Abstract
Aim:
To investigate the effects of 2′-hydroxy-4′-methoxyacetophenone (paeonol) on the electrophysiological behavior of a central neuron (right parietal 4; RP4) of the giant African snail (Achatina fulica Ferussac).
Methods:
Intracellular recordings and the two-electrode voltage clamp method were used to study the effects of paeonol on the RP4 neuron.
Results:
The RP4 neuron generated spontaneous action potentials. Bath application of paeonol at a concentration of ≥500 μmol/L reversibly elicited action potential bursts in a concentration-dependent manner. Immersing the neurons in Co2+-substituted Ca2+-free solution did not block paeonol-elicited bursting. Pretreatment with the protein kinase A (PKA) inhibitor KT-5720 or the protein kinase C (PKC) inhibitor Ro 31-8220 did not affect the action potential bursts. Voltage-clamp studies revealed that paeonol at a concentration of 500 μmol/L had no remarkable effects on the total inward currents, whereas paeonol decreased the delayed rectifying K+ current (IKD) and the fast-inactivating K+ current (IA). Application of 4-aminopyridine (4-AP 5 mmol/L), an inhibitor of IA, or charybdotoxin 250 nmol/L, an inhibitor of the Ca2+-activated K+ current (IK(Ca)), failed to elicit action potential bursts, whereas tetraethylammonium chloride (TEA 50 mmol/L), an IKD blocker, successfully elicited action potential bursts. At a lower concentration of 5 mmol/L, TEA facilitated the induction of action potential bursts elicited by paeonol.
Conclusion:
Paeonol elicited a bursting firing pattern of action potentials in the RP4 neuron and this activity relates closely to the inhibitory effects of paeonol on the IKD.
Keywords: paeonol, neuron, snail, action potential bursts, fast-inactivating K+ current, delayed rectifying current
Introduction
The Chinese have used herbs to treat a wide variety of medical ailments for several thousand years. Moutan cortex, the root bark of Paeonia suffruticosa Andrews, has long been used for its antipyretic and anti-inflammatory effects in traditional Chinese medicine1, 2. One of the major components of Moutan cortex is 2'-hydroxy-4'-methoxyacetophenone (paeonol), which has been reported to possess analgesic, antipyretic and antibacterial properties as well as anti-inflammatory and antioxidant activities and an ability to suppress ADP or collagen-induced human blood platelet aggregation1, 3, 4.
Recent pharmacological experiments have shown that paeonol protects against reperfusion-induced myocardial damage5. Paeonol has also been reported to block the L-type calcium current in cardiac myocytes, thereby decreasing the excitability of cardiac tissue6. In a recent study carried out in guinea pig ventricular myocytes using patch-clamp techniques, paeonol decreased the action potential upstroke phase, an action associated with the blockade of the voltage-gated fast sodium channel7.
Other research indicates that paeonol has neuroprotective effects. For example, paeonol has been shown to protect rat neurons from oxygen-glucose deprivation-induced injury by alleviating morphological damage and increasing neuron viability8. Paeonol has ameliorated neuronal damage in both the hippocampus and temporal cortex in D-galactose-treated mice9. These results suggest that paeonol possesses anti-aging properties and may have potential in the treatment of neurodegenerative diseases. However, scant evidence exists as to the effects of paeonol on neuronal excitability.
The central nervous system of the gastropod snail contains large, identifiable neurons with known pharmacological profiles and synaptic inputs. The size, accessibility and relative simplicity of the molluscan neuronal network makes it an ideal in vitro preparation for electrophysiological and neuropharmacological studies10, 11, 12. Identifiable neurons in the ganglia can undergo repeated investigations into drug-related effects on the same neuron. Snail ganglia contain many identifiable neurotransmitters and receptors, and their neurons are used for biological studies11, 13, 14. In our previous studies, CNS stimulants, including d-amphetamine, cocaine and methamphetamine, elicited in vitro action potential bursts in the central right parietal 4 (RP4) neuron of the African snail Achatina fulica Ferussac13, 15, 16, 17.
Few studies have examined the effects of paeonol on neuronal excitability. The present study aimed to determine the effects of paeonol on membrane potentials and ionic currents in the central RP4 neuron using the conventional two-electrode voltage clamp technique.
Materials and methods
Experiments were performed on identified central RP4 neurons from the subesophageal ganglia of the African snail Achatina fulica Ferussac. The ganglia were pinned to a Sylgard-coated perfusion chamber base (volume=2 mL) and removed from the connective tissue sheath to allow easy identification and penetration by microelectrodes13, 16, 17.
Intracellular recordings were made with a Gene clamp 500 amplifier (Axon Instruments, Foster City, CA, USA). Membrane potentials were recorded with microelectrodes (5–6 MΩ) filled with 3 mol/L potassium chloride (KCl). The experimental chamber was perfused with the control solution, ie, (in mmol/L) NaCl (85), KCl (4), CaCl2 (8), MgCl2 (7), and Tris-HCl (10) at pH 7.6, at a room temperature of 23–24 °C with a perfusion speed of 8 mL/min. Neurons were studied only if the negativity of their resting membrane potentials (RMPs) exceeded -50 mV, the time constant was around 5–8 ms and the rate of rise of the action potentials was around 5–8 V/s18.
The ionic currents of the central snail neurons were measured using the two-electrode voltage clamp method. Two microelectrodes were inserted into the neuron for current injection and voltage clamp studies. The recording electrode (5–6 MΩ) and current injection electrode (1–5 MΩ) were filled with 3 mol/L KCl. All potentials and currents were recorded on tape via a digitalizing unit (Digidata 1440, Axon Instruments) and analyzed using pCLAMP software.
Na+-free solution was used to measure the fast-inactivating K+ current (IA). The currents were elicited by 200 ms test potentials of -60, -50, -40, and -30 mV from a holding potential of -100 mV19. A P/4 leak subtraction subpulse routine supplied by the pCLAMP software was used to measure the IA.
For measuring the IKD, Na+-free and Co2+-substituted Ca2+-free solutions were used to remove Ca2+ currents (ICa), Na+ currents (INa) and the Ca2+-activated K+ current (IK(Ca)). RP4 neuron currents were elicited with 500-ms-long voltage clamp steps from a holding potential of -60 mV to test potentials between -70 and +50 mV at intervals of 10 mV. The potential recording and current injection electrodes were filled with 3 mol/L KCl to measure both types of K+ current.
The ganglia were perfused with the following solutions: (a) physiological solution; (b) Na+-free solution: equimolar amounts (85 mmol/L) of Tris were added to replace Na+ ions; (c) Co2+-substituted Ca2+-free solution: equimolar amounts (8 mmol/L) of Co2+ were added to replace Ca2+ ions; and (d) Na+-free and Co2+-substituted Ca2+-free solution: equimolar amounts of Tris (85 mmol/L) and Co2+ (8 mmol/L) were added to replace Na+ and Ca2+ ions, respectively. The tetraethylammonium chloride (TEA)-containing solution was prepared by replacing equimolar quantities of NaCl or Tris-Cl with TEA20.
Paeonol, 4-aminopyridine (4-AP), TEA, KT-5720, Ro 31-8220 and charybdotoxin were purchased from the Sigma Chemical Company (St Louis, MO, USA). All drug stocks were made with double-distilled water, except for KT-5720 and Ro 31-8220, which were prepared in dimethyl sulfoxide (DMSO), and paeonol, which was prepared in ethanol. The presence of DMSO (≤0.1%) and ethanol (≤1%) did not affect the RMPs, amplitude or frequency of the spontaneous firing of action potentials in the RP4 neuron.
Tests of significance regarding any differences between the amplitude and frequency of the action potentials, RMPs and currents post-treatment compared with the pre-drug controls were determined by the Student's paired t-test. Differences were considered significant at P<0.05.
Results
Effects of extracellular application of paeonol on spontaneous action potentials of the RP4 neuron
The RMP, amplitude and frequency of spontaneously generated action potentials of the identified RP4 neuron are shown in Table 1. The electrical characteristics of the RP4 neuron were similar to those detailed in a previous report15.
Table 1. Effects of paeonol, Ro 31-8220, KT-5720, Co2+-substituted Ca2+-free solution, 4-AP, TEA, and charybdotoxin on the resting membrane potential, amplitude and frequency of spontaneously generated action potentials of RP4 neurons, and effects of KT-5720, Ro 31-8220, Co2+-substituted Ca2+-free solution and TEA on paeonol-elicited action potential bursts. Values are expressed as the mean±SEM. (n being the number of neurons tested). bP<0.05 vs paeonol (500 μmol/L)-elicited action potential bursts. eP<0.05 vs the data in physiological solution (control).
| Variable | n | RMP (mV) | Amplitude of action potential (mV) | Frequency of single spikes (pulse/min) | Frequency of bursts (burst/min) | Number of action potential/burst | |
|---|---|---|---|---|---|---|---|
| Paeonol | Control | 7 | −57.7±1.8 | 85.1±3.3 | 50.1±11.2 | ||
| Paeonol (150 μmol/L) | 4 | −58.5±2.7 | 86.1±6.9 | 47.3±11.8 | |||
| Paeonol (500 μmol/L) | 6 | −55.1±1.4 | 80.8±5.1 | 8.1±1.6 | 5.0±0.6 | ||
| Paeonol (1.5 mmol/L) | 3 | −59.3±2.3 | 83.3±9.6 | 5.0±1.5 | 8.7±1.7b | ||
| Ro 31-8220+Paeonol | Control | 5 | −57.0±1.1 | 96.9±2.1 | 28.5±5.0 | ||
| Ro 31-8220 (20 μmol/L) | 4 | −57.7±1.2 | 98.8±2.2 | 30.0±6.7 | |||
| Ro 31-8220 (10 μmol/L)+ Paeonol (500 μmol/L) | 3 | −54.0±2.3 | 100.3±4.5 | 7.0±1.0 | 4.5±2.5 | ||
| KT-5720+Paeonol | Control | 3 | −63.0±0.6 | 96.1±3.7 | 25.3±3.3 | ||
| KT-5720 (10 μmol/L) | 3 | −61.3±1.2 | 97.8±4.3 | 30.7±3.8 | |||
| KT-5720 (10 μmol/L)+ Paeonol (500 μmol/L) | 3 | −61.7±3.5 | 96.1±7.3 | 7.0±1.5 | 4.3±0.3 | ||
| Paeonol in Ca2+-free | Control | 6 | −63.0±2.1 | 98.2±3.2 | 26.2±4.9 | ||
| Ca2+-free | 3 | −49.8±1.3b | 65.7±3.5e | 22.7±5.4 | |||
| Paeonol (500 μmol/L) in Ca2+-free | 3 | −58.3±1.5 | 73.5±5.3e | 2.7±1.2e | 17.3±5.3e | ||
| Paeonol+TEA | Control | 3 | −59.3±3.3 | 82.3±5.2 | 51.3±5.3 | ||
| TEA (5 mmol/L) | 4 | −57.5±1.6 | 86.0±3.2 | 29.5±3.0e | |||
| Paeonol (150 μmol/L)+ TEA (5 mmol/L) | 3 | −56.3±0.7 | 92.7±5.6 | 14.7±6.7 | 3.7±0.9 | ||
| 4-AP | Control | 4 | −55.3±2.8 | 90.2±6.0 | 48.8±16.4 | ||
| 4-AP (5 mmol/L) | 4 | −56.3±4.2 | 91.5±6.1 | 49.0±14.8 | |||
| TEA | Control | 4 | −60.5±1.0 | 79.9±3.2 | 23.5±9.2 | ||
| TEA (50 mmol/L) | 4 | −58.0±4.0 | 84.4±5.6 | 10.5±5.5 | 3.1±0.7 | ||
| Charybdotoxin | Control | 3 | −59.0±1.7 | 87.3±2.6 | 49.6±6.0 | ||
| Charybdotoxin (250 nmol/L) | 3 | −57.6±1.2 | 88.7±3.2 | 39.3±1.5 |
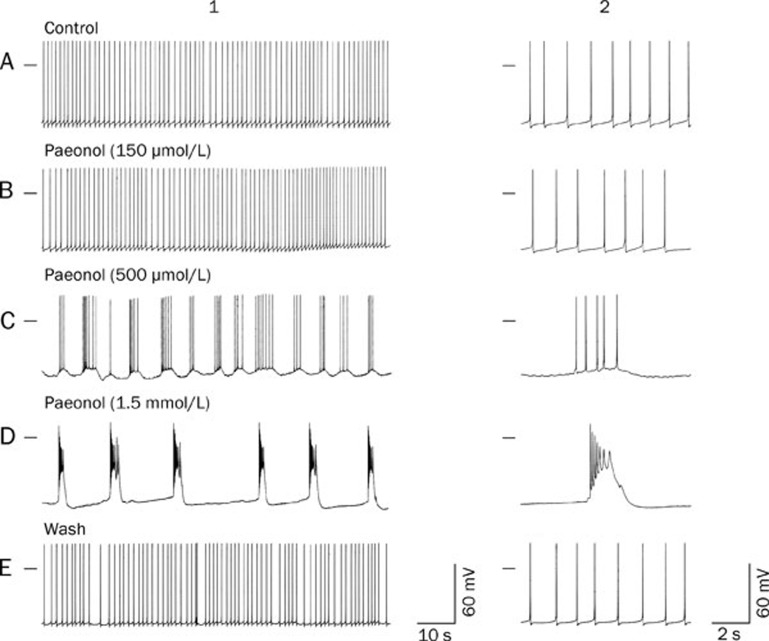
The effects of paeonol (150 μmol/L, 500 μmol/L, and 1.5 mmol/L) on the spontaneous firing action potentials of the RP4 neuron are shown in Figure 1 and Table 1. At a concentration of 150 μmol/L, paeonol did not alter the action potential firing pattern. As shown in Table 1, at 20 min after extracellular perfusion of paeonol (150 μmol/L), the frequency of spontaneously firing action potentials and the RMP remained unchanged. No action potential bursts were observed even after 1 h of incubation. However, higher concentrations of paeonol did change the action potential firing pattern. Twenty minutes after extracellular perfusion of paeonol (500 μmol/L), the firing pattern changed from regularly spaced single spikes to one in which bursts of between 2 and 10 action potentials were separated by large hyperpolarizations of the membrane potentials (up to 9 mV) that lasted for 5–15 s each. The RMP, action potential amplitude and bursting frequency and the number of action potentials for each burst are shown in Table 1. These effects of paeonol continued throughout its application (for up to 3 h).
Figure 1.
Effects of paeonol on the RP4 neuron. A, B, C, D, and E were recorded from the same neuron. A1: Control; the neuron shows spontaneous firing of action potentials. B1, C1, and D1: Membrane potentials recorded 20 min after the administration of paeonol (150, 500 and 1500 μmol/L), respectively. E1: Membrane potentials measured at 30 min after the washing off of paeonol from D1. A2, B2, C2, D2, and E2: Expanded pictures showing individual action potentials related to A1, B1, C1, D1, and E1, respectively. The horizontal bar on the upper left side indicates the membrane potential at 0 mV. Notably, action potential bursts were not elicited by paeonol at 150 μmol/L but were reversibly elicited at concentrations of 500 μmol/L and 1.5 mmol/L.
At the highest concentration of 1.5 mmol/L, paeonol enhanced the pattern of action potential bursts. The membrane potential underwent a phasic depolarization followed by a sustained depolarization. The number of action potentials for each burst was increased compared with those for the bursts elicited by paeonol 500 μmol/L (Table 1 and Figure 1). After 30 min of continuous washing, the spontaneously generated spikes of the central neuron returned to control levels.
Effects of Co2+-substituted Ca2+-free solution on paeonol-elicited potential changes of the RP4 neuron
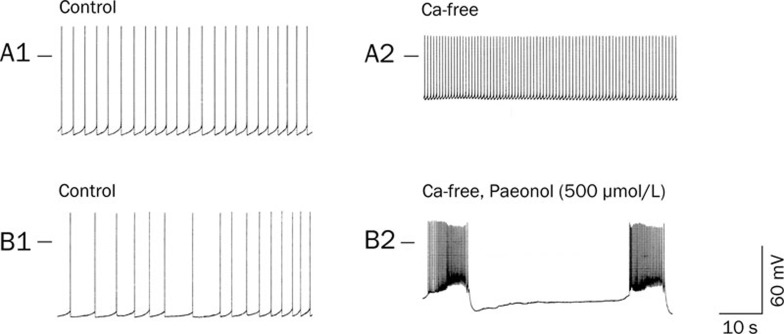
To test the effects of Co2+-substituted Ca2+-free solution on paeonol-elicited changes in action potential bursts, the RP4 neuron underwent 30 min of treatment with Co2+-substituted Ca2+-free solution alone (Figure 2A2) or in combination with paeonol (Figure 2B2). Co2+-substituted Ca2+-free solution decreased both the RMP and the amplitude of action potentials (Table 1). No action potential bursts were elicited (Figure 2A2).
Figure 2.
Effects of Co2+-substituted Ca2+-free solution on paeonol (500 μmol/L)-elicited action potential bursts in the RP4 neuron. A1 and A2 were recorded from one neuron, while B1 and B2 were recorded from another. A1: Spontaneous action potentials recorded from an RP4 neuron in normal physiological solution. A2: The potentials at 30 min after perfusion with Co2+-substituted Ca2+-free solution. B1: Spontaneous action potentials recorded from an RP4 neuron in normal physiological solution. B2: Membrane potentials recorded at 20 min after perfusion with Co2+-substituted Ca2+-free solution containing paeonol (500 μmol/L).
In contrast, perfusion with the Co2+-substituted Ca2+-free solution containing paeonol (500 μmol/L) elicited action potential bursts (Figure 2B2 and Table 1). The RMP and the amplitude of action potentials were decreased, as shown in Table 1. The number of action potentials for each burst was higher, and the frequency of bursts lower, compared with the bursting elicited by paeonol at 500 μmol/L in normal physiological solution.
The removal of extracellular calcium ions did not abolish the paeonol (500 μmol/L)-induced bursting pattern of action potentials, but it reduced the action potential amplitudes.
Effects of a PKA inhibitor on paeonol-elicited potential changes of the RP4 neuron
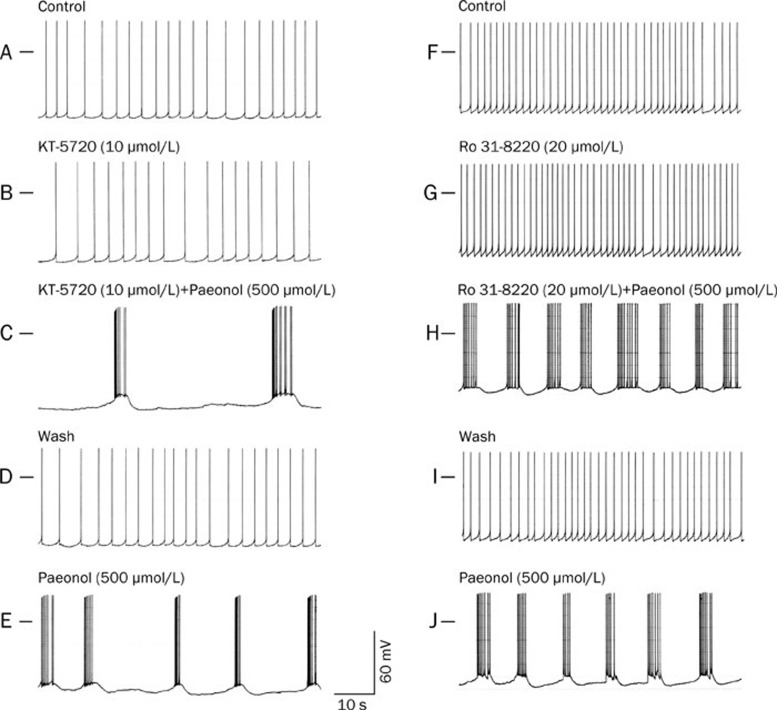
We then sought to determine whether the cAMP-protein kinase A (PKA) signaling pathway is implicated in the generation of paeonol-elicited action potential bursts. As shown in Table 1 and Figure 3B, after 40 min of incubation with the PKA inhibitor KT-5720 (10 μmol/L)21, the frequency of the spontaneous action potentials, the RMP and the amplitude of the action potentials did not differ from those of the control RP4 neuron.
Figure 3.
Effects of KT-5720 (10 μmol/L; A-E) and Ro 31-8220 (20 μmol/L; F–J) on paeonol-elicited action potential bursts in the RP4 neuron. A, B, C, D, and E were recorded from the same neuron, while F, G, H, I, and J were recorded from another. A and F: Controls; spontaneous action potentials. B and G: Potentials at 40 min after perfusion with KT-5720 (10 μmol/L) and Ro 31-8220 (20 μmol/L), respectively. C and H: Potentials at 20 min after further incubation of the neuron with paeonol (500 μmol/L) plus KT-5720 (10 μmol/L) and paeonol (500 μmol/L) plus Ro 31-8220 (20 μmol/L), respectively. D and I: Potentials at 30 min after washing from C and H, respectively. E and J: Potentials at 20 min after incubation of the neuron with paeonol (500 μmol/L) from D and I, respectively. The horizontal bar on the upper left side indicates the membrane potential at 0 mV. Note that paeonol elicited action potential bursts in the presence of KT-5720 (10 μmol/L) and also Ro 31-8220 (20 μmol/L).
As mentioned earlier, paeonol (500 μmol/L) elicited action potential bursts in the RP4 neuron. When KT-5720 10 μmol/L-pretreated RP4 neurons were further incubated with paeonol (500 μmol/L) for 20 min, action potential bursts were elicited, as shown in Table 1 and Figure 3C.
Pretreatment with KT-5720 failed to prevent paeonol-elicited action potential bursts.
Effects of a PKC inhibitor on paeonol-elicited potential changes of the RP4 neuron
To determine whether protein kinase C (PKC) is involved in the generation of action potential bursts elicited by paeonol, we tested the effects of PKC inhibition with Ro 31-8220 (20 μmol/L)22. After a 40-min incubation, the RMP, amplitude and frequency of single spikes in the RP4 neuron were not significantly different from values obtained from neurons in normal physiological solution (Table 1 and Figure 3G). Further incubation of Ro 31-8220-pretreated RP4 neurons with paeonol (500 μmol/L) for 20 min resulted in action potential bursts (Table 1 and Figure 3H).
Pretreatment with Ro 31-8220 failed to abolish paeonol-generated action potential bursts.
Effects of paeonol on the fast ionic currents of the RP4 neuron
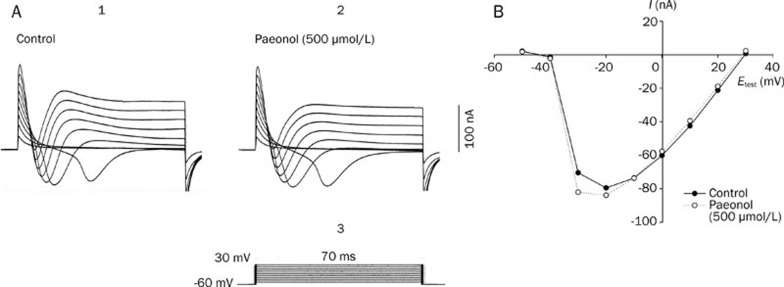
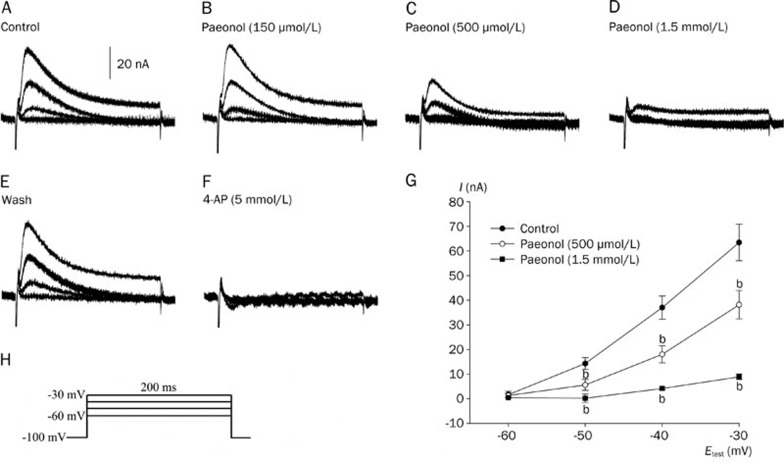
The fast ionic currents of the RP4 neuron clamped at 70 ms durations are shown in Figure 4. The membrane potentials were held at -60 mV and stepped to the testing potentials of -50, -40, -30, -20, -10, 0, 10, 20, and 30 mV in 70-ms-long durations. The total inward currents observed in various voltage clamping command steps are shown in Figure 4A. The relationships between the peak inward currents and the test potentials are shown in Figure 4B. The inward current was obvious when the potential was stepped to positive levels that exceeded -40 mV. The maximum peak inward current was observed after voltage stepping to test potentials between -20 mV and -10 mV. As shown in our previous study, the removal of either extracellular Na+ ions or Ca2+ ions decreased the amplitude of the total inward currents13, 23. Both the ICa and the INa contributed to the total inward currents of the RP4 neuron. As shown in Figure 4B, paeonol (500 μmol/L) did not significantly decrease the fast inward currents in a series of voltage steps.
Figure 4.
(A) Effects of paeonol on the total inward currents of the RP4 neuron. The membrane currents were elicited from a holding potential of -60 mV to test potentials of -50, -40, -30, -20, -10, 0, 10, 20, and 30 mV of 70-ms-long durations in normal physiological solution. A1: Control; total inward and outward currents recorded in normal physiological solution. A2: Total inward and outward currents recorded at 20 min after incubation with paeonol (500 μmol/L). A3: Voltage step commands. (B) Current-voltage relationships of the peak total inward currents before (•), and at 20 min after (○), paeonol (500 μmol/L) application. Note that paeonol (500 μmol/L) did not decrease the total inward currents.
Effects of paeonol on the IA current
Measurement of the IA followed Thompson's method19. Currents were elicited by 200 ms test potentials of -60 mV, -50, -40, and -30 mV from a holding potential of -100 mV. An example of the IA current is shown in Figure 5; the IA current was completely abolished if 4-AP was applied to the Na+-free solution for 20 min (Figure 5F).
Figure 5.
Effects of paeonol on the peak amplitude of the IA current of the RP4 neuron. A: Control IA currents recorded in Na+-free solution. B, C, and D: IA currents recorded at 20 min after paeonol (150 μmol/L), paeonol (500 μmol/L) and paeonol (1.5 mmol/L) administration, respectively. E: IA currents recorded at 30 min after washing off from D. F: IA currents recorded at 20 min after 4-AP (5 mmol/L) administration from E. G: Current-voltage relationships of the IA currents before (•) and after paeonol administration at 500 μmol/L (○) and 1.5 mmol/L (▪), respectively. H: Voltage step commands. Note that paeonol (500 μmol/L and 1.5 mmol/L) significantly decreased the IA at 20 min after treatment (bP<0.05 vs control. n=3−4).
As shown in Figure 5, perfusion of neurons for 20 min with paeonol (150 μmol/L) did not affect the peak amplitude of the IA. However, the IA was decreased after a 20 min perfusion with paeonol at higher concentrations of 500 μmol/L and also 1.5 mmol/L (by 40.0%±6.7%, n=4 and 85.7%±0.6%, n=3, respectively; at a test potential of -30 mV). Paeonol 150 μmol/L had no effect on the IA, whereas higher concentrations of paeonol (500 μmol/L and 1.5 mmol/L) successfully decreased the IA.
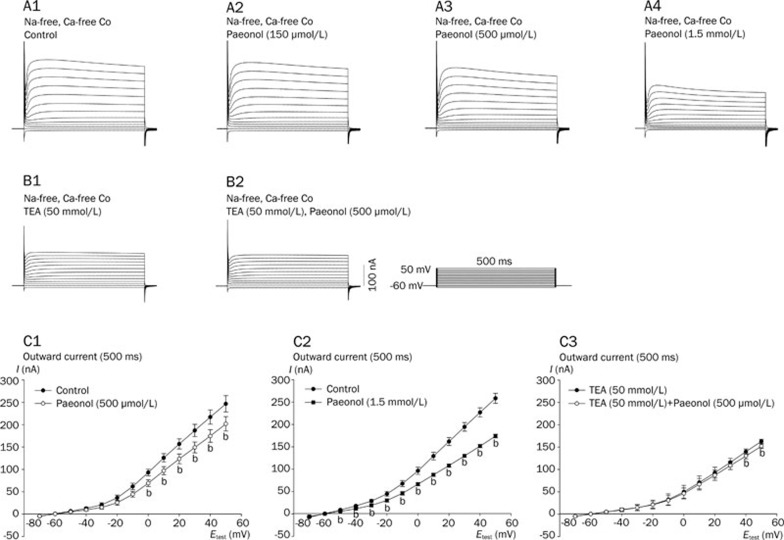
Effects of paeonol on the steady-state outward K+ current of the RP4 neuron
The steady-state outward current of the RP4 neuron was measured by 500-ms-long voltage clamp steps in Na+-free and Co2+-substituted Ca2+-free solution (Figure 6A). In this condition, the IA and delayed rectifying K+ (IKD) components of the outward currents were identified by 4-AP and TEA, as in our previous study13. The amplitude of the outward current at 500 ms was significantly reduced (by 40.7%±3.1%, n=3; at a test potential of 50 mV) after perfusion with TEA (50 mmol/L) for 40 min (Figure 6B). If 4-AP (5 mmol/L) was applied to the bath solution for 20 min, the outward current remained unchanged. The sensitivity of the outward current to TEA indicates that this is a type of IKD. However, there was a sustained residual TEA-insensitive current13.
Figure 6.
Effects of paeonol on steady-state outward currents of the RP4 neuron in Na+-free and Co2+-substituted Ca2+-free saline in the absence and presence of TEA (50 mmol/L). A and B were recorded from two different RP4 neurons. Currents were elicited by 500-ms-long command steps from holding potentials of -60 mV to test potentials (ie, from -70 to 50 mV at intervals of 10 mV). A1: Control; steady-state outward currents of the RP4 neuron in Na+-free and Co2+-substituted Ca2+-free saline. A2, A3 and A4: 20 min after administration of paeonol 150 μmol/L, 500 μmol/L and 1.5 mmol/L, respectively. B1: Steady-state outward currents of the RP4 neuron in TEA (50 mmol/L)-containing Na+-free and Co2+-substituted Ca2+-free saline. B2: 20 min after administration of paeonol (500 μmol/L), from B1. C1 and C2: Effects of paeonol (500 μmol/L and 1.5 mmol/L) on the current-voltage relationships of the steady-state outward currents measured at 500 ms of the RP4 neuron in Co2+-substituted Ca2+-free and Na+-free solution, respectively. The closed circle (•) in C1 and C2 represents the I–V relationship before paeonol (500 μmol/L) application. The open circle (○) in C1 and closed square (▪) in C2 represent the I–V relationship at 20 min after paeonol (500 μmol/L and 1.5 mmol/L) application, respectively (bP<0.05 vs control, n=5). C3: Effects of paeonol (500 μmol/L) on the current-voltage relationships of the steady-state outward currents measured at 500 ms of the RP4 neuron in TEA (50 mmol/L)-containing Co2+-substituted Ca2+-free and Na+-free solution. The closed circle (•) and open circle (○) in C3 represent the I–V relationship before and at 20 min after paeonol (500 μmol/L) application, respectively (bP<0.05 vs the data in TEA 50 mmol/L). However, when neurons were pretreated with TEA, paeonol was less effective at decreasing the outward current.
The effects of paeonol on the steady-state outward current in Na+-free and Co2+-substituted Ca2+-free solution are shown in Figure 6A. The corresponding amplitudes of the steady-state outward currents, as measured at 500 ms of the outward current, and the steady-state current-voltage (I–V) relationships are shown in Figures 6C1 and 6C2. When neurons underwent 20 min of treatment with paeonol at 150 μmol/L, the steady-state outward current was not affected, whereas a 20-min treatment with paeonol at 500 μmol/L significantly decreased the steady-state outward current when subjected to test potentials in the range of 0 mV to 50 mV. The current amplitude measured at 500 ms was reduced by 44.8±5.2 nA (by 18.2%±1.8% vs controls, P<0.05, n=5) at a test potential of 50 mV. A further 20-min treatment with paeonol at 1.5 mmol/L significantly decreased the current under test potentials ranging from -50 mV to 50 mV; the current amplitude measured at 500 ms was reduced by 84.4±0.7 nA (by 32.3%±2.6% vs controls, paired t-test, P<0.05, n=5) at a test potential of 50 mV.
The effects of paeonol (500 μmol/L) on the steady-state outward current in Na+-free and Co2+-substituted Ca2+-free solution containing TEA (50 mmol/L) are shown in Figure 6B. The corresponding amplitudes of the steady-state outward current were measured after 500 ms of the outward current, and the steady-state current-voltage (I–V) relationships are shown in Figure 6C3. After a 20-min application of paeonol (500 μmol/L), the currents at test potentials between -50 and 30 mV were not significantly affected, but the currents at test potentials of 40 and 50 mV were slightly decreased. The current amplitude at 500 ms was reduced by 10.7±0.5 nA (by 6.6%±0.4% vs controls, paired t-test, P<0.05, n=4) at a test potential of 50 mV.
Paeonol (500 μmol/L) decreased the steady-state current in a Na+-free and Co2+-substituted Ca2+-free solution, but only slightly decreased the steady-state current in RP4 neurons pretreated with TEA (50 mmol/L) (Figure 6). These data suggest that the paeonol-elicited decrease in the steady-state current in the Na+-free and Co2+-substituted Ca2+-free solution is mainly to a decrease in the TEA-sensitive current.
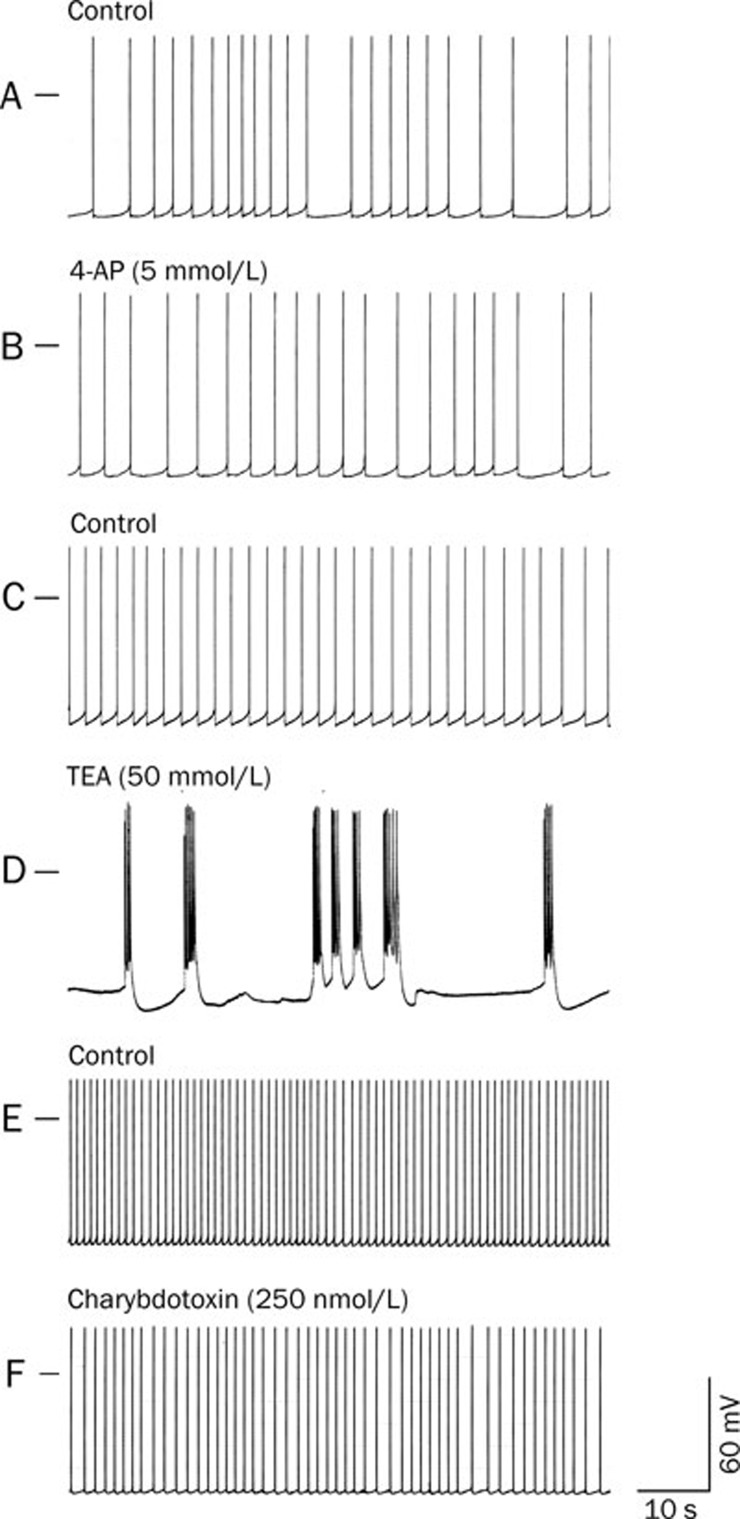
Effects of 4-AP, TEA and charybdotoxin on the spontaneous action potentials of the RP4 neuron
To elucidate the role of IKD, IA, and IK(Ca) in the generation of the action potential bursts elicited by paeonol, we tested the effects of 4-AP, TEA and charybdotoxin on spontaneous action potentials. An example of the effects of 4-AP (5 mmol/L) is shown in Figure 7B. Application of 4-AP, an inhibitor of IA24, failed to elicit action potential bursts. Table 1 details the RMPs, frequencies and amplitudes of spontaneously generated action potentials after a 40-min treatment with 4-AP (5 mmol/L).
Figure 7.
Effects of 4-AP (5 mmol/L), TEA (50 mmol/L) and charybdotoxin (250 nmol/L) on spontaneous action potentials of the RP4 neuron. A and B, C, and D, E, and F were recorded from 3 different RP4 neurons. A, C, and E: Control; the spontaneous action potentials of the RP4 neuron. B, D, and F: The potentials at 40 min after perfusion with 4-AP (5 mmol/L), TEA (50 mmol/L) and charybdotoxin (250 nmol/L), respectively. The upper left-hand horizontal bar indicates the membrane potential at 0 mV. Note that while action potential bursts were not elicited by 4-AP (5 mmol/L) or charybdotoxin (250 nmol/L), they were elicited by TEA (50 mmol/L).
TEA (50 mmol/L) elicited bursts of action potentials in the RP4 neurons, as shown in Table 1. An example of the effects of TEA on the spontaneous firing of action potentials is shown in Figure 7D.
Charybdotoxin is a IK(Ca) inhibitor and reportedly inhibits IK(Ca) in the Aplysia neuron at concentrations between 100 nmol/L and 300 nmol/L25. We therefore treated RP4 neurons with 250 nmol/L of charybdotoxin. After 40 min, the RMPs, the frequencies and amplitudes of spontaneously generated action potentials remained unchanged from baseline. No bursting activity of potentials was observed (Figure 7F).
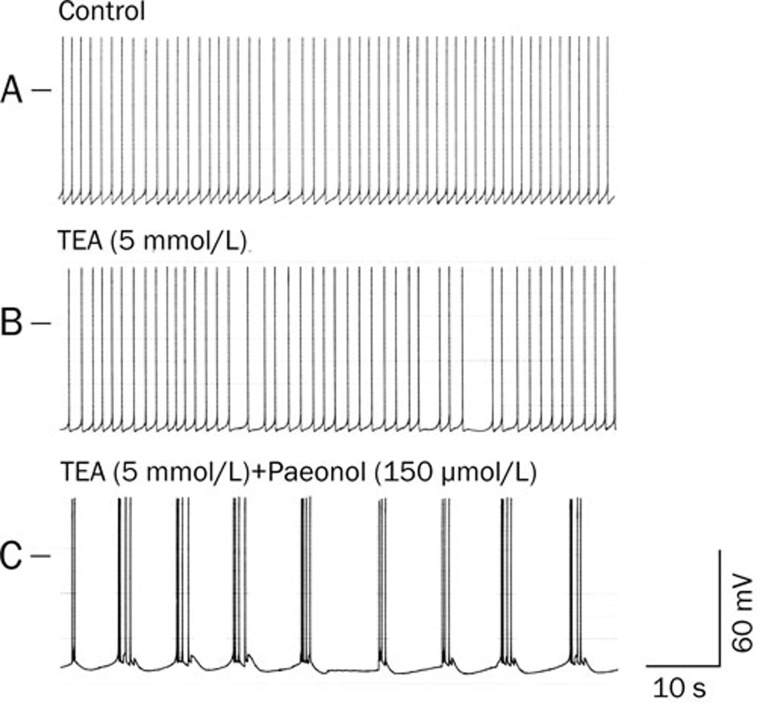
Effects of TEA on paeonol-elicited action potential bursts
To test whether the IKD is involved in the generation of action potential bursts elicited by paeonol (500 μmol/L), we assessed the effects of TEA (5 mmol/L), an IKD blocker. As shown in Table 1 and Figure 8, TEA (5 mmol/L) reduced the frequency of action potentials but failed to elicit action potential bursts.
Figure 8.
Effects of co-application of TEA (5 mmol/L) plus paeonol (150 μmol/L) in the RP4 neuron. (A) Control; spontaneous action potentials of the RP4 neuron. (B) Potentials at 30 min after administration of TEA (5 mmol/L). (C) Potentials at 20 min after further incubation with paeonol (150 μmol/L) and TEA (5 mmol/L). The left-hand horizontal bar indicates the membrane potential at 0 mV. Note that co-application of TEA (5 mmol/L) plus paeonol (150 μmol/L) elicited action potential bursts.
The facilitative effects of TEA were tested in RP4 neurons pretreated with a low concentration of paeonol (150 μmol/L) (Figure 8). Whereas paeonol at 150 μmol/L alone failed to elicit action potential bursts, the coadministration of paeonol (150 μmol/L) with TEA (5 mmol/L) elicited action potential bursts after 20 min.
Discussion
This study investigated the effects of paeonol on the electrophysiological behavior of the central RP4 neuron in the giant African snail (Achatina fulica Ferussac). The neuron exhibited spontaneous regular firing of action potentials. No bursts of action potential activity were found in control RP4 neurons, whereas extracellular application of paeonol (500 μmol/L and 1.5 mmol/L) reversibly elicited bursts of action potential spikes in a dose-dependent manner (Figure 1). The highest concentration of paeonol (1.5 mmol/L) elicited more remarkable bursting behavior patterns compared with those observed after 500 μmol/L. The bursting pattern elicited by paeonol (500 μmol/L) was not abolished after continuous perfusion with Co2+-substituted Ca2+-free solution (Figure 2B2), although the amplitudes of the action potentials were reduced. These findings suggest that paeonol-elicited bursts of potential firing are not directly associated with calcium fluxes of the neuron.
It has been suggested that both PKA and PKC play a key role in the plasticity of the nervous system in vertebrates and invertebrates. PKA and PKC modulate the function of ion channels26, 27, 28. In previous studies, we found that PKA and PKC activity were associated with the generation of action potential bursts in the central RP4 snail neuron16, 17.
The PKA inhibitor KT-5720 is used at concentrations of approximately 10 μmol/L as a research tool in snail and Aplysia neurons12, 29. The bisindolylmaleimide Ro 31-8220 is a structurally-related staurosporine analog, which acts as an ATP-competitive inhibitor of PKC and has been used extensively for studying the role of PKC in cell signaling22. Recently, Ro 31-8220 (1–10 μmol/L)22 has been used in snail30 and Aplysia neurons27. In our previous work, pretreatment with KT-5720 (10 μmol/L) and Ro 31-8220 (20 μmol/L) prevented the induction of action potential bursts elicited by methamphetamine (METH) and 3,4-methylenedioxymethamphetamine (MDMA), respectively16. In the present study, the action potential bursts elicited by paeonol (500 μmol/L) were not affected by KT-5720 (10 μmol/L; Figure 3) or Ro 31-8220 (20 μmol/L; Figure 3). These results suggest that the action potential bursts elicited by paeonol are not associated with PKA and PKC activity.
Ionic currents play an important role in the firing of action potential bursts, as seen in the neurons of Aplysia california31, Euhadra peliomphala32, and Drosophila33. To understand the mechanism underlying paeonol-elicited potential bursts, the effects of paeonol on ionic currents were investigated at a concentration of 500 μmol/L. As shown in Figure 4, paeonol had no remarkable effects on the total inward currents.
In the RP4 neuron, the IA is sensitive to 4-AP, the IKD is sensitive to TEA, and the IK(Ca) is sensitive to extracellular Co2+ ions13. These findings are consistent with other research involving snail or Aplysia neurons 19, 34, 35.
The measurement of the IA followed Thompson's method19. The currents were elicited by 200 ms test potentials of -60 mV, -50, -40, and -30 mV from a holding potential of -100 mV. At 20 min after paeonol application (500 μmol/L and 1.5 mmol/L), the peak IA current was decreased (Figure 5). The IA current was completely abolished if the bath solution was infused with 4-AP (5 mmol/L) for 20 min. Notably, perfusion with 4-AP (5 mmol/L) elicited no bursts of action potentials in the RP4 neuron (Figure 7B), a finding that is consistent with our previous study13. These results suggest that the effects of paeonol on the IA current may not be directly related to its effects on bursts of action potentials.
To measure the IKD current, the steady-state outward current was elicited with 500-ms-long voltage clamp steps in a Na+-free and Co2+-substituted Ca2+-free solution36. Under this condition, the IK(Ca), ICa, and INa were removed. To identify the IKD, TEA was applied. The amplitude at 500 ms of outward current was significantly reduced after perfusion with TEA (50 mmol/L). The sensitivity of the outward current to TEA indicated that this is a type of IKD. Notably, a sustained residual current was insensitive to TEA19.
We also sought to determine whether paeonol affected the IKD. Our results indicate that paeonol at 500 μmol/L decreased the steady-state current in Na+-free and Co2+-substituted Ca2+-free solution but only slightly decreased the steady-state current when the RP4 neurons were pretreated with TEA 50 mmol/L (Figure 6). This result suggests that paeonol decreases the TEA-sensitive IKD. In our experiments, paeonol significantly decreased the outward current, including the IA and the IKD, but did not affect the total inward current. It may be that paeonol has greater potency on potassium currents in this preparation.
To further test whether inhibition of IKD is involved in the generation of action potential bursts, we examined the effects of TEA on action potential bursting. As in our previous work, we found that TEA elicited action potential bursts at a concentration of 50 mmol/L (Figure 7D), but not at 5 mmol/L (Figure 8B)13. In the present study, paeonol (150 μmol/L) alone failed to elicit action potential bursts (Figure 1B), whereas its co-application with TEA (5 mmol/L) did elicit action potential bursts (Figure 8C). These data suggest that inhibition of IKD facilitates the effects of paeonol; the TEA-sensitive IKD helps to generate paeonol-elicited action potential bursts.
The IK(Ca) current plays an important role in the regulation of neuronal excitability37. To further clarify the role of the IK(Ca) current in the generation of action potential bursts, we tested the effects of charybdotoxin; this agent failed to elicit action potential bursts (Figure 7F). Notably, perfusion with Co2+-substituted Ca2+-free solution failed to elicit action potential bursts in the RP4 neuron (Figure 2). These findings suggest that the IK(Ca) current may not be involved in the generation of action potential bursts.
In animal models, paeonol showed anxiolytic-like effects at a dose of 17.5 mg/kg (po), and at doses of 50 and 100 mg/kg (po) in mice successfully reduced oxidative stress, cognitive impairment and neurotoxicity induced by D-galactose (50 mg·kg-1·d-1)9, 38. At doses of 30 and 100 mg/kg (ip), paeonol inhibited carrageenan-evoked thermal hyperalgesia in rats39. In mice, maximum plasma levels were around 16.3 μmol/L after oral dosing with 20 mg/kg paeonol40.
In vitro studies have used varying concentrations. At concentrations up to 500 μg/mL (around 3 mmol/L), paeonol has not shown any cytotoxicity in normal human endothelial cells as assessed by a trypan blue dye exclusion test. In the concentration range of 125 to 500 μg/mL (around 750 μmol/L to 3 mmol/L), paeonol significantly inhibited proliferation of basic fibroblast growth factor (bFGF)-stimulated human umbilical vein endothelial cells (HUVECs)41. At concentrations ranging from 24 μmol/L to 1.5 mmol/L, paeonol inhibited cell proliferation in HT-29 cells42 and protected rat neurons from oxygen-glucose deprivation-induced injury at 0.2–5 μmol/L8. In addition, paeonol (15.63–62.5 mg/L) in combination with cisplatin has shown a synergistic growth-inhibitory and apoptosis-inducing effect in two human hepatoma cell lines43.
In investigations seeking clarification of how paeonol affects ionic function in excitable tissue, concentrations of 160 μmol/L and 640 μmol/L successfully decreased the action potential upstroke phase in guinea pig ventricular cells7. At concentrations ranging from 25 μg/mL to 400 μg/mL (150 μmol/L–2.4 mmol/L), paeonol inhibited the delayed outward K+ current and, to a lesser extent, the INa in NG108-15 cells44. The data from our present study indicate that paeonol at a concentration of ≥500 μmol/L reversibly elicits action potential bursts of the central RP4 neuron. The concentration used in the present study may be higher than that of the plasma concentration in vivo and may be associated with toxic effects.
An identifiable unit of epileptiform activity in the mammalian central nervous system (CNS) is the interictal spike. The intracellular correlate of the interictal spike is an overt depolarization, termed the paroxysmal depolarizing shift (PDS), that results in the initiation of a high-frequency burst of action potentials followed by a period of hyperpolarization45, 46, 47. Research has revealed that convulsants, such as pentylenetetrazol (PTZ), induce bursts of action potentials in snail central neurons32, 48, 49, a response that closely resembles the PTZ-induced PDS in the cerebral cortical neurons of mammals50. In our previous studies, CNS stimulants, such as d-amphetamine, cocaine and methamphetamine, elicited in vitro action potential bursts in the central RP4 neuron of the African snail, Achatina fulica Ferussac13, 15, 16. Interestingly, high doses of these substances induce convulsions in animals and in humans. The present study demonstrated that paeonol at 500 μmol/L induced bursts of action potential in neurons. Our data indicate that paeonol at high doses may have a pro-epileptic effect. Patients should be closely supervised if the Moutan cortex of Paeonia suffruticosa Andrews (MC) is combined with a CNS stimulant.
Conclusion
The present study aimed to elucidate the effects of paeonol on membrane potentials and ionic currents in the central RP4 neuron using the conventional two-electrode voltage clamp technique. Our results indicate that paeonol elicits a bursting firing pattern in action potentials that is closely related to the inhibitory effects on the IKD.
Author contribution
Yi-hung CHEN: collection and assembly of data, manuscript writing; Pei-lin LIN: conception and design; Hui-yu HSU: collection and assembly of data; Ya-ting WU: revision of the manuscript; Dah-yuu LU: conception and design; Han-yin YANG: collection and assembly of data; Shiang-suo HUANG: collection and assembly of data; Ching-liang HSIEH: conception and design; Jaung-geng LIN: conception and design, manuscript writing.
Acknowledgments
We are grateful to Ms Iona MacDonald, a native English speaker, for checking the English in this paper. This work was supported by grants NSC 98-2320-B-039-016 and 98-2622-B-039-001-CC3, issued by the National Science Council, Taipei, Taiwan, China.
References
- Hirai A, Terano T, Hamazaki T, Sajiki J, Saito H, Tahara K, et al. Studies on the mechanism of antiaggregatory effect of Moutan Cortex. Thromb Res. 1983;31:29–40. doi: 10.1016/0049-3848(83)90005-1. [DOI] [PubMed] [Google Scholar]
- Lin HC, Ding HY, Ko FN, Teng CM, Wu YC. Aggregation inhibitory activity of minor acetophenones from Paeonia species. Planta Med. 1999;65:595–9. doi: 10.1055/s-1999-14030. [DOI] [PubMed] [Google Scholar]
- Harada M, Yamashita A, Aburada M. Pharmacological studies on the root bark of Paeonia moutan. II. Anti-inflammatory effect, preventive effect on stress-induced gastric erosion, inhibitory effect on gastric juice secretion and other effects of paeonol. Yakugaku Zasshi. 1972;92:750–6. doi: 10.1248/yakushi1947.92.6_750. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Ge N, Zhang ZY. Theoretical elucidation of activity differences of five phenolic antioxidants. Acta Pharmacol Sin. 1999;20:363–6. [PubMed] [Google Scholar]
- Zhang WG, Zhang ZS. Anti-ischemia reperfusion damage and anti-lipid peroxidation effects of paeonol in rat heart. Yao Xue Xue Bao. 1994;29:145–8. [PubMed] [Google Scholar]
- Zhang GQ, Hao XM, Zhou PA, Wu CH. Effect of paeonol on L-type calcium channel in rat ventricular myocytes. Methods Find Exp Clin Pharmacol. 2003;25:281–5. doi: 10.1358/mf.2003.25.4.769676. [DOI] [PubMed] [Google Scholar]
- Ma YL, Bates S, Gurney AM. The effects of paeonol on the electrophysiological properties of cardiac ventricular myocytes. Eur J Pharmacol. 2006;545:87–92. doi: 10.1016/j.ejphar.2006.06.064. [DOI] [PubMed] [Google Scholar]
- Wu JB, Song NN, Wei XB, Guan HS, Zhang XM. Protective effects of paeonol on cultured rat hippocampal neurons against oxygen-glucose deprivation-induced injury. J Neurol Sci. 2008;264:50–5. doi: 10.1016/j.jns.2007.06.057. [DOI] [PubMed] [Google Scholar]
- Zhong SZ, Ge QH, Qu R, Li Q, Ma SP. Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by d-galactose in ICR mice. J Neurol Sci. 2009;277:58–64. doi: 10.1016/j.jns.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, Lambert JD, Gayton RJ, Loker JE, Walder RJ. Mapping of nerve cells in the suboesophageal ganglia of Helix aspersa. Comp Biochem Physiol A Comp Physiol. 1975;50:1–25. doi: 10.1016/s0010-406x(75)80194-0. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Araki Y, Emaduddin M, Zhang W, Han XY, Salunga TL, et al. Identifiable Achatina giant neurones: their localizations in ganglia, axonal pathways and pharmacological features. Gen Pharmacol. 1996;27:3–32. doi: 10.1016/0306-3623(95)00113-1. [DOI] [PubMed] [Google Scholar]
- Lin CH, Tsai MC. The modulation effects of d-amphetamine and procaine on the spontaneously generated action potentials in the central neuron of snail, Achatina fulica Ferussac. Comp Biochem Physiol C Toxicol Pharmacol. 2005;141:58–68. doi: 10.1016/j.cca.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Chen YH, Lin CH, Lin PL, Tsai MC. Cocaine elicits action potential bursts in a central snail neuron: the role of delayed rectifying K+ current. Neuroscience. 2006;138:257–80. doi: 10.1016/j.neuroscience.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Onizuka S, Kasaba T, Takasaki M. The effect of lidocaine on cholinergic neurotransmission in an identified reconstructed synapse. Anesth Analg. 2008;107:1236–42. doi: 10.1213/ane.0b013e31818064f6. [DOI] [PubMed] [Google Scholar]
- Tsai MC, Chen YH. Bursting firing of action potentials in central snail neurons elicited by d-amphetamine: role of the electrogenic sodium pump. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1995;111:131–41. doi: 10.1016/0742-8413(94)00097-d. [DOI] [PubMed] [Google Scholar]
- Lin PL, Tsai MC, Lu GL, Lu DY, Chuang CM, Yang HY, et al. Ecstasy and methamphetamine elicit action potential bursts via different mechanisms in a central snail neuron. Neurotoxicology. 2010;31:26–41. doi: 10.1016/j.neuro.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Tsai MC, Chen YH. (+/−)3,4-Methylenedioxyamphetamine elicits action potential bursts in a central snail neuron. Exp Neurol. 2007;203:423–44. doi: 10.1016/j.expneurol.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liou HH, Chang YC, Chen RC, Peng FC, Ling KH, et al. Interactions of anticholinesterases with Achatina fulica acetylcholine responses and electrogenic sodium pump. Neuroscience. 1994;62:581–6. doi: 10.1016/0306-4522(94)90390-5. [DOI] [PubMed] [Google Scholar]
- Thompson SH. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977;265:465–88. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XP, Funase K, Takeuchi H. Ionic current of an identifiable giant neurone, d-RPLN, of an African giant snail (Achatina fulica Ferussac), measured under voltage clamping — II. Outward currents. Comp Biochem Physiol A Comp Physiol. 1987;88:707–14. doi: 10.1016/0300-9629(87)90687-6. [DOI] [PubMed] [Google Scholar]
- Kim MS, Cheong YP, So HS, Lee KM, Son Y, Lee CS, et al. Regulation of cyclic AMP-dependent response element-binding protein (CREB) by the nociceptin/orphanin FQ in human dopaminergic SH-SY5Y cells. Biochem Biophys Res Commun. 2002;291:663–8. doi: 10.1006/bbrc.2002.6503. [DOI] [PubMed] [Google Scholar]
- Beltman J, McCormick F, Cook SJ. The selective protein kinase C inhibitor, Ro-31-8220, inhibits mitogen-activated protein kinase phosphatase-1 (MKP-1) expression, induces c-Jun expression, and activates Jun N-terminal kinase. J Biol Chem. 1996;271:27018–24. doi: 10.1074/jbc.271.43.27018. [DOI] [PubMed] [Google Scholar]
- Chen YH, Tsai MC. Action potential bursts in central snail neurons elicited by d-amphetamine: roles of ionic currents. Neuroscience. 2000;96:237–48. doi: 10.1016/s0306-4522(99)00513-8. [DOI] [PubMed] [Google Scholar]
- Bukanova JV, Solntseva EI, Skrebitsky VG. Selective suppression of the slow-inactivating potassium currents by nootropics in molluscan neurons. Int J Neuropsychopharmacol. 2002;5:229–37. doi: 10.1017/S1461145702002997. [DOI] [PubMed] [Google Scholar]
- Hermann A, Erxleben C. Charybdotoxin selectively blocks small Ca-activated K channels in Aplysia neurons. J Gen Physiol. 1987;90:27–47. doi: 10.1085/jgp.90.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Strong JA, Azhderian EM, Nairn AC, Greengard P, Kaczmarek LK. Protein kinase inhibitors selectively block phorbol ester- or forskolin-induced changes in excitability of Aplysia neurons. J Neurosci. 1989;9:473–9. doi: 10.1523/JNEUROSCI.09-02-00473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manseau F, Sossin WS, Castellucci VF. Long-term changes in excitability induced by protein kinase C activation in Aplysia sensory neurons. J Neurophysiol. 1998;79:1210–8. doi: 10.1152/jn.1998.79.3.1210. [DOI] [PubMed] [Google Scholar]
- Su TR, Chen CH, Huang SJ, Lee CY, Su MC, Chen GH, et al. Functional study of the effect of phosphatase inhibitors on KCNQ4 channels expressed in Xenopus oocytes. Acta Pharmacol Sin. 2009;30:1220–6. doi: 10.1038/aps.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Harada N, Asano M, Nomura N, Saito T, Mishima A, et al. Atrial natriuretic peptide reduces ischemia/reperfusion-induced spinal cord injury in rats by enhancing sensory neuron activation. J Pharmacol Exp Ther. 2007;322:582–90. doi: 10.1124/jpet.107.120725. [DOI] [PubMed] [Google Scholar]
- Nikitin VP, Kozyrev SA. Protein kinase C is selectively involved in the mechanisms of long-term synaptic plasticity. Bull Exp Biol Med. 2005;139:639–42. doi: 10.1007/s10517-005-0365-4. [DOI] [PubMed] [Google Scholar]
- Smith TG Jr, Barker JL, Gainer H. Requirements for bursting pacemaker potential activity in molluscan neurones. Nature. 1975;253:450–2. doi: 10.1038/253450a0. [DOI] [PubMed] [Google Scholar]
- Onozuka M, Kubo KY, Ozono S. The molecular mechanism underlying pentylenetetrazole-induced bursting activity in Euhadra neurons: involvement of protein phosphorylation. Comp Biochem Physiol C. 1991;100:423–32. [PubMed] [Google Scholar]
- Zhao ML, Wu CF. Alterations in frequency coding and activity dependence of excitability in cultured neurons of Drosophila memory mutants. J Neurosci. 1997;17:2187–99. doi: 10.1523/JNEUROSCI.17-06-02187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staras K, Gyori J, Kemenes G. Voltage-gated ionic currents in an identified modulatory cell type controlling molluscan feeding. Eur J Neurosci. 2002;15:109–19. doi: 10.1046/j.0953-816x.2001.01845.x. [DOI] [PubMed] [Google Scholar]
- Sakakibara M, Okuda F, Nomura K, Watanabe K, Meng H, Horikoshi T, et al. Potassium currents in isolated statocyst neurons and RPeD1 in the pond snail, Lymnaea stagnalis. J Neurophysiol. 2005;94:3884–92. doi: 10.1152/jn.01163.2004. [DOI] [PubMed] [Google Scholar]
- Kraliz D, Singh S. Selective blockade of the delayed rectifier potassium current by tacrine in Drosophila. J Neurobiol. 1997;32:1–10. [PubMed] [Google Scholar]
- Latorre R, Oberhauser A, Labarca P, Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–99. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Mi XJ, Chen SW, Wang WJ, Wang R, Zhang YJ, Li WJ, et al. Anxiolytic-like effect of paeonol in mice. Pharmacol Biochem Behav. 2005;81:683–7. doi: 10.1016/j.pbb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Chou TC. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br J Pharmacol. 2003;139:1146–52. doi: 10.1038/sj.bjp.0705360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Chen H, Chen X, Hu Z. Determination of paeonol in rat plasma by high-performance liquid chromatography and its application to pharmacokinetic studies following oral administration of Moutan cortex decoction. Biomed Chromatogr. 2003;17:504–8. doi: 10.1002/bmc.259. [DOI] [PubMed] [Google Scholar]
- Kim SA, Lee HJ, Ahn KS, Lee EO, Choi SH, Jung SJ, et al. Paeonol exerts anti-angiogenic and anti-metastatic activities through downmodulation of Akt activation and inactivation of matrix metalloproteinases. Biol Pharm Bull. 2009;32:1142–7. doi: 10.1248/bpb.32.1142. [DOI] [PubMed] [Google Scholar]
- Ye JM, Deng T, Zhang JB. Influence of paeonol on expression of COX-2 and p27 in HT-29 cells. World J Gastroenterol. 2009;15:4410–4. doi: 10.3748/wjg.15.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SP, Sun GP, Shen YX, Peng WR, Wang H, Wei W. Synergistic effect of combining paeonol and cisplatin on apoptotic induction of human hepatoma cell lines. Acta Pharmacol Sin. 2007;28:869–78. doi: 10.1111/j.1745-7254.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- Hu Q, Shi YL. Inhibition of voltage-gated K+, Na+, and Ca2+ currents in neuroblastoma x glioma hybrid cells by paeonol. Sheng Li Xue Bao. 1994;46:575–80. [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–46. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Jefferys JG. Experimental neurobiology of epilepsies. Curr Opin Neurol. 1994;7:113–22. doi: 10.1097/00019052-199404000-00007. [DOI] [PubMed] [Google Scholar]
- Onozuka M, Imai S, Sugaya E. Pentylenetetrazole-induced bursting activity and cellular protein phosphorylation in snail neurons. Brain Res. 1986;362:33–9. doi: 10.1016/0006-8993(86)91395-8. [DOI] [PubMed] [Google Scholar]
- Sugaya E, Furuichi H, Takagi T, Kajiwara K, Komatsubara J. Intracellular calcium concentration during pentylenetetrazol-induced bursting activity in snail neurons. Brain Res. 1987;416:183–6. doi: 10.1016/0006-8993(87)91515-0. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Shouse MN, McGinty D. Brainstem stimulation during sleep evokes abnormal rhythmic activity in thalamic neurons in feline penicillin epilepsy. Brain Res. 1996;713:253–60. doi: 10.1016/0006-8993(95)01549-3. [DOI] [PubMed] [Google Scholar]