Abstract
Aim:
To determine the efficacy and toxicities of sorafenib in the treatment of patients with multiple recurrences of hepatocellular carcinoma (HCC) after liver transplantation in a Chinese population.
Methods:
Twenty patients with multiple recurrences of HCC after liver transplantation were retrospectively studied. They received either transarterial chemoembolization (TACE) or TACE combined with sorafenib.
Results:
The median survival times (MST) after multiple recurrences was 14 months (TACE+sorafenib group) and 6 months (TACE only group). The difference was significant in MST between the two groups (P=0.005). The TACE + sorafenib group had more stable disease (SD) patients than the TACE group. The most frequent adverse events of sorafenib were hand–foot skin reaction and diarrhea. In the univariate analysis, preoperative bilirubin and CHILD grade are found to be significantly associated with tumor-free survival time, the survival time after multiple recurrences and overall survival time. TACE+sorafenib group showed a better outcome than single TACE treatment group. In the multivariate COX regression modeling, the preoperative high CHILD grade was found to be a risk factor of tumor-free survival time. In addition, the preoperative high bilirubin grade was also found to be a risk factor of survival time after recurrence and overall survival time. Furthermore, survival time after recurrence and overall survival time were also associated with therapeutic schedule, which was indicated by the GROUP.
Conclusion:
Treatment with TACE and sorafenib is worthy of further study and may have more extensive application prospects.
Keywords: hepatocellular carcinoma, sorafenib, transarterial chemoembolization, survival analysis, molecular targeted therapy
Introduction
Hepatocellular carcinoma (HCC) is a major health problem, with more than 620,000 new cases every year worldwide. HCC is the sixth most common carcinoma worldwide with more than 0.6 million deaths annually. Additionally, it is the third highest cause of cancer-related death globally, behind lung and stomach cancers1. Because of alcohol abuse, flavacin, environmental contamination and the prevalence of Hepatitis B, the incidence and mortality of HCC in China is higher than in any other country in the world2. Presently, the incidence rates for HCC in China are approximately 347,000 people per year, accounting for 55% of the world; in addition, the mortalities total 323,000 people per year, accounting for 45% of the world.
As the early symptoms of HCC are atypical and difficult to diagnose, most patients are diagnosed as advanced and metastatic, and only about 15% of patients are eligible for surgical resection3. Despite recent improvements in surveillance programs and diagnostic tools allowing identification of small suspicious nodules, only 30% to 40% of patients with HCC are eligible for curative treatments4. In well-selected patients, resection and liver transplantation provide 5-year survival rates of 70%, whereas local ablation with radio frequency results in a 5-year survival rate of 50%5. These treatments change the natural course of the disease. Tumor relapse complicates half of the patients at 3 years, and none of the 14 randomized controlled trial (RCT)s published thus far provide a strong rationale for the establishment of a standard adjuvant therapy6.
For patients with advanced HCC who are not eligible for surgery, drug therapy is the main treatment method7. In addition, patients who have recurrence after liver transplantation and surgical resection require drug treatment. However, since the patients often have liver diseases (hepatitis, cirrhosis and liver dysfunction), the treatment of advanced HCC is very difficult8. Thus far, no systematic therapy has been shown to improve survival in patients with advanced HCC9, 10, 11, 12, 13. Therefore, patients with advanced HCC have a poor prognosis. The median survival time (MST) of advanced HCC in China is only 3 to 6 months, and a slightly longer MST of 6 to 9 months in Europe and the United States.
Sorafenib (Nexavar, Bayer Pharmaceuticals) is a small molecule that can inhibit tumor cell proliferation and tumor angiogenesis as well as increase the rate of apoptosis in a wide range of tumor models14, 15. Sorafenib works by inhibiting the serine/threonine kinases Raf-1, B-Raf, the receptor tyrosine kinase activity of vascular endothelial growth factor receptors (VEGFRs) 1, 2, and 3 and platelet-derived growth factor receptor β (PDGFR-β)14, 15. Cellular signaling pathways mediated by Raf-1 and vascular endothelial growth factor (VEGF) have been implicated in the molecular pathogenesis of HCC16, 17, 18, 19, providing a rationale for investigating sorafenib for this indication. In preclinical experiments, sorafenib had anti-proliferative activity on liver cancer cell lines, and it reduced tumor angiogenesis and tumor-cell signaling. Moreover, sorafenib increased tumor-cell apoptosis in a mouse xenograft model of human HCC20. In 2005, sorafenib was approved for therapy of advanced renal carcinoma by the FDA. In this article, patients with multiple recurrences of HCC after liver transplantation were studied. They received either transarterial chemoembolization (TACE) combined with sorafenib or TACE only. The efficacy and treatment-induced toxicities of sorafenib were studied.
Materials and methods
Patients and staging evaluation
Subject recruitment
From November 2004 to December 2009, patients with multiple recurrences of HCC after liver transplantation from Shanghai Eastern Hepatobiliary Surgery Hospital with the following selection criteria were enrolled.
Inclusion criteria
Patients were included based on the following criteria:
1) Patients with multiple recurrences of HCC after liver transplantation and they were not eligible for surgery; 2) Clinical diagnosis of recurrent tumor and/or metastatic lesions of hepatocellular carcinoma according to the EASL diagnostic criteria; 3) Liver function was Child A or Child B; 4) Recurrent tumor and/or metastatic lesions with at least one lesion confirmed by CT or MRI.
Exclusion criteria
Patients were excluded based on the following criteria:
1) Drug allergy history; 2) Other tumors or a history of heart disease; 3) Brain metastasis or digestive tract hemorrhage history in the previous 30 days.
Adverse effects assessment grading
Hand-foot skin reaction grading
1) Hand and/or foot numbness, insensitivity/paresthesia, tingling, discomfort, swelling or redness, where these anomalies do not affect normal activities.
2) Hand and/or foot pain in erythema and edema that affect the patients' activities.
3) Hands and/or feet appear wet, scaling, ulcers, bullae, severe pain, and/or severe discomfort, where patients are unable to perform daily activities.
Hypertension grading
1) Asymptomatic and transient (<24 h) increase, increased amplitude >20 mmHg (diastolic blood pressure), or blood pressure >150/100. No treatment required.
2) Recrudescent and persistent (>24 h) increase, increased amplitude >20 mmHg (diastolic blood pressure), or blood pressure >150/100. Requires single drug treatment.
3) Requires more than one high intensity medical treatment.
4) Life crisis occurs (for example, hypertensive crisis).
5) Death.
Diarrhea grading
1) Compared with the baseline, number of defecation <4; stoma discharge increased slightly.
2) Compared with the baseline, number of defecation is 4-6; Intravenous infusion <24 h; moderate increase in stoma discharge.
3) Compared with the baseline, number of defecation ≥7; Fecal incontinence; intravenous infusion ≥ 24 h; hospitalization; stoma discharge has severe increase.
4) Life crisis occurs (for example, hemodynamic disturbance).
5) Death.
Treatment
Informed consent was required and obtained from all patients prior to the initiation of treatment. Ten patients were assigned to the TACE group, and ten patients were assigned to the TACE + sorafenib group. In the TACE + sorafenib group, patients received 400 mg of sorafenib orally twice daily, spaced 12 h apart, on a continuous basis. Dose modification to 400 mg once daily was permitted if grade 3 or 4 hematologic toxicity, skin toxicity, hypertension, and/or hepatic dysfunction defined by the NCI-CTCAE 3.0 occurred. The treatment continued until a liver function of Child C grade occurred. The start point of the study was defined as the time of liver cancer recrudescence after liver transplantation; the endpoint was the time of patient death.
Therapeutic effect evaluations
The main therapeutic measurement is overall survival. According to the RECIST standard, we evaluated therapeutic effects after 4 to 6 weeks for patients treated with TACE and sorafenib. The results were divided into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). CR, PR and SD (surviving 12 weeks or more) were regarded as positive results.
Follow up
In the first three months, all patients were required to be evaluated monthly during their treatment. Three months later, patients had to be monitored every 2 months. As a requirement of the protocol, each follow-up session included a complete history and physical examination, routine laboratory tests including liver function tests, blood routine, CT scan (1 and 3 months), AFP, and B-Mode Ultrasonography. Concentration of FK506 and Hepatitis B surface antibody titer were measured on a monthly basis during the treatment and at every follow-up visit after the termination of the treatment. Tumor response was measured from one month after the initiation of the treatment using the RECIST criteria. The radiological responses were evaluated by diagnostic radiologists independent of this study and verified by the investigators. The adverse effects were evaluated at each visit during and after the treatment.
Prognostic parameters
This study selected nine pathological parameters to explore factors influencing the prognosis of patients with multiple recurrences of HCC after liver transplantation. These parameters include preoperative tumor size, preoperative vascular tumor thrombi, preoperative bilirubin, preoperative Child-Pugh grade, preoperative white protein, multiple tumor before liver transplantation, hepatic cirrhosis background, preoperative alpha-fetoprotein (AFP) level (grade 1: ≤20, grade 2: 20-400, grade 3: 400-1000, grade 4: >1000) and age.
Statistical analysis
Independent-sample T test, Fisher's Exact Test and Chi-square Test were performed using SPSS software version 15.0 (SPSS, Chicago, IL, USA) to investigate whether there were significant differences in indicated parameters between the two groups with different therapeutic schedules (Table 1).
Table 1. Data of the 20 patients with multiple recurrences of HCC after liver transplantation.
| Variable | TACE | TACE+Sorafenib | P |
|---|---|---|---|
| Gender | |||
| Male | 10 | 10 | - |
| Age(year) | 43.40±7.31 | 46.30±5.95 | 0.327 |
| AFP(ng/mL) | 0.785 | ||
| ≤ 20 | 1 | 1 | |
| 20-400 | 4 | 2 | |
| 400-1000 | 1 | 1 | |
| > 1000 | 4 | 6 | |
| Tumor size(cm) | 9.98±1.96 | 9.52±4.63 | 0.778 |
| Multiple recrudescence | 0.656 | ||
| No | 6 | 4 | |
| yes | 4 | 6 | |
| Vascular tumor thrombi | 1.000 | ||
| No | 3 | 4 | |
| yes | 7 | 6 | |
| Hepatic cirrhosis | 1.000 | ||
| No | 3 | 2 | |
| yes | 7 | 8 | |
| Child-Pugh score | 1.000 | ||
| A | 6 | 5 | |
| B | 4 | 5 | |
| Bilirubin (μmol/L) | 0.350 | ||
| ≤ 34 | 5 | 8 | |
| > 34 | 5 | 2 | |
| White protein (g/L) | 0.582 | ||
| ≤ 35 | 1 | 3 | |
| > 35 | 9 | 7 | |
| Tumor free survival time (month) | 1.000 | ||
| ≤ 12 | 6 | 5 | |
| > 12 | 4 | 5 | |
Three types of survival time were analyzed: tumor-free survival time (from operation to recurrence), survival time after multi-recurrence (from recurrence or therapy of sorafenib to the end of the study) and overall survival time (from operation to the end of the study). Survival curves were generated by the Kaplan-Meier method and the lifetime of the two treatment groups were compared by the Log-rank method of SPSS 15.0. Univariate analysis was performed by the Log-rank method while multivariate analysis was performed by the Cox regression method. All reported P values are two-sided. P<0.05 was considered statistically significant.
Results
Study patients
From November 2004 to December 2009, we enrolled patients with HCC from Shanghai Eastern Hepatobiliary Hospital. The study enrolled a total of 20 patients. After nearly 5 years of tracking, we collected nearly 20 cases of liver transplant patients in the surgery clinic. Of those, the percent of female patients was 10% and the overall 3-year recurrence rate was 50%, but no female patients had recrudescence. Patient characteristics are listed in Table 1. There was no statistically significant difference of the collected parameters(including: preoperative tumor size, hepatic cirrhosis background, tumor free survival time after liver transplantation, etc.) between the two groups.
Treatment outcome
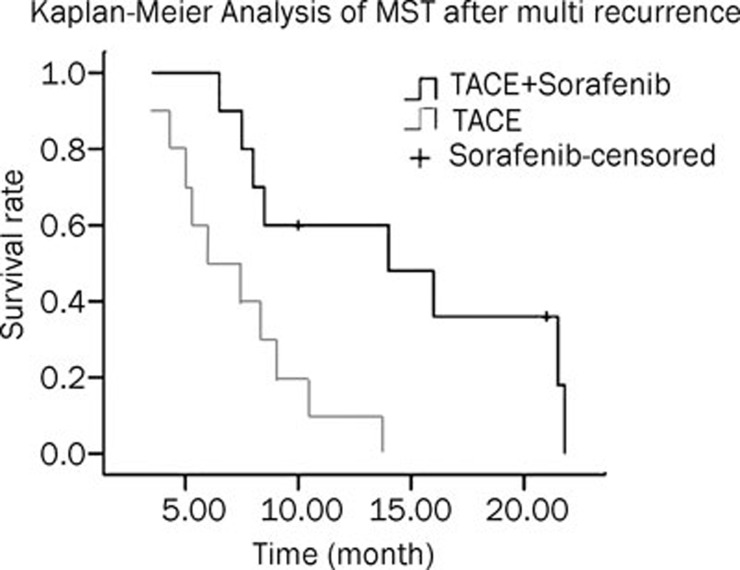
The median survival time (MST) after multiple recurrences in the TACE and TACE+Sorafenib group were 6 months (95% CI=2.59–9.41) and 14 months (95%CI=5.30–22.7), respectively (Table 2). This survival was significantly different as determined by the Log-rank method (P=0.005) (Figure 1). At the time of this analysis, 18 patients (90%) were deceased: 5 patients died of liver failure, 2 patients died of myelosuppression, 10 patients died of respiratory failure that was caused by lung metastasis, and 1 patient died of psychoactive stimulus.
Table 2. Summary of survival statistics of patients with multiple recurrences of HCC after liver transplantation.
| Treatment Group a | MST(month) b | 95% CI | P |
|---|---|---|---|
| 1 | 6 | 2.59-9.41 | 0.005 |
| 2 | 14 | 5.30-22.70 |
Group 1 was treated with TACE, group 2 was treated with TACE combined with sorafenib. aMST: median survival time, month.
Figure 1.
Kaplan–Meier analysis of MST after multi recurrence. n=10.
Therapeutic effect
In the TACE plus sorafenib group, seven (70%) patients had stable disease (SD) (according to RECIST), whereas in the TACE group, one patient (10%) had a partial response (PR) and three patients (30%) had stable disease (SD) (Table 3). There were no complete responses in either group. There was no significant difference between the two groups.
Table 3. Summary of therapeutic effect.
| Treatment group | CR | PR(%) | SD(%) | PD(%) |
|---|---|---|---|---|
| 1 | 0 | 1(10%) | 3(30%) | 6(60%) |
| 2 | 0 | 0 | 7(70%) | 3(30%) |
CR: complete responses; PR: partial response; SD: stable disease; PD: progressive disease.
Adverse effects
Adverse events that were reported for patients receiving sorafenib were hypertension, weight loss, rash, hand–foot skin reaction, alopecia, anepithymia, mucositis (oral cavity), diarrhea, anemia, and voice changes, among others. In our study, all patients in the sorafenib group experienced some type of adverse effect; however, the studied treatment protocol was well tolerated and most patients experienced grade 1 or 2 toxicities (Table 4). Only one (10%) patient required dose reduction due to grade 3 or 4 adverse effects, and one (10%) patient discontinued his treatment due to severe adverse effects.
Table 4. Incidence of drug-related adverse events in sorafenib group.
| Adverse event | Toxicity grade | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Hypertension | 1 | 1 | 0 | 0 |
| Agrypnia | 1 | 0 | 0 | 0 |
| Hand–foot skin reaction | 3 | 1 | 1 | 0 |
| Diarrhea | 2 | 1 | 0 | 0 |
The remaining eight (80%) patients tolerated and continued their treatment with standard dose of sorafenib at the time of this analysis or until disease progression. The most commonly observed severe toxicities were hand-foot syndrome, hypertension and diarrhea. Treatment-induced grade 4 toxicity was not observed in the patients.
Univariate analysis for survival time
Univariate analysis was performed by Log-rank test with variants, preoperative tumor size, preoperative vascular tumor thrombi, preoperative bilirubin, preoperative Child-Pugh grade, and other parameters. The tumor-free survival time was significantly associated with preoperative bilirubin and CHILD grade, with scores of χ2 = 3.977 (P = 0.046) for bilirubin and χ2 = 4.345 (P = 0.037) for CHILD score. High CHILD grade and low bilirubin obtained longer tumor free survival time. For the survival time after multi-recurrence, the two groups with different treatments showed significant differences , with χ2 = 7.957 (P=0.005), while preoperative bilirubin was still associated with the survival time after multi-recurrence (χ2 =11.246, P=0.001) , patients in TACE plus sorafenib group with low bilirubin level obtained longer survival time after multi-recurrence. Moreover, the variants of groups and preoperative bilirubin were significant predictors of overall survival time with χ2 =4.751 (P=0.029) and χ2 =9.465 (P =0.002), patients in TACE plus sorafenib group with low bilirubin level obtained longer overall survival time.
Multivariate analysis for survival time
In the multivariate COX regression modeling, preoperative high CHILD grade was found to be a risk factor of tumor-free survival time [Exp (B) = 2.752 (95%CI=1.004-7.546, P=0.049)]. Preoperative high bilirubin grade was found to be a risk factor of survival time after recurrence [Exp (B) =7.935 (95% CI=1.945-32.376, P=0.004)] and overall survival time [Exp (B) =5.875 (95%CI=1.724-20.021, P=0.005)]. Furthermore, survival time after recurrence and overall survival time were also associated with therapeutic schedule, which was indicated by GROUP, with Exp(B)=0.195, (95%CI=0.057-0.669, P=0.009) and Exp(B)=0.305, (95%CI=0.104-0.890, P=0.03), patients in TACE plus sorafenib group lived longer than single TACE treatment group indicating sorafenibs benefits for survival.
Discussion
Although liver transplantation is the most common treatment for advanced HCC patients, recurrence rates after transplantation remain as high as 60% to 100%. Because micro-metastases remain that can migrate to peripheral tissues, the recurrences are sometimes accompanied by widespread metastatic carcinoma, and partial or palliative treatments are usually used 6. TACE is the most widely used neo-adjuvant treatment for patients with HCC. The 3-year survival rate of this treatment is 20% to 40%. Recent randomized and controlled analysis along with Meta analysis have shown that TACE can significantly improve the 2-year survival rate and extend the median survival time to 20 months 12. Thus, TACE is considered the standard treatment for patients with advanced HCC after liver transplantation. TACE utilizes the excessive angiogenesis of HCC and obtains arterial blood supply from the HCC. TACE treatments use chemotherapeutic drugs and iodized oil emulsifier, combined with embolization agents such as gelatin sponges and others, to induce tumor necrosis through blocking angiogenesis. TACE treatment usually cannot cause complete necrosis, so survival is still unsatisfactory, especially for larger tumors or those with no capsule. This is due to the fact that HCC is a hypervascular malignant tumor and has a high recurrence and fast growth rate. Metabolism of tumor growth, invasion, metastasis and recurrence of the tumor are closely interrelated with blood supply. Some studies have confirmed that there are multiple angiogenesis factors expressed in the tumors, of which VEGF is the strongest factor. A number of studies have shown that VEGF over-expression and the activation of its signaling pathway play an important role in tumor progression and angiogenesis. In vitro experiments have shown that hypoxia can upregulate VEGF and stimulate liver tumor cell growth. Although TACE treatment will inevitably lead to hypoxia of the liver cancer cells and the surrounding liver tissue, TACE not only blocks the blood vessels but can also increase VEGF, which causes residual tumor progression, metastasis, and even the generation of new tumors21. Therefore, blocking VEGF-mediated angiogenesis after TACE is likely to be an effective way to optimize the efficacy of TACE.
Sorafenib (Nexavar, Bayer Pharmaceuticals) is an oral multikinase inhibitor that blocks tumor cell proliferation by targeting the Raf/mitogen-activated protein kinase/extracellular signal regulated kinase (Raf/MEK/ERK) signaling pathway and exerts an anti-angiogenic effect by targeting the tyrosine kinases (TKs) VEGF receptor 2 (VEGFR-2), VEGFR-3, and platelet-derived growth factor receptor (PDGFR-b). In preclinical models, evidence of dose-dependent activity against a wide range of tumor types, including HCC, was observed. Sorafenib exhibited growth inhibition, induction of apoptosis, and down-regulation of the anti-apoptotic protein Mcl-1 through a Raf/MEK/ERK-independent mechanism. This was reported in the SHARP clinical trials recently12. The study, which included 602 advanced (phase 3) HCC patients, demonstrated in a double-blind and placebo-controlled trial that sorafenib can significantly increase the overall survival of patients with advanced hepatocellular carcinoma or primary liver cancer. It suggested that sorafenib would be the first drug that is well tolerated and can significantly extend overall survival for patients with advanced HCC. It is a breakthrough for molecular targeted therapy in the treatment of liver cancer4. With the breakthrough of sorafenib in liver cancer treatment, studies focused on the combination treatment of sorafenib and other chemotherapy drugs in clinical and basic research. Sorafenib combined with TACE, radiofrequency therapy or microwave therapy became another area of focus. In one study using TACE plus sorafenib treatment, Strebel22 suggested that an anoxic environment was caused by TACE treatment, which increased the expression of VEGF. TACE combined with sorafenib can effectively inhibit the regeneration of blood vessels, reduce tumor recurrence and metastasis and improve the therapeutic effects for liver cancer.
In this study, TACE combined with sorafenib had no CR or PR patients, but seven patients had stable disease (SD), accounting for (70%, According to RECIST criteria). In the TACE alone group, one patient (10%) had a PR and three (30%) had SD. Patients with multiple recurrences of HCC after liver transplantation that received sorafenib treatment had nearly an eight month median survival benefit, compared with those who received TACE alone. The effect of sorafenib on survival time was significantly better than that of TACE (P=0.005). Adverse events were more common in the sorafenib treatment (eg, diarrhea, weight loss, and hand–foot skin reaction) but were mild to moderate. The most frequent adverse events in this study were hand–foot skin reaction and diarrhea, which were in agreement with previous reports6. In the multivariate Cox regression analysis, preoperative high bilirubin grade was found to be a risk factor of survival time, both after recurrence and overall survival time. In addition, the therapeutic strategy marked by GROUP showed that TACE and sorafenib combination therapy benefited patients by prolonging their lifetime.
This trial showed that sorafenib prolonged median survival in patients with multiple recurrences of HCC after liver transplantation. This is an important finding given the increasing incidence of the disease around the world and the lack of efficacious therapeutic options for advanced HCC after liver transplantation. The number of cases in our study is relatively small compared to other studies, which is one flaw of this study. However, because the aim of this report was to study patients with multiple recurrences after liver transplantation with rare liver defects, expensive surgery cost and the high drug prices for sorafenib resulted in a small patient number. However, appropriate statistical methods and small P values demonstrated the reliability of our findings. Furthermore, more cases are being collected and we also conducted a clinical trial with pre-liver transplantation patients. In summary, we expect that sorafenib will become the first-line treatment drug for patients with advanced HCC who had recurrence after liver transplantation. The TACE plus sorafenib group in this study has longer stable disease status and survival time. The sorafenib was well tolerated, making it worthy of further studies with a large group of cases.
Author contribution
Wei-feng TAN, Zhi-quan QIU designed the research, collected the data and carried out part of the operations. Xiao-qing JIANG and Xiang-ji LUO planned and coordinated the research. Yong YU, Bing YI, Chen LIU, Ying-he QIU, Fei-ling FENG and Bai-he ZHANG collected the data and carried out part of the operations. Jing-han WANG and Pei-ning YAN carried out case follow-up. Rong-zheng RAN analyzed the data. Meng-chao WU and Wan-yee LAU planned, oversaw the research project and drafted the paper.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Yates MS, Kensler TW. Keap1 eye on the target: chemoprevention of liver cancer. Acta Pharmacol Sin. 2007;28:1331–42. doi: 10.1111/j.1745-7254.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143–54. doi: 10.1055/s-2005-871194. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- Mi JX, Wang GF, Wang HB, Sun XQ, Ni XY, Zhang XW, et al. Synergistic antitumoral activity and induction of apoptosis by novel pan Bcl-2 proteins inhibitor apogossypolone with adriamycin in human hepatocellular carcinoma. Acta Pharmacol Sin. 2008;29:1467–77. doi: 10.1111/j.1745-7254.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- Pan QW, Zhong SY, Liu BS, Liu J, Cai R, Wang YG, et al. Enhanced sensitivity of hepatocellular carcinoma cells to chemotherapy with a Smac-armed oncolytic adenovirus. Acta Pharmacol Sin. 2007;28:1996–2004. doi: 10.1111/j.1745-7254.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–9. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892–9. doi: 10.1200/JCO.2005.03.196. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma- an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–47. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59:561–74. doi: 10.1007/s00280-006-0393-4. [DOI] [PubMed] [Google Scholar]
- Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, et al. Activation of mitogen-activated protein kinases/ extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951–8. doi: 10.1002/hep.510270409. [DOI] [PubMed] [Google Scholar]
- Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55–76. doi: 10.1055/s-2006-960171. [DOI] [PubMed] [Google Scholar]
- Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–28. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864–80. doi: 10.1016/j.jhep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–21. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- Strebel BM, Dufour JF. Combined approach to hepatocellular carcinoma: a new treatment concept for nonresectable disease. Expert Rev Anticancer Ther. 2008;8:1743–9. doi: 10.1586/14737140.8.11.1743. [DOI] [PubMed] [Google Scholar]