Abstract
To explore the significance of cancerous immunoglobulin (Ig) in cancer cell growth, HeLa cervical cancer cells were stably transfected with small interfering RNA (siRNA) that specifically, efficiently and consistently silences the expression of heavy chain genes of all immunoglobulin isotypes. This stable cell line was used to examine cell viability, colony formation and tumor growth in athymic nude mice. The results of these experiments indicated that siRNA-mediated knockdown of cancerous Ig inhibited cell growth in vitro and suppressed tumor cell growth in immune-deficient nude mice in vivo. Similarly, this siRNA also inhibited the growth of MGC gastric cancer cells and MCF-7 breast cancer cells. Furthermore, the presence of cancerous Ig specifically reduced antibody-dependent cell-mediated cytotoxicity (ADCC) induced by an anti-human epithelial growth factor receptor (EGFR) antibody in a dose-dependent manner, suggesting that the cancerous Ig-Fc receptor interaction inhibits natural killer cell (or NK cell) effector function. The prevalent expression of Ig in human carcinomas and its capacity to promote growth and inhibit immunity might have important implications in growth regulation and targeted therapy for human cancers.
Keywords: antibody-dependent cell-mediated cytotoxicity (ADCC), cancerous immunoglobulin, cell proliferation, small interfering RNA (siRNA)
Introduction
In 1991, we cloned the transforming gene Tx (GenBank accession no. AF276073) from the genomic DNA library of the human nasopharyngeal carcinoma cell line CNE2. Upon transfection with this gene, the mouse epithelial JB6 P+ cell line undergoes neoplastic transformation in vitro and in vivo, demonstrating that the Tx gene is associated with cell transformation.1 Blast analysis demonstrated that Tx was a human immunoglobulin (Ig) kappa light chain gene lacking the variable region, suggesting that cancer cells express the Ig kappa light chain.2 Accumulating evidence has recently revealed that epithelial cancer cells and cancer tissues express ‘Ig-like' molecules, such as the Ig kappa light chain, as well as Ig heavy chains, including α, γ and µ, in both cytoplasmic and secreted forms.3, 4, 5, 6, 7, 8, 9, 10 In our previous studies addressing the significance of cancerous Ig expression, we found that depletion of tumor-derived Ig by either antisense RNA or a human IgG or IgA antibody resulted in an increase in apoptosis and inhibition of cancer cell growth in vitro and in vivo.3, 11 Moreover, we extended our findings by using in situ hybridization to detect Ig kappa light chain expression in clinical cervical tissue samples, including cervicitis, dysplasia and cancer samples. In these experiments, we detected a remarkable increase in Ig kappa light chain expression that paralleled the escalating severity of epithelial transformation. This finding suggests that the expression of Ig kappa is as an early molecular event in cervical cancer progression;9 however, further exploration of the functions of cancerous Ig are necessary.
Elevated levels of serum IgG, IgA or IgM antibodies are frequently observed in patients with cancers of epithelial origin, including carcinoma of the breast, gastric system and liver.12, 13 These tumor-derived Igs suppress the host's humoral response to cancer growth, which is mediated by secreted antibodies.14 Many of the effector functions of secreted antibodies are mediated by the heavy chain constant regions of Ig molecules. For example, nature killer cells (or NK cells) and other leukocytes bind antibody-coated target cells through interactions between the Fc receptor and the Fc domain of the specific antibody and destroy the target cells. This process is referred to as antibody-dependent cell-mediated cytotoxicity (ADCC). The ability of cancerous Ig to inhibit humoral immunity against tumor growth is thus hypothesized to be due to its ability to inhibit ADCC by competitively binding to the Fc receptor on NK cells.
In this study, we constructed a cell line stably expressing a small interfering RNA (siRNA) that specifically and efficiently silenced the expression of all isotypes of cancerous Ig heavy chains. The growth of this cell line was assessed in vitro and in immunodeficient nude mice. The effectiveness of this siRNA was further demonstrated in gastric and breast cancer cell lines. Moreover, to explore the capacity of cancerous Ig to inhibit the humoral response, the ability of purified and labeled cancerous Ig to bind the Fc receptor on NK cells and the effect of cancerous Ig on ADCC induced by an anti-human epithelial growth factor receptor (EGFR) antibody were assessed.
Materials and methods
siRNA design
The siRNA insert sequence designed to silence expression of all types of Ig heavy chains is as follows: CATGCAGCGCGTAGGGACA. This siRNA was predicted to inhibit Ig heavy chain expression by binding to the VDJ region of the Ig genes. As a negative control, a mock (scrambled) vector was designed with the sequence TTCTCCGAACGTGTCACGT. This siRNA did not target any human genes. Sense and antisense oligonucleotides were annealed and subcloned into the small hairpin RNA (shRNA) expression vector pSM2 (OpenBiosystems; Thermo Fisher Scientific, Huntsville, AL, USA) at XhoI and BamHI sites according to the manufacturer's instructions. The correct insertions of the siRNA cassettes were confirmed by direct DNA sequencing. The resulting plasmids were designated as psi-IgH and psi-scrambled, respectively. Plasmid DNA was extracted from Escherichia coli transformants using the Fastfilter Endo-free Plasmid Midi Kit (Omega Bio-Tek Inc., Doraville, GA, USA).
Transfection of plasmids and establishment of stable cell lines
HeLa cells were transfected with psi-IgH or psi-scrambled using Arrest-In (OpenBiosystems; Thermo Fisher Scientific) and were selected after 1 week by culturing cells in the presence of 500 µg/ml puromycin (Gibco-BRL, Gaithersburg, MD, USA) in RPMI 1640 medium containing 10% fetal bovine serum. The cells were then maintained in normal RPMI 1640 medium containing 200 µg/ml puromycin. Individual clones were selected and referred to as psi-IgH-HeLa and psi-scrambled-HeLa. Silencing of Ig heavy chain genes was confirmed by RT-PCR. Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Grand Island, NY, USA). Total RNA (1 µg) from each cell line was used to synthesize cDNA using Superscript RTII (Invitrogen). The resulting cDNA was used as a template, and PCR was performed using Taq polymerase (Promega, Madison, WI, USA) and the following primers: 5′-GTGACCAGGGTNCCTTGGCCCCAG-3′ and 5′-GACACGGCTGTGTATTACTGTGCG-3′. These primers amplify a 100-bp product that represents part of the VDJ coding region of the Ig heavy chain. β-actin, which was used as an internal loading control, was amplified using the following primer pair: 5′-TTGCCGTCAGGAGGAGCAAT-3′ (forward) and 5′-TTCCAGCCTTCCTTCCTGGG-3′ (reverse). The resulting product was 200 bp.
MTS assay
HeLa, psi-IgH-HeLa, and psi-scrambled-HeLa cells (100 µl, 104 cells/ml) were separately seeded at the same time into 96-well plates and routinely cultured for 6 days. MTS (20 µl) was added to each well, and after incubation at 37 °C in a 5% CO2 incubator for 1 h, the OD value at 472 nm was read. A time-course experiment was performed to monitor cell viability, and all experiments were performed in triplicate and repeated 3 times. The viability of MGC and MCF-7 cells transiently transfected with psi-IgH or psi-scrambled plasmids was also assessed by MTS assay.
Plate colony formation assay
HeLa, psi-IgH-HeLa, and psi-scrambled-HeLa cells (1×103) were individually and concurrently seeded into six-well culture plates and then routinely cultured for 2 weeks. The medium was changed every 4 days. Finally, the cells were stained with crystal violet, and colonies larger than 50 cells were counted. All experiments were performed in triplicate and repeated 3 times. Colony formation capacity was also assessed by plate colony formation assay in MGC and MCF-7 cells transiently transfected with psi-IgH or psi-scrambled plasmids.
Tumor growth in nude mice
Nude mice (BALB/c) were inoculated subcutaneously with HeLa, psi-IgH-HeLa and psi-scrambled-HeLa cells (5×107/0.1 ml). Tumor growth was assessed by two-dimensional measurements using a caliper every 3 days. Tumor volume was estimated using the formula a×b2/2, where a is the longest dimension and b is the width. On day 30, the tumors were removed, and the volumes of the tumors were measured and calculated.
Flow cytometry assay
A mouse anti-human EGFR antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was labeled with FITC using the FluoReporter FITC protein labeling kit (Molecular Probes; Invitrogen). Cells (1×106) were fixed in 70% ethanol for 30 min and centrifuged at 5000 g for 3 min. The cell pellet was harvested and incubated with the FITC-labeled mouse anti-human EGFR antibody in phosphate buffer solution (PBS) containing 1% fetal bovine serum for 30 min at room temperature. The cells were then washed with PBS and analyzed by flow cytometry.
Separation and purification of antibodies
Purification of the cancerous IgG from the cell culture medium was carried out using a Montage antibody purification kit and a spin column containing PROSEP-A medium (Millipore, Bedford, MA, USA) following the manufacturer's instructions. Briefly, the sample was pre-filtered through a 0.22-µm Steriflip-GP device to remove any debris. The filtered sample was diluted 1:1 (v/v) in binding buffer A (1.5 M glycine/NaOH buffer, 3 M NaCl, pH 9.0). The diluted sample (20 ml) was pipetted into a PROSEP-A spin column, which was already equilibrated with 10 ml of binding buffer A. After centrifugation at 150 g for 20 min, the spin column was washed 2 times with 10 ml binding buffer A to remove unbound contaminants. The bound IgG in the spin column was eluted with 10 ml of elution buffer B2 (0.2 M glycine/HCl buffer, pH 2.5) and neutralized with buffer C (1 M Tris/HCl buffer, pH 9.0). Finally, the purified sample was concentrated with an Amicon Ultra-15 centrifugal filter device.
FITC labeling of cancerous IgG
The purified cancerous IgG was labeled with FITC using the FluoReporter FITC protein labeling kit (Invitrogen) according to the manufacturer's instructions. Briefly, 200 µl of purified cancerous IgG was added to a reaction tube containing 20 µl of freshly prepared 1 M sodium bicarbonate solution. The appropriate amount of reactive dye solution was added while stirring the protein solution in the reaction tube. After adding the dye solution, stirring was continued at room temperature for 1 h without exposure to light. The labeled Ig was purified in a spin column.
Antibody-dependent cell-mediated cytotoxicity assay
ADCC was evaluated by using the CytoTox96 non-radioactive assay (Promega) to measure the release of lactate dehydrogenase (LDH) into the medium after treatment with an anti-human EGFR antibody and cancerous IgG exposure. LDH activity was quantified by measuring absorbance at 490 nm. Peripheral blood mononuclear cells (PBMCs) were purified by standard Ficoll-Hypaque gradient centrifugation of healthy human peripheral blood, cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and plated (2×105/well) in round-bottomed, 96-well plates. HeLa cells (1×104/well) were also plated in the same plates. For the ADCC reaction, the effector and target cells were incubated with 1 µg/ml of a mouse anti-human EGFR antibody and 0, 0.1 or 1 µg/ml of purified cancerous IgG at 37 °C for 4 h. Then, 45 min prior to harvesting the supernatant fraction, lysis solution was added to the target cells as a maximal LDH release control, and the plate was centrifuged at 250 g for 4 min. The supernatant fraction (50 µl) from each well of the assay plate was transferred to the corresponding well of a flat-bottomed 96-well enzymatic assay plate, 50 µl of the reconstituted substrate was added, and the samples were incubated at room temperature for 30 min and protected from light. After stopping the reaction with 50 µl of the ‘stop' solution, absorbance at 490 nm was recorded. The corrected values in the following formula were used to compute the percent cytotoxicity. The LDH activity detected in supernatants from cocultured PBMCs and HeLa cells, PBMCs cultured alone, and HeLa cells cultured alone were corrected to account for the baseline LDH activity detected in the culture medium, resulting in Corrected Experimental, Corrected Effector and Corrected Target values, respectively. The maximum LDH activity detected in the supernatant of coculutred cells treated with anti-EGFR antibody (100%) was corrected for the volume change caused by the addition of lysis solution, resulting in a Corrected Target Maximum Value. The corrected values were used to calculate the percent cytotoxicity according to the following formula:
 |
All experiments were performed in triplicate and repeated 3 times.
Results
Ig heavy chain-specific siRNA inhibits cancer cell growth in vitro and in vivo
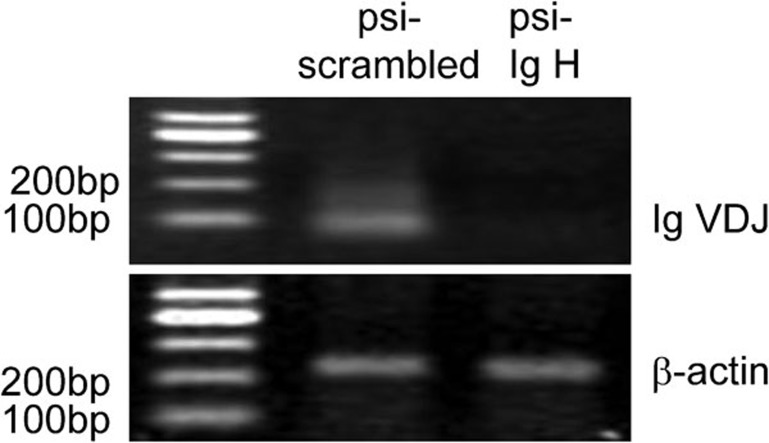
Immunoglobulins are normally expressed only in B lymphocytes. Therefore, we used an antibody against CD19, which is a cell surface marker specific for B lymphocytes, to confirm that the cell lines used in the present study were not contaminated by B lymphocytes. Flow cytometry analysis demonstrated that none of the cell lines were contaminated with B cells.15 To explore the effects of the cancerous Ig heavy chain on HeLa human cervical cancer cell growth, we constructed an siRNA plasmid to silence the expression of all Ig heavy chain genes (psi-IgH). HeLa cells were stably transfected with this plasmid or a scrambled control plasmid to establish psi-IgH-HeLa cells or psi-scrambled-HeLa cells, which were used as a negative control. Silencing of all Ig heavy chain genes in HeLa cells was confirmed by RT-PCR analysis (Figure 1), and β-actin was used as an internal loading control. The mRNA levels of the VDJ domain of all types of Ig heavy chains in the psi-IgH-HeLa cell line were much lower than those in the psi-scrambled-HeLa cells (Figure 1).
Figure 1.
HeLa cells transfected with psi-IgH or psi-scrambled constructs were selected, and gene expression was confirmed by PCR analysis. The expression of Ig VDJ fragments at the mRNA level was analyzed by RT-PCR in HeLa cells transfected with a psi-IgH or psi-scrambled construct. β-actin was used as an internal template control.
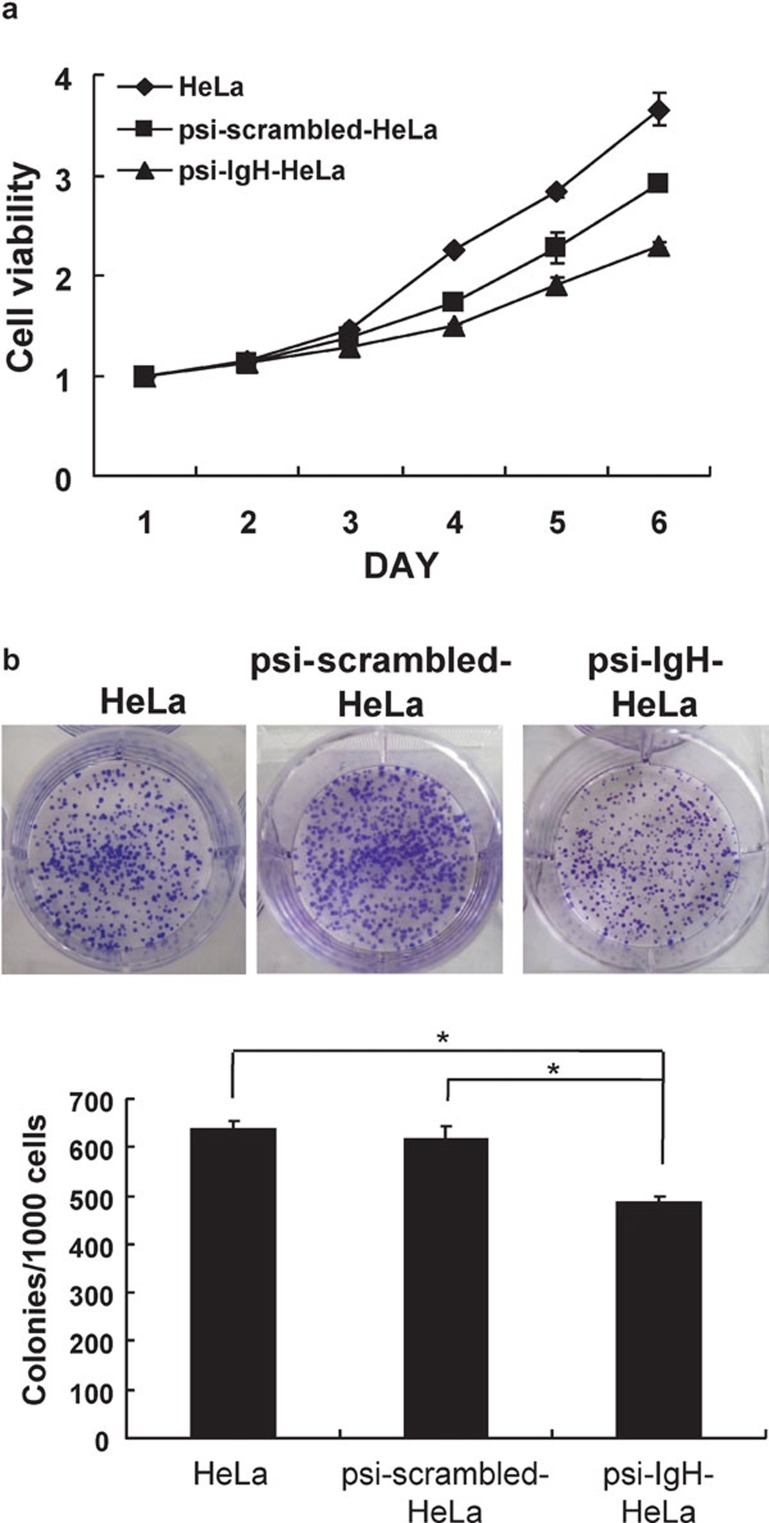
We next used an MTS assay to test the ability of siRNA–Ig heavy chains to influence cell viability. The results of this assay demonstrated that silencing of all types of Ig heavy chains substantially inhibited HeLa cell viability (Figure 2a). This finding was further confirmed by measuring colony formation on culture plates. Cells expressing siRNA directed against Ig heavy chains displayed significantly less colony formation (48%±1.05%) than HeLa cells (64%±1.23%) or cells expressing the psi-scrambled plasmid (62%±2.52%) (P<0.05) (Figure 2b).
Figure 2.
siRNA-Ig heavy chain inhibits cell growth. (a) Cell viability was measured in HeLa, psi-scrambled-HeLa and psi-IgH-HeLa cells. (b) Cell proliferation was estimated by counting the colonies formed by HeLa, psi-scrambled-HeLa and psi-IgH-HeLa cells on culture plates. Top, representative photomicrographs; bottom, data are presented as the mean ±s.d. of values obtained from triplicate experiments. The asterisk (*) indicates that significantly (P<0.05) fewer colonies were formed by psi-IgH-HeLa cells than by psi-scrambled-HeLa or HeLa cells (Student's t-test).
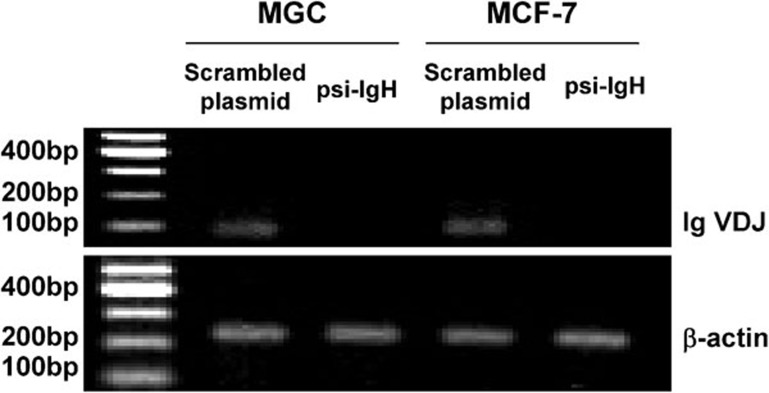
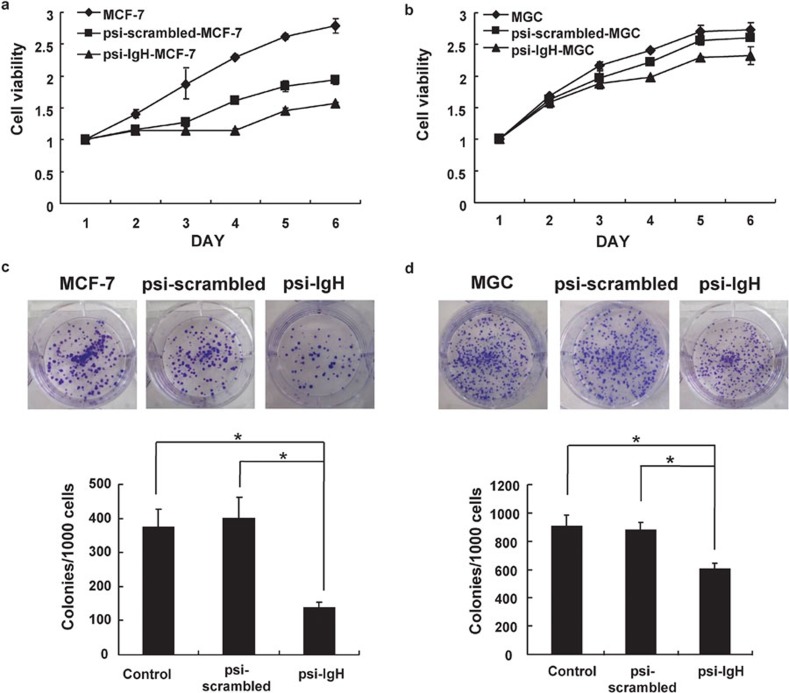
Many cancer cells other than HeLa cells express Ig-like heavy chains; therefore, to explore the functions of cancerous Ig in other cancer cell types, we transiently transfected other cancer cell lines, including the MGC gastric cancer cell line and the MCF-7 breast cancer cell line, with the psi-IgH or psi-scrambled plasmids. The ability of the psi-IgH plasmid to decrease the expression of Ig VDJ mRNA in these cell lines was confirmed by RT-PCR (Figure 3). The cells expressing psi-IgH exhibited varying degrees of decreased cell viability and growth as determined by MTS assay and plate colony formation assay, respectively (Figure 4). Overall, silencing cancerous Ig heavy chain gene expression clearly suppressed cancer cell proliferation in vitro.
Figure 3.
MGC and MCF-7 cells transiently transfected with psi-IgH exhibit substantially decreased Ig heavy chain mRNA levels. The expression of Ig VDJ fragments at the mRNA level was analyzed by RT-PCR in MCG and MCF-7 cells transfected with a psi-IgH or psi-scrambled construct. β-actin was used as an internal template control.
Figure 4.
Cell viability is inhibited by siRNA–Ig heavy chain in vitro. Cell viability was measured in MCF-7 (a) and MGC (b) cells transiently transfected with psi-IgH or psi-scrambled plasmids. Cell proliferation was estimated by counting the colonies formed by the transiently transfected MCF-7 (c) or MGC (d) cells on culture plates. (c, d) top, representative photomicrographs; bottom, data are shown as the mean±s.d. of values obtained from triplicate experiments. The asterisk (*) indicates that significantly (P<0.05) fewer colonies were formed by MCF-7 or MGC cells transfected with psi-IgH than by cells transfected with the psi-scrambled plasmid (Student's t-test).
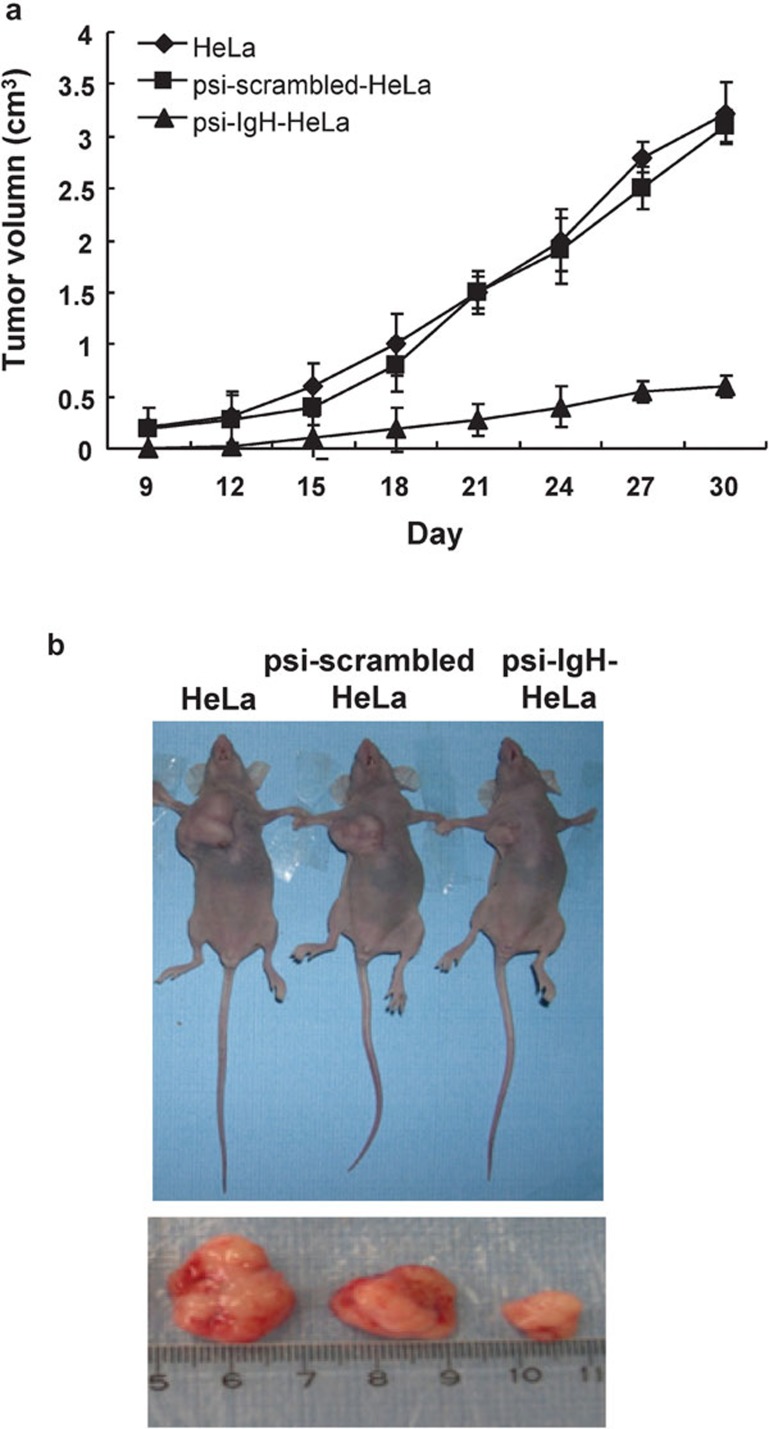
Next, the effects of siRNA–Ig heavy chain expression on cell proliferation in vivo were examined in immune-deficient nude mice. Tumors were first observed on day 9 after the injection of mice with HeLa cells or psi-scrambled-HeLa cells, whereas tumors were not observed until day 12 after injection of mice with psi-IgH-HeLa cells. The growth rates of tumors were markedly different among the 3 groups. Tumors in mice injected with HeLa or psi-scrambled-HeLa cells grew rapidly. In contrast, tumor growth in mice injected with psi-IgH-HeLa cells was significantly (P<0.05) delayed. The tumors were removed, and their volumes were measured on day 30 after the injection of cells (Figure 5b). The tumors from mice injected with HeLa or psi-scrambled-HeLa cells had average volumes of 3.3±2.1 and 3.1±1.3 cm3, respectively, whereas tumors from mice injected with psi-IgH-HeLa cells had an average tumor volume of 0.6±0.1 cm3 (Figure 5).
Figure 5.
siRNA–Ig heavy chain inhibits cancer cell growth in athymic nude mice. HeLa cells or HeLa cells transfected with the psi-scrambled or psi-IgH plasmids were injected into athymic nude mice. (a) Tumor growth curves. Tumors were measured by caliper every 3 days, and volume was calculated using the formula a×b2/2, where a is the longest dimension and b is the width. (b) On day 30, the tumors were removed from the mice, and volumes were measured.
Cancerous Ig competitively inhibits ADCC induced by an anti-EGFR antibody
We next evaluated the hypothesis that cancerous Ig inhibits ADCC by competitively binding to the Fc receptor on NK cells using LDH release assays. In this assay, PBMCs were used as effector cells, and HeLa cells were used as target cells. The EGFR is generally expressed in cancer cells. Antibodies that bind this receptor can interact with the Fc receptor on NK cells, resulting in NK cell activation and killing of the cancer cells through ADCC. In the present study, PBMCs (containing an average of 11% CD56+/CD16+ NK cells) and HeLa cells were incubated at an effector/target ratio of 20:1 in the presence of different concentrations of cancerous Ig and mouse anti-human EGFR IgG2a, and ADCC was assessed by measuring LDH release. Because IgG is the predominant isotype involved in ADCC, cancerous IgG was purified and labeled with FITC. After demonstrating that the purified and labeled cancerous IgG bound to the Fc receptor on NK cells, we assessed the ability of cancerous IgG to inhibit ADCC.
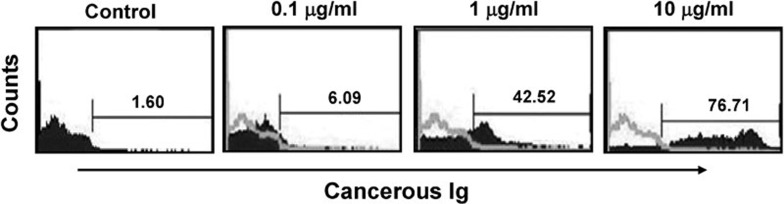
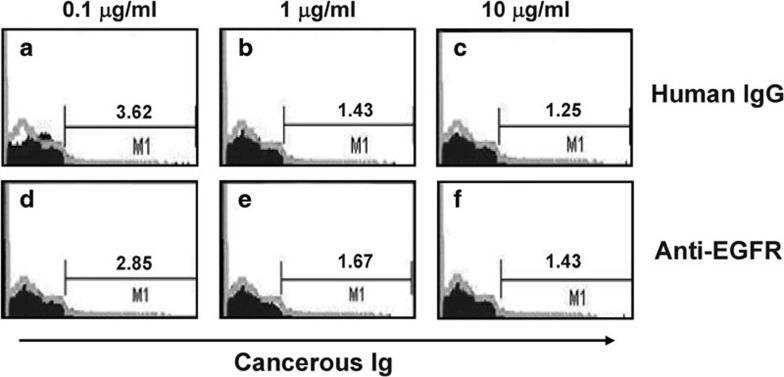
Free cancerous IgG was purified from cell culture media, concentrated and labeled with FITC and then incubated with PBMCs. Flow cytometry analysis demonstrated that after incubation with 0, 0.1, 1 or 10 µg/ml cancerous IgG, 1.60, 6.09, 42.52 or 76.71% of PBMCs were bound to cancerous IgG, respectively. These results suggest that cancerous IgG is bound by the Fc receptor on killer cells in a dose-dependent manner (Figure 6). Next, an excess of normal human IgG (10 µg/ml) was used as a competitive inhibitor of cancerous IgG binding to PBMCs. PBMCs were first incubated with normal human IgG and then incubated with FITC-labeled cancerous IgG. After incubation with 0.1, 1 or 10 µg/ml cancerous IgG, 3.62, 1.43 or 1.25% of PBMCs were bound to cancerous IgG, respectively (Figure 7a–c). These results indicate that an excess of normal IgG blocked the binding site of the Fc receptor on the PBMCs, preventing the interaction of the Fc receptor with cancerous IgG. The Fc fragment of mouse anti-human EGFR IgG2a is highly homologous to that of human IgG1 and IgG3 and binds to the Fc receptor on NK cells with high affinity. Therefore, we used an excess of mouse anti-human EGFR IgG2 (10 µg/ml) to compete with cancerous IgG for binding to the Fc receptor on the PBMCs. After incubation with 0.1, 1 or 10 µg/ml of cancerous IgG, 2.85, 1.67 or 1.43% of PBMCs were bound cancerous IgG. These results suggest that the cancerous IgG only weakly bound to the Fc receptor due to the presence of excess mouse EGFR antibody (Figure 7d–f). Overall, we concluded that cancerous Ig might bind to the Fc receptor on NK cells.
Figure 6.
Cancerous Ig binds to human PBMCs in a dose-dependent manner. Cancerous Ig was purified from culture medium and labeled with FITC. PBMCs were incubated with different concentrations of the purified and labeled cancerous Ig. The percentage of PBMCs bound by the cancerous Ig was detected by flow cytometry. The concentrations of cancerous Ig were 0.1 µg/ml, 1 µg/ml and 10 µg/ml. Ig, immunoglobulin; PBMC, peripheral blood mononuclear cell.
Figure 7.
Incubation with an excess of human normal IgG or an excess of a mouse anti-human EGFR antibody blocked the binding site of the Fc receptor from interacting with cancerous IgG. PBMCs were incubated with different concentrations of purified and labeled cancerous IgG in the presence of normal IgG (10 µg/ml) or the mouse anti-human EGFR antibody (10 µg/ml), and the percentage of PBMCs bound by cancerous IgG was measured by flow cytometry. The concentrations of cancerous IgG were 0.1, 1 and 10 µg/ml.
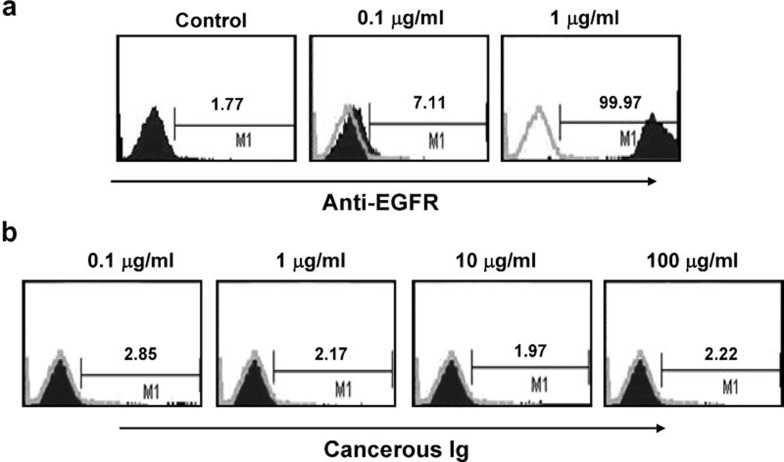
Next, to demonstrate that the mouse anti-human EGFR IgG2a antibody bound to the EGFR antigen on the target cells, the antibody was labeled with FITC and incubated with HeLa cells. After incubation with 0, 0.1 or 1 µg/ml EGFR antibody, 1.77, 7.11 or 99.97% of HeLa cells were bound by the antibody, respectively. These findings demonstrate that the antibody and the antigen interacted effectively (Figure 8a). In contrast, after incubation with 0.1, 1, 10 or 100 µg/ml of FITC-labeled cancerous IgG, 1.97%–2.85% of HeLa cells were bound by the antibody (Figure 8b), demonstrating that cancerous Ig did not bind the target cells.
Figure 8.
An anti-EGFR antibody binds to the EGFR on HeLa cells in a dose-dependent manner. An anti-EGFR antibody was labeled with FITC and then incubated with HeLa cells. (a) The percentage of HeLa cells bound by anti-EGFR was detected by flow cytometry. Negative control without any anti-EGFR antibody. The concentrations of the anti-EGFR antibody were 0.1 and 1 µg/ml. (b) HeLa cells were incubated with different concentrations of purified and FITC-labeled cancerous IgG. The percentage of PBMCs bound by cancerous Ig was determined by flow cytometry. The concentrations of cancerous IgG were 0.1, 1, 10 and 100 µg/ml.
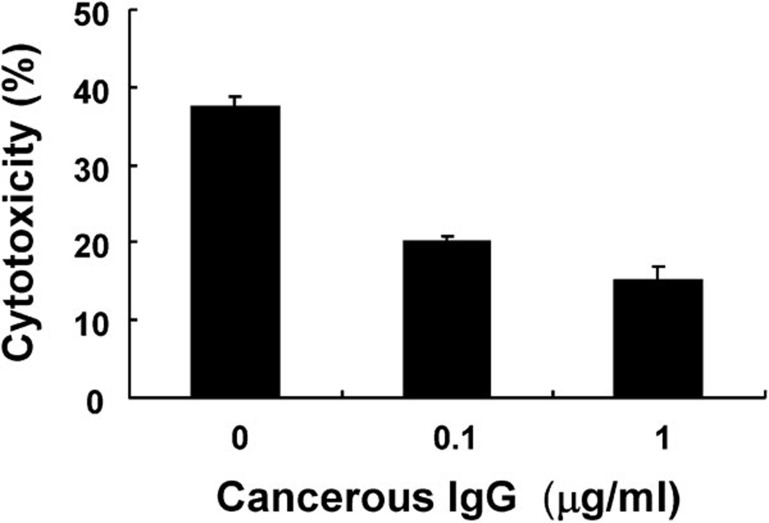
Finally, standard ADCC assays were performed by coculturing PBMCs and HeLa cells at an effector/target ratio of 20:1. All reactions were conducted in the presence of mouse anti-human EGFR IgG2a and purified cancerous IgG. Several controls were designed (Figure 9). Incubation with 0, 0.1 or 1 µg/ml of cancerous IgG resulted in 37.5%±1.2%, 20.0%±0.8% or 15%±1.8% cytotoxicity, respectively. Notably, incubation with 10 µg/ml of cancerous Ig completely inhibited ADCC.
Figure 9.
Cancerous IgG suppresses anti-EGFR-induced ADCC. PBMCs and HeLa cells (E/T=20:1) were incubated with an anti-human EGFR antibody (1 µg/ml) in the presence of different concentrations (0, 0.1 and 1 µg/ml) of cancerous IgG. Cytotoxicity was measured by detecting the release of LDH using the CytoTox96 non-radioactive assay. The percent cytotoxicity in the presence of various concentrations (0, 0.1 or 1 µg/ml) of cancerous IgG was evaluated. Data are shown as mean±s.d. of values obtained from triplicate experiments.
Discussion
The unexpected finding that epithelial-derived cancer cells express and secrete several types of Ig has been confirmed by many laboratories;3, 4, 5, 6, 7, 8, 9, 10 however, the biological effects of the cancerous Ig on the cancer cell itself and its effects on the immune response have not been extensively studied. siRNA is a type of RNA interference technology that specifically, efficiently and consistently induces gene silencing in cell lines after stable transfection with the siRNA plasmid. siRNA is widely used to study gene functions and is also used as an anti-viral agent and a method of delivering gene therapies in cancer. Cancer-derived Ig is reported to contain IgA, IgG and IgM;3, 10 therefore, in this study, stable cancer cell lines expressing siRNA targeting all the Ig heavy chain genes were constructed. Using these stable cell lines, we performed MTS assays and plate colony formation assays to demonstrate that silencing of the Ig heavy chains partially inhibits cancer cell viability and proliferation, respectively. In addition, silencing of the Ig heavy chain resulted in decreased xenograft tumor size. Overall, our data indicate that cancerous Ig promoted cell proliferation in vitro and in vivo. Qiu et al.3 demonstrated that cancer-derived Ig contributed to the survival and growth of epithelial tumor cells through experiments showing that anti-human IgG and antisense oligodeoxynucleotide specific for Ig-induced apoptosis and growth inhibition of cancer cells in vitro and in vivo. In addition, blocking the function of cancer-derived Ig alpha has been shown to inhibit the growth and malignant proliferation of cancer cells and to decrease the percentage of cells in S phase.11 These results indicate that cancer-derived Ig plays a role in the growth and proliferation of cancer cells. Furthermore, our past studies have demonstrated that cancerous Ig not only exists in the cytoplasm but is also distributed on the cell surface and secreted into the cell culture medium.3, 4, 5, 6, 7, 8, 9, 10 Based on these studies, cancer-derived Ig alpha might engage signaling pathways leading to cell proliferation or cell cycle progression in cancer cells and promote increased entry to the S phase and growth of cancer cells. Further research is required to address this issue. Together, these data reveal a novel mechanism mediating the growth of cancer cells. Interestingly, cancerous Ig has already become a potential marker for tumor diagnosis and a specific target for cancer therapy.16, 17
Although the structure of cancerous Ig is not clear, native western blot analysis of cell lysates from cancer cell lines showed that cancerous Ig contains abnormal Ig heavy chain and kappa light chain. Together with our previous finding that the Tx gene lacks the variable region of the Ig gene, these data suggest that cancerous Ig is likely very different from regular Ig and raise the question of whether cancerous Ig inhibits human humoral immunity. Normal antibodies induce humoral responses through complement-dependent cytotoxicity and ADCC. Thus, we hypothesized that a fragment of the cancerous Ig heavy chain might compete with normal Ig for binding to the Fc receptor on NK cells, thereby interfering with antibody function. In this study, a mouse anti-human EGFR antibody was used to induce ADCC. Adding cancerous Ig resulted in a dose-dependent decrease in anti-EGFR-induced ADCC of target cells. Thus, we concluded that cancerous Ig plays a role in the ability of cancer to escape from humoral immunity. Notably, some blocking factors expressed by tumor cells have been shown to promote tumor growth rather than damage tumor cells.14 Although these blocking factors are antigens or antigen-antibody complexes, the nature of the blocking factors is not clear. We hypothesize that cancerous Ig is a novel type of blocking factor that indirectly inhibits ADCC of tumor cells, resulting in increased tumor proliferation; however, more studies are needed to test this hypothesis.
Together, our findings demonstrate that cancerous Ig contributes to the growth of epithelial tumor cells. First, we showed that silencing cancerous Ig inhibited cancer cell growth in vitro and in vivo. Second, we demonstrated that cancerous Ig inhibited ADCC by blocking antibody binding to the Fc receptor on NK cells. The development of methods to selectively block cancerous Ig might constitute a new approach for cancer therapy and prevention.
Acknowledgments
This work was supported by the Major Program of National Natural Science Foundation of China (39830410), National High Technology Research and Development Program (863) of China (2006AA02A404), CMB project (99665) and National Natural Science Foundation of China (30772465).
References
- Cao Y, Sun Y, Poirier S, Winterstein D, Hegamyer G, Seed J, et al. Isolation and partial characterization of transformation associated sequence from human nasopharyngeal carcinoma. Mol Carcinogenesis. 1991;4:297–302. doi: 10.1002/mc.2940040408. [DOI] [PubMed] [Google Scholar]
- Li M, Ren W, Weng XX, Liao W, Xia L, Deng X, et al. Nucleotide sequence analysis of a transforming gene isolated from human nasopharyngeal carcinoma cell line CNE2: an aberrant human immunoglobulin kappa light chain which lacks variable region. DNA Sequence. 2001;12:331–335. doi: 10.3109/10425170109084456. [DOI] [PubMed] [Google Scholar]
- Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao P, et al. Human epithelial cancers secrete immunoglobulin G with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003;63:6488–6495. [PubMed] [Google Scholar]
- Zhu X, Li C, Sun X, Mao Y, Li G, Liu X, et al. Immunoglobulin mRNA and protein expression in human oral epithelial tumor cells. Appl Immunohistochem Mol Morphol. 2008;16:232–238. doi: 10.1097/PAI.0b013e31814c915a. [DOI] [PubMed] [Google Scholar]
- Chen Z, Qiu X, Gu J. Immunoglobulin expression in non-lymphoid lineage and neoplastic cells. Am J Pathol. 2009;174:1139–1148. doi: 10.2353/ajpath.2009.080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gu J. Immunoglobulin G expression in carcinomas and cancer cell lines. FASEB J. 2007;21:2931–2938. doi: 10.1096/fj.07-8073com. [DOI] [PubMed] [Google Scholar]
- Babbage G, Ottensmeier VH, Blaydes J, Stevenson FK, Sahota SS. Immunoglobulin heavy chain locus events and expression of activation-induced cytidine deaminase in epithelial breast cancer cell lines. Cancer Res. 2006;66:3990–4000. doi: 10.1158/0008-5472.CAN-05-3704. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wu L, Zhang L, Hao P, Zhang S, Huang J, et al. Distinct regulatory mechanism of immunoglobulin gene transcription in epithelial cancer cells. Cell Mol Immunol. 2010;7:279–287. doi: 10.1038/cmi.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Feng D, Ren W, Zeng L, Zheng H, Tang M, et al. Expression of immunoglobulin kappa light chain constant region in abnormal human cervical epithelial cells. Int J Biochem Cell Biol. 2004;36:2250–2257. doi: 10.1016/j.biocel.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Li M, Tang M, Deng X, Cao Y. Tumor cells derived from epithelial express immunoglobulin. Chin J Oncol. 2001;23:451–453. [PubMed] [Google Scholar]
- Zheng H, Li M, Liu H, Ren W, Hu D, Shi Y, et al. Immunoglobulin alpha heavy chain derived from human epithelial cancer cells promotes the access of S phase and growth of cancer cells. Cell Biol Int. 2007;31:82–87. doi: 10.1016/j.cellbi.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Olubuyide IO, Salimonu LS, Adeniran SO. Soluble immune complexes and immunoglobulin IgG, IgA, and IgM levels in Nigerians with primary liver cell carcinoma. Afr J Med Sci. 1993;22:57–62. [PubMed] [Google Scholar]
- Manjula S, Aroor AR, Raja A, Rao SN, Rao A. Serum immunoglobulins in brain tumors. Acta Neurochir. 1992;115:103–105. doi: 10.1007/BF01406366. [DOI] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C. Tumor-reactive immunoglobulins in ovarian cancer. Diagnostic and therapeutic significance. Oncol Rep. 1998;5:1519–1524. doi: 10.3892/or.5.6.1519. [DOI] [PubMed] [Google Scholar]
- Zheng H, Li M, Ren W, Zeng L, Liu H, Hua D, et al. Expression and secretion of immunoglobulin alpha heavy chain with diverse VDJ recombinations by human epithelial cancer cells. Mol Immunol. 2007;44:2221–2227. doi: 10.1016/j.molimm.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Lee G, Chu RA, Ting HH. Preclinical assessment of anti-cancer drugs by using RP215 monoclonal antibody. Cancer Biol Ther. 2009;8:161–166. doi: 10.4161/cbt.8.2.7117. [DOI] [PubMed] [Google Scholar]
- Lee G. Cancer cell-expressed immunoglobulins: CA215 as a pan cancer marker and its diagnostic applications. Cancer Biomarkers. 2009;5:137–142. doi: 10.3233/CBM-2009-0610. [DOI] [PubMed] [Google Scholar]