Abstract
Dendritic cell (DC)-based vaccines continue to be considered an attractive tool for cancer immunotherapy. DCs require an additional signal from the environment or other immune cells to polarize the development of immune responses toward T helper 1 (Th1) or Th2 responses. DCs play a role in natural killer (NK) cell activation, and NK cells are also able to activate and induce the maturation of DCs. We investigated the types of NK cells that can induce the maturation and enhanced function of DCs and the conditions under which these interactions occur. DCs that were activated by resting NK cells in the presence of inflammatory cytokines exhibited increased expression of several costimulatory molecules and an enhanced ability to produce IL-12p70. NK cell-stimulated DCs potently induced Th1 polarization and exhibited the ability to generate tumor antigen-specific cytotoxic T lymphocyte responses. Our data demonstrate that functional DCs can be generated by coculturing immature DCs with freshly isolated resting NK cells in the presence of Toll-like receptor agonists and proinflammatory cytokines and that the resulting DCs effectively present antigens to induce tumor-specific T-cell responses, which suggests that these cells may be useful for cancer immunotherapy.
Keywords: cancer immunotherapy, dendritic cells, natural killer cells, signal
Introduction
Dendritic cells (DCs) are key antigen-presenting cells, capable of initiating primary T cell responses and linking innate immunity and adaptive immunity in antitumor immune responses.1 To initiate a productive T-cell response, immature DCs must undergo a maturation and activation process, which results in the expression of high levels of major histocompatibility complex (MHC) and costimulatory molecules, allowing the DCs to present antigens and activate T cells.1 DCs stimulate T cells through antigen-specific and costimulatory signals, both of which are required for the activation and expansion of pathogen-specific T cells.1, 2 In addition, DCs require an additional signal to polarize developing immune responses toward T helper 1 (Th1) or Th2 responses.3, 4 Several factors or cytokines can directly induce the maturation of DCs toward type 1 or type 2 DC fates, resulting in the polarization of naive T cells to Th1 or Th2 cell fates, respectively.3, 4, 5, 6 The presence of interferon (IFN)-γ or IFN-γ-producing cells, such as CD8+ T cells or natural killer (NK) cells, at the final stages of DC maturation, leads to the development of a type 1-polarized DC subset, which produces increased levels of IL-12p70 after contact with naive Th cells and induces Th1-like differentiation in naive Th cells.7, 8
NK cells are the major lymphocyte population of the innate immune response and function through the production of cytokines and lysis of virus-infected cells or tumor cells.9 The activation of NK cells relies on a combination of interactions with cytokine receptors, adhesion molecules and activating receptors ligated via interactions with tumors, pathogen-infected cells or other immune cells.10, 11 Interactions between NK cells and DCs result in activation of and cytokine production by both cell types, including NK cell proliferation, cytokine production and cytotoxicity and further DC maturation and activation.12, 13, 14, 15 Recently, NK cells have also been shown to play immunoregulatory ‘helper' roles, by activating DCs and enhancing their ability to produce pro-inflammatory cytokines, as well as to stimulate Th1 and cytotoxic T lymphocyte (CTL) responses of tumor-specific CD4+ and CD8+ T cells, respectively.7, 16, 17 In our previous study, we proposed that potent DCs were generated by NK cell-mediated maturation in the presence of Toll-like receptor (TLR) 4 agonist, and vaccination with these DCs demonstrated strong efficacy in a mouse colon cancer model.18 Although the above data suggest that NK cells and DCs can reciprocally activate each other during the immune response, which type of NK cells (freshly isolated resting cells or previously activated cells) are most effective in inducing the activation and maturation of DCs remains unknown. In addition, the optimal conditions for NK cell-mediated DC maturation require further investigation.
Therefore, we investigated the ability of different types of NK cells and different culture conditions to enhance the maturation and function of DCs. Our data demonstrate that functional DCs can be generated by coculturing immature DCs with freshly isolated resting NK cells in the presence of TLR agonist and pro-inflammatory cytokines. Importantly, DCs generated in this manner showed an enhanced capacity to induce T-cell responses.
Materials and methods
Generation of monocyte-derived immature DCs
All experiments were performed after obtaining informed consent from the subjects, according to a protocol approved by the Chonnam National University Hwasun Hospital Institutional Review Board. Monocytes were isolated from the peripheral blood of healthy donors using two-step density gradient centrifugation, followed by plastic adherence as previously described.19 The adherent monocytes, which were more than 95% pure, were cultured for 6 days in 24-well plates (BD Biosciences-Labware, San Jose, CA, USA) at a density of 5×105 cells per well in complete medium containing RPMI-1640 supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin (all from Gibco-BRL, Grand Island, NY, USA) in the presence of 50 ng/ml GM-CSF (LG Biochemical, Daejeon, Korea) and 10 ng/ml IL-4 (R&D Systems, Minneapolis, MN, USA) to generate immature DCs (iDCs).
Preparation of activated NK cells
CD56+ NK cells were isolated from the lymphocyte fraction from the same donor using the magnetic activated cell sorting system (Miltenyi Biotec, Auburn, CA, USA). NK cells were used when >95% were CD56-positive. For preparation of activated NK cells, purified NK cells were cultured in complete medium for 48 h in the presence of IL-2 (50 U/ml) (R&D Systems), IL-2 and poly(I:C) (10 µg/ml) (Sigma-Aldrich, St Louis, MO, USA) or IL-2, poly(I:C) and IFN-α (10 000 U/ml) (LG Life Science, Chonbuk, Korea). Before coculturing cells with DCs, activated NK cells were harvested and washed three times to remove all excess reagents.
iDC and NK cell coculture conditions
To assess the optimal conditions for DC and NK cell coculture, iDCs (2.0×105) were either directly cocultured with NK cells (4.0×105) or separated from NK cells by transwell membranes with 0.4-µm pore diameters in 24-well plates in the presence of IL-2 and/or poly(I:C). iDCs were placed in the lower chamber, and NK cells were placed in the upper chamber (Costar, Cambridge, MA, USA). After 48 h, the cells were harvested and analyzed for the expression of costimulatory molecules and the ability to produce IL-12.
NK cell-mediated DC maturation
Based on the results of our preliminary experiments, iDCs and NK cells were cocultured at a ratio of 1∶2. For DC maturation, iDCs were directly cocultured with either freshly isolated autologous resting NK cells or activated NK cells in 24-well plates. When indicated, IL-2, poly(I:C) and IFN-α were added to cocultures containing iDCs and resting NK cells to induce DC maturation. Alternatively, iDCs were matured with lipopolysaccharide (LPS) to compare the migration capacity of NK cell-matured DCs to that of DCs that were maturated with TLR ligand only.
To test the killing activity of NK cells, matured DCs were stained with nonyl acridine orange (NAO) on day 8, and the frequency of apoptotic cells was analyzed by flow cytometry. Briefly, DCs (5×105 cells) were stained with 0.5 µg/ml NAO (Molecular Probes; Invitrogen, Paisley, UK) in RPMI-1640 for 15 min at 37 °C in 5% CO2. The cells were then washed and immediately analyzed using a FACSAria cell sorter (Becton Dickinson, San Jose, CA, USA). A light scatter gate was used to exclude events with very low forward and side scatter from the NAO samples on the assumption that they were cell debris. Data were analyzed with WinMDI Version 2.9 software (Biology Software Net).
Phenotypic and cytokine analysis of DCs
Fluorescence-activated cell sorting (FACS) analysis was performed using phycoerythrin-labeled monoclonal antibodies (mAbs) specific for human CD80, CD86 or CD38 or using fluorescein isothiocyanate-labeled mAbs specific for CD83 or CCR7 (all from BD Biosciences Pharmingen). Isotype matched controls were analyzed in parallel. Cell debris was eliminated from the analysis by forward and side scatter gating. Data were acquired on a FACSAria cell sorter (Becton Dickinson) and analyzed with FACSDiva (Becton Dickinson) and Win MDI Version 2.9 software (Biology Software Net).
To analyze cytokine production, we measured the levels of IFN-γ and IL-12p70 in the culture supernatants using a BD OptEIA ELISA Set (BD Bioscience). The mature DCs were harvested on day 8, plated in 96-well flat-bottomed plates at a density of 2×104 cells/well, and stimulated for 24 h with 5×104 CD40 ligand (CD40L)-transfected J558 cells (kindly provided by Dr Kalinski at the University of Pittsburgh, PA, USA), which mimic the interaction with CD40L-expressing Th cells, to measure the ability of DCs matured under different conditions to secrete IL-12p70 upon secondary activation.
T-cell stimulatory capacities of DCs
Allogeneic CD3+ T cells obtained from healthy volunteers were isolated using the magnetic activated cell sorting system (Miltenyi Biotec). T cells (2×104/well) were cocultured in 96-well U-bottomed plates with graded doses (3.12×102–1.25×103) of irradiated (30 Gy) mature DCs. On day 5, the cultured cells were pulsed with 1 µCi of [3H]-methylthymidine per well during the last 18 h of culture and analyzed in a liquid scintillation counter (Beckman, Fullerton, CA, USA). The results are expressed as the mean c.p.m.±s.d. of values obtained from triplicate samples. Unprimed T cells were used as a control.
Differentiation of naive T cells by DCs
Naive allogeneic CD4+ T cells were obtained from peripheral blood mononuclear cells of healthy volunteers by negative selection with a naive CD4+ T-cell isolation kit (Miltenyi Biotec). The purity of isolated CD45RA+ cells was >95% by FACS. CD4+CD45RA+ naive T cells (2×104) were stimulated with 2×104 matured DCs that were pre-incubated with 1 ng/ml of Staphylococcus aureus enterotoxin B in 96-well flat-bottomed plates. On day 5, 10 U/ml rhIL-2 was added to each well. On day 10, the expanded CD4+ Th cells were washed, counted, plated in 96-well plates, and stimulated with CD3 and CD28 mAbs (Dynabeads CD3/CD28 T Cell Expander; Invitrogen). The supernatants from restimulated CD4+ Th cells were collected after 24 h, and the concentrations of the Th1 cytokine IFN-γ and the Th2 cytokine IL-13 were measured using a BD OptEIA ELISA Set (BD Bioscience).
Migration assays
The ability of DCs to undergo chemotaxis toward the chemokines CCL19 and CCL21 was measured by migration through a polycarbonate filter of 5 µm pore size in 24-well transwell chambers (Corning Costar), as described previously.8 Briefly, the lower chamber was filled with 600 µl of medium containing 250 ng/ml of CCL19 and CCL21 (BD Biosciences) or medium alone as a control. DCs (1×105) were added to the upper chamber in 100-µl medium. Migration chambers were incubated for 3 h at 37 °C. A 500-µl aliquot of the cells that migrated to the bottom chamber was assessed by flow cytometry using a FACSAria cell sorter (BD Biosciences). Events were acquired for a fixed period of 60 s using CellQuest software (BD Biosciences). The results are expressed as the number of migrated cells, which was defined as follows: the number of cells that migrated into the lower chamber containing chemokines−the number of cells that migrated into the lower chamber containing medium alone.
In vitro tumor-specific CTL expansion
CTLs were generated as previously described.20 Autologous CD3+ T cells obtained from the lymphocyte fraction from the same donor were isolated using the magnetic activated cell sorting system (Miltenyi Biotec). CD3+ T cells (1×106) were stimulated with irradiated DCs (2×105 cells) that had previously been loaded with ultraviolet B-irradiated ARH-77 tumor cells. On day 3, 10 ng/ml rhIL-2 and 5 ng/ml IL-7 (both from R&D Systems) were added. The CTLs were restimulated with the same DCs on day 10. After 20 days, the CTLs were harvested, and 51Cr release assays were performed to measure the cytotoxic activities of the CTLs.
51Cr release assays
Standard 4-h 51Cr release assays were performed as described previously.21 ARH77 cells were used as target cells with or without pre-incubation with MHC I antibodies to assess MHC-restricted cytotoxicity in CTLs. The target tumor cells (1×106) were labeled with 200 µCi (3.7 MBq) of Na251CrO4 (Amersham Pharmacia Biotech Benelux, Roosendaal, The Netherlands) for 1 h. The 51Cr-labeled target cells (1×104 cells/well) were cocultured with CTLs at various effector-to-target (E/T) ratios for 4 h. Spontaneous release and maximal release were assessed in parallel. Supernatants were collected, and the release of 51Cr was measured in an automatic gamma counter (WizarD3; PerkinElmer, Waltham, MA, USA). The percentage of specific lysis was calculated as follows: % specific cytotoxicity=(experimental release (c.p.m.)−spontaneous release (c.p.m.))/(maximal release (c.p.m.)−spontaneous release (c.p.m.))×100%.
Statistical analysis
All statistical analyses were performed with SPSS 13.0 for Windows. The Mann–Whitney U test was performed to analyze the statistical significance of non-parametric differences between the groups. P<0.05 was considered statistically significant.
Results
Direct cell-to-cell contact is required for NK cell-mediated DC maturation
To determine whether NK cell-mediated DC maturation requires direct cell-to-cell contact or involves soluble factors, we compared the maturation status of DCs following coculture with NK cells with or without separation by a transwell membrane in the presence of IL-2 and/or poly(I:C). On day 8 of the coculture, DCs that were cocultured in direct contact with NK cells displayed a more activated phenotype than DCs that were separated from NK cells by a transwell membrane (Figure 1). Immature DCs expressed moderate levels of CD86 and very low levels of CD83. The activation of NK cells with IL-2 alone induced upregulation of CD86 and CD83 expression; however, when NK cells were stimulated with a combination of IL-2 and poly(I:C), the cocultured DCs showed enhanced expression of CD86 and CD83. As we expected, the expression levels of these molecules were significantly higher in DCs cocultured in direct contact with NK cells than in DCs separated from NK cells by a transwell membrane (Figure 1a). Furthermore, direct coculture of NK cells with immature DCs in the presence of either IL-2 alone or IL-2 and poly(I:C) significantly increased production of IL-12p40 (Figure 1b) and IL-12p70 (Figure 1c), both during maturation and after subsequent CD40L stimulation (*P<0.05). Thus, NK cell-mediated DC maturation required direct cell-to-cell contact, and the effective induction of DC maturation in the bystander model suggests a key role for NK cell-produced soluble factors in this process.
Figure 1.
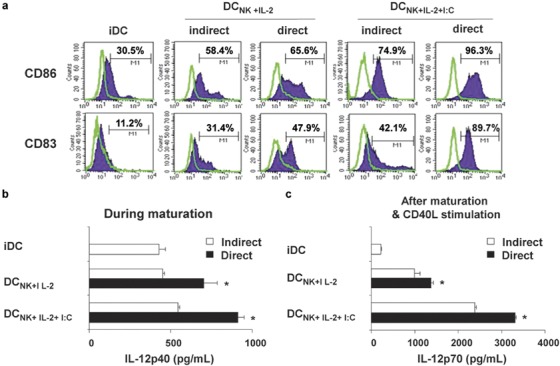
Optimal culture conditions for natural killer (NK) cell-mediated dendritic cell (DC) maturation. Immature DCs (iDCs) were cocultured with NK cells or separated from NK cells by transwell membranes in the presence of IL-2 (DCNK+IL-2) or IL-2 and poly(I:C) (DCNK+IL-2+I:C). (a) DC maturation was determined by measuring CD86 and CD83 expression by flow cytometry. DCs in direct coculture expressed high levels of CD86 and CD83. Shown are representative histograms demonstrating the expression of surface markers (shaded histogram) compared with isotype controls (open histogram). These figures are representative of three independent experiments. (b) Cytokine production was measured in the supernatants from cultures of iDCs (gray bars) and from cocultures of DCs and NK cells in indirect contact (white bars) or direct contact (black bars). IL-12p40 levels were significantly higher in supernatants from cells cocultured in direct contact (*P<0.05). (c) To assess IL-12p70 production, all DCs were further stimulated with CD40L-transfected J558 cells. IL-12p70 levels were significantly higher in supernatants from cells cocultured in direct contact (*P<0.05).
NK cells induce type 1-polarized DCs with the capacity to produce IL-12p70
To determine whether resting or activated NK cells could enhance DC maturation, we analyzed the phenotype of DCs that were cocultured with either freshly isolated resting NK cells in the presence of IL-2, poly(I:C) and IFN-α or NK cells that had been activated with these stimuli before coculturing. On day 6 of culture, iDCs expressed intermediate levels of CD86 and low levels of CD83, CCR7 and CD38. Coculturing iDCs with resting NK cells in the presence of various stimuli induced upregulation of CD86, CD83 and CCR7 on DCs more efficiently than coculturing iDCs with activated NK cells. In addition, DCs cocultured with resting NK cells in the presence of stimuli also expressed high levels of CD38, a recently identified DC activation marker that orchestrates migration, survival and induction of Th1 immune response by DCs.8, 22 The expression of these molecules in this study was dependent not only on the type of NK cells used but also on the stimulation conditions (IL-2+poly(I:C)+IFN-α>IL-2+poly(I:C)>IL-2) (Figure 2a).
Figure 2.
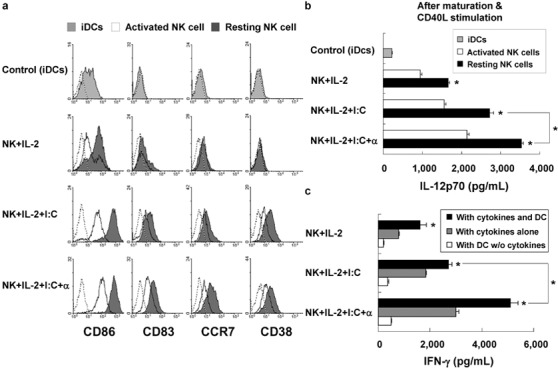
Resting natural killer (NK) cells potently induce dendritic cell (DC) maturation. (a) Cultured immature DCs (iDCs) were incubated alone (iDC); with NK cells and IL-2 (NK+IL-2); with NK cells, IL-2 and poly(I:C) (NK+IL-2+I:C); with NK cells, IL-2, poly(I:C) and interferon (IFN)-α (NK+IL-2+I:C+α). The expression of CD86, CD83, CCR7 and CD38 on DCs that were induced by resting NK cells (shaded histogram) was significantly higher than that on DCs induced by activated NK cells (open histogram). Open histograms (dotted line) show the background staining with the isotype control. The data shown were obtained in a single experiment and are representative of three independent experiments. (b) IL-12p70 production after subsequent stimulation of DCs with CD40L-transfected J558 cells. Resting NK cells cultured DCs to produce significantly higher levels of IL-12p70 than did activated NK cells (*P<0.05). (c) IFN-γ production in NK cells. Purified NK cells were cultured with DCs alone or with cytokines and/or DCs. IFN-γ production during cultivation was measured. The data are shown as the mean (pg/ml)±s.d. of values from triplicate cultures obtained in a single experiment and are representative of four independent experiments.
To determine whether coculturing iDCs with resting NK cells in the presence of different stimuli could enhance DC maturation, we measured the production of IL-12p70 after subsequent stimulation of the cocultured DCs with CD40L-transfected J558 cells. In accord with the surface phenotype of DCs cocultured with resting NK cells in the presence of stimuli, we found that these DCs demonstrated enhanced IL-12p70 production when compared to DCs cocultured with activated NK cells or control iDCs (Figure 2b) (P<0.05). In addition, the production of IL-12p70 was also dependent on the stimuli present during the coculture period (IL-2+poly(I:C)+IFN-α>IL-2+poly(I:C)>IL-2).
Previous studies have shown that soluble factors affect DC maturation, including IFN-γ and tumor-necrosis factor (TNF)-α, are produced during DC–NK cell interactions.17 We found that IFN-γ production was significantly higher in NK cells that were activated with both DCs and cytokines than in those cells activated with cytokines alone or DCs alone (P<0.05) (Figure 2c). The same pattern was observed for TNF-α (data not shown). Interestingly, the production of IFN-γ and TNF-α was also dependent on the stimuli present during the coculture period (IL-2+poly(I:C)+IFN-α>IL-2+poly(I:C)>IL-2).
NK cells induce apoptosis of un-matured DCs to further enhance DC maturation
Theoretically, NK cell activation results in the killing of un-matured DCs.13, 14, 15 We therefore evaluated the capacity of NK cells to induce apoptosis in un-matured DCs. Because decreased levels of cardiolipin in the mitochondrial inner membrane are indicative of apoptosis,23 we stained harvested DCs with NAO to detect the loss of mitochondrial cardiolipin, and thus mitochondrial damage, by flow cytometry. NK cell activation resulted in a number of apoptotic cells under all culture conditions. A slight increase in apoptotic DCs was observed when DCs were cocultured with NK cells in the presence of poly(I:C) with or without IFN-α however, no significant difference in apoptosis was observed between DCs cocultured with activated or resting NK cells in the absence of stimuli (Figure 3). These results suggest that coculturing DCs with NK cells may result in the killing of un-matured DCs, allowing for the selection of mature DCs.
Figure 3.
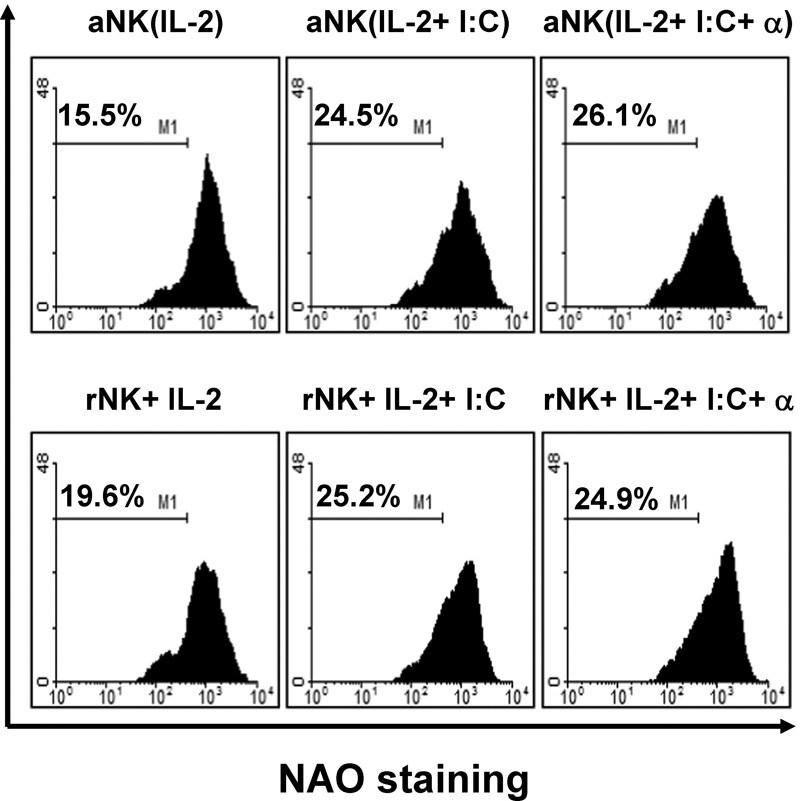
Natural killer (NK) cells induce apoptosis of un-matured dendritic cells (DCs). Apoptosis of DCs during coculture with NK cells was evaluated by detecting nonyl acridine orange (NAO) staining by flow cytometry. The number of dying cells was increased in all DCs cultured with NK cells. This figure is representative of three independent experiments.
Resting NK cell-stimulated DCs strongly enhance T-cell proliferation and drive Th1 polarization of naive T cells
To evaluate the proliferation of T cells, allogeneic CD3+ T cells obtained from healthy volunteers were stimulated with various types of DCs, and allogeneic mixed lymphocyte reactions were performed in three independent experiments using [3H]-methylthymidine incorporation assays. As shown in Figure 4a, DCs cocultured with resting NK cells in the presence of poly(I:C) and/or IFN-α demonstrated a more potent allostimulatory capacity than other DCs (P<0.05), and this capacity was dependent on the DC/T cell ratio. Stimulation with IL-2 did not induce potent allostimulatory capacity in DCs cocultured with either resting or activated NK cells.
Figure 4.
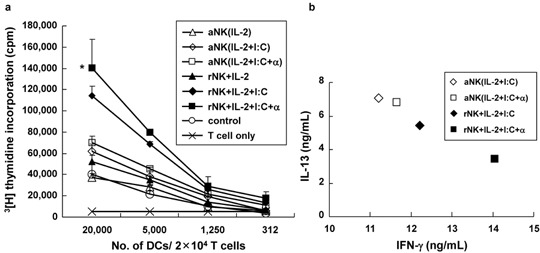
Resting natural killer (NK) cells act as activating helper cells to induce T helper 1 (Th1) polarization of dendritic cells (DCs). (a) Allogeneic mixed lymphocyte reactions. (MLRs) were performed using a [3H]-methylthymidine incorporation assay. CD3+ T cells (20 000 cells/well) obtained from healthy donors were stimulated with graded doses of irradiated DCs for 5 days. Incorporation of [3H]-methylthymidine was used to measure the proliferative response. The stimulatory capacity of DCs that were cocultured with resting NK cells was dependent on the DC/T cell ratio and was significantly higher than that of DCs that were co-cultured with activated NK cells (*P<0.05). The data are shown as the mean c.p.m. (±s.d.) of values from triplicate cultures from three independent experiments. (b) Resting NK cells induce Th1 polarization of DCs. Naive CD4+CD45RA+ T cells were primed with DCs and restimulated with CD3/CD28 antibodies. Culture supernatants were analyzed for IL-13 and interferon (IFN)-γ levels by ELISA. DCs cocultured with resting NK cells induced higher levels of Th1 cytokine (IFN-γ) expression than Th2 cytokine (IL-13) expression. The filled symbol represents an individual experiment using resting NK cell-stimulated DCs, while the open symbol represents an individual experiment using activated NK cell-stimulated DCs. The results were obtained in a single experiment and are representative of three similar independent experiments.
To assess the ability of DCs to induce Th1 polarization, we compared the levels of Th1 cytokine (IFN-γ) and Th2 cytokine (IL-13) production in naive T cells primed by DCs cocultured with NK cells under different conditions. DCs cocultured with resting NK cells in the presence of poly(I:C) and IFN-α induced strong Th1 responses in naive T cells, resulting in the production of high levels of IFN-γ and low levels of IL-13 after restimulation with CD3/CD28 mAbs.
Resting NK cells enhance the migration of DCs toward CCL19 and CCL21
As migration is a key element of DC biology, we also investigated whether coculture with NK cells influenced the migratory response of DCs in vitro. Coculturing DCs with NK cells in the presence of TLR agonists and IFN-α upregulated CCR7 and CD38 on DCs, resulting in DC homing to lymph nodes.8, 22 Recent reports have suggested that CCR7 expression does not always guarantee CCL19/CCL21 responsiveness.24 In addition, the upregulation of CD38 has been shown to regulate DC migration in vitro.8 Resting NK cell-stimulated DCs enhanced the expression of CCR7 and CD38, compared to activated NK cell-stimulated DCs; we thus examined the migratory capacity of these DCs. LPS-stimulated DCs were assessed in parallel as a control. As we expected, the capacity of DCs cocultured with resting NK cells to migrate towards CCL19 and CCL21 was greater than those of either LPS-stimulated DCs or DCs cocultured with activated NK cells (P<0.05) (Figure 5).
Figure 5.
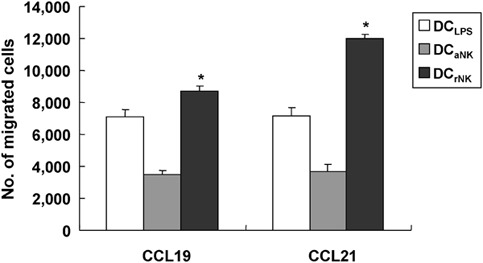
Migration in response to CCL19 and CCL21 by natural killer (NK) cell-stimulated dendritic cells (DCs). Migration of resting NK cell-stimulated DCs (DCrNK) was enhanced in response to CCL19 (250 ng/ml) and CCL21 (250 ng/ml), compared to that of either lipopolysaccharide (LPS)-stimulated DCs (DCLPS) or activated NK cell-stimulated DCs (DCaNK). The results are presented as the mean number of migrated cells±s.d. from one of three independent experiments (*P<0.05).
Resting NK cell-polarized DCs have an enhanced ability to induce potent tumor-specific CTL responses
To determine the ability of NK cell-stimulated DCs to induce potent tumor-specific CTL responses, we performed a standard 4-h [51Cr] release assay in three independent experiments. As shown in Figure 6, the CTLs generated by DCs that were stimulated with resting NK cells in the presence of stimuli, but not those simulated with activated NK cells, exhibited highly specific killing activity against ARH77 cells. While the cytotoxic activity of CTLs induced by activated NK cell-stimulated DCs was inhibited by anti-MHC class I antibody only at an E/T ratio of 20∶1, the cytolytic activity of CTLs induced by resting NK cell-stimulated DCs against ARH77 cells was markedly inhibited at E/T ratios of both 20∶1 and 10∶1. Thus, experiments using an MHC class I blocking antibody verified that CTL responses induced by resting NK cell-stimulated DCs were HLA class I-restricted. None of the DCs generated CTL responses against K562 cells (data not shown).
Figure 6.
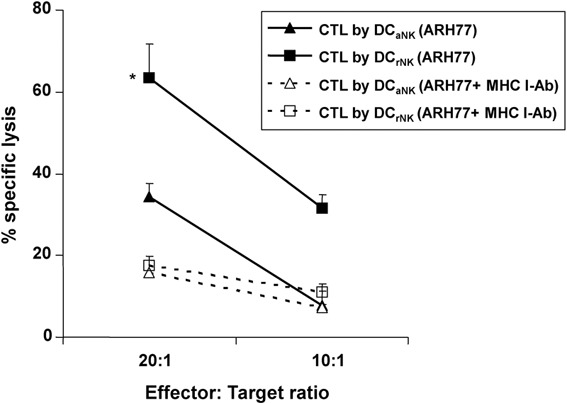
Specific killing activity of cytotoxic T lymphocytes (CTLs) was measured by 51Cr release assays. The CTLs induced by ARH77-loaded DCs were incubated with 51Cr-labeled target cells at an effector-to-target ratio (E/T) of 20∶1 or 10∶1. The amount of 51Cr released from the lysed target cells was measured. The CTLs stimulated by resting natural killer (NK) cell-stimulated DCs exhibited higher specific killing activity against ARH77 cells than those stimulated by activated NK cell-stimulated DCs (*P<0.05). Anti-major histocompatibility complex (MHC) I-blocking antibody was used to confirm the MHC class I dependence of target cell recognition. Data are shown as the means of values from triplicate cultures from a single experiment and are representative of three similar independent experiments.
Discussion
This study demonstrates that freshly isolated resting NK cells obtained from peripheral blood are capable of stimulating the maturation of DCs in the presence of IL-2, poly(I:C) and IFN-α. In the presence of such NK cells, immature DCs matured to form phenotypically and functionally mature DCs. Importantly, these DCs expressed high levels of CD38, a molecule typically associated with DC migration, survival and induction of Th1 immune responses. These DCs efficiently produced IL-12p70 and stimulated T-cell proliferation and Th1 polarization of naive CD4+ T cells. Resting NK cell-induced DCs were also potent antigen-presenting cells because they demonstrated an enhanced ability to induce tumor-specific CTL responses. In addition, we defined two culture conditions required for an efficient NK cell–DC interaction: (i) direct cell-to-cell contact; and (ii) coculture of DCs with freshly isolated resting NK cells in the presence of stimuli, which resulted in more efficient DC maturation than did coculture with pre-activated NK cells. Several cytokines, such as type I IFN, IL-12, TNF-α and IL-15, are potent inducers of NK cell activation, and all of them are secreted by human DCs, following culture with maturation stimuli.25 Reciprocally, DCs can be activated by interaction with NK cells in the presence of several cytokines that can be secreted by activated NK cells.12, 13, 14, 15 In accordance with recent reports,13, 14, 15 our results show that although coculturing DCs and NK cells separated by a transwell insert in the presence of stimuli can induce the maturation of DCs, direct cell-to-cell contact is necessary for NK cell-mediated DC maturation. In addition, NK cells enhanced the maturation of iDCs in both the presence and the absence of TLR3 agonist (poly(I:C)) in direct contact conditions; however, culturing DCs in direct contact with NK cells in the presence of a TLR3 agonist resulted in increased expression of CD86 and CD83 and enhanced cytokine production.
In our study, the crucial factor for the successful generation of potent DCs via NK cell-mediated maturation was the use of resting NK cells in the presence of stimuli rather than activated NK cells. The two-signal requirement for the induction of NK “helper” activity was met by using freshly isolated NK cells in the presence of an additional costimulatory signal.7, 16 The presence of IFN-γ (which can be induced by TLR ligands) during DC maturation overcomes maturation-associated DC ‘exhaustion', yielding stable type 1-polarized DCs that produce higher levels of IL-12p70 and exhibit a dramatically enhanced capacity to induce Th1 responses.26 It has been reported that IFN-γ is produced by NK cells after 17 h of stimulation with poly(I:C), and it peaks at 48 h.27 In our study, the level of IFN-γ production in resting NK cells after 48 h of stimulation in the presence of TLR agonists and cytokines was higher than that observed in either activated NK cells or resting NK cells in the absence of costimulatory signals. This finding may explain why resting NK cells in the presence of stimuli induced the maturation and function of DCs more efficiently than activated NK cells. Furthermore, the cross-talk between immature DCs and resting NK cells leads to cell activation only in the presence of additional stimuli.13, 14, 15 A second reason for the enhanced ability of resting NK cells in the presence of stimuli to induce DC maturation may be the killing activity of activated NK cells. Although we did not detect any significant killing of DCs by resting NK cells or activated NK cells, it is possible that depending on the cytolytic activity of activated NK cells, the DC/NK cell ratio or the presence of particular NK cell subsets, NK cells will mainly kill or inactivate DCs, resulting in suppression of the immune response.7
The key to the initiation of immune responses to pathogens is the recognition of pathogen associated ligands that are not normally expressed by host cells. This recognition is mediated by members of the TLR family, several of which are expressed by immature DCs28 and NK cells.27, 29 The maturation of DCs (which is considered crucial for functions, including survival, migration, secretion of cytokines and chemokines and expression of costimulatory molecules) is influenced by signaling through TLRs.28 Human NK cells express functional TLR3, enabling them to respond to viral infection through enhanced cytokine production and cytotoxic activity.29 To initiate DC activation and maturation, NK cells must be activated, and our data show that NK cells produced higher levels of IFN-γ and TNF-α upon stimulation by exogenous cytokines. The production of cytokines by NK cells was dramatically enhanced when NK cells were cocultured with DCs in the presence of exogenous cytokines. These results suggest that NK cells efficiently enhance DC maturation in coculture systems.
To investigate the mechanism underlying the DC maturation induced by resting NK cells in the presence of stimuli, we used IL-2 as the first activator of NK cells. IL-2-activated human NK cells can trigger maturation of immature DCs by upregulating the costimulatory molecule CD86, resulting in enhancement of the T-cell stimulatory capacity of DCs.15 In accord with previous reports, our results showed that only resting NK cells efficiently induced the maturation of DCs, as indicated by the upregulation of maturation-related markers and the increased production of IL-12. In contrast, IL-2-activated NK cells induced DC maturation less efficiently. Therefore, our findings suggest that efficient interactions between NK cells and DCs required conditions in which both cells were activated together.
Previous studies have shown that IFN-α has important functions in DC maturation, NK cell activation, Th1 polarization and CTL priming in vitro.30, 31 The presence of IFN-α during DC maturation results in the development of type 1-polarized effector DCs with an enhanced ability to produce IL-12 and a strong Th1-inducing capacity.3 Therefore, by providing a ‘signal 3', the addition of exogenous IFN-α is the DC-dependent component of the initial polarization of naive Th cells to preferentially produce Th1 cytokines. Our data demonstrate that potent DCs can be generated by coculture with resting NK cells in the presence of TLR3 agonist, IL-2 and IFN-α. Although IFN-α can be secreted by human DCs upon maturation stimulus, the addition of exogenous IFN-α increases the production of IL-12p70 and may induce Th1 immune responses in our system. Coculturing DCs with resting NK cells in combination with these stimuli increased the expression of the surface molecule (CD86, CD83, CD38 and CCR7) on the DCs, the production of IL-12, the potential to induce Th1 polarization and the induction of tumor-specific CTLs. Our data suggest the existence of a positive feedback loop that augments both NK cell activation and DC maturation.
IL-2-activated human NK cells can trigger maturation of immature DCs by upregulating costimulatory molecules, resulting in enhancement of the T-cell stimulatory capacity of DCs. TLRs are expressed in both NK cells and DCs, and TLR ligands play a role in both the activation of NK cells and the maturation of immature DCs. TLR-dependent stimulation that results in full activation of DCs induces IFN-γ production by NK cells. This function is dependent on both exogenous IL-2 and IL-2 secreted by activated DCs. It has been reported that TLR ligands do not induce IFN-γ production by NK cells in the absence of IL-12.32 IFN-α, which is secreted by virus-infected cells, can induce both DC maturation and NK cell activation. In the presence of an inflammatory stimulus (TLR ligands, type I IFN or bacterial products), DC/NK cross-talk leads to not only NK cell activation but also DC maturation.14, 15 Therefore, we propose the following model to explain the ability of DC-NK cell coculture in the presence of IL-2, poly(I:C) and IFN-α to induce DC maturation. After recognizing the microbial TLR ligand poly(I:C), NK cells become activated. At the same time, immature DCs are activated by poly(I:C) to release abundant IL-12. The secreted IL-12 and the additional IL-2 further induce NK cell activation. Stimulated NK cells produce increased levels of IFN-γ to promote further maturation of DCs, resulting in increased production of IL-12p70 and acquisition of the capacity to induce Th1 immune responses. In addition, IL-12-stimulated NK cells become highly susceptible to other stimuli. This process forms a positive feedback loop between TLR-induced production of IL-12 and IL-12-mediated enhanced TLR ligand responsiveness in NK cells. Furthermore, NK cells exposed to IL-12 are further activated and can kill immature DCs, thus favoring the survival of mature DCs. In addition, IFN-α provides a ‘signal 3' that can enhance both the activation of NK cells and the maturation of DCs, thus further promoting cross-talk between DCs and NK cells.
DCs induced by NK cells and NK cell-related products are powerful inducers of tumor-specific immune responses.18, 33 These observations, which have been made both in vitro and in mice, suggest a potential method to improve the efficacy of DC immunotherapy by using NK-stimulated DCs. Examination of allogenic T-cell responses and tumor-specific T-cell cytotoxicity showed that resting NK cell-stimulated DCs induced more effective and functional T-cell responses than activated NK cell-stimulated DCs. Our results suggest that NK cell-stimulated DCs could be potentially useful for immunotherapy against cancer.
In conclusion, the results of our study suggest that potent DCs can be generated by resting NK cells in the presence of TLR agonist and pro-inflammatory cytokines, and these DCs can be used to induce tumor-specific CTLs that exert an effective anti-tumor immune response for cancer immunotherapy.
Acknowledgments
This study was financially supported by grant no. RTI05-01-01 from the Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy, Republic of Korea.
References
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- Moser M, Murphy KM. Dendritic cell regulation of TH1–TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, et al. CD40 triggering of heterodimeric IL-12p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- Frasca L, Fedele G, Deaglio S, Capuano C, Palazzo R, Vaisitti T, et al. CD38 orchestrates migration, survival, and Th1 immune response of human mature dendritic cells. Blood. 2006;107:2392–2399. doi: 10.1182/blood-2005-07-2913. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. . Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski P, Giermasz A, Nakamura Y, Basse P, Storkus WJ, Kirkwood JM, et al. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol Immunol. 2005;42:535–539. doi: 10.1016/j.molimm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Kalinski P, Mailliard RB, Giermasz A, Zeh HJ, Basse P, Bartlett DL, et al. Natural killer-dendritic cell cross-talk in cancer immunotherapy. Expert Opin Biol Ther. 2005;5:1303–1315. doi: 10.1517/14712598.5.10.1303. [DOI] [PubMed] [Google Scholar]
- Pham TN, Hong CY, Min JJ, Rhee JH, Nguyen TA, Park BC, et al. Enhancement of antitumor effect using dendritic cells activated with natural killer cells in the presence of Toll-like receptor agonist. Exp Mol Med. 2010;42:407–419. doi: 10.3858/emm.2010.42.6.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Choi BH, Kang HK, Park MS, Park JS, Kim SK, et al. Induction of multiple myeloma-specific cytotoxic T lymphocyte stimulation by dendritic cell pulsing with purified and optimized myeloma cell lysates. Leuk Lymphoma. 2007;48:2022–2031. doi: 10.1080/10428190701583975. [DOI] [PubMed] [Google Scholar]
- Kim SK, Nguyen Pham TN, Nguyen Hoang TM, Kang HK, Jin CJ, Nam JH, et al. Induction of myeloma-specific cytotoxic T lymphocytes ex vivo by CD40-activated B cells loaded with myeloma tumor antigens. Ann Hematol. 2009;88:1113–1123. doi: 10.1007/s00277-009-0721-y. [DOI] [PubMed] [Google Scholar]
- Fedele G, Frasca L, Palazzo R, Ferrero E, Malavasi F, Ausiello CM. CD38 is expressed on human mature monocyte-derived dendritic cells and is functionally involved in CD83 expression and IL-12 induction. Eur J Immunol. 2004;34:1342–1350. doi: 10.1002/eji.200324728. [DOI] [PubMed] [Google Scholar]
- King MA, Eddaoudi A, Davies DC. A comparison of three flow cytometry methods for evaluating mitochondrial damage during staurosporine-induced apoptosis in jurkat cells. Cytometry A. 2007;71:668–674. doi: 10.1002/cyto.a.20428. [DOI] [PubMed] [Google Scholar]
- Sanchez-Sanchez N, Riol-Blanco L, Rodriguez-Fernandez JL. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J Immunol. 2006;176:5153–5159. doi: 10.4049/jimmunol.176.9.5153. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- Schmidt KN, Leung B, Kwong M, Zarember KA, Satyal S, Navas TA, et al. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Akira S. Regulation of dendritic cell function through Toll-like receptors. Curr Mol Med. 2003;3:373–385. doi: 10.2174/1566524033479726. [DOI] [PubMed] [Google Scholar]
- Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: Induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. . Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- Farrar JD, Murphy KM. Type I interferons and T helper development. Immunol Today. 2000;21:484–489. doi: 10.1016/s0167-5699(00)01710-2. [DOI] [PubMed] [Google Scholar]
- Chace JH, Hooker NA, Mildenstein KL, Krieg AM, Cowdery JS. Bacterial DNA-induced NK cell IFN-gamma production is dependent on macrophage secretion of IL-12. Clin Immunol Immunopathol. 1997;84:185–193. doi: 10.1006/clin.1997.4380. [DOI] [PubMed] [Google Scholar]
- Yang DH, Kim MH, Hong CY, Lee YK, Jin CJ, Pham TN, et al. Alpha-type 1-polarized dendritic cells loaded with apoptotic allogeneic myeloma cell line induce strong CTL responses against autologous myeloma cells. Ann Hematol. 2010;89:795–801. doi: 10.1007/s00277-010-0931-3. [DOI] [PubMed] [Google Scholar]


