Abstract
Aim:
To investigate the changing resistance to nondepolarizing muscle relaxants (NDMRs) during the first month after denervation.
Methods:
The denervated and innervated skeletal muscle cells were examined on days 1, 4, 7, 14, 21, and 28 after denervation. Individual denervated and innervated cells were prepared from the flexor digitorum brevis of the surgically denervated and contralateral hind feet, respectively. Nicotinic acetylcholine receptors (nAChRs) in the cells were activated with 30 μmol/L acetylcholine, either alone or in combination with various concentrations of vecuronium. Currents were recorded using a whole-cell patch-clamp technique.
Results:
The concentrations of vecuronium resulting in half-maximal inhibitory responses (IC50) increased 1.2- (P>0.05), 1.7-, 3.7-, 2.5-, 1.9-, and 1.8-fold (P<0.05) at Days 1, 4, 7, 14, 21, and 28 after denervation, respectively, compared to the innervated control. Resistance to vecuronium appeared at Day 4, peaked at Day 7, and declined at Day 14 after denervation. Nevertheless, IC50 values at Day 28 remained significantly higher than those for the innervated control, suggesting that the resistance to vecuronium had not disappeared at Day 28.
Conclusion:
The NDMR doses required to achieve satisfactory clinical effects differ at different times after muscle denervation.
Keywords: denervation, nondepolarizing muscle relaxants, nicotinic acetylcholine receptors, skeletal muscle cells, vecuronium
Introduction
At the neuromuscular junction (NMJ), the motor end-plate (sarcolemma of the postsynaptic surface) contains a high density of nicotinic acetylcholine receptors (nAChRs). When these receptors are activated by presynaptically released acetylcholine (ACh), cations are permitted to flow through the nAChR pore, leading to depolarization of the muscle membrane and creating an end-plate potential. If the end-plate potential is large enough, an action potential is evoked and ultimately causes muscle contraction. Clinically used nondepolarizing muscle relaxants (NDMRs), typified by d-tubocurarine (dTC), competitively occupy the αδ and αɛ (or αγ) sites of nAChRs to achieve muscle relaxation during surgery and anesthesia.
nAChRs include two subtypes: the adult form (ɛ-AChR), composed of α2βδɛ subunits, and the fetal form (γ-AChR), containing α2βδγ subunits1, 2. The two subtypes have different electrophysiological properties: ɛ-AChR manifests a larger slope conductance and a much shorter mean open channel time than γ-AChR3. γ-AChR is initially expressed in fetal muscle and is then replaced by ɛ-AChR when muscles are innervated. However, after denervation of adult muscles, γ-AChR is expressed de novo throughout the muscle membrane4.
Thermal injury and other forms of critical tissue damage can cause denervation changes in skeletal muscle. Denervated skeletal muscle displays aberrant responses to muscle relaxants, including lethal hyperkalemia in response to the depolarizing muscle relaxant succinylcholine and resistance to NDMRs, which coincide with the up-regulation of nAChRs and γ-AChRs on the muscle membrane5, 6, 7, 8, 9. After denervation, the amounts of nAChR and γ-AChR change with time; furthermore, the IC50 of dTC correlates positively with nAChR and γ-AChR mRNA levels at different times after denervation9, 10, 11. All of these results indicate that the NDMR doses required to achieve satisfactory clinical effects may differ at different times after denervation. The aim of this study was to measure the changes in the potency of NDMRs during the first month after denervation in a mouse model.
Materials and methods
Denervation
This study was approved by the Animal Care and Use Committee of the Shanghai JiaoTong University School of Medicine. Balb/c mice (35 days old) were anesthetized with pentobarbital, 40 mg/kg ip. A few mm of the right sciatic nerve was excised through a small (3–5 mm) incision over the hip. The incision was sutured with a single stitch. Animals were killed at 1, 4, 7, 14, 21, or 28 days after denervation by pentobarbital anesthesia and cervical dislocation. The left leg of each mouse served as the innervated control.
Isolation of muscle fibers
Single skeletal muscle cells from the flexor digitorum brevis (FDB) muscle were obtained from the hind feet of each mouse. The muscles were incubated for 3 h with Dulbecco's modified Eagle's medium (DMEM) (InvitrogenTM, Grand Island, NY, USA) containing 10% fetal calf serum (InvitrogenTM, Grand Island, NY, USA), 100 units/mL penicillin, 100 μg/mL streptomycin, and 0.2% collagenase 1A (Sigma Chemical Co, Saint Louis, USA) in a shaking bath at 37 °C. After 3 h of enzymatic treatment, the muscles were dissociated into single muscle cells using Pasteur pipettes with different tip sizes.
Electrophysiology
FDB muscle cells were voltage-clamped using a whole-cell patch-clamp technique12. All experiments were performed at room temperature (20–24 °C). Patch pipettes were pulled from borosilicate glass using a Flaming Brown micropipette puller (P97; Sutter Instrument Co, Novato, CA, USA), ranging from 1 to 2 MΩ. The pipette electrode was filled with the following solution (mmol/L): 140 KCl, 10 HEPES, 10 EGTA, 1 CaCl2, and 1 MgCl2; the pH was adjusted to 7.2 with KOH. The external solution contained (mmol/L) 5 KCl, 140 NaCl, 1 CaCl2, 1.25 MgCl2, 10 HEPES, and 10 glucose, with 0.5 μmol/L atropine sulfate, and the pH was adjusted to 7.4 with NaOH. The cells were voltage-clamped at -80 mV in a whole-cell configuration after obtaining GΩ seals. Much (60%–80%) of the series resistance was compensated. Currents were recorded with an EPC10 amplifier (HEKA Elektronik, Germany) and PatchMaster software (HEKA Elektronik, Germany), sampled at 10 kHz, and stored on a computer.
ACh and atropine sulfate were purchased from Sigma (Saint Louis, USA). Vecuronium (NV Organon, The Netherlands) was obtained from a preparation for clinical use. All drugs were dissolved in the external solution and were applied by a gravity-driven perfusion system. Solutions and subsequent dilutions were prepared immediately before the experiments.
Test solution applications containing either ACh alone or in combination with various concentrations of vecuronium were applied for 10 s to the skeletal muscle cells, and the peak current was determined. To determine the effect of vecuronium on the ACh-elicited current, the solution containing the vecuronium perfused the skeletal muscle cells for 3 min prior to the application of ACh. The washout time between each drug application was at least 60 s in order to minimize the amount of desensitization throughout the course of an experiment. Currents were acquired from five skeletal muscle cells taken from at least two mice. The control current caused by ACh alone was measured again after washout of the vecuronium. Taking the mean value of these two ACh applications as the average control current, the antagonist response (percentage inhibition of average control current) was calculated using the following equation:
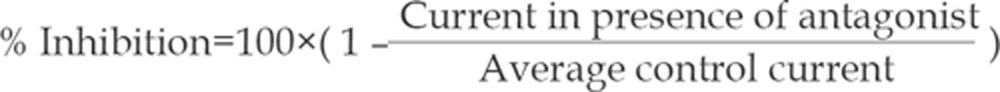 |
Statistical analysis
Data analysis was performed off-line using Origin 8 (OriginLab, Northampton, MA, USA) and GraphPad Prism 4 (GraphPad software, Inc, San Diego, CA, USA). Concentration-response curves were fitted to the four-parameter logistic equation by nonlinear regression analysis, and IC50 values were determined. Statistical significance was assessed with one-way analysis of variance followed by Tukey's test. P<0.05 was considered significant.
Results
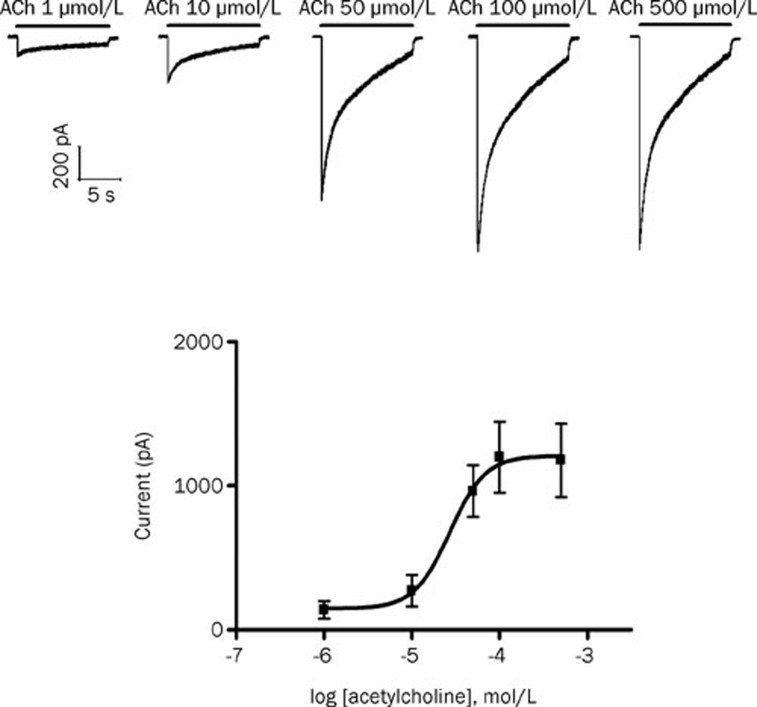
ACh at different concentrations (1–500 μmol/L) was applied for 10 s to mouse skeletal muscle cells without denervation. ACh elicited concentration-dependent inward currents. The data were fitted to a logistic equation. The ACh concentration producing 50% of the maximal response (EC50) was 27.1 μmol/L for the nAChR in the innervated skeletal muscle (Figure 1; Hill coefficient=2.14). In all following experiments, a concentration of 30 μmol/L was used to activate nAChR because this concentration was close to the EC50 concentration and produced large and robust responses.
Figure 1.
Acetylcholine (ACh) produced concentration-dependent inward currents in the innervated skeletal muscles. (Top) Representative recording of currents produced by 1, 10, 50, 100, and 500 μmol/L ACh (horizontal bars). (Lower) Concentration-response curve for the action of ACh on adult-type nicotinic acetylcholine receptors (ɛ-nAChRs). Data points show means±SD (error bars) of five muscle cells taken from at least two mice.
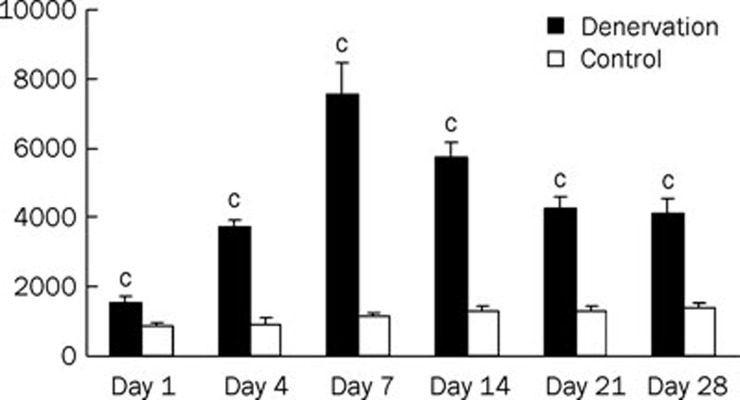
Application of 30 μmol/L ACh produced inward currents with different amplitudes at the nAChRs of denervated and control (innervated) skeletal muscle cells on Days 1, 4, 7, 14, 21, and 28 after denervation. Compared with the responses of control cells, responses induced by 30 μmol/L ACh after denervation increased 1.8-, 4.0-, 6.7-, 4.5-, 3.3-, and 3.0-fold (P<0.01) at days 1, 4, 7, 14, 21, and 28, respectively (Figure 2).
Figure 2.
Responses were elicited by the same concentration of acetylcholine (30 μmol/L) on denervated and control (innervated) skeletal muscle cells at Day 1, 4, 7, 14, 21, and 28 after denervation (n=5). cP<0.01 vs Control.
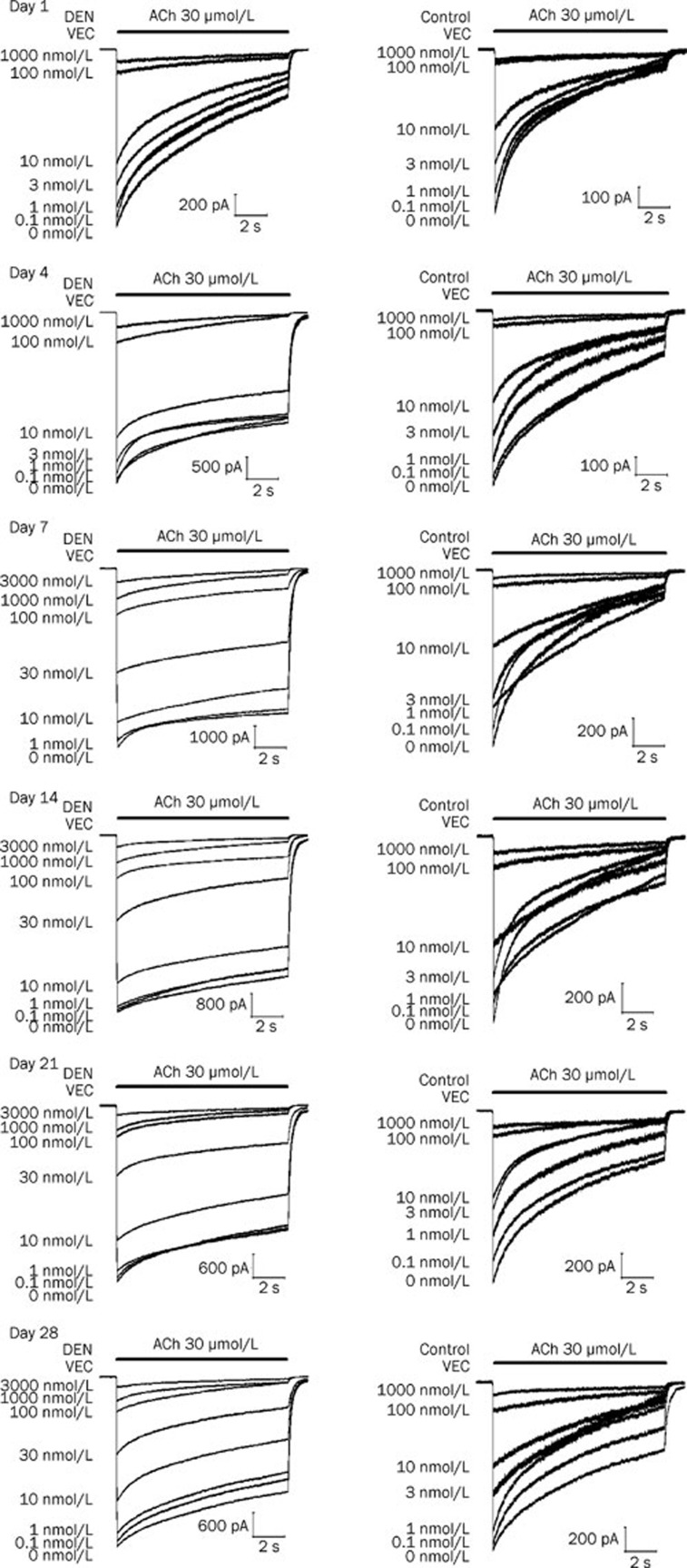
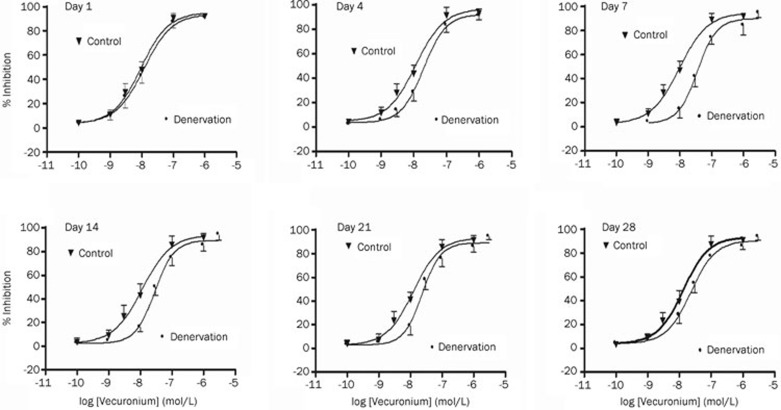
Vecuronium reversibly inhibited inward currents elicited by the application of ACh (30 μmol/L) on denervated and control skeletal muscle cells in a concentration-dependent manner at days 1, 4, 7, 14, 21, and 28 after denervation (Figure 3 and 4). Compared with the IC50 values of vecuronium for the control, the IC50 values were 1.2- (P>0.05), 1.7-, 3.7-, 2.5-, 1.9-, and 1.8-fold greater (P<0.05) at Days 1, 4, 7, 14, 21, and 28 after denervation, respectively (Table 1).
Figure 3.
Concentration-dependent effects of vecuronium on nicotinic acetylcholine receptors (nAChRs) in denervated (DEN) and innervated (Control) muscle cells at Day 1, 4, 7, 14, 21, and 28 after denervation.
Figure 4.
Concentration-response effects of vecuronium looking at inhibition of acetylcholine-induced currents in denervated and control (innervated) skeletal muscle cells at Day 1, 4, 7, 14, 21, and 28 after denervation. Data points show means±SD (error bars) of five muscle cells taken from at least two mice. Error bars not visible are smaller than symbols.
Table 1. Potencies of vecuronium at nicotinic acetylcholine receptors in denervated and innervated skeletal muscle cells at different time points after denervation.
| Group | IC50 (nmol/L) (95% CI) | P value | Hill coefficient | ||
|---|---|---|---|---|---|
| Denervation | Control | Denervation | Control | ||
| Day 1 | 11.7 (4.6–29.9) | 9.4 (3.7–23.8) | >0.05 | 0.99 | 1 |
| Day 4 | 20.0 (10.8–37.0) | 11.5 (3.4–38.2) | <0.01 | 1.27 | 1.15 |
| Day 7 | 35.7 (16.2–78.5) | 9.6 (3.9–23.7) | <0.01 | 1.33 | 0.97 |
| Day 14 | 28.8 (18.1–45.6) | 11.5 (5.4–24.5) | <0.01 | 1.22 | 0.95 |
| Day 21 | 23.5 (13.8–40.4) | 12.5 (5.6–27.6) | <0.01 | 1.19 | 1.03 |
| Day 28 | 23.3 (15.6–34.7) | 13.2 (4.8–36.1) | <0.05 | 1.12 | 1.07 |
Potencies of vecuronium at nicotinic acetylcholine receptors (nAChRs) are expressed as the value achieving 50% of maximal inhibition (IC50) of the inward currents induced by coapplication of 30 μmol/L acetylcholine (95% confidence interval [CI]). For Denervation or Control group, drug IC50 values that do not share a superscript are statistically different from each other by one-way analysis of variance and Tukey's multiple comparison method.
Discussion
Determination of the altered potencies of NDMRs on nAChRs in the short-term after denervation of skeletal muscle is important from a clinical viewpoint because the amount of membrane nAChR and the proportion of γ-AChR after denervation change with time, which may lead to altered dosing requirements of NDMRs to achieve the desired effect.
This study involved the dynamic observation of the potencies of NDMRs at different times after muscle denervation. We first established the action of acetylcholine on nAChR in the skeletal muscle of the innervated controls. Interestingly, the EC50 value of ACh we determined was greater than values obtained in previous studies13, 14. This disagreement may result from a discrepancy in the endogenous nature of the nAChR between mouse skeletal muscle and the Xenopus laevis oocytes used in other research. The process by which the adult mouse muscle nAChR was heterologously expressed in Xenopus laevis oocytes may lead to minor differences in glycosylation or other posttranslational modifications. Looking at previous reports of single channel recordings of ɛ-AChR, we found that the slope conductance of ɛ-AChR expressed in Xenopus laevis oocytes was different from that in skeletal muscle15, 16.
We applied a holding voltage of -80 mV because the antagonistic effects of NDMRs are independent of holding voltages ranging from -100 to -40 mV17, 18. Jonsson Fagerlund et al19 reported that repeated applications of 10 μmol/L acetylcholine desensitized the receptor, and they suggested that the decrease in the IC50 values of NDMRs could be at least partly explained by receptor desensitization. However, in Paul's work13 and in this study, 10 μmol/L or 30 μmol/L acetylcholine concentrations were selected because these concentrations were close to the EC50 concentration for the innervated skeletal muscle and thus more physiologically relevant than lower acetylcholine concentrations. Additionally, measurement of the control current caused by ACh alone was repeated after washout of the vecuronium in both Paul's work13 and in this study. After taking the mean value of these two ACh applications and using this value as the average control current, the antagonist response was calculated by use of the above equation. Through this method, we can minimize the effect of receptor desensitization on the results.
The same ACh concentration applied here produced larger inward currents in nAChRs in skeletal muscle after denervation than in the innervated control. This leads us to suggest, in accordance with the views of others20, 21, that skeletal muscles deprived of their motor nerves develop an increased sensitivity to ACh.
Consistent with previous reports9, 22, the IC50 values of vecuronium increased after denervation, indicating that denervation caused resistance to NDMRs. One potential mechanism for this resistance could be that denervation induces the up-regulation of mature and immature nAChR on the muscle membrane, which would increase the amount of NDMR required to competitively block ACh3. However, reports of changes in nAChR numbers at Day 1 have been conflicting. Tsay and Schmidt23 reported a sharp increase in the transcriptional activity of AChR subunit genes beginning after approximately half a day after denervation of chick skeletal muscle. However, Ibebunjo and Martyn9, 24 found no changes in nAChR numbers at Day 1 after a burn, and they also observed that skeletal muscle at Day 1 post-burn was resistant to dTC in rats. However, our study showed that skeletal muscle did not become resistant to vecuronium at Day 1 after denervation. Wang et al25 observed that post-denervation resistance to atracurium was greater than to vecuronium, which is due to the different potencies of atracurium and vecuronium on nAChR subunits. In addition to having a similar structure to that of atracurium, dTC also has less potency on γ-AChR than on ɛ-AChR and has partial agonist effects on γ-AChR13, 18, 26. Thus, the occurrence of these differences in resistance to NDMRs at Day 1 after denervation may be associated with the different potencies of the NDMRs on nAChR subtypes and not with changes in the amount of nAChRs after denervation.
Resistance to vecuronium appeared at Day 4, peaked at Day 7, and declined by Day 14. These findings are paralleled by changes in nAChRs and γ-AChR on the same days after denervation, as shown by other research9,10,24. Although the IC50 values of vecuronium further decreased after Day 14, the IC50 values at Day 28 remained significantly higher for denervated cells than for the innervated control cells, which indicated that the resistance to NDMRs had not disappeared at Day 28. Consistent with our results, Adams et al11 observed an increase in nAChR RNA levels during the first month of denervation, and the level began to return to the innervated level beyond one month after denervation. However, Ma et al10 found that the expression of most of the nAChR subunits returned to the control level within one month after denervation.
In summary, short-term muscle denervation leads to a changing pattern of resistance to NDMRs that can be attributed to up-regulation of mature and immature nAChRs on the muscle membrane. After denervation, resistance to vecuronium appeared by Day 4, peaked at Day 7, declined by Day 14, and persisted through Day 28. Our findings suggest that the dose requirement of NDMRs after denervation to achieve clinically appropriate muscle relaxation changes during the first month after denervation.
Author contribution
Hong WANG and Shi-tong LI designed the research; Hong WANG and Bin YANG performed the research; Hong WANG and Guang-wei HAN analyzed the data; and Hong WANG and Shi-tong LI wrote the paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 30571796).
We thank Dr Xin-qiu LIU (Department of Neurobiology, School of Medicine, Shanghai JiaoTong University) for technical assistance and strong support.
Glossary
- NDMRs
nondepolarizing muscle relaxants
- nAChR
nicotinic acetylcholine receptor
- γ-AChR
fetal-type nicotinic acetylcholine receptor
- ɛ-AChR
adult-type nicotinic acetylcholine receptor
- IC50
inhibitory concentration resulting in a half-maximal response
- dTC
d-tubocurarine
- FDB
flexor digitorum brevis
- DMEM
Dulbecco's modified Eagle's medium
- ACh
Acetylcholine
- VEC
vecuronium
References
- Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, et al. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986;321:406–11. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Gu Y, Hall ZW. Characterization of acetylcholine receptor subunits in developing and in denervated mammalian muscle. J Biol Chem. 1988;263:12878–85. [PubMed] [Google Scholar]
- Martyn JA, White DA, Gronert GA, Jaffe RS, Ward JM. Up-and-down regulation of skeletal muscle acetylcholine receptors: effects on neuromuscular blockers. Anesthesiology. 1992;76:822–43. doi: 10.1097/00000542-199205000-00022. [DOI] [PubMed] [Google Scholar]
- Witzemann V, Brenner HR, Sakmann B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular synapses. J Cell Biol. 1991;114:125–41. doi: 10.1083/jcb.114.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue CW Jr, Itani MS, Martyn JA. Resistance to d-tubocurarine in lower motor neuron injury is related to increased acetylcholine receptors at the neuromuscular junction. Anesthesiology. 1990;73:703–9. doi: 10.1097/00000542-199010000-00016. [DOI] [PubMed] [Google Scholar]
- Kim C, Martyn J, Fuke N. Burn injury to trunk of rat causes denervation-like responses in the gastrocnemius muscle. J Appl Physiol. 1988;65:1745–51. doi: 10.1152/jappl.1988.65.4.1745. [DOI] [PubMed] [Google Scholar]
- Yanez P, Martyn JA. Prolonged D-tubocurarine infusion and/or immobilization causes upregulation of acetylcholine receptors and hyperkalemia to succinylcholine. Anesthesiology. 1996;84:384–91. doi: 10.1097/00000542-199602000-00017. [DOI] [PubMed] [Google Scholar]
- Nosek MT, Martyn JA. Na+ channel and acetylcholine receptor changes in muscle at sites distant from burns do not simulate denervation. J Appl Physiol. 1997;82:1333–9. doi: 10.1152/jappl.1997.82.4.1333. [DOI] [PubMed] [Google Scholar]
- Ibebunjo C, Martyn JA. Thermal injury induces greater resistance to d-tubocurarine in local rather than in distant muscles in the rat. Anesth Analg. 2000;91:1243–9. doi: 10.1097/00000539-200011000-00036. [DOI] [PubMed] [Google Scholar]
- Ma J, Shen J, Garrett JP, Lee CA, Li Z, Elsaidi GA, et al. Gene expression of myogenic regulatory factors, nicotinic acetylcholine receptor subunits, and GAP-43 in skeletal muscle following denervation in a rat model. J Orthop Res. 2007;25:1498–505. doi: 10.1002/jor.20414. [DOI] [PubMed] [Google Scholar]
- Adams L, Carlson BM, Henderson L, Goldman D. Adaptation of nicotinic acetylcholine receptor, myogenin, and MRF4 gene expression to long-term muscle denervation. J Cell Biol. 1995;131:1341–9. doi: 10.1083/jcb.131.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Siqworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Paul M, Kindler CH, Fokt RM, Dresser MJ, Dipp NC, Yost CS. The potency of new muscle relaxants on recombinant muscle-type acetylcholine receptors. Anesth Analg. 2002;94:597–603. doi: 10.1097/00000539-200203000-00022. [DOI] [PubMed] [Google Scholar]
- Kindler CH, Verotta D, Gray AT, Gropper MA, Yost CS. Additive inhibition of nicotinic acetylcholine receptors by corticosteroids and the neuromuscular blocking drug vecuronium. Anesthesiology. 2000;92:821–32. doi: 10.1097/00000542-200003000-00026. [DOI] [PubMed] [Google Scholar]
- Yost CS, Wineqar BD. Potency of agonists and competitive antagonists on adult- and fetal-type nicotinic acetylcholine receptors. Cell Mol Neurobiol. 1997;17:35–50. doi: 10.1023/A:1026325020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A, Sakmann B. Calcium permeability increase of endplate channels in rat muscle during postnatal development. J Physiol. 1996;496:331–8. doi: 10.1113/jphysiol.1996.sp021688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland CM, Foreman RC, Chad JE, Hold-Dye L, Walker RJ. The actions of muscle relaxants at nicotinic acetylcholine receptor isoforms. Eur J Pharmacol. 1988;357:83–92. doi: 10.1016/s0014-2999(98)00542-1. [DOI] [PubMed] [Google Scholar]
- Fletcher GH, Steinbach JH. Ability of nondepolarizing neuromuscular blocking drugs to act as partial agonists at fetal and adult mouse muscle nicotinic receptor. Mol Pharmacol. 1996;49:938–47. [PubMed] [Google Scholar]
- Jonsson Fagerlund M, Dabrowski M, Eriksson LI. Pharmacological characteristics of the inhibition of nondepolarizing neuromuscular blocking agents at human adult muscle nicotinic acetylcholine receptor. Anesthesiology. 2009;110:1244–52. doi: 10.1097/ALN.0b013e31819fade3. [DOI] [PubMed] [Google Scholar]
- Axelsson J, Thesleff S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959;147:178–93. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn JA, Richtsfeld M. Succinylcholine-induced hyperkalemia in acquired pathologic states: etiologic factors and molecular mechanisms. Anesthesiology. 2006;104:158–69. doi: 10.1097/00000542-200601000-00022. [DOI] [PubMed] [Google Scholar]
- Han T, Kim H, Bae J, Kim K, Martyn JA. Neuromuscular pharmacodynamics of rocuronium in patients with major burns. Anesth Analg. 2004;99:386–92. doi: 10.1213/01.ANE.0000129992.07527.4B. [DOI] [PubMed] [Google Scholar]
- Tsay HJ, Schmidt J. Skeletal muscle denervation activates acetylcholine receptor genes. J Cell Biol. 1989;108:1523–6. doi: 10.1083/jcb.108.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibebunjo C, Martyn J. Disparate dysfunction of skeletal muscles located near and distant from burn site in the rat. Muscle Nerve. 2001;24:1283–94. doi: 10.1002/mus.1146. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang B, Xu YF, Yan T, Li ST. Magnitude differences of resistance to different nondepolarizing muscle relaxants in the denervated mouse skeletal muscle. Acta Pharmacol Sin. 2010;31:399–404. doi: 10.1038/aps.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach JH, Chen Q. Antagonist and partial agonist action of d-tubocurarine at mammalian muscle acetylcholine receptors. J Neurosci. 1995;15:230–40. doi: 10.1523/JNEUROSCI.15-01-00230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]