Abstract
Aim:
To investigate the ability of drug-loaded N,O-carboxymethyl chitosan (CMCS) hydrogels to modulate wound healing after glaucoma filtration surgery.
Methods:
The drug-loaded CMCS hydrogels were in situ synthesized using genipin as the crosslinker in the presence of 5-fluorouracil (5FU) or bevacizumab. Their structures were characterized by FTIR, ultraviolet-visible (UV-vis) spectroscopy and scanning electron microscopy (SEM). In-vitro drug release experiments and in vivo evaluation in rabbits were performed.
Results:
The results of FTIR, UV-vis spectroscopy and SEM analyses indicated that 5FU was encapsulated into the CMCS hydrogels that were crosslinked by genipin. The in vitro drug release experiments showed that nearly 100% of 5FU was released from the drug-loaded hydrogels within 8 h, but less than 20% bevacizumab was released after 53 h. The in vivo evaluation in rabbits indicated that the drug-loaded CMCS hydrogels were nontoxic to the cornea and were gradually biodegraded in the eyes. Furthermore, the drug-loaded CMCS hydrogels effectively inhibited conjunctival scarring after glaucoma filtration surgery and controlled postoperative intraocular pressure (IOP).
Conclusion:
The drug-loaded CMCS hydrogels provide a great opportunity to increase the therapeutic efficacy of glaucoma filtration surgery.
Keywords: N,O-carboxymethyl chitosan; genipin; hydrogel; glaucoma; intraocular pressure; 5-fluorouracil; bevacizumab
Introduction
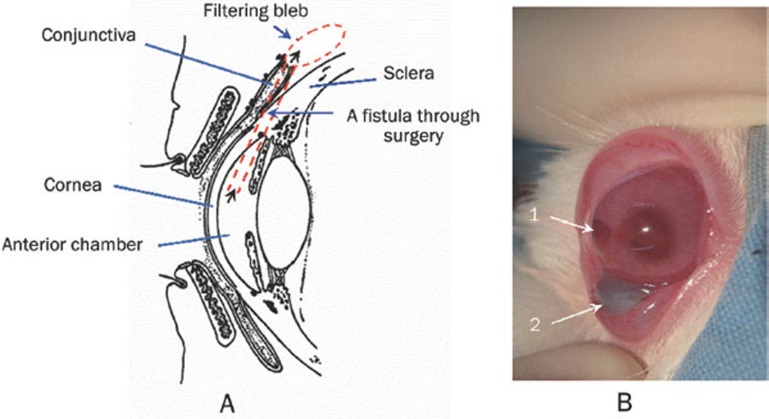
Glaucoma is one of the major causes of irreversible blindness in the world, which is usually associated with increased intraocular pressure (IOP)1, 2. At present, glaucoma filtration surgery is the most effective therapy1. It is performed by creating a fistula between the anterior chamber and the subconjunctival space of the eye to allow the drainage of aqueous humor into the filtering bleb (Scheme 1A), resulting in reduced IOP3. However, glaucoma filtration surgery fails in 30% to 50% of patients because the scarred conjunctiva adheres to the episcleral tissue, preventing further drainage of aqueous humor4. Therefore, to maximize the therapeutic effect of glaucoma filtration surgery, the wound-healing process must be modulated5. 5-Fluorouracil (5FU), a potent antimetabolic agent, can play an important role in preventing proliferation of fibroblasts, which is crucial for reducing scar formation5, 6. Recently, bevacizumab (Avastin), a synthetic monoclonal antibody against vascular endothelial growth factor (anti-VEGF), was reported to limit scar formation after glaucoma filtration surgery and effectively decreased IOP of patients4, 7, 8. However, multiple subconjunctival injections of 5FU or bevacizumab after surgery are required to maintain a high drug concentration4, 9, 10. This process is usually associated with several undesirable complications such as conjunctival wound leaks, corneal epithelial defects, endophthalmitis, cataracts and other ocular diseases6. Controlled release of 5FU and bevacizumab is thus important to achieve a sustained pharmacologic effect and overcome the drawbacks of this procedure.
Scheme 1.
(A) Representation of the eyeball structure and the glaucoma filtration surgery and (B) a photo of the postoperative rabbit eye after injecting the drug-loaded hydrogels (1: the surgery section, 2: the drug-loaded CMCS hydrogel).
Carboxymethyl chitosan is an important water-soluble polysaccharide with low toxicity, good biocompatibility and biodegradability11. It has attracted much attention due to its ability to prolong ophthalmic drug retention12. In addition, genipin, a naturally water-soluble compound with good biocompatibility and low cytotoxicity13, 14, has been widely reported as a crosslinker for creating hydrogels in biomedical fields15. Various genipin-crosslinked N,O-carboxymethyl chitosan (CMCS) hydrogels have been developed for drug delivery11, 12. To our knowledge, however, there are few reports in the literature on the use of carboxymethyl chitosan-genipin hydrogels in glaucoma therapy.
In this work, genipin-crosslinked CMCS hydrogels loaded with 5FU in the presence or absence of bevacizumab were in situ synthesized to improve the therapeutic efficacy of glaucoma filtration surgery. The hydrogels were characterized by Fourier transform infrared spectroscopy (FTIR), ultraviolet-visible (UV-vis) spectroscopy, and scanning electron microscopy (SEM). Swelling properties and in vitro drug release behaviors of the hydrogels were assessed. The drug-loaded hydrogels were injected into the subconjunctival space surrounding the filtration surgery section in rabbits (Scheme 1B). Then the in vivo evaluations were performed, including evaluation of the toxicity and biodegradability of the hydrogels in the anterior ocular tissues, histological assessment of the subconjunctival tissues surrounding the surgery section, evaluation of the filtering blebs and measurement of the IOP values.
Materials and methods
Reagents
Water-soluble CMCS (deacetylation degree=90%, substitution degree of carboxymethyl ≥80%, and viscosity=10–1000 mpa·s) was obtained from Qingdao Heppe Biotechnology, Ltd (Qingdao, Shandong, China). Genipin (purity measured by HPLC >98.0%) was from Wako Pure Chemical Industries, Ltd (Osaka, Japan). 5FU was bought from Shanghai Xudong-Haipu Pharmaceutical CO, Ltd (Shanghai, China). Bevacizumab, with a molecular weight 149 000 Daltons, was originally created by Genentech Inc (South San Francisco, California, USA) and was purchased from F Hoffmann-La Roche Ltd (Basel, Switzerland). Mitomycin C (MMC) was obtained from Zhejiang Hisun Pharmaceutical Co Ltd (Taizhou, Zhejiang, China). Coomassie brilliant blue G-250 (CBBG) was obtained from Beyotime Institute of Biotechnology (Shanghai, China). Bovine serum albumin (BSA) was purchased from Shanghai Bio Life Science & Technology Co, Ltd (Shanghai, China).
In situ synthesis and characterization of the drug-loaded CMCS hydrogels
The drug-loaded CMCS hydrogels were in situ synthesized as illustrated in Table 1. Generally, 10.0 mg of CMCS was dissolved in 1 mL of water and stirred at 37 °C for approximately 2 h. 5FU (10.0 mg) in the presence or absence of bevacizumab (2.5 mg) and different amounts of genipin were then added in sequence under stirring. The resulting viscous mixture was allowed to set aside at room temperature for 24 h, after which became dark blue, resulting in the formation of elastic hydrogel. The CMCS hydrogel was synthesized according to the method described above without adding drugs. Some hydrogels were freeze-dried for characterization.
Table 1. Conditions of in situ synthesis of the drug-loaded CMCS/genipin hydrogels.
| Hydrogels | WCChit (mg) (WCChit/WT%) | W5-Fu (mg) (W5-Fu/WT%) | WGenip (mg) (WGenip/WT%) | WBev (mg) (WBev/WT%) |
|---|---|---|---|---|
| CMCS-5FU-1 | 10.0 (47.5) | 10.0 (47.5) | 1.0 (5.0) | – |
| CMCS-5FU-2 | 10.0 (38.5) | 10.0 (38.5) | 6.0 (23.0) | – |
| CMCS-5FU-3 | 10.0 (31.0) | 10.0 (31.0) | 12.0 (38.0) | – |
| CMCS-5FU-Bev | 10.0 (29.0) | 10.0 (29.0) | 12.0 (35.0) | 2.5 (7.0) |
| CMCS/genipin | 10.0 (45.0) | – | 12.0 (65.0) |
WT: the total weight of all materials.
The chemical structures of the synthesized hydrogels were characterized by FTIR and UV-vis spectroscopy. Using the freeze-dried hydrogel samples, FTIR measurement was performed with an FTIR Analyzer (Nicolet/Nexus 670, USA) at a resolution of 4 cm-1 using the KBr method. After the hydrogels were dispersed in water by stirring, UV-vis spectroscopy experiments were conducted with a UV-vis-NIR Spectrophotometer (UV-3150, Shimadzu, Japan) from 190–700 nm. The microstructures of freeze-dried hydrogels were observed on an S-4800 scanning electron microscope (HI-9056-0003, Hitachi, Japan), after they were sputter-coated with gold in an E-1045 ion sputter (Hitachi, Japan).
Swelling property of the drug-loaded CMCS hydrogels
The swelling properties were evaluated by immersing the freeze-dried hydrogels (10 mg) to swell in 10 mL of a PBS solution (pH 7.4) at 37 °C, simulating the tear conditions of human bodies16. At different intervals, the swollen hydrogels were withdrawn and weighed after removal of excess surface water with a filter paper. Each experiment was performed in triplicate. The swelling ratio was defined as:
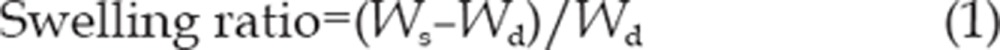 |
where Ws and Wd are the weights of swollen and dried hydrogel samples, respectively.
In vitro evaluation of drug release
Release studies were conducted at 37 °C under slow magnetic stirring. Approximately 1 mL of drug-loaded hydrogel was enclosed in a dialysis bag (molecular weight cutoff 14 000), which was immersed in 120 mL of PBS (pH 7.4). At predetermined time intervals, 0.5-mL aliquots of buffer medium were removed and replaced with 0.5 mL of fresh buffer solution. Using a UV-vis-NIR Spectrophotometer as above, the amount of released 5FU was determined from the 5FU standard calibration at 266 nm, and the amount of released bevacizumab was determined from the standard calibration of BSA dyed by CBBG at 595 nm according to the literature17. All release studies were performed in triplicate.
Rabbit model of glaucoma filtration surgery
Animal experiments complied with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Male and female New Zealand White rabbits weighing 2.5 to 3.2 kg were purchased from the Guangdong Medical Laboratory Animal Center, China.
Glaucoma filtration surgery was performed on one eye of each rabbit using a standard clinical model of trabeculectomy as illustrated in Scheme 1A. Generally, the rabbits were anesthetized by intramuscular injection of a mixture of 40 to 80 mg/kg of ketamine and 20 to 40 mg/kg of chloropromazine. Following local anesthesia, a lid speculum was inserted to expose the eyeball following the local anesthesia with 2% lidocaine. A limbus-based conjunctival flap and scleral flap were made in sequence. A block of tissue containing inner sclera, trabeculum and peripheral cornea and measuring approximately 2 mm×1 mm was excised at the limbus. A peripheral iridectomy was then performed. The conjunctiva was repositioned, and the wound was closed with a 10–0 nylon suture in a continuous fashion. At the end of each surgery, 0.5 % erythromycin ointment was applied to the conjunctival sac, and 0.5% neomycin eye drops were instilled four times for three days. The animals were then randomly divided into four groups based on the different treatments in the subconjunctival section as summarized in Table 2. The drug-loaded or -unloaded CMCS hydrogels were injected into the subconjunctival space surrounding the filtration surgery section in rabbits as shown in Scheme 1B.
Table 2. Random divided animal groups.
| Groups | n* | Treatment in the subconjunctival section undergoing the filtration surgery |
|---|---|---|
| Control | 8 | Only undergoing the filtration surgery. |
| I | 8 | The CMCS/genipin hydrogel (0.3 mL) was postoperatively injected. |
| II | 8 | A sponge (3 mm×4 mm) soaked with MMC solution (0.25 mg/mL) was placed under the conjunctival flap intraoperation. After 5 min, it was removed and all the exposed tissues were irrigated with 10 mL of physiological saline solution. |
| III | 9 | The CMCS-5FU-3 hydrogel (0.3 mL) containing 3.3 mg of 5-Fu was postoperatively injected. |
| IV | 9 | The CMCS-5FU-Bev hydrogel (0.3 mL) containing 3.3 mg of 5-Fu and 0.8 mg of bevacizumab was postoperatively injected. |
* Numbers of rabbit eyes.
Corneal and conjunctival biopsy
One rabbit in each group was sacrificed by air embolism at postoperative days 7, 14, 21, and 28. Their eyes were then enucleated carefully. The isolated cornea of each rabbit was placed endothelial side up in a concave slide and was stained by a trypan blue solution (0.25%) for 1 min and an alizarin red solution (0.2%, pH 4.2) for 5 min consecutively according to the literature18. The stained cornea was rinsed twice in saline after pouring away the staining reagent and then was fixed by 2.5% glutaraldehyde solution for 10 min. The morphologies of the corneal cells were observed on an Axioplan2 imaging microscope (Carl Zeiss, Germany) at ×200 magnification.
The enucleated eyes were immersed in 10% formalin solution for 48 h to perform the histological assessment. The tissues surrounding the filtration surgery were cut down, stained with hematoxylin and eosin (H&E) and then were observed on a Nikon Eclipse Ti-s optical microscope (Nikon, Japan) at ×40 magnification.
Postoperative evaluation
The heights of the filtering blebs for the four groups, defined in terms of the Indiana Bleb Appearance Grading Scale19, were evaluated with a nonparametric Mann-Whitney U test as appropriate. The IOP values of rabbits were measured at predetermined time intervals by a non-contact tonometer (Canon TX-F, Japan). One-way ANOVA was used to compare the preoperative IOP values, in which the P value was shown to be 0.232 (>0.05), indicating that there was no statistical difference between groups. The general linear model was used to compare the IOP values within each group and a level of P< 0.05 was considered statistically significant.
Results
Synthesis and structural analysis of the drug-loaded CMCS hydrogels
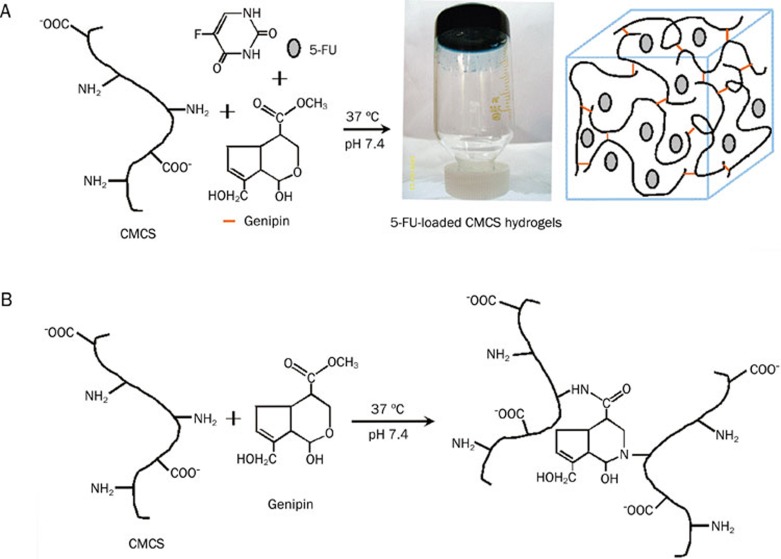
After reacting CMCS with genipin in the presence of 5FU, dark bluish hydrogels were obtained as shown in Figure 1A. The FTIR and UV-vis spectra of the synthesized hydrogels are shown in Figures 2 and 3, respectively.
Figure 1.
Possible representations for (A) in situ synthesis of the drug-loaded CMCS hydrogel and its photo and (B) crosslinking mechanism between CMCS and genipin.
Figure 2.
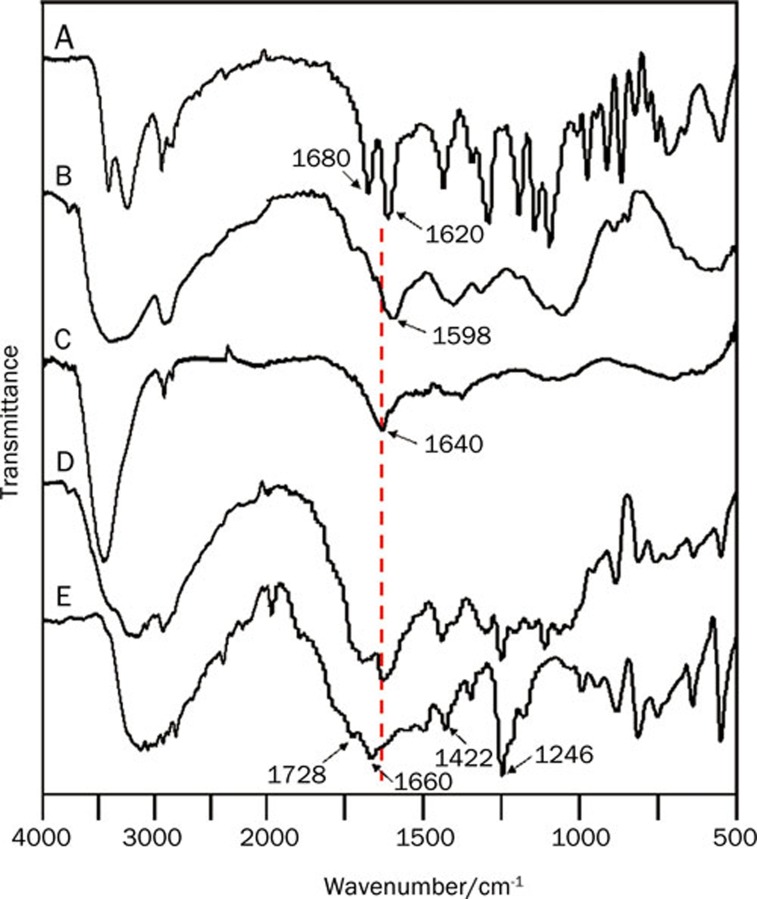
FTIR spectra of (A) genipin, (B) CMCS, (C) the CMCS hydrogel, (D) CMCS-5FU-3 and (E) 5FU.
Figure 3.
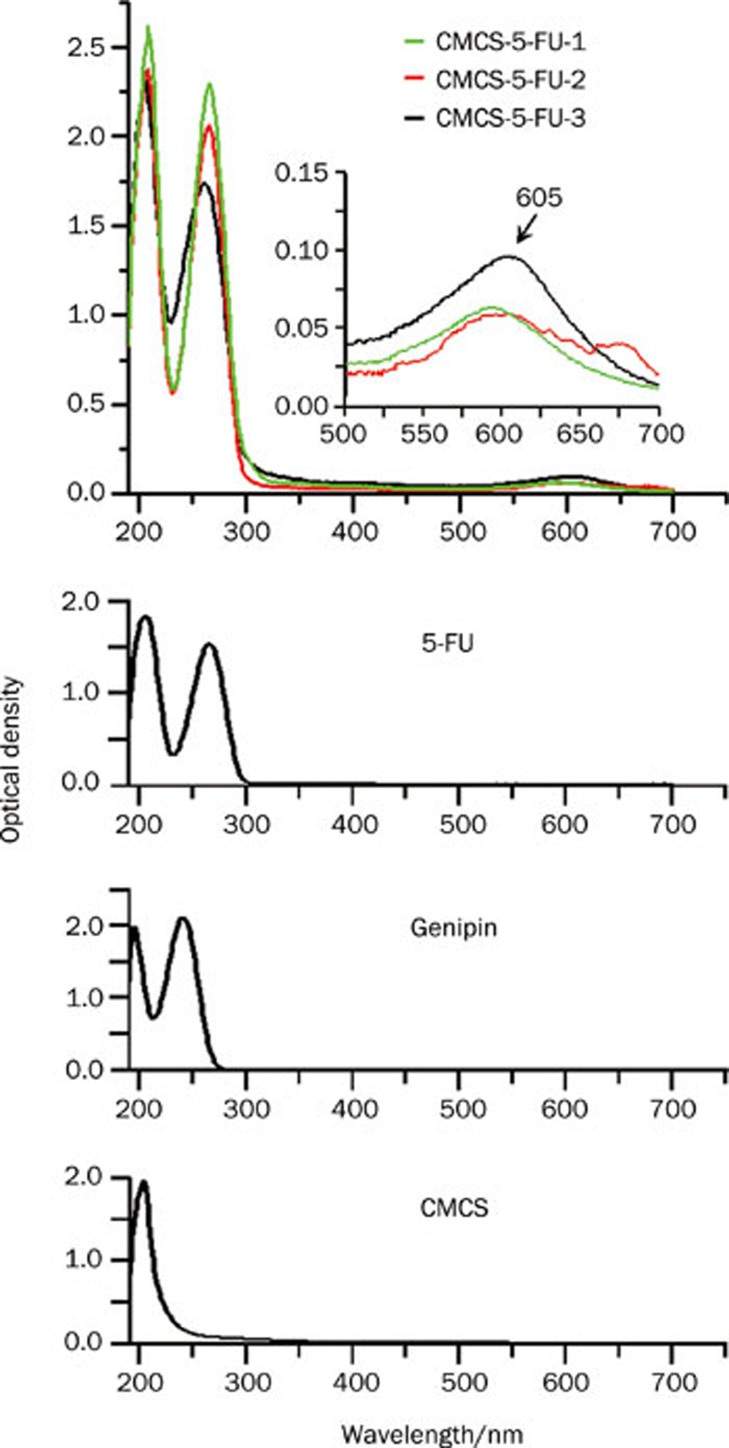
UV-vis spectra of the 5FU-loaded CMCS hydrogels, 5FU, genipin, and CMCS.
The morphology of 5FU-loaded CMCS hydrogels was then observed by SEM as shown in Figure 4. The lamellar crystals of 5FU were embedded in the porous CMCS hydrogels. In addition, the crystals of CMCS-5FU-1 were more than that of CMCS-5FU-2 and CMCS-5FU-3.
Figure 4.
SEM images of (A) CMCS-5FU-1, (B) enlarged image of CMCS-5FU-1, (C) CMCS-5FU-2 and (D) CMCS-5FU-3.
Swelling properties of the drug-loaded CMCS hydrogels
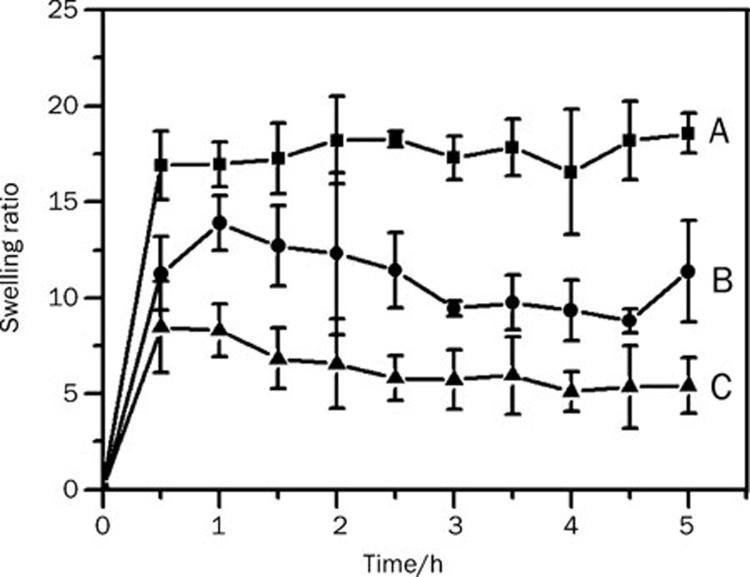
Figure 5 illustrates the swelling kinetic curves of the 5FU-loaded CMCS hydrogels in the pH 7.4 PBS solution at 37 °C. All hydrogels arrived at equilibrium process after swelling for about half an hour. Furthermore, the swelling ratios of CMCS-5FU-3 hydrogel decreased approximately three-fold and two-fold compared with those of CMCS-5FU-1 and CMCS-5FU-2 hydrogels, respectively. Generally, with increasing amounts of genipin, the swelling ratios of the hydrogel decreased.
Figure 5.
Swelling behaviors of 5FU-loaded hydrogels in PBS solution (pH 7.4): (A) CMCS-5FU-1, (B) CMCS-5FU-2, and (C) CMCS-5FU-3.
In vitro drug release behaviors of the drug-loaded CMCS hydrogels
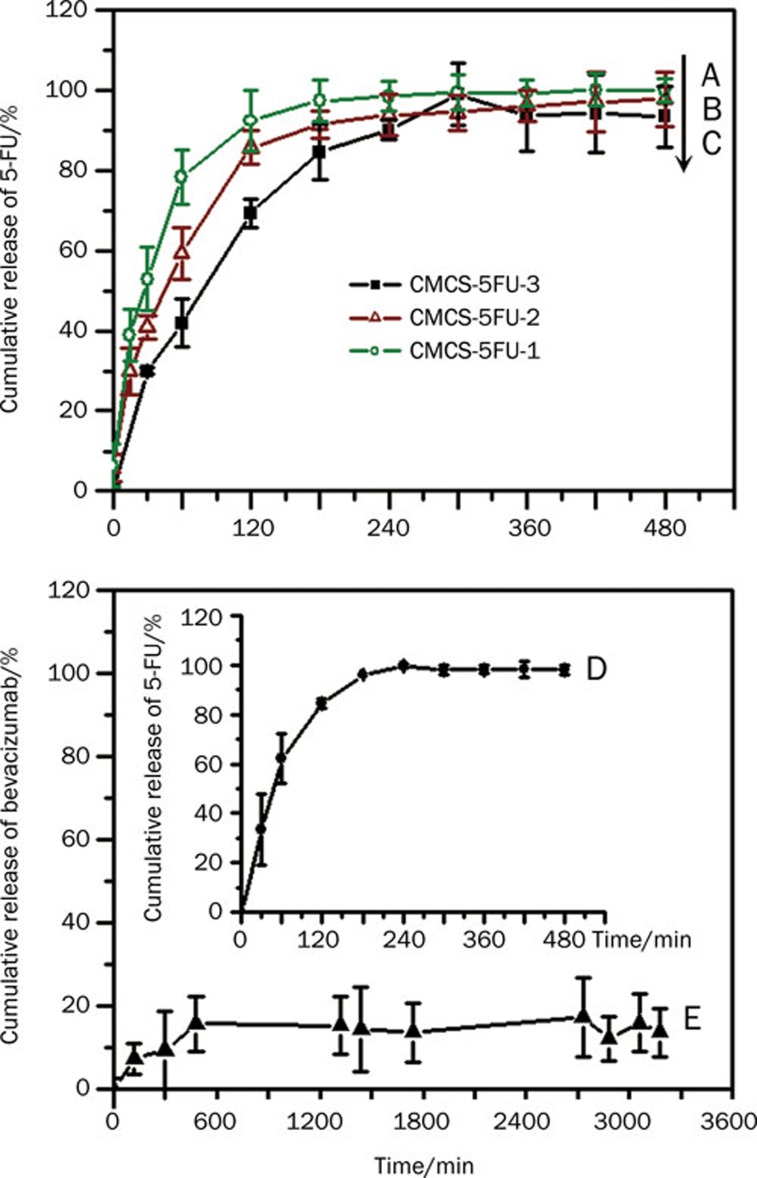
The 5FU in vitro release profiles of the drug-loaded hydrogels are shown in curves A–D in Figure 6. The results indicated that 5FU was released within 8 h, and the cumulative amounts of 5FU were close to 100% at equilibrium. To some extent, it is likely that increasing amounts of genipin prolonged the releasing equilibrium time; however, the cumulative release of 5FU decreased with increasing amounts of genipin. By contrast, less than 20% bevacizumab was released even after 53 h (curve E).
Figure 6.
In vitro drug release profiles of 5FU and bevacizumab from different hydrogels in PBS solution (pH 7.4): (A) 5FU release from CMCS-5FU-1 (○ olive), (B) 5FU release from CMCS-5FU-2 (Δ wine), (C) 5FU release from CMCS-5FU-3 (▪ black), (D) 5FU release from CMCS-5FU-Be (• black) and (E) bevacizumab released from CMCS-5FU-Bev hydrogel (▴ black).
Drug release kinetics was further analyzed by fitting the data to an exponential Peppa's model described as Eq (2)20:
 |
where Mt and M∞ are the cumulative amount of drug released at time t and infinite time (to be approximated by the equilibrium time)21, respectively. The exponent constant n indicates the type of drug release mechanism21, 22: (i) a value of n=0.5 represents Fickian or case I diffusion; (ii) a value of n=1.0 suggests non-Fickian or case II diffusion; (iii) 0.5<n<1.0 indicates anomalous transport, exhibiting the rates of Fickian diffusion and polymer relaxation are comparable; and (iv) if n<0.5, the diffusion process exhibits a pseudo-Fickian behavior, but the approach to final equilibrium is very slow. The parameter k is a kinetic rate constant that is independent of the geometric and structural properties of the polymer23.
By plotting log10 (Mt/M∞) versus log10 (t), the exponent constant (n) and rate constant (k) as well as the corresponding coefficient (R2) were estimated, as summarized in Table 3.
Table 3. Release kinetic parameters of the drug-loaded CMCS/genipin hydrogels.
| Drug | Hydrogel | n | k | R2 | Transport mechanism |
|---|---|---|---|---|---|
| 5-Fu | CMCS-5FU-1 | 0.51 | 0.097 | 0.99 | Fickian diffusion |
| 5-Fu | CMCS-5FU-2 | 0.51 | 0.074 | 0.99 | Fickian diffusion |
| 5-Fu | CMCS-5FU-3 | 0.56 | 0.048 | 0.99 | Fickian diffusion |
| 5-Fu | CMCS-5FU-Bev | 0.52 | 0.066 | 0.94 | Fickian diffusion |
| Bevacizumab | CMCS-5FU-Bev | 0.62 | 0.022 | 0.99 | Anomalous transport |
In vivo evaluation of toxicity and biodegradability of the drug-loaded CMCS hydrogels
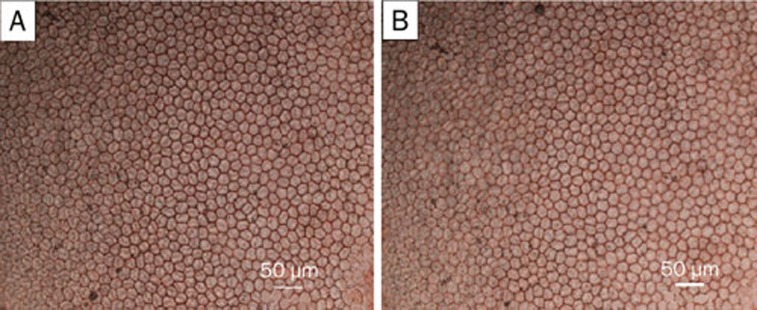
From the images shown in Figure 7, it is clearly observed that the corneal endothelial cells exhibited some regularly hexagonal cells with the sizes of 20 to 30 μm that joined with each other tightly. Additionally, the blue nuclei did not appear in the images.
Figure 7.
Images of stained corneal endothelial cells surrounding the surgery section after injection of the drug-loaded CMCS hydrogels on postoperative day 28: (A) CMCS-5FU-3 and (B) CMCS-5FU-Bev (×200 magnification).
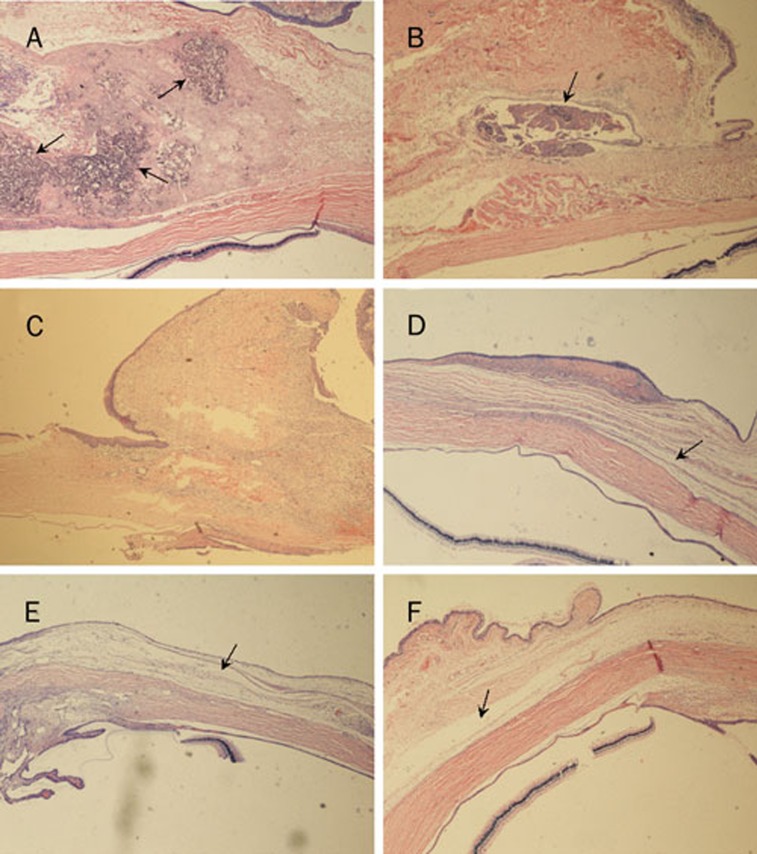
Figures 8A–8B illustrate the histologic sections of the subconjunctival tissue surrounding the CMCS-5FU-3 hydrogel on postoperative days 7 and 28, respectively. The regions of CMCS-5FU-3 marked by arrows decreased with prolonged postoperative time.
Figure 8.
Histologic sections of: (A) and (B) the subconjunctival tissues surrounding CMCS-5FU-3 hydrogel on postoperative days 7and 28; and the subconjunctival tissues in the surgery regions: (C) group I on postoperative day 7 and (D) group II, (E) group III and (F) group IV on postoperative day 28 (×40 magnification)
Effects of the drug-loaded CMCS hydrogels in modulating wound healing and reducing the IOP values
As shown in Figure 8C, following postoperative injection of the CMCS hydrogel, dense connective tissue formed in the subconjunctival region only on postoperative day 7. Obviously, there were great differences in the subconjunctival tissues in the surgery regions undergoing treatment with MMC and the CMCS-5FU-3 and CMCS-5FU-Bev hydrogels (Figures 8D–8F). The connective tissues were loosely arranged and cleft-like spaces marked by arrows were clearly observed on postoperative day 28.
Table 4 illustrates that the heights of blebs were in range of grades 1–3 on postoperative day 7. With prolonged postoperative time, the height grades of blebs decreased to 0 in the control group and group I. However, the height grades of blebs were maintained at 0.5–1 grade in groups II, III, and IV until postoperative day 21. A characteristically avascular and thin bleb surrounded by no hyperemia was observed in Figure 9D, but the blebs were flat in Figures 9A–9C.
Table 4. Height grades of blebs following the filtration surgery. bP<0.05 vs control group.
| Postoperative days | Control group | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|---|
| 7 | 1 (1,1) | 1 (1,2.5) | 3 (3,3)b | 2 (1.25, 2.75) | 2 (2,2)b |
| 14 | 0 (0,0.75) | 0 (0,0) | 2.5 (2,3)b | 1 (1, 1.75)b | 2 (1.25,2)b |
| 21 | 0 (0,0.75) | 0.5 (0,1) | 1 (1,1)b | 0.5 (0,1) | 1 (0.25,1) |
| 28 | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0 (0,0) | 1 (0.25,1)b |
Data were expressed as mdian (25 percentile,75 percentile).
Figure 9.
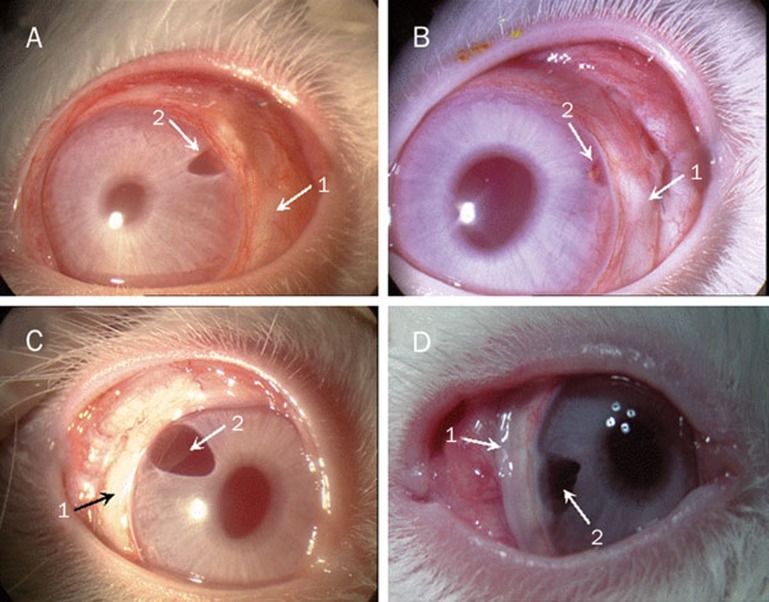
The morphologic features of blebs after the filtration surgery: (A) group I on postoperative day 14 and (D) group II, (C) group III and (D) group IV on postoperative day 28 (1: blebs, 2: the surgery sections).
Table 5 illustrates the postoperative IOP values following the filtration surgery for different animal groups.
Table 5. Postoperative intraocular pressures following the filtration surgery (mmHg). bP<0.05 vs control.
| Postoperative days | Control group | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|---|
| 0 | 6.55±0.21 | 6.50±0.19 | 6.62±0.16 | 7.00±0.11 | 6.67±0.24 |
| 1 | 5.12±0.36b | 4.50±0.22b | 5.40±0.04b | 5.85±0.07b | 5.57±0.36b |
| 3 | 4.85±0.07b | 4.80±0.22b | 5.30±0.13b | 6.00±0.08b | 5.65±0.33b |
| 5 | 4.95±0.09b | 5.50±0.22b | 5.50±0.22b | 6.13±0.19b | 5.67±0.33b |
| 7 | 4.88±0.36b | 6.15±0.38 | 5.55±0.25b | 5.77±0.18b | 5.50±0.19b |
| 14 | 6.35±0.29 | 6.65±0.16 | 5.12±0.31b | 5.57±0.19b | 5.67±0.21b |
| 21 | 6.22±0.14 | 6.40±0.23 | 5.35±0.21b | 5.90±0.13b | 5.87±0.19b |
| 28 | 6.05±0.09 | 6.45±0.26 | 5.35±0.29b | 6.33±0.21 | 5.33±0.31b |
Discussion
Structure of the drug-loaded CMCS hydrogels
Many previous works have reported that genipin may form blue pigments upon spontaneous reaction with amino groups of some biopolymer, such as chitosan24, 25, 26, 27, CMCS11, 12, and casein21. It was thus speculated that the dark bluish color of the hydrogels in Figure 1A resulted from the crosslinking reaction between genipin and the amino groups of CMCS. For further characterization of the structure of the synthesized hydrogels, FTIR and UV-vis analyses were performed.
Upon comparison of the FTIR spectra of the CMCS hydrogel, CMCS-5FU-3 and genipin (curves C, D, and A in Figure 2, respectively), it was found that amide bands at 1640 cm−1 appeared, while the absorption at 1680 cm-1 assigned to the vibration of the carboxymethyl group of genipin disappeared from the spectra of the synthesized hydrogels (curves C and D). Moreover, the amino peak at 1598 cm-1 in the FTIR spectrum of CMCS (curve B) disappeared after CMCS reacted with genipin (curves C and D). These features suggest that the carboxymethyl group of genipin reacted with the amino group of CMCS to form a secondary amide according to the literature25, 26.
On the other hand, a broad absorption peak at about 605 nm appeared in the UV-vis spectra of 5FU-loaded CMCS hydrogels in Figure 3, corresponding to the characteristic absorption of the crosslinking reaction between amino groups of CMCS and genipin24. This further confirmed that the crosslinking reaction happened. Additionally, the absorbance at λ=605 nm of CMCS-5FU-3 was higher than those of the other two hydrogels, suggesting its higher crosslinking degree. The possible crosslinking mechanism for the reaction of CMCS with genipin was thus speculated as illustrated in Figure 1B.
Compared with the FTIR spectrum of 5FU (Figure 2E), some characteristic absorptions of 5FU appeared in the FTIR spectrum of CMCS-5FU-3 (Figure 2D), such as 1246, 1422, and 1730–1655 cm-1, in agreement with the previous works28. Furthermore, the UV-vis spectra of the CMCS-5FU hydrogels also exhibited the 5FU absorption peaks in Figure 3, implying that 5FU was encapsulated in the CMCS hydrogel. The SEM images indicated that the lamellar crystals of 5FU were embedded in the porous CMCS hydrogels. Therefore, the structure of drug-loaded CMCS hydrogel was speculated as illustrated in Figure 1A, in which 5FU was encapsulated into the CMCS hydrogels.
Swelling properties of the drug-loaded CMCS hydrogels
In Figure 5, CMCS-5FU hydrogels reached the swelling equilibrium only after about half an hour. This might be explained by a large swelling force created by the electrostatic repulsion between the negatively charged carboxymethyl groups substituted on CMCS at pH 7.411, leading to water molecules promptly penetrating into the hydrogels. A higher amount of genipin used in the synthesis process could bring about a greater extent of chemical crosslinking of CMCS, which greatly restricts the mobility and hydration of the macromolecular chains in the hydrogel21. This is advantageous for retardation of 5FU diffusing from the hydrogel and to realize sustained release of drugs. Based on these results, the in vitro drug release behavior and in vivo evaluation of CMCS-5FU-3 were further investigated.
In vitro drug release behaviors of the drug-loaded CMCS hydrogels
The result of in vitro drug release behaviors indicated that 5FU was almost released within 8 h. However, a longer drug release time is anticipated in vivo because the drug-loaded hydrogels were injected in the subconjunctival section (Scheme 1B), in which has a much lower water content.
By contrast, the release time of bulky bevacizumab molecules became longer. In addition, the amount of released bevacizumab was less than the amount of released 5FU. It is possible that some bevacizumab molecules crosslinked with the hydrogel network induced by genipin, and they cannot be released unless the CMCS matrices are degraded. According to the chemical reaction mechanism between genipin and protein reported by Song et al21, as a protein, bevacizumab contains primary amine groups, which may be crosslinked by genipin. Therefore, the crosslinked bevacizumab molecules may be released until the drug-loaded hydrogel is biodegraded in vivo. This result coincided with previous works, which reported that some protein molecules crosslinked with the hydrogel of CMCS and alginate could not be totally released in vitro11.
By fitting the exponential Peppa's model in Eq (2), the obtained parameters are summarized in Table 3. The n values of 5FU released from the drug-loaded hydrogels are close to 0.5, indicating that Fickian diffusion plays an important role during the release period. For bevacizumab released from the CMCS-5FU-Bev hydrogel, the n value increased to 0.62, which is in the range of 0.5–1.0, implying anomalous transport.
Additionally, the k values of 5FU release from the hydrogels of CMCS-5FU-1, CMCS-5FU-2, and CMCS-5FU-3 decreased with increasing the amounts of added genipin, implying that the 5FU release rate was related to the extent of chemical crosslinking of the hydrogels. Hydrogel with high crosslinking exhibited the worse swelling property as mentioned above, which tended to inhibit 5FU from diffusing. However, the k value of released bevacizumab was lower than that of released 5FU, suggesting that bevacizumab is more slowly diffused from the hydrogel than 5FU. This might be attributed to the dense network structure and low swelling ratio, which hindered the diffusion of bulky bevacizumab molecules29.
In vivo evaluation of toxicity and biodegradability of the drug-loaded CMCS hydrogels
Subconjunctival injection of antifibroblastic drugs remains the most appropriate technique for inhibition of wound-healing following glaucoma filtration surgery30. Considering that a fistula exists between the subconjunctival space and the anterior chamber, it is necessary to assess the toxicity of the injected drug-loaded hydrogels to the anterior ocular tissues, especially for important visual tissues such as the cornea.
Corneal endothelial integrity was evaluated by combined staining with two reagents, alizarin red S and trypan blue. These staining reagents provide a simple and quick technique for visualization of both damaged and healthy endothelial cells18. The shapes of cells may be reflected by staining the intercellular borders red with alizarin red S; meanwhile, damaged nuclei are stained blue by trypan blue, but healthy nuclei are not stained.
The cell characteristics shown in Figure 7 are consistent with those of corneal endothelial cells described in the literature16, confirming that these cells are healthy and the drug-loaded CMCS hydrogels are nontoxic to cornea. Moreover, the regions of CMCS-5FU-3 decreased with prolonged postoperative time in Figure 8B, proving that the hydrogel was gradually biodegraded in the eyes of rabbits.
Effects of the drug-loaded CMCS hydrogels in modulating wound healing and reducing the IOP values
It is known that, compared with other antifibrosis agents, MMC is highly effective in increasing the success rate of filtration surgery5, 31. Therefore, MMC has been considered the most effective antifibrotic agent for standard trabeculectomy despite its toxicities and side effects5. Here, the effect of the drug-loaded CMCS hydrogel on modulation of wound healing was analyzed in contrast to that associated with MMC.
Histological studies were performed to evaluate the modulation of wound healing after glaucoma filtration surgery on rabbits. The images in Figures 8C–8F indicated that the CMCS-5FU-3 and CMCS-5FU-Bev hydrogels inhibited conjunctival scarring as effectively as MMC. The modulation of wound healing by the drug-loaded hydrogels may have resulted from the sustained release of 5FU and bevacizumab from the hydrogels, which prolonged their antiscarring effects. The modulation of wound healing by the drug-loaded hydrogels was further evaluated by the morphologic appearance of the filtering bleb and the IOP.
It was illustrated in Table 4 that the heights of blebs were in range of grades 1–3 on postoperative day 7, suggesting that the aqueous humor was drained from the anterior chamber into the filtering bleb as illustrated in Scheme 1. With prolonging the postoperative time, the height grades of blebs decreased to 0 grade in the control group and group I, hinting that there was little aqueous humor in the blebs. However, the height grades of blebs remained at 0.5–1 grade in group II, III, and IV until postoperative day 21. These results indicated that, for the eyes undergoing treatment with MMC and the drug-loaded CMCS hydrogels, the aqueous humor could be drained from the anterior chamber for a long time after the glaucoma filtration surgery. In addition, it should be noted that the height grade of bleb in group IV still remained at 1 grade on postoperative day 28, but those of groups II and III decreased to 0. From the morphologic features of blebs shown in Figure 9, a characteristically avascular and thin bleb surrounded by no hyperemia was observed in Figure 9D, but the blebs were flat in Figures 9A–9C. This indicated that the effect of drainage into functional bleb produced by the CMCS-5FU-Bev hydrogel is more obvious.
In the control group and group I, IOP returned to preoperative levels after approximately two weeks as shown in Table 5. By contrast, the IOP values of groups II, III, and IV gradually decreased to a lower level compared with their preoperative IOP values from postoperative day 1 until day 21. This demonstrated that CMCS-5FU-3 and CMCS-5FU-Bev hydrogels could reduce postoperative IOP as effectively as MMC. In particular, the IOP of group IV continued to decrease, indicating that the CMCS-5FU-Bev hydrogel exhibited a stronger ability of remaining to lower IOP.
In brief, the results of postoperative evaluation revealed that the CMCS-5FU-Bev hydrogel exhibited strong antifibrotic effects and still controlled postoperative IOP values. It thus has potential for increasing the therapeutic effect of glaucoma filtration surgery.
In conclusion, the CMCS hydrogels loaded with 5FU and bevacizumab were in situ synthesized using genipin as the crosslinker. The results of FTIR, UV-vis spectroscopy and SEM analysis indicated that 5FU was encapsulated into the genipin-crosslinked CMCS hydrogels. In vitro drug release experiments showed that bevacizumab was more slowly released from the hydrogel than 5FU in a pH 7.4 PBS solution. The in vivo evaluation showed that the drug-loaded CMCS hydrogels were nontoxic to the cornea and were gradually biodegraded in the eyes of rabbits. Additionally, the drug-loaded CMCS hydrogels effectively delayed subconjunctival scar formation after glaucoma filtration surgery and controlled postoperative IOP. Further experiments on ocular pharmacokinetics will deepen the mechanistic understanding of antiscarring effects of the drug-loaded CMCS hydrogels.
Author contribution
Li-qun YANG, Yu-qing LAN, Li-ming ZHANG, and Ru-fu CHEN designed the research; Liang-zheng CHENG and Hui GUO prepared the drug-loaded CMCS hydrogels; Ji-zhou FAN and Xiang CAI analyzed the structures of the drug-loaded hydrogels; Hui GUO and Huai-sheng ZHOU performed in vitro and in vivo evaluations of the drug-loaded hydrogels; and Li-qun YANG and Yu-qing LAN wrote the paper.
Acknowledgments
We are grateful for support from the National Natural Science Foundation of China (No 20974130, 20574089), the Fundamental Research Funds for the Central Universities of China (No 09lgpy14), the Natural Science Foundation of Guangdong Province, China (No 6021311) and the Science and Technology Planning Project of Guangdong Province, China (No 2009B020313001, 2007B031504006).
References
- Li JY, Fu P, Yang Q. Experimental study of TGF-β2 antisense oligodeo-xynucleotide as an anti-scarring agent in glaucoma surgery. Int J Ophthalmol. 2007;7:10–4. [Google Scholar]
- Volotinen M, Maenpaa J, Kautiainen H, Tolonen A, Uusitalo J, Ropo A, et al. Ophthalmic timolol in a hydrogel vehicle leads to minor inter-individual variation in timolol concentration in aqueous humor. Eur J Pharm Sci. 2009;36:292–6. doi: 10.1016/j.ejps.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Chang MR, Cheng Q, Lee DA. Basic science and clinical aspects of wound healing in glaucoma filtering surgery. J Ocul Pharmacol. 1998;14:75–95. doi: 10.1089/jop.1998.14.75. [DOI] [PubMed] [Google Scholar]
- Li Z, Van Bergen T, Van de Veire S, Van de Vel I, Moreau H, Dewerchin M, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2009;50:5217–25. doi: 10.1167/iovs.08-2662. [DOI] [PubMed] [Google Scholar]
- Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003;48:314–46. doi: 10.1016/s0039-6257(03)00038-9. [DOI] [PubMed] [Google Scholar]
- Cui L, Sun N, Li X, Huang J, Yang J. Subconjunctival sustained release 5-fluorouracil for glaucoma filtration surgery. Acta Ophthalmol Sin. 2008;29:1021–8. doi: 10.1111/j.1745-7254.2008.00833.x. [DOI] [PubMed] [Google Scholar]
- Horsley MB, Kahook MY. Anti-VEGF therapy for glaucoma. Curr Opin Ophthalmol. 2010;21:112–7. doi: 10.1097/ICU.0b013e3283360aad. [DOI] [PubMed] [Google Scholar]
- Grewal DS, Jain R, Kumar H, Grewal SPS. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy: a pilot study. Ophthalmology. 2008;115:2141–5. doi: 10.1016/j.ophtha.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Lattanzio FA, Jr, Sheppard JD, Jr, Allen RC, Baynham S, Samuel P, Samudre S. Do injections of 5-fluorouracil after trabeculectomy have toxic effects on the anterior segment. J Ocul Pharmacol Ther. 2005;21:223–35. doi: 10.1089/jop.2005.21.223. [DOI] [PubMed] [Google Scholar]
- Zignani M, Einmahl S, Baeyens V, Varesio E, Veuthey JL, Anderson J, et al. A poly(ortho ester) designed for combined ocular delivery of dexamethasone sodium phosphate and 5-fluorouracil: subconjunctival tolerance and in vitro release. Eur J Pharm Biopharm. 2000;50:251–5. doi: 10.1016/s0939-6411(00)00107-7. [DOI] [PubMed] [Google Scholar]
- Chen SC, Wu YC, Mi FL, Lin YH, Yu LC, Sung HW. A novel pH sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate crosslinked by genipin for protein drug delivery. J Control Release. 2004;96:285–300. doi: 10.1016/j.jconrel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Colo GD, Zambito Y, Burgalassi S, Nardini I, Saettone MF. Effect of chitosan and of N-carboxymethylchitosan on intraocular penetration of topically applied ofloxacin. Int J Pharm. 2004;273:37–44. doi: 10.1016/j.ijpharm.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Mi FL. Synthesis and characterization of a novel chitosan-gelatin bioconjugate with fluorescence emission. Biomacromolecules. 2005;6:975–87. doi: 10.1021/bm049335p. [DOI] [PubMed] [Google Scholar]
- Jin J, Song M, Hourston DJ. Novel chitosan-based films cross-linked by genipin with improved physical properties. Biomacromolecules. 2004;5:162–8. doi: 10.1021/bm034286m. [DOI] [PubMed] [Google Scholar]
- Trevor SJ, Butler MF, Adams S, Laity PR, Burley JC, Cameron RE. Structure and phase transitions of genipin, an herbal medicine and naturally occurring crosslinker. Cryst Growth Des. 2008;8:1748–53. [Google Scholar]
- Ge J, Zhao J.OphthalmologyBeijing: Higher Education Press; 2004. p65p 20
- Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal Biochem. 1996;236:302–8. doi: 10.1006/abio.1996.0171. [DOI] [PubMed] [Google Scholar]
- Ide T, Yoo SH, Goldman JM. Descemet-stripping automated endothelial keratoplasty effect of inserting forceps on DSAEK donor tissue viability by using an in vitro delivery model and vital dye assay. Cornea. 2007;26:1079–81. doi: 10.1097/ICO.0b013e318142bdde. [DOI] [PubMed] [Google Scholar]
- Cantor LB, Mantravadi A, WuDunn D, Swamynathan K, Cortes A. Morphologic classification of filtering blebs after glaucoma filtration surgery: the Indiana bleb appearance grading scale. J Glaucoma. 2003;12:266–71. doi: 10.1097/00061198-200306000-00015. [DOI] [PubMed] [Google Scholar]
- Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release. 1987;5:37–42. [PubMed] [Google Scholar]
- Song F, Zhang LM, Yang C, Yan L. Genipin-crosslinked casein hydrogels for controlled drug delivery. Int J Pharm. 2009;373:41–7. doi: 10.1016/j.ijpharm.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Gudasi KB, Vadavi RS, Shelke NB, Sairam M, Aminahbavi TM. Synthesis and characterization of novel polyorganophosphazenes substituted with 4-methoxybenzylamine and 4-methoxyphenethylamine for in vitro release of indomethacin and 5-fluorouracil. React Funct Polym. 2006;66:1149–57. [Google Scholar]
- Hu SH, Liu TY, Liu DM, Chen SY. Nano-ferrosponges for controlled drug release. J Control Release. 2007;121:181–9. doi: 10.1016/j.jconrel.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Butler MF, Ng YF, Pudney DA. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J Polym Sci A: Polym Chem. 2003;41:3941–53. [Google Scholar]
- Mi FL, Sung HW, Shyu SS. Synthesis and characterization of a novel chitosan-based network prepared using naturally occurring crosslinker. J Polym Sci A: Polym Chem. 2000;38:2804–14. [Google Scholar]
- Mi FL, Shyu SS, Peng CK. Characterization of ring-opening polymerization of genipin and pH-dependent crosslinking reactions between chitosan and genipin. J Polym Sci A: Polym Chem. 2005;43:1985–2000. [Google Scholar]
- Muzzarelli RAA. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr Polym. 2009;77:1–9. [Google Scholar]
- Rao KSVK, Chung I, Reddy KM, Ha CS. PMMA-based microgels for controlled release of an anticancer drug. J Appl Polym Sci. 2009;111:845–53. [Google Scholar]
- Abrishami M, Ganavati SZ, Soroush D, Rouhbakhsh M, Jaafari MR, Malaekeh-Nikouei M. Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (Avastin) for intravitreal administration. Retina. 2009;29:699–703. doi: 10.1097/IAE.0b013e3181a2f42a. [DOI] [PubMed] [Google Scholar]
- Einmahl S, Behar-Cohen F, Tabatabay C, Savoldelli M, Hermies FD, Chauvaud D, et al. A viscous bioerodible poly(ortho ester) as a new biomaterial for intraocular application. J Biomed Mater Res. 2000;50:566–73. doi: 10.1002/(sici)1097-4636(20000615)50:4<566::aid-jbm12>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Carlson DW, Alward LM, Barad JP, Zimmerman MB, Carney BL. A random study of mitomycin augmentation in combined phacoemulsification and trabeculectomy. Ophthalmology. 1997;104:719–24. doi: 10.1016/s0161-6420(97)30246-2. [DOI] [PubMed] [Google Scholar]