γδ T cells in vitro and in vivo
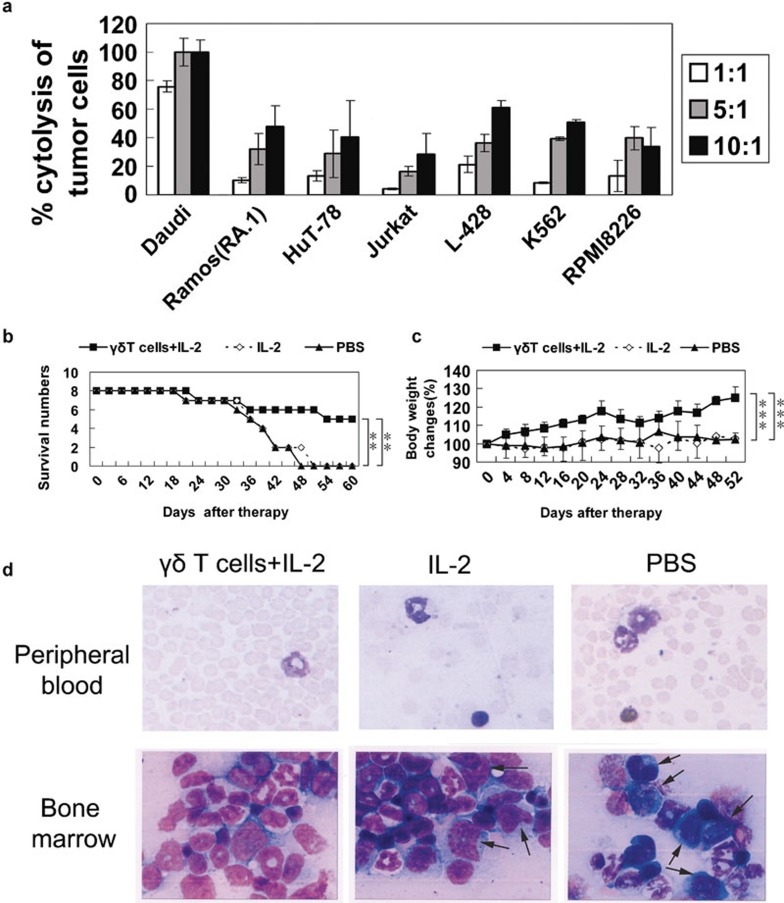
Anti-tumour efficacy is an important parameter for successful adoptive immunotherapy. We examined the cytotoxicity of Ab-expanded γδ T cells towards various haematological tumour cell lines. Ex vivo-expanded γδ T cells showed potent cytotoxicity against all of the tested lymphoma cell lines, including Daudi, Ramos (RA.1), HuT-78, Jurkat and L-428, as well as against the chronic myelogenous leukaemia cell line K562 and the myeloma cell line RPMI 8226 (Figure 1a).
Figure 1.
Human anti-γδ TCR-expanded peripheral blood γδ T cells exhibited significant anti-tumour activity in vitro and in Daudi lymphoma-bearing nude mice. (a) Cytotoxicity of γδ T cells from four individuals on various haematological tumour cell lines (b) Daudi lymphoma-bearing nude mice were treated with γδ T cells plus IL-2 (n=8), IL-2 alone (n=8) or PBS (n=8). Treatment with γδ T cells plus IL-2 significantly prolonged the survival time of Daudi lymphoma-bearing nude mice. (c) Body weight was significantly increased in mice treated with γδ T cells plus IL-2 compared to PBS or IL-2. (d) After 48 days of treatment, two mice from each treatment group were killed. Blood films and bone marrow smears were prepared, fixed using absolute methanol, stained with May–Grunwald–Giemsa stain and evaluated by light microscopy for the presence of lymphoma cells. A manual differential count was performed in each blood film by counting 200 white blood cells per film. A differential count was also performed by counting 1000 nucleated cells in each bone marrow smear. Lymphoma cells, with large size, irregular nucleus shape and dark purple cytoplasm, were included in the differential counts. Although no lymphoma cells were detected in the blood films (magnification: ×1000) from any of the three groups, lymphoma cells (indicated by the black arrows) were found in the bone marrow smears (magnification: ×1000) from the PBS and IL-2 treatment groups. One representative microscopic view is shown. **P<0.01; ***P<0.005. PBS, phosphate-buffered saline; TCR, T-cell receptor.
To determine the anti-tumour efficacy of adoptively transferred γδ T cells, Daudi lymphoma-bearing nude mice were treated with γδ T cells plus IL-2, IL-2 alone or PBS. As shown in Figure 1b, the survival rate of mice treated with γδ T cells plus IL-2 was significantly higher than the PBS- or IL-2-treated group, as was the change in body weight (Figure 1c). Lymphoma cells were not observed in the murine blood films from any of the tested groups. Whereas lymphoma cells accounted for 7% and 8% of the nucleated cells in the bone marrow smears from two IL-2-treated mice, these cells represented 21% and 24% of the nucleated cells in the bone marrow smears from two PBS-treated mice. No lymphoma cells were found in the bone marrow smears from mice in the γδ T cells plus IL-2 treatment group (Figure 1d).
Phenotypic and functional comparison of anti-γδ TCR Ab- and PAM-expanded γδ T cells
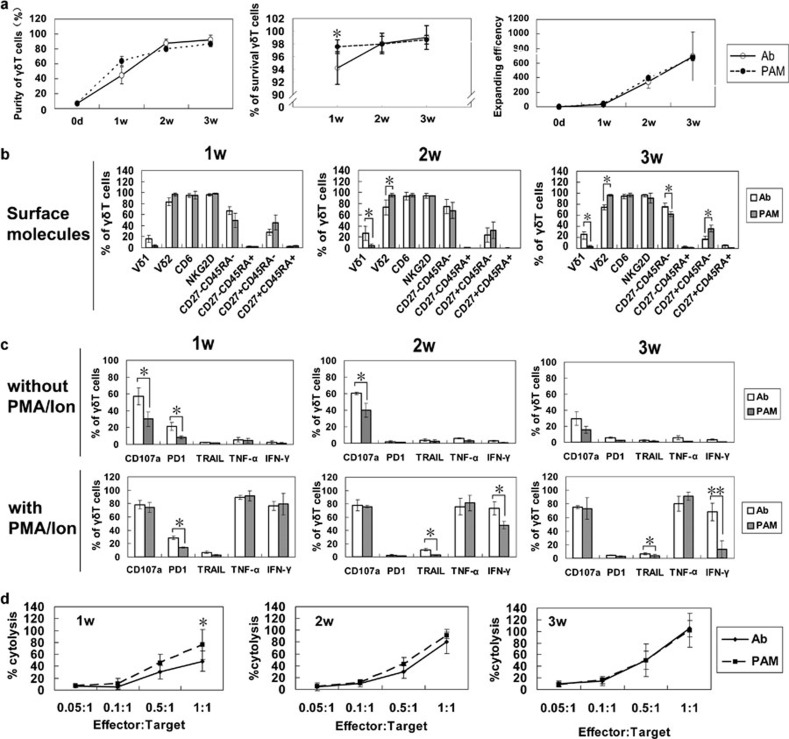
The quantity and quality of the transferred cells are important for adoptive immunotherapy. Therefore, we compared the proliferation and survival of γδ T cells expanded by anti-γδ TCR Ab with those expanded by PAM. Human peripheral blood γδ T cells were expanded by anti-γδ TCR Ab or PAM for 3 weeks. The purities, survival rates and expanding efficiencies at different time points (1, 2 and 3 weeks) were recorded and compared. Both strategies caused expansion of γδ T cells at ∼90% purity at the 3-week time point. Although the purity and survival rate of γδ T cells expanded in response to Ab were lower than cells expanded by PAM during the first two weeks, they reached the same or even higher levels at 3 weeks. A similar expanding efficiency was observed in the Ab- and PAM-stimulated γδ T cells during the entire culture process (Figure 2a).
Figure 2.
Proliferation, phenotypic and functional comparison of anti-γδ TCR Ab- and pamidronate-expanded γδ T cells. Peripheral blood γδ T cells from four healthy donors were expanded by immobilized anti-γδ TCR Ab or pamidronate. (a) The purity of the expanded γδ T cells after 3 weeks of culture and the survival rates of γδ T cells at 1, 2 and 3 weeks are shown. The expansion efficiency was calculated as the absolute number of expanded γδ T cells divided by the number of γδ T cells in the PBMCs. (b) Surface molecules of anti-γδ TCR Ab- and pamidronate-expanded γδ T cells at different time points. (c) The function-related molecules and cytokines without or with PMA/Ion restimulation. (d) Anti-γδ TCR Ab- and pamidronate-expanded cells were collected at 1, 2 or 3 weeks, counted and compared for their cytotoxicity against Daudi lymphoma cells. *P<0.05; **P<0.01. Ab, antibody; IFN, interferon; Ion, ionomycin; PBMC, peripheral blood mononuclear cell; PD1, programmed-death receptor 1; PMA, phorbol 12-myristate-13-acetate; TCR, T-cell receptor; TNF, tumour-necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand.
To determine the function-related phenotype and cytokine release profile of γδ T cells during the expansion process, we analysed the Ab- or PAM-activated γδ T cells at different time points. We found that culture time had little impact on the expression levels of most analysed molecules. The majority of γδ T cells expanded by PAM were Vδ2 T cells, whereas Ab-expanded γδ T cells contained 25±5% Vδ1 T cells. Almost all of the γδ T cells expressed NKG2D and CD6 on their surface. Ab-expanded γδ T cells contained a higher percentage of CD27−CD45RA− cells and a lower percentage of CD27+CD45RA− cells than PAM-expanded γδ T cells. The difference was significant at 3 weeks (Figure 2b). In addition, we examined the expression of CD107a, PD1 and TRAIL expression, as well as TNF-α and IFN-γ production, in γδ T cells expanded by both methods following PMA/ionomycin stimulation. Before stimulation, more of the Ab-expanded γδ T cells expressed CD107a and PD1. No TRAIL, TNF-α or IFN-γ was detected. Following PMA/ionomycin stimulation, both Ab- and PAM-expanded γδ T cells showed markedly elevated expression of CD107a, TRAIL, TNF-α and IFN-γ. Interestingly, although the proportions of TNF-α- and IFN-γ-secreting Ab-expanded γδ T cells remained constant over the entire culture period, the proportion of IFN-γ-secreting cells in PAM-expanded γδ T cell population was reduced from 1 to 3 weeks. Moreover, the Ab-expanded γδ T cells showed increased TRAIL expression on their surface compared to PAM-expanded cells. We also observed an elevated frequency of PD1+ γδ T cells in the Ab-expanded group at 1 week. As expected, the frequency decreased over time and became similar to the frequency of PD1+ γδ T cells in the PAM-expanded group (Figure 2c).
The cytotoxic activities of the γδ T cells expanded by both methods were determined as well. Consistent with the purity of γδ T cells in the culture, the cytotoxicity of the cells expanded by both methods was gradually enhanced over time. The PAM-expanded γδ T cells exhibited slightly stronger cytotoxic activity than the Ab-expanded cells at 1 week, probably resulting from their higher purity at that time. At 2 and 3 weeks, similar cytotoxic activity towards Daudi cells was observed (Figure 2d).
Phenotypic and functional comparison of Ab-expanded Vδ1 and Vδ2 subsets
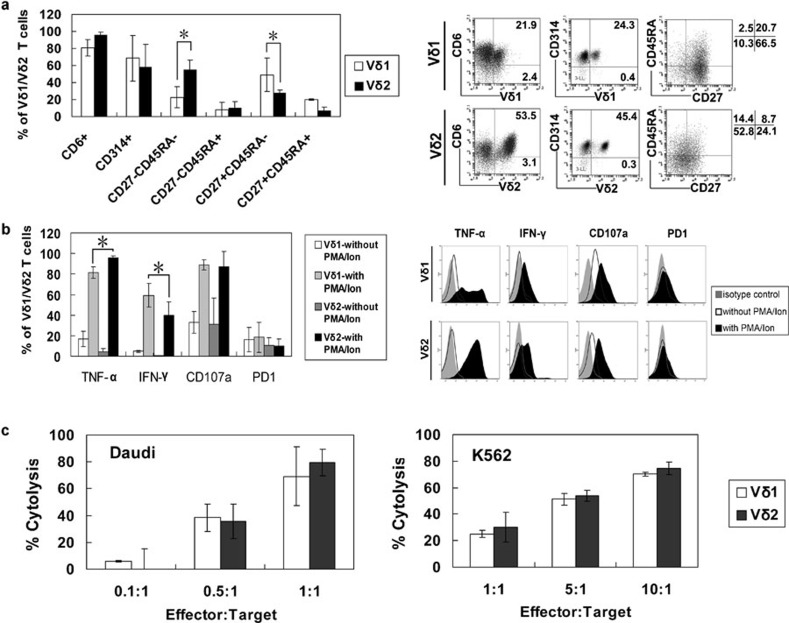
The most significant difference between the Ab- and PAM-expanded γδ T cells was the presence of the Vδ1 subset in the Ab expanded cells. To determine the differences between the Vδ1 and Vδ2 subsets, we compared their phenotypes and cytotoxicity at the end of the 2-week culture period.
The Vδ1 and Vδ2 subsets contained almost equal proportions of CD6+ and NKG2D+ cells, while the Vδ1 subset contained more CD27+CD45RA− cells, but fewer CD27−CD45RA− cells (Figure 3a). The Vδ1 subset contained fewer TNF-α-producing, but more IFN-γ-producing cells than the Vδ2 subset upon PMA/ionomycin stimulation. Cells in both subsets had similar CD107a and PD1 expression (Figure 3b). To compare the cytotoxic activity between them, we purified each subset by magnetic bead separation at 2 weeks and tested their cytotoxicity against Daudi and K562 cells. As shown in Figure 3c, Vδ1 and Vδ2 subset exhibited similar cytotoxicity against these tumour cells.
Figure 3.
Phenotypic and functional comparisons of Vδ1 and Vδ2 subsets. PBMCs from four healthy donors were cultured in the presence of immobilized anti-γδ TCR antibody for 2 weeks, then either the Vδ1 or Vδ2 subset was obtained via magnetic cell sorting, and the expression of CD6, NKG2D, CD27 and CD45RA (a), the expression of CD107a, PD1 and production of TNF-α and IFN-γ without and with PMA/Ion restimulation (b), and the cytotoxic effects against Daudi and K562 cells (c) of both subsets were compared. *P<0.05. IFN, interferon; Ion, ionomycin; PBMC, peripheral blood mononuclear cell; PD1, programmed-death receptor 1; PMA, phorbol 12-myristate-13-acetate; TCR, T-cell receptor; TNF, tumour-necrosis factor.
Vδ1 T cells manifested a superior tendency to migrate towards CCL17 and CCL22, primarily via CCR4
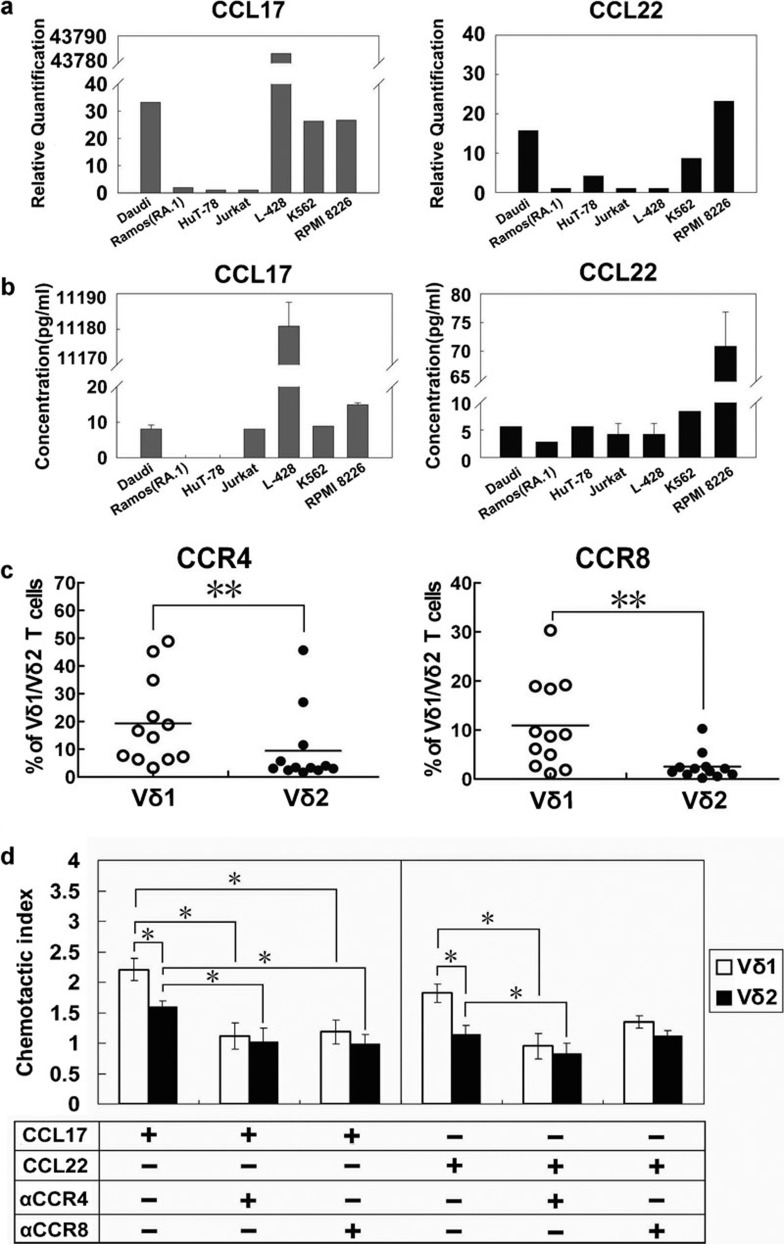
Chemokines and chemokine receptors play vital roles in lymphocyte function in vivo. High levels of TARC/CCL17 (thymus- and activation-regulated chemokine) and MDC/CCL22 (macrophage-derived chemokine) have been detected in various lymphomas. In this study, the mRNA expression levels of CCL17 and CCL22 were determined in five lymphoma cell lines, the myeloma cell line RPMI 8226 and the chronic myelogenous leukaemia cell line K562. It was found that the non-Hodgkin's lymphoma cell line Daudi expressed a certain level of CCL17 and that the Hodgkin's lymphoma cell line L-428 exhibited extremely high expression of CCL17 mRNA. Additionally, the Daudi cells and the cutaneous T lymphoma HuT-78 cells also expressed CCL22. In contrast, the lymphoma cell line Ramos (RA.1) and Jurkat cells had very low levels of CCL17 and CCL22 gene expression (Figure 4a). It was meaningful that both chemokines were also detected in K562 and RPMI 8226 cells. The PCR results were further confirmed by ELISA testing of the cell culture supernatants (Figure 4b).
Figure 4.
Migration tendency of anti-γδ TCR antibody-expanded γδ T cells. (a) Detection of chemokine expression in various haematological tumour cell lines by real-time PCR. (b) Detection of chemokine expression in various haematological tumour cell lines by ELISA. (c) Percentages of CCR4+ and CCR8+ cells among the Vδ1 and Vδ2 T cells (n=12). (d) CCL17- and CCL22-mediated chemotaxis of Vδ1 and Vδ2 T cells (n=5), which was partially blocked by anti-CCR4 antibody and anti-CCR8 antibody. Data are presented as mean±s.d. *P<0.05; **P<0.01. CCL, C–C chemokine ligand; CCR, C–C chemokine receptor; TCR, T-cell receptor.
Both CCL17 and CCL22 are ligands for CCR4. CCL17 is also a ligand for CCR8. Therefore, we examined CCR4 and CCR8 expression in the Vδ1 and Vδ2 subsets. Our data showed that more Vδ1 T cells expressed CCR4 and CCR8 than Vδ2 T cells (Figure 4c). The results of an in vitro migration assay showed that CCL17 and CCL22 had an apparent impact on the migration of γδ T cells, especially Vδ1 T cells (P<0.05 compared with Vδ2 T cells). To determine whether CCR4 or CCR8 was responsible for recruitment, anti-CCR4 Ab- and anti-CCR8 Ab-mediated blocking assays were carried out. We found that the anti-CCR4 Ab could significantly block the chemoattraction caused by CCL17 and CCL22 in both the Vδ1 and Vδ2 subsets. In contrast, the anti-CCR8 Ab could only block CCL17 mediated chemotaxis but not CCL22-mediated chemotaxis (Figure 4d).
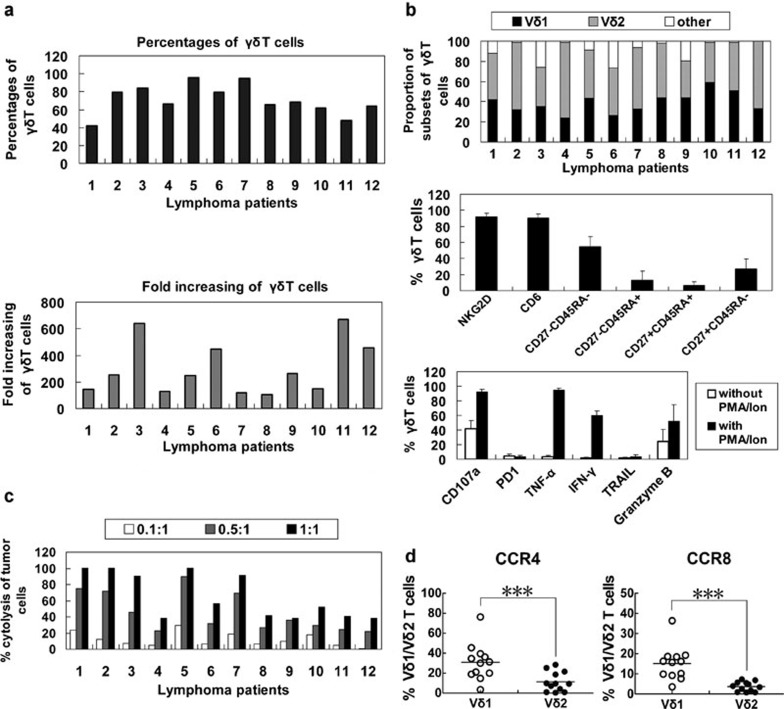
Proliferation, phenotype and cytotoxicity of γδ T-cells from the peripheral blood of lymphoma patients
To test the feasibility of employing Ab-expanded γδ T cells for lymphoma immunotherapy, we expanded γδ T cells from peripheral blood drawn from 12 lymphoma patients. Peripheral blood γδ T cells proliferated in response to immobilized anti-TCR γδ Ab in all the patients. The purities of the expanded γδ T cells at the end of a 2-week culture period ranged from 40% to 96%. Proliferation efficiencies, calculated by dividing the number of expanded to initial γδ T cells, ranged from 100 to 700 (Figure 5a). The expanded cells belonged to the Vδ1 (25%–60%) or Vδ2 (36%–80%) subsets. Most cells expressed NKG2D and CD6, and 54±13% were CD27−CD45RA−. The expression of CD107a, granzyme B, TNF-α and IFN-γ in the expanded γδ T cells markedly increased following PMA/ionomycin stimulation. In contrast, PD1 expression remained low upon PMA/ionomycin treatment (Figure 5b). All of the γδ T cells showed some degree of cytotoxicity towards the lymphoma cell line Daudi (Figure 5c). Consistent with the data from normal donors, significantly more Vδ1 T cells were CCR4- or CCR8-positive (Figure 5d).
Figure 5.
Proliferation, phenotype and cytotoxicity of γδ T cells from the peripheral blood of lymphoma patients. (a) The purity and proliferation efficiency of in vitro expanded γδ T cells of lymphoma patients (n=12). (b) Phenotype expression and cytokine release by γδ T cells (n=12). (c) Cytotoxicity of γδ T cells from 12 lymphoma patients. (d) Percentages of CCR4+ and CCR8+ cells among the Vδ1 and Vδ2T cells from patients. ***P<0.005. CCR, C–C chemokine receptor; IFN, interferon; PD1, programmed-death receptor 1; TNF, tumour-necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand.
Discussion
In human peripheral blood, γδ T cells account for only 1%–10% of CD3+ T cells. According to their δ-chain usage, γδ T cells can be divided into two major groups, the Vδ1 and Vδ2 T cells. The Vδ2 subset is dominant in the peripheral blood, whereas the Vδ1 subset is frequently found in epithelial tissues and in epithelial tumour-infiltrating lymphocytes (TILs).17 Neither subset responds to peptide antigens presented by major histocompatibility complex. Instead, they can recognize non-peptide antigens, such as phosphoantigens18 and stress-induced proteins such as MICA/B,19 ULBPs,20 heat shock proteins and F1F0-ATPase,21 via TCR and/or non-TCR receptors. In vitro and in vivo studies have demonstrated that human γδ T cells can kill various haematological and solid tumour cells expressing these antigens. In addition to direct killing of tumour targets, γδ T cells also display an anti-tumour effect in vivo by activating other innate cells and promoting the adaptive immune response. For example, recent data have demonstrated that γδ T lymphocytes can induce robust natural killer cell-mediated anti-tumour cytotoxicity.22 Additionally, increasing evidence has demonstrated that γδ T cells play an important role in the cross-talk between innate and adaptive immunity. γδ T cells actively regulate the adaptive immune response through interactions with antigen presenting cells, such as dendritic cells.23 They can even directly present antigens to adaptive immune cells.24 Currently, most clinical studies are aimed at increasing the γδ T cell population in patients by administering certain phosphoantigens intravenously to expand γδ T cells in vivo, or by adoptive transfer of self γδ T cells stimulated with phosphoantigen in vitro.6, 7, 8, 9, 10, 11
We have previously established conditions for expanding peripheral blood γδ T cells with immobilized anti-γδ TCR antibodies over a 2-week culture period.16 The in vitro- expanded γδ T cells displayed potent cytotoxicity against various tumours. In the present study, anti-γδ TCR Ab-expanded γδ T cells showed potent anti-tumour effects against various types of haematological cell lines, including the non-Hodgkin's lymphoma cell lines Daudi, Ramos (RA.1), HuT-78 and Jurkat, as well as the Hodgkin's lymphoma cell line L-428. Furthermore, they also demonstrated a potent anti-lymphoma effect in Daudi lymphoma-bearing nude mice, indicating the feasibility of this regimen for lymphoma treatment.
Phosphoantigens are widely used to stimulate γδ T cells. Previous studies have shown that lymphoid malignancies can be recognized and killed by phosphoantigen-expanded γδ T cells in animal models.25, 26 Aminobisphosphonates, which inhibit the mevalonate pathway by reducing the activity of farnesyl pyrophosphate synthase, promote the intracellular accumulation of isopentenyl pyrophosphate, and activate γδ T cells in vitro and in vivo.5, 27 As one of the new-generation aminobisphosphonates, PAM was proven to be a more potent γδ T cell stimulus.5 Compared to PAM-activated cells, γδ T cells stimulated by the anti-γδ TCR Ab manifested a relatively delayed activation response, followed by a mild but sustained proliferation process. The Ab-expanded cells constantly expressed anti-tumour activity-related biomolecules, including CD6, NKG2D, CD107a, TNF-α and IFN-γ. Our data indicate that Ab stimulation supported a 3-week-long expansion with adequate purity and quantity without apparent function loss. In contrast, PAM stimulated rapid γδ T-cell activation and proliferation. Although these seemingly rapidly activated γδ T cells displayed similar proliferation and cytotoxic activity against tumour cells during the 3-week culture period, they produced less IFN-γ than Ab-expanded T cells. It is noteworthy that IFN-γ plays an important role in in vivo anti-tumour activity by enhancing the Th1 immune response.28 Hence, this difference might make sense. In contrast, Ab-expanded γδ T cells contained more CD27−CD45RA− cells and fewer CD27+CD45RA− cells than PAM-expanded γδ T cells. It has been proposed that γδ T cells differentiate in a sequence of naive (CD27+CD45RA+), central memory (CD27+CD45RA−), effector memory (CD27−CD45RA−) and terminally differentiated (CD27−CD45RA+) cells. CD27+CD45RA− cells and CD27+CD45RA+ cells express lymph node homing receptors and lack immediate effector functions. Conversely, CD27−CD45RA− and CD27−CD45RA+ cells express receptors for homing to inflamed tissues and display immediate effector functions.29, 30 Taken together, Ab-expanded γδ T cells may lead to an immediate and more potent anti-tumour effect in vivo.
CD166 (also called activated leukocyte-cell adhesion molecule) is a member of the immunoglobulin superfamily expressed on a variety of normal and tumour cells, including lymphoma cells.31, 32 CD166 functions as a ligand for the CD6 receptor, which is expressed on T cells and some B cells.33, 34 The CD6–CD166 interaction facilitates stable cell–cell binding and is involved in the activation of human γδ T cells.31 NKG2D, which is expressed on natural killer cells, CD8+ lymphocytes and γδ T cells, can recognize MICA/B or ULBPs expressed on malignant haematological cells. It is closely related to γδ T cell-mediated cytotoxicity.15, 35 In the present study, expanded γδ T cells expressed comparable levels of CD6 and NKG2D over the culture time.
PD1 is expressed on peripheral T and B lymphocytes upon activation.36 It negatively regulates activated T cells upon interaction with its ligands PD-L1 and PD-L2. Many tumours, including lymphoma, express PD1 ligands.37, 38 The PD1 ligand/PD1 signalling pathway mediates one of the tumour immune evasion strategies.37, 38, 39 Following the initial activation, PD1 expression is transiently elevated, especially on Ab-expanded cells, in the first week. Fortunately, PD1 expression gradually decreased with time and reached a considerably low level over the next 2 weeks of the culture period.
It must be noted that only the peripheral Vδ2, but not the Vδ1 subset, can be expanded by phosphoantigens. In contrast, the anti-γδ TCR Ab is a potent stimulus that could expand both Vδ1 and Vδ2 subsets. It has been reported that some MICA/B or ULBPs expressing malignant haematological cells were susceptible to Vδ1 T cell-mediated cytotoxicity.15, 40 In this study, we found that the expanded Vδ1 T cells contained relatively fewer TNF-α-, but more IFN-γ-, producing cells upon PMA/ionomycin stimulation; however, they had similar levels of CD107a and PD1 expression and displayed comparable cytotoxic effects against Daudi cells and K562 cells as the Vδ2 T cells. Recently, it was reported that both the Vδ1 and Vδ2 subsets could be expanded using concanavalin A41 or anti-CD3 Ab.42 Both of the protocols resulted in considerable percentages of the Vδ1 and Vδ2 subsets. However, because both concanavalin A and anti-CD3 Ab are unspecific activators of γδ T cells, γδ T cells must be purified before expansion. In contrast, according to our protocol, PBMCs can directly be expanded by anti-γδ TCR Ab to obtain both the Vδ1 and Vδ2 subsets. This makes our protocol simple and easy to operate for most laboratories. It reduces costs and is more suitable for clinical use.
Recruiting effector lymphocytes to the tumour site is an essential prerequisite for an effective anti-tumour immune response. The migration of effector lymphocytes greatly depends on chemokines and their receptors. In this study, several lymphoma cell lines expressed CCL17 and/or CCL22. It has also been reported that high levels of CCL17 and CCL22 were detected in various tumours, such as lung cancer,43 gastric cancer,44 B-cell non-Hodgkin lymphoma,45 Hodgkin's lymphoma46 and peripheral T-cell lymphomas.47 Among the lymphomas, CCL17 is specifically expressed in classical Hodgkin's lymphoma,46, 48 while CCL22 is expressed in nodular lymphocyte-predominant Hodgkin′s lymphoma and B-cell non-Hodgkin's lymphoma.49 Both CCL17 and CCL22 are ligands for CCR4, but CCL17 is also a ligand for CCR8. We found that significantly more Vδ1 T cells expressed CCR4 and CCR8 than Vδ2 T cells. CCL17 and CCL22 could significantly induce migration of Vδ1 T cells, suggesting that Vδ1 T cells have distinctive and non-redundant anti-lymphoma effects in vivo.
Importantly, peripheral blood γδ T cells from all patients proliferated in response to immobilized anti-γδ TCR Ab. Expanded γδ T cells expressed function-related phenotype markers, such as CD107a and granzyme B, and released cytokines including TNF-α and IFN-γ. Additionally, they showed potent cytotoxicity against the lymphoma cell line Daudi. Consistent with the results from healthy donors, significantly more Vδ1 T cells expressed CCR4 and CCR8 than Vδ2 T cells in lymphoma patients. It is noteworthy that Vδ1 T cells constituted 25%–60% of the expanded γδ T cells in lymphoma patients. Vδ1 T cells will consequently be necessary in fighting against lymphoma in vivo. Therefore, adoptive transfer of anti-γδ TCR Ab-expanded autologous γδ T cells for the treatment of lymphoid malignancies is a feasible and promising strategy.
Currently, a phase I clinical trial carried out by our research group using anti-γδ TCR Ab-expanded γδ T cells for adoptive immune therapy for hepatocellular carcinoma has just been completed, and a phase II clinical trial is underway. Our present data indicate that Ab-expanded γδ T cells, which comprise both Vδ1 and Vδ2 subsets, can be an excellent candidate for adoptive immune therapy in patients with lymphoma. Further studies will focus on optimizing the current γδ T-cell preparation, establishing individualized therapeutic regimens, and choosing the appropriate indications for the adoptive γδ T-cell immunotherapy.
Acknowledgments
This work was supported by two grants (No. 2006AA02Z480 and No. 2007AA021109) from the National High Technology Research and Development Program (863 Program), and one grant (No. 30972776) from the National Natural Science Foundation of China.
References
- Long HM, Parsonage G, Fox CP, Lee SP. Immunotherapy for Epstein–Barr virus-associated malignancies. Drug News Perspect. 2010;23:221–228. doi: 10.1358/dnp.2010.23.4.1439500. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human γδ T cells by nonpeptide antigens. J Immunol. 2001;167:5092–5098. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Bauer E, Wilhelm M. γ/δ T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- Bennouna J, Levy V, Sicard H, Senellart H, Audrain M, Hiret S, et al. Phase I study of bromohydrin pyrophosphate (BrHPP, IPH 1101), a Vgamma9Vdelta2 T lymphocyte agonist in patients with solid tumors. Cancer Immunol Immunother. 2010;59:1521–1530. doi: 10.1007/s00262-010-0879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, et al. Safety profile and antitumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi A, Kaneko T, Kamigaki T, Fujimoto K, Ozawa M, Saito M, et al. Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy. 2011;13:92–97. doi: 10.3109/14653249.2010.515581. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Nakajima J, Murakawa T, Fukami T, Yoshida Y, Murayama T, et al. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded γδT cells: a phase I clinical study. J Immunother. 2011;34:202–211. doi: 10.1097/CJI.0b013e318207ecfb. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K. Complete remission of lung metastasis following adoptive immunotherapy using activated autologous gammadelta T-cells in a patient with renal cell carcinoma. Anticancer Res. 2010;30:575–579. [PubMed] [Google Scholar]
- Qi J, Zhang J, Zhang S, Cui L, He W. Immobilized MICA could expand human Vdelta1 gammadelta T cells in vitro that displayed major histocompatibility complex class I chain-related A-dependent cytotoxicity to human epithelial carcinomas. Scand J Immunol. 2003;58:211–220. doi: 10.1046/j.1365-3083.2003.01288.x. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MIC-A and MIC-B. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi A, Venturino C, Catellani S, Clavio M, Miglino M, Gobbi M, et al. Vδ1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res. 2004;64:9172–9179. doi: 10.1158/0008-5472.CAN-04-2417. [DOI] [PubMed] [Google Scholar]
- Kang N, Zhou J, Zhang T, Wang L, Lu F, Cui Y, et al. Adoptive immunotherapy of lung cancer with immobilized anti-TCRgammadelta antibody-expanded human gammadelta T-cells in peripheral blood. Cancer Biol Ther. 2009;8:1540–1549. doi: 10.4161/cbt.8.16.8950. [DOI] [PubMed] [Google Scholar]
- Maeurer MJ, Martin D, Walter W, Liu K, Zitvogel L, Halusczcak K, et al. Human intestinal Vdelta1+ lymphocytes recognize tumor cells of epithelial origin. J Exp Med. 1996;183:1681–1696. doi: 10.1084/jem.183.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sireci G, Espinosa E, Di Sano C, Dieli F, Fournié JJ, Salerno A. Differential activation of human gammadelta cells by nonpeptide phosphoantigens. Eur J Immunol. 2001;31:1628–1635. doi: 10.1002/1521-4141(200105)31:5<1628::AID-IMMU1628>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Zhao J, Huang J, Chen H, Cui L, He W. Vdelta1 T cell receptor binds specifically to MHC I chain related A: molecular and biochemical evidences. Biochem Biophys Res Commun. 2006;339:232–240. doi: 10.1016/j.bbrc.2005.10.198. [DOI] [PubMed] [Google Scholar]
- Cao W, Xi X, Wang Z, Dong L, Hao Z, Cui L, et al. Four novel ULBP splice variants are ligands for human NKG2D. Int Immunol. 2008;20:981–991. doi: 10.1093/intimm/dxn057. [DOI] [PubMed] [Google Scholar]
- Scotet E, Martinez LO, Grant E, Barbaras R, Jenö P, Guiraud M, et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A–I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, et al. Human γδ T lymphocytes induce robust NK cell mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116:1726–1733. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha N, Ida JA, Lubinski AS, Pallin M, Kaplan G, Haslett PA. Regulation of acquired immunity by gamma delta T-cell/dendritic-cell interactions. Ann NY Acad Sci. 2005;1062:79–94. doi: 10.1196/annals.1358.011. [DOI] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Bioley G, Lévy N, Eberl M, Luo M, et al. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci USA. 2009;106:2307–2312. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone F, Pende D, Burgio VL, Castelli C, Spada M, Venditti M, et al. Effect of human natural killer and gammadelta T cells on the growth of human autologous melanoma xenografts in SCID mice. Cancer Res. 2004;64:378–385. doi: 10.1158/0008-5472.can-03-1501. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, Pitters E, Zöller M. Characterization of tumor reactivity of human Vgamma9Vdelta2 gammadelta T cells in vitro and in SCID mice in vivo. . J Immunol. 2004;173:6767–6776. doi: 10.4049/jimmunol.173.11.6767. [DOI] [PubMed] [Google Scholar]
- Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, de Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- Angelini DF, Borsellino G, Poupot M, Diamantini A, Poupot R, Bernardi G, et al. FcγRIII discriminates between 2 subsets of Vγ9Vδ2 effector cells with different responses and activation pathways. Blood. 2004;104:1801–1807. doi: 10.1182/blood-2004-01-0331. [DOI] [PubMed] [Google Scholar]
- Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, et al. Differentiation of effector/memory Vδ2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Tanaka Y, Hayashi M, Okawa K, Minato N. Involvement of CD166 in the activation of human gamma delta T cells by tumor cells sensitized with nonpeptide antigens. J Immunol. 2006;177:877–884. doi: 10.4049/jimmunol.177.2.877. [DOI] [PubMed] [Google Scholar]
- Ma Y, Visser L, Roelofsen H, de Vries M, Diepstra A, van Imhoff G, et al. Proteomics analysis of Hodgkin lymphoma: identification of new players involved in the cross-talk between HRS cells and infiltrating lymphocytes. Blood. 2008;111:2339–2346. doi: 10.1182/blood-2007-09-112128. [DOI] [PubMed] [Google Scholar]
- Aruffo A, Melnick MB, Linsley PS, Seed B. The lymphocyte glycoprotein CD6 contains a repeated domain structure characteristic of a new family of cell surface and secreted proteins. J Exp Med. 1991;174:949–952. doi: 10.1084/jem.174.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A, Bowen MA, Patel DD, Haynes BF, Starling GC, Gebe JA, et al. CD6-ligand interactions: a paradigm for SRCR domain function. Immunol Today. 1997;18:498–504. doi: 10.1016/s0167-5699(97)01130-4. [DOI] [PubMed] [Google Scholar]
- Lança T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C, et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to γδ T-cell cytotoxicity. Blood. 2010;115:2407–2411. doi: 10.1182/blood-2009-08-237123. [DOI] [PubMed] [Google Scholar]
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M, et al. PD-1–PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111:3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- Catellani S, Poggi A, Bruzzone A, Dadati P, Ravetti JL, Gobbi M, et al. Expansion of Vdelta1 T lymphocytes producing IL-4 in low-grade non-Hodgkin lymphomas expressing UL-16-binding proteins. Blood. 2007;109:2078–2085. doi: 10.1182/blood-2006-06-028985. [DOI] [PubMed] [Google Scholar]
- Siegers GM, Dhamko H, Wang XH, Mathieson AM, Kosaka Y, Felizardo TC, et al. Human Vδ1 γδ T cells expanded from peripheral blood exhibit specific cytotoxicity against B-cell chronic lymphocytic leukemia-derived cells Cytotherapy 201111in press. [DOI] [PubMed] [Google Scholar]
- Dokouhaki P, Han M, Joe B, Li M, Johnston MR, Tsao MS, et al. Adoptive immunotherapy of cancer using ex vivo expanded human gammadelta T cells: a new approach. Cancer Lett. 2010;297:126–136. doi: 10.1016/j.canlet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Imaizumi K, Hasegawa Y, Kawabe T, Hashimoto N, Okamoto M, et al. Expression of macrophage-derived chemokine (MDC)/CCL22 in human lung cancer. Cancer Immunol Immunother. 2006;55:1320–1329. doi: 10.1007/s00262-006-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peh SC, Kim LH, Poppema S. TARC, a CC chemokine, is frequently expressed in classic Hodgkin's lymphoma but not in NLP Hodgkin's lymphoma, T-cell-rich B-cell lymphoma, and most cases of anaplastic large cell lymphoma. Am J Surg Pathol. 2001;25:925–929. doi: 10.1097/00000478-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Thielen C, Radermacher V, Trimeche M, Roufosse F, Goldman M, Boniver J, et al. TARC and IL-5 expression correlates with tissue eosinophilia in peripheral T-cell lymphomas. Leuk Res. 2008;32:1431–1438. doi: 10.1016/j.leukres.2008.02.016. [DOI] [PubMed] [Google Scholar]
- van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed–Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin's lymphoma. Am J Pathol. 1999;154:1685–1691. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio E, van den Berg A, Diepstra A, Kluiver J, Visser L, Poppema S. Chemokines, cytokines and their receptors in Hodgkin's lymphoma cell lines and tissues. Ann Oncol. 2002;13 Suppl 1:52–56. doi: 10.1093/annonc/13.s1.52. [DOI] [PubMed] [Google Scholar]