Abstract
Aim:
To characterize the metabolism of nuciferine by P450 enzymes and uridine diphosphate glucuronosyltransferase (UGT) in liver microsomes from humans and several other animals including rats, mice, dogs, rabbits and monkeys.
Methods:
Nuciferine was incubated with both human and animal liver microsomal fractions containing P450 or UGT reaction components. Ultra performance liquid chromatography coupled with mass spectrometry was used to separate and identify nuciferine metabolites. Chemical inhibition was used to identify the involved isozymes. Species difference of nuciferine metabolism in human and various animals were investigated in the liver microsomal incubation system.
Results:
Among the nuciferine metabolites detected and identified, seven were catalyzed by P450 and one by UGT. Ketoconazole inhibited the formation of M292, M294 and M312. Furafylline, 8-methoxypsoralen and quercetin inhibited the formation of M282. Hecogenin showed a significant inhibitory effect on nuciferine glucuronidation. While the P450-catalyzed metabolites showed no species differences, the glucuronidation product was only detected in microsomes from humans and rabbits.
Conclusion:
The isozymes UGT 1A4, CYP 3A4, 1A2, 2A6 and 2C8 participated in the hepatic metabolism of nuciferine. Based on the observed species-specific hepatic metabolism of nuciferine, rats, mice, dogs and even monkeys are not suitable models for the pharmacokinetics of nuciferine in humans.
Keywords: nuciferine, metabolism, cytochrome P450, UGT, species differences, rats, mice, rabbits, monkeys, human
Introduction
Lotus is a common plant that grows worldwide. This plant has long been used as an herb in traditional Chinese medicine1. In recent years, significant pharmacological activities have been observed for lotus, which displays beneficial effects on hyperlipidemia2, obesity3, arrhythmia4 and atherosclerosis 2. Most of the beneficial effects have been attributed to the main constituent of lotus, the aporphine alkaloid nuciferine4. Given its salutary effects, nuciferine is a promising drug candidate. However, the metabolism and in vivo kinetics of nuciferine have not been investigated until now.
Cytochrome P450 (CYP) enzymes participate in 70%–80% of the known phase I metabolism of drugs5 and, as such, are among the most important drug-metabolizing enzymes. In recent years, there has been increased interest in in vitro metabolism studies involving P450 enzymes during preclinical drug development6, 7, 8 in order to identify drugs with undesirable metabolites and to prevent failure in clinical trials. In addition to CYP enzymes, UDP-glucuronosyltransferases (UGTs) participate in nearly half of the known phase II metabolism of the top 200 prescribed drugs in the United States9. Thus, like CYPs, UGTs are important drug-metabolizing enzymes.
In the present study, both P450- and UGT-mediated metabolism of nuciferine were investigated in order to learn the general structure of the metabolites, to identify the isozymes involved in nuciferine metabolism and to examine the species differences in nuciferine metabolism in humans and other commonly used experimental animals.
Materials and methods
Chemicals and materials
Nuciferine, glucose-6-phosphate (G-6-P), G-6-P dehydrogenase, alamethicin, uridine diphosphate glucuronic acid, β-nicotinamide-adenine dinucleotide phosphate (NADP), sulfaphenazole, quinidine, clomethiazole, furafylline, 8-methoxypsoralen, hecogenin, fluconazole, androsterone and phenylbutazone were purchased from Sigma-Aldrich (St Louis, MO, USA). Ketoconazole and S-mephenytoin were obtained from ICN Biomedicals Inc (Aurora, OH, USA) and Toronto Research Chemicals Inc (North York, Canada), respectively. Pooled human liver microsomes (HLM) were prepared using tissue from 13 Chinese donors and stored in phosphate buffer (100 mmol/L, pH 7.4). Except the pooled HLM, 6 HLM samples (male) and male cynomolgus monkey liver microsomes were purchased from Rild Research Institute for Liver Diseases (Shanghai, China). Experiments involving human subjects were approved by the local governmental ethics authorities and were pursuant to the Helsinki Declaration. All other reagents were of HPLC grade or the highest grade commercially available.
Preparation of liver microsomes
Livers from rats (Sprague-Dawley, male, n=6), mice (Swiss, male, n=6), rabbits (New Zealand white, male, n=6) and dogs (Beagle, male, n=6) were obtained from healthy animals at the Experimental Animal Center of Shanghai University of Traditional Chinese Medicine (SUTCM, Shanghai, China). The use of livers in the present study was approved by the ethics committee of SUTCM. Liver samples were pooled by species and stored in liquid nitrogen immediately after being harvested. Microsomes were prepared from pooled frozen liver tissue by differential ultracentrifugation as described previously10. Protein concentration was determined using bovine serum albumin as reference11. Liver microsomes were diluted to 10 mg/mL and stored at -80 °C.
Parameters for chromatography and mass spectrometry
An ultra performance liquid chromatography (UPLC) system coupled with triple quadrupole mass spectrometry (Acquity-Premier, Waters, Milford, MA) and an electrospray ionization (ESI) source was used in the present study. A Waters bridged ethyl hybrid (BEH) C18 (50×2.1 mm, 1.7 μm) column was used for separation. Acetonitrile and formic acid solution (0.1% in water) were used as mobile phase components A and B, respectively. The mobile phase elution gradient was as follows: 0 to 0.5 min, 5% A; 0.5 to 1.5 min, 5% to 50% A; 1.5 to 3 min, 50% to 80% A; 3 to 4 min, 80% to 95% of A. The mobile-phase flow rate was 0.3 mL/min.
The positive ion monitor mode was adopted, and mass parameters for UPLC-MS were set as follows: capillary voltage, 3.2 kV; cone voltage, 40 V; extractor voltage, 1.59 V; source and desolvation temperatures, 100 and 350 °C, respectively; cone and desolvation gas flow, 50 and 550 L/h, respectively. Multiple reaction monitor mode (MRM) was used to determine the metabolites and ion transitions were set as follows: 296 to 235 for nuciferine; 294 to 248 and 294 to 279 for M294-1 and M294-2, respectively; 292 to 246 for M292; 282 to 191 and 282 to 250 for M282-1 and M282-2, respectively; 312 to 250 and 312 to 248 for M312-1 and M312-2, respectively; 472 to 265 for M472.
Another alkaloid, senecionine, was used as the internal standard, which was also detected in MRM mode with ion transition of 336 to 138. The accuracy for determination of nuciferine was more than 90% and less than 105%. The precision for determination of nuciferine and metabolites was measured, and the residual standard deviation (RSD) values were less than 15%.
Microsomal incubation system for P450-mediated metabolism
A standard incubation system for P450-mediated metabolism (System P450) included HLM (0.5 g/L, 10 μL), G-6-P (1 mmol/L, 20 μL), G-6-P dehydrogenase (1 unit/mL, 20 μL), phosphate buffer (100 mmol/L, pH 7.4, 108 μL), MgCl2 (4 mmol/L, 20 μL), and nuciferine (200 μmol/L, 2 μL). Nuciferine was dissolved in methanol; all other reagents were dissolved in phosphate buffer. The total volume of the incubation system was 200 μL, and the total organic volume was less than 1% of the system. The reaction was initiated by adding NADP (1 mmol/L, 20 μL). After incubation for 60 min, 200 μL of acetonitrile was added to stop the reaction. The stopped incubation system was centrifuged for 10 min at 20 000 g (4 °C) and a 2-μL aliquot of the supernatant was analyzed as described above.
Microsomal incubation system for UGT-mediated metabolism
A standard incubation system for UGT-mediated metabolism (system UGT) with a total volume of 200 μL contained HLM (0.5 g/L, 10 μL), alamethicin (25 μg/mg protein, 10 μL), UDPGA (5 mmol/L, 20 μL), MgCl2 (4 mmol/L, 20 μL), Tris-HCl buffer (50 mmol/L, pH 7.4) and nuciferine (200 μmol/L, 2 μL). Reactions were started by adding UDPGA at 37 °C and stopped after 60 min by adding ice-cold 10% trichloroacetic acid (200 μL). After stopping the reaction, the incubation mixture was centrifuged for 10 min at 20 000 g (4 oC). Aliquots of the supernatant were subsequently analyzed.
Identification of metabolites
Three experimental groups were used in the present study to identify P450-catalyzed nuciferine metabolites: a reaction group, which included nuciferine, HLM, NADP and the other components described above; a negative control group, which included nuciferine and the other reaction components except NADP (which was replaced by phosphate buffer); and a blank group, which included all reaction components without nuciferine (but with the appropriate volume of methanol).
Similarly, three experimental groups were used in the present study to identify glucuronidated nuciferine metabolites: a reaction group, which included nuciferine, HLM, UDPGA and the other components described above; a negative control group, which included nuciferine and the other reaction components except UDPGA (which was replaced by phosphate buffer); and a blank group, which included all reaction components without nuciferine (but with the appropriate volume of methanol).
Peaks appearing only in the reaction group, but not in the negative control and blank groups, were considered to correspond to metabolites of nuciferine. The molecular weight and the MS/MS spectra of the metabolites were compared with those of nuciferine to determine their structures.
Chemical inhibition of P450-mediated metabolism of nuciferine in HLM
Selective inhibitors, including furafylline (10 μmol/L), 8-methoxypsoralen (2.5 μmol/L), quercetin (10 μmol/L), sulfaphenazole (10 μmol/L), quinidine (10 μmol/L), clomethiazole (50 μmol/L), and ketoconazole (1 μmol/L), were used to inhibit CYP 1A2, 2A6, 2C8, 2C9, 2D6, 2E1, and CYP3A4, respectively, in HLM12, 13, 14. All of the inhibitors added to the incubation system were dissolved in 1 μL of methanol, which was less than 0.5% of the total incubation volume; other components in the incubation system were the same as those described above. An incubation without any inhibitor, but with the dissolving medium was used as a solvent control. The reaction system was pre-incubated for 3 min at 37 °C, and the reaction was initiated by the addition of nuciferine. The quantities of metabolites in the inhibited incubations were compared with those in the solvent control incubations, which were normalized to 100%. The compared value was defined as the remaining enzyme activity (% of control) and used as a parameter to evaluate the catalytic capacity of an isozyme.
Chemical inhibition of glucuronidation of nuciferine in HLM
Fluconazole15, phenylbutazone16, 17, androsterone16, 17, and hecogenin17 were used to inhibit nuciferine glucuronidation in HLM. Fluconazole and hecogenin showed a potent and selective inhibitory effect on UGT 2B7 and UGT 1A4, respectively15, 17. Androsterone showed a non-selective inhibitory effect on several UGT isozymes including UGT 2B7, 2B15, 1A1, 1A3, 1A4, and 1A9, with UGT 1A9 being inhibited strongly17. Phenylbutazone showed non-selective effect on several isozymes including UGT 1A1, 1A3, 1A6, 1A7, 1A8, 1A9, 1A10, and 2B15; however, the compound showed no inhibitory effect on UGT 1A417. A range of concentrations including 1, 10, 50, and 200 μmol/L were used for all of the inhibitors. The concentration of nuciferine in the reactions was 100 μmol/L. The reaction time and protein concentrations were set at 60 min and 0.5 g/L, respectively. All other reaction parameters were the same as those in the standard incubation system described above. The concentration of organic solvent dissolving inhibitors was less than 0.5% of the total incubation volume. An experimental group without inhibitors but dissolving solvents was used as the solvent control, in which the activities of nuciferine glucuronidation were normalized to 100%. The activities of nuciferine glucuronidation in the inhibited samples were compared with the solvent control to calculate the remaining enzyme activity (% of control).
Species differences in nuciferine metabolism in liver microsomes from humans and other animals
To compare the species differences in nuciferine metabolism and to identify a suitable animal species for pharmacokinetic studies, nuciferine was incubated in the P450 and UGT systems described above but with liver microsomes from human, rat, mouse, dog, rabbit, or monkey. The concentrations of nuciferine used in the incubation systems were 50, 100, and 200 μmol/L. The protein concentration of the microsomes used in the incubation systems was 0.5 g/L. The incubation time was 60 min. The quantities of metabolites formed by human and the various non-human microsomes were compared. Microsomes used to determine species differences in nuciferine metabolism were different from other experiments of the present study. The microsomes were from six animals, and their preparation was the same as for the pooled HLMs purchased from the Rild Institute for Liver Disease in Shanghai. Average values of the six individuals were used.
Statistical analysis
All data were acquired from three replicates. Student's t-tests (group) were performed to evaluate the statistical significance of differences between two experimental groups using a probability value (P) threshold of 0.05 or 0.01. Inhibitory potency (IC50) values were obtained by non-linear regression of inhibition-versus-log (inhibitor) plots using the “sigmoidal fit” function built into Origin (OriginLab Corporation, Northampton, MA) software.
Results
Identification of metabolites
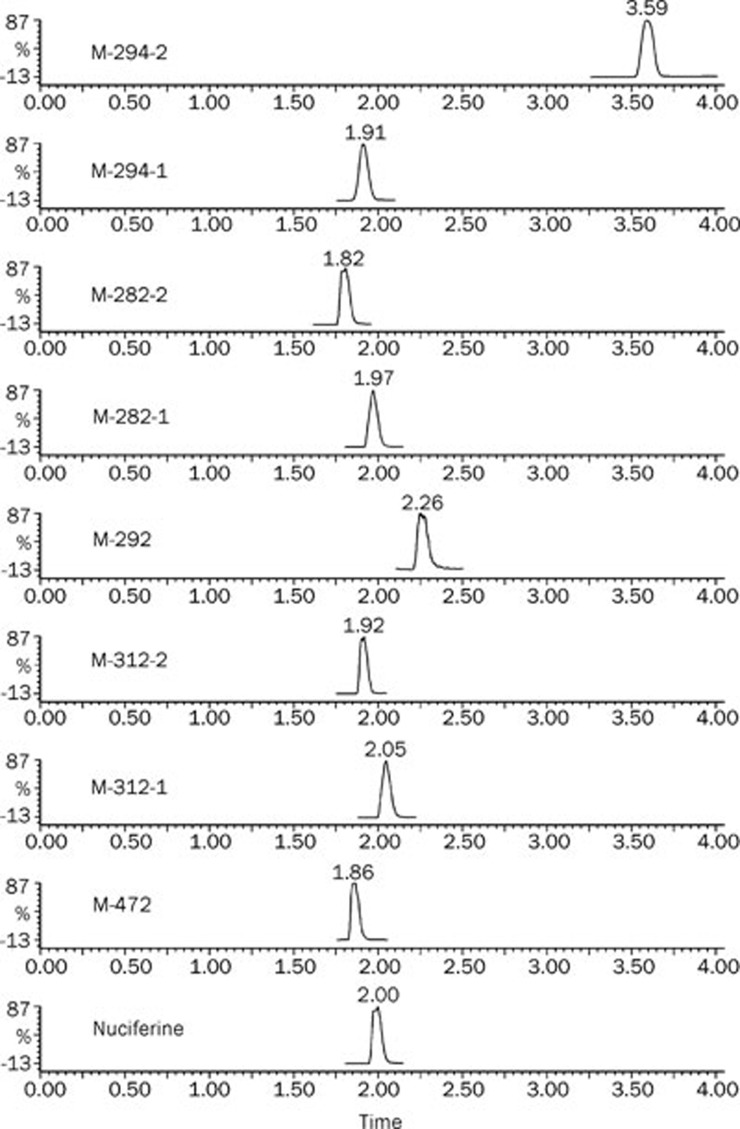
In the HLM P450 system, seven NADPH-dependent metabolites were observed (Figure 1). According to the molecular weight values, these metabolites were identified as M-312-1, M-312-2, M-282-1, M-282-2, M-294-1, M-294-2, and M-292. In the HLM UGT system, a unique UDPGA-dependent metabolite was detected and identified as M-472.
Figure 1.
Chromatograms of nuciferine and its metabolites.
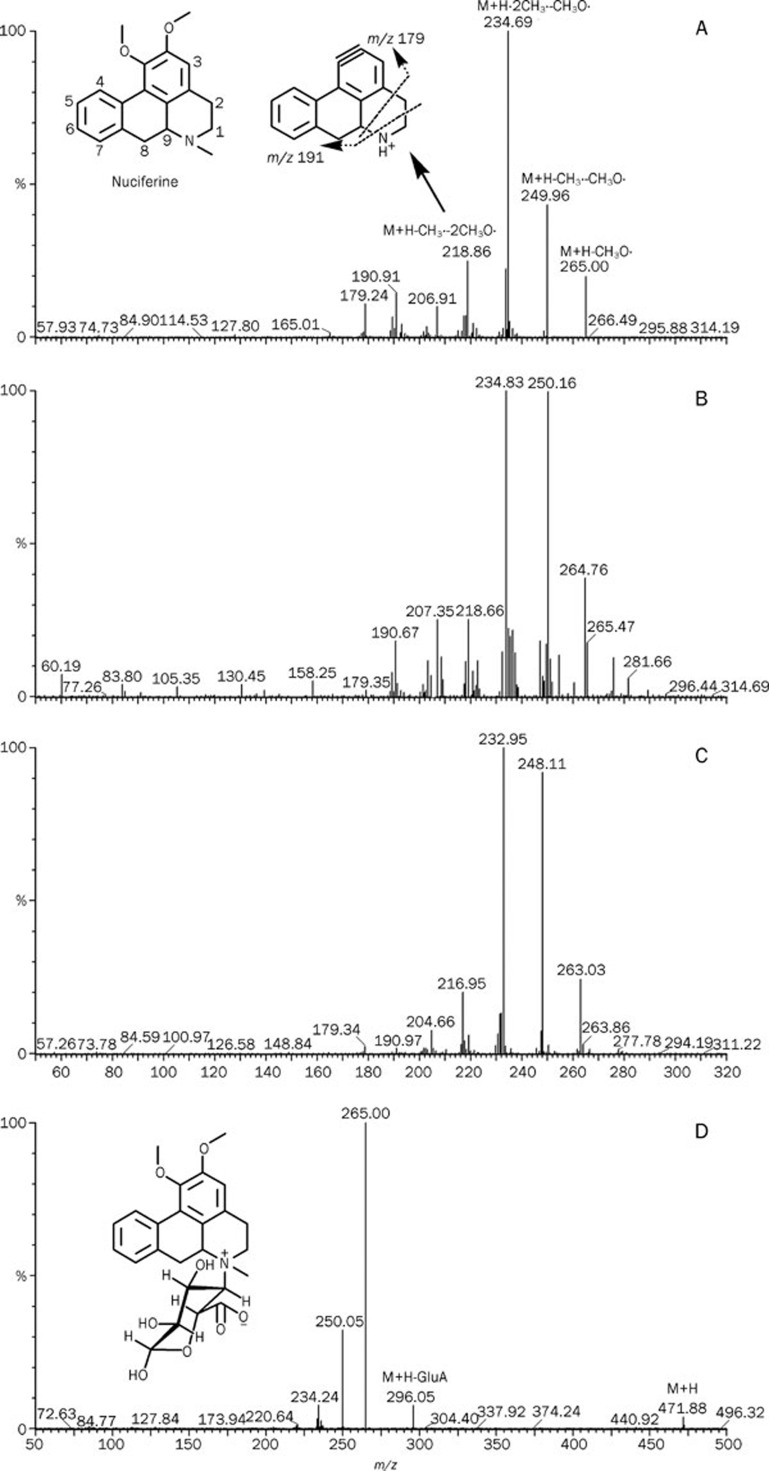
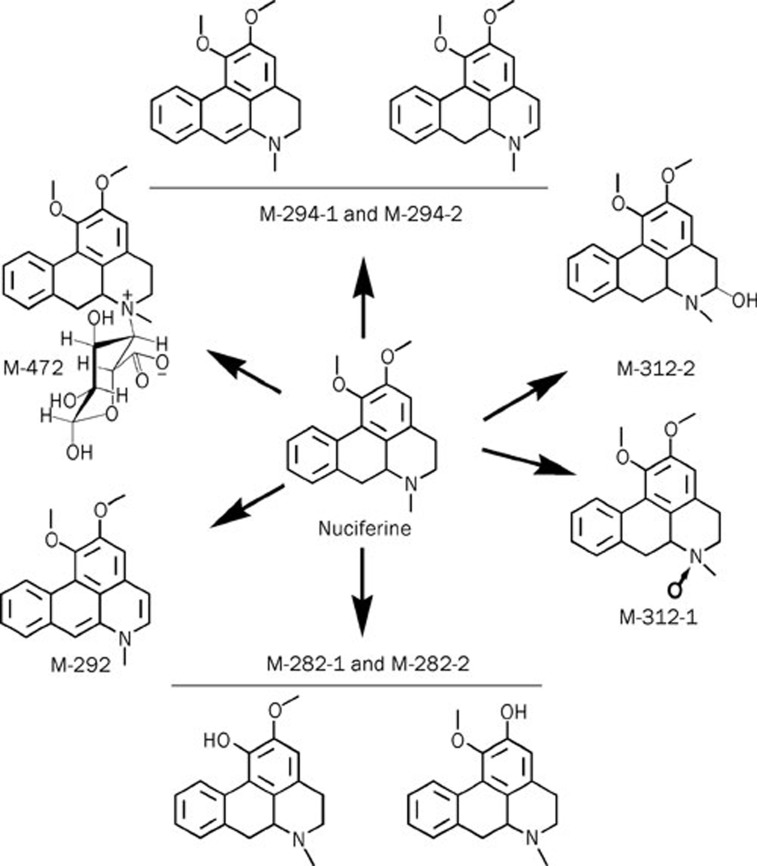
The molecular weight value indicated that M-312-1 and M-312-2 were probably hydroxylated nuciferine or nuciferine N-oxide. In the nuciferine molecule (Figure 2, A), C1 to C9 and the N-atom are the most likely sites for oxidation. The MS/MS spectrum showed that the fragment ions at m/z 179 and 191 were observed for nuciferine, M-312-1 and M-312-2, indicating that the hydroxylation sites were not C2 to C9. Hydroxylation increases water-solubility and thus shortens the retention time of a compound on a C18 column; however, M-312-1 was retained slightly longer than nuciferine, indicating that M-312-1 was not a hydroxylated product but an N-oxide (Figure 3). Thus, M-312-2 was a C1-hydroxylated product of nuciferine (Figure 3).
Figure 2.
MS/MS spectrum of nuciferine (A), M-312-1 (B), M-312-2 (C), and M-472 (D). GluA, glucuronic acid residue.
Figure 3.
Structures of nuciferine and its metabolites.
The molecular weight value indicated that M-282-1 and M-282-2 were demethylated products (Figure 3). However, the demethylated sites could not be distinguished between M-288-1 and M-288-2 based on the present data. The metabolites M-294-1 and M-294-2 had a molecular weight 2 Da less than that of nuciferine, indicating they were dehydrogenated products (Figure 3). In the nuciferine molecule, the likely dehydrogenation sites are C1-C2 and C8-C9. However, the dehydrogenation sites could not be distinguished between M-294-1 and M-294-2. The product M-292 had a molecular weight 4 Da smaller than that of nuciferine, indicating it was a di-dehydrogenation product. The dehydrogenation sites were both C1-C2 and C8-C9 as they were the only possible sites for dehydrogenation (Figure 3).
The metabolite M-472 was a unique UDPGA-dependent product of nuciferine. Both its molecular weight value and its MS/MS fragment ions indicated it was a glucuronidation product of nuciferine (Figure 2D). Because the N-atom in nuciferine was the only possible glucuronic acid conjugation site, M-472 was identified as nuciferine N-glucuronide (Figure 3).
Chemical inhibition of P450-mediated metabolism of nuciferine in HLM
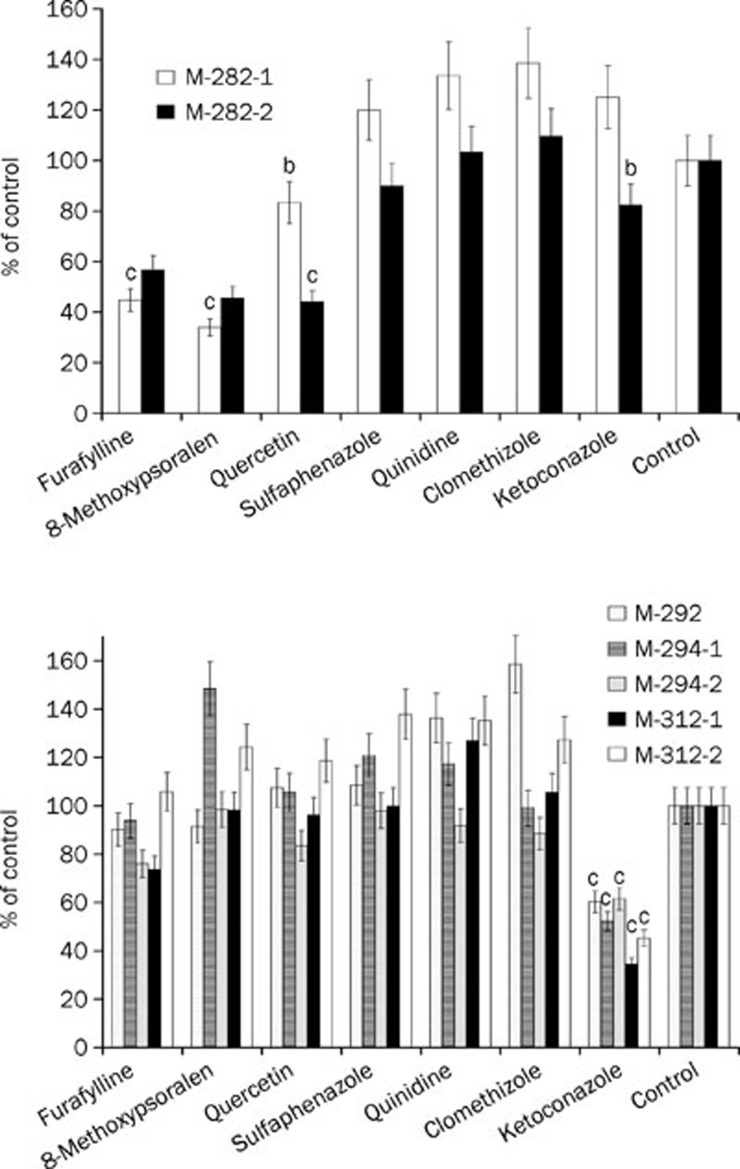
Furafylline, 8-methoxypsoralen, and quercetin significantly inhibited the formation of M-282-1 and M-282-2 (Figure 4A), indicating that CYP 1A2, 2A6, and 2C8 participated in the demethylation of nuciferine. As for the formation of M-282-1, quercetin inhibited it slightly, indicating that CYP 2C8 contributed less to its production than did CYP 1A2 and 2A6. Ketoconazole was the most effective and selective inhibitor of CYP 3A4. In the present study, the formation of M-312-1, M-312-2, M-294-1, M-294-2, and M-292 was significantly inhibited by ketoconazole (Figure 4B), indicating that CYP 3A4 participated in the N-oxidation, hydroxylation and dehydrogenation of nuciferine.
Figure 4.
Chemical inhibition of P450-catalyzed nuciferine metabolism by selective inhibitors of major P450 isozymes. The x-axis contains the names of the inhibitors, and the y-axis contains the percentage of metabolite formation compared with the control, which was normalized to 100%. In the control, the inhibitors were replaced by their corresponding solvents. Data are expressed as the mean and SD values. bP<0.05, cP<0.01 vs the control group.
Chemical inhibition of nuciferine N-glucuronidation in HLM
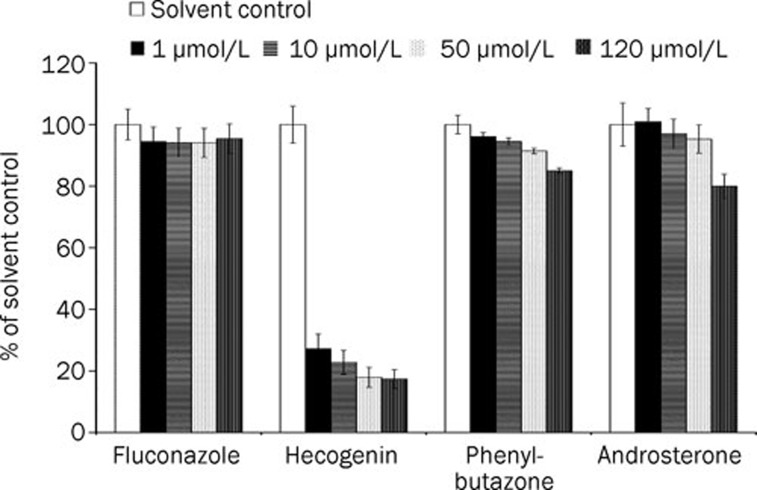
Four potent chemical inhibitors of major UGT isozymes including fluconazole, androsterone, phenylbutazone and hecogenin were tested for inhibition of nuciferine N-glucuronidation. Each drug was assayed at four concentrations: 1, 10, 50 and 200 μmol/L. As shown in Figure 5, hecogenin was the most potent and concentration-dependent inhibitor of nuciferine N-glucuronidation, reducing it more than 75% at 1 μmol/L. The IC50 for hecogenin inhibition of nuciferine N-glucuronidation was calculated to be 0.63±0.15 μmol/L. Fluconazole was an effective and selective inhibitor of UGT 2B7. In the present study, this inhibitor showed no inhibitory effect on nuciferine N-glucuronidation at concentrations up to 200 μmol/L, indicating that UGT 2B7 did not participate in this metabolism. Androsterone and phenylbutazone, two additional UGT inhibitors with a broad inhibitory effect on several UGT isozymes, did not significantly inhibit nuciferine N-glucuronidation. Together, the results showed that UGT 1A4 was the main enzyme involved in the N-glucuronidation of nuciferine.
Figure 5.
Chemical inhibition of nuciferine N-glucuronide formation by potent UGT inhibitors. The inhibitor concentrations are labeled in the figure. In the solvent control, the inhibitors were replaced by their corresponding solvents. Data are expressed as the mean and SD values. The IC50 value for hecogenin is 0.63±0.15 μmol/L.
Species differences of nuciferine metabolism in human and animal liver microsomes
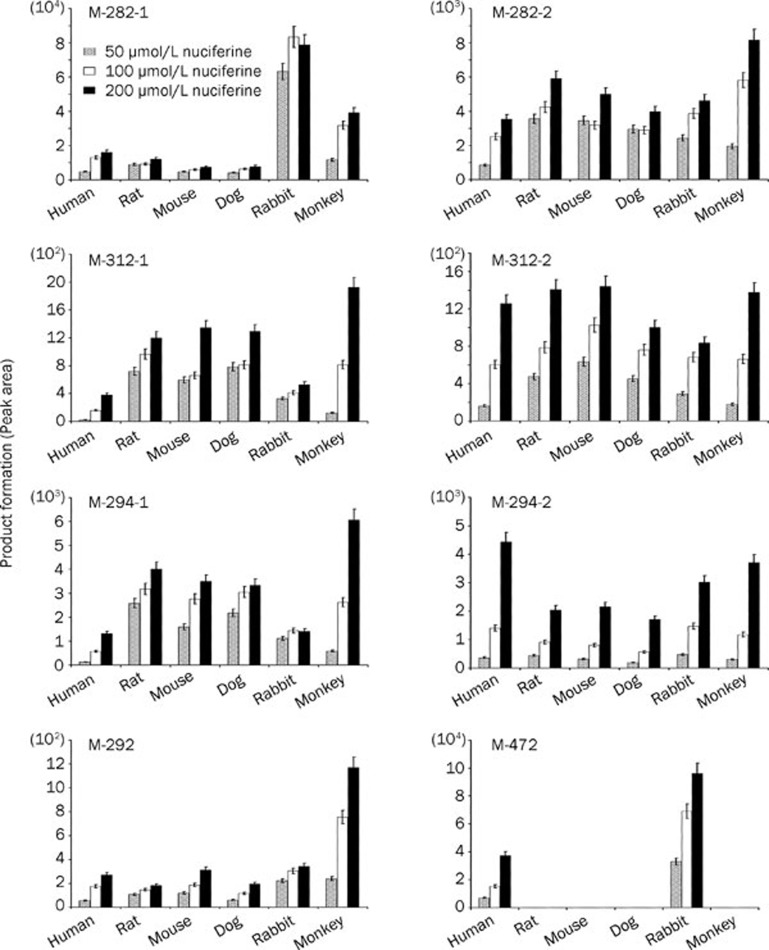
To investigate the species differences in nuciferine metabolism, nuciferine (at 50, 100, and 200 μmol/L) was incubated with human, rat, mouse, dog and rabbit liver microsomes in both the P450 and the UGT systems. In the P450 system, microsomes from all species generated the metabolites identified in the HLM incubation system described above (Figure 6). For most of the P450-catalyzed metabolites, microsomes from all species showed comparable levels. However, for the metabolite M-282-1, rabbit liver microsomes generated significantly higher production than did microsomes from humans, rats, mice and dogs. As concerns the N-glucuronidation product M-472, only human and rabbit liver microsomes generated that nuciferine metabolite.
Figure 6.
Species differences in the formation of P450- and UGT-catalyzed metabolites by microsomes from humans, rats, mice, dogs, rabbits and monkeys. Data are expressed as the mean and SD values.
Discussion
Identification of the structure of M-312-1 was based on its chromatographic characteristics. Usually, in the acidic mobile phase, the alkaloid compound would show high water solubility and would be eluted from the C18 column early. However, when the N-atom was blocked, the basic portion of the alkaloid could no longer accept a proton, leading to a longer retention time than that of the free alkaloid. This phenomenon has already been reported for other alkaloids18. Thus, based on its longer retention time (Figure 1) compared with nuciferine, M-312-1 was identified as nuciferine N-oxide. In addition, hydroxylation would increase the water-solubility of a compound, leading to a shorter retention time than the parent compound. Thus, M-312-2 (Figure 1) was reasoned to be a hydroxylated product of nuciferine. Theoretically, both the C1 (Figure 2A) and the N-methyl group of nuciferine could be hydroxylated; however, N-methyl hydroxylation would generate an extremely unstable product19. Because M-312-2 was stable for more than 6 days in our incubation system (data not shown), we reasoned that the metabolite was hydroxylated at C1.
In addition to the two selective inhibitors fluconazole (for UGT 2B7)15 and hecogenin (for UGT 1A4)17, the inhibitors androsterone and phenylbutazone were also used to inhibit nuciferine N-glucuronidation in the present study. Androsterone was a potent inhibitor of UGT 1A9, 2B7, and 2B1517. However, even at a high concentration, androsterone only slightly inhibited nuciferine glucuronidation. Thus, UGT 1A9, 2B7, and 2B15 are not likely to participate in glucuronidation of nuciferine. Phenylbutazone inhibited UGT 1A1, 1A3, 1A6, 1A7, 1A8, 1A9, 1A10, and 2B1517 significantly, but did not inhibit UGT 1A4, suggesting that nuciferine N-glucuronidation was not likely catalyzed by the phenylbutazone-sensitive isozymes. Combining these results, UGT 1A4 is the sole isozyme responsible for nuciferine N-glucuronidation, which is consistent with reports that UGT 1A4 is the most effective isozyme for N-glucuronidation of compounds with tertiary amine groups20. In addition, because UGT1A4 was shown to be deficient in human intestines20, 21, the liver is likely the predominant organ responsible for glucuronidation of nuciferine in humans.
Metabolism can alter the activity or even toxicity of drugs in vivo. To avoid failure in clinical studies, a suitable animal model with metabolic properties similar to those of humans should be useful. In vitro metabolism studies could also be used to help rapidly and conveniently screen animal species. Rats, mice and dogs are commonly used experimental animals, and their P450 enzyme systems are very similar to that of humans. Indeed, P450 enzymes are highly conserved in these mammals22. However, N-glucuronidation of tertiary amines, an important phase II metabolic process, is deficient in the aforementioned animal models23. Of the UGT isozymes, UGT 1A4 is known to have the greatest effect on N-glucuronidation of compounds with tertiary amine groups23, 24, 25 and the corresponding UGT 1A4 genes in rats, mice and dogs are pseudogenes20. Thus, microsomes from these animals did not catalyze the N-glucuronidation of nuciferine, as demonstrated in Figure 6. Among all of the animal species investigated in the present study, rabbits are the only species possessing the same nuciferine metabolic activity as humans. Thus, rabbits may be more suitable than rats, mice and dogs for further pharmacokinetic studies of nuciferine in vivo. However, potential differences in nuciferine absorption between humans and rabbits should be considered.
In the present study, two demethylated metabolites, two dehydrogenated products, a hydroxylated species, an N-oxide and a di-dehydrogenated derivative of nuciferine were observed in the HLM P450 system, and a unique N-glucuronidation product of nuciferine was observed in the HLM UGT system. The isozymes CYP 3A4, 1A2, 2A6, and 2C8 were responsible for the P450-mediated metabolism of nuciferine, and UGT 1A4 was the most effective isozyme involved in nuciferine N-glucuronidation. The species difference studies on the metabolism of nuciferine showed that rabbits, with the nuciferine metabolism most similar to that in humans, might be useful for advanced pharmacokinetic studies in vivo. Furthermore, the present study demonstrated that rats, mice and dogs are not suitable for such studies because they lack the important phase II metabolism of nuciferine that occurs in humans.
Author contribution
Yan-liu LU, Yu-qi HE and Guang JI designed the research. Yan-liu LU and Yu-qi HE performed the main experiments and wrote the paper. Miao WANG, Li ZHANG, and Chang-hong WANG prepared the animal liver microsomes, Li YANG participated in the UPLC-MS analysis of the metabolites, Zheng-tao WANG participated in the identification of the metabolite structures.
Acknowledgments
The authors are grateful for financial support from the National Natural Science Foundation of China (No 30873260), the Talents Scheme from the Science and Technology Commission of Shanghai Municipality (No 09XD1403800), the China Postdoctoral Science Foundation (No 20090460645), the Supporting Project for Elitists in the New Century from the Ministry of Education (No NCET-07563) and the Shanghai Leading Academic Discipline Project (No J50305 and E3008).
References
- Commission CP (China). Pharmacopoeia of the People's Republic of ChinaBeijing: People's Medical Publishing House; 2005
- Ho HH, Hsu LS, Chan KC, Chen HM, Wu CH, Wang CJ. Extract from the leaf of nucifera reduced the development of atherosclerosis via inhibition of vascular smooth muscle cell proliferation and migration. Food Chem Toxicol. 2010;48:159–68. doi: 10.1016/j.fct.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Ono Y, Hattori E, Fukaya Y, Imai S, Ohizumi Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J Ethnopharmacol. 2006;106:238–44. doi: 10.1016/j.jep.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Xu Q, Guo RX, Wang CY, Hu XY. Application of activated glassy carbon electrode for the detection of nuciferine in lotus leaves. Talanta. 2007;73:262–8. doi: 10.1016/j.talanta.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nature. 2006;6:947–60. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- Bachmann KA, Ghosh R. The use of in vitro methods to predict in vivo pharmacokinetics and drug interactions. Curr Drug Metab. 2001;2:299–314. doi: 10.2174/1389200013338504. [DOI] [PubMed] [Google Scholar]
- Howgate EM, Rowland Yeo K, Proctor NJ, Tucker GT, Rostami-Hodjegan A. Prediction of in vivo drug clearance from in vitro data. I: impact of inter-individual variability. Xenobiotica. 2006;36:473–97. doi: 10.1080/00498250600683197. [DOI] [PubMed] [Google Scholar]
- Obach RS, Walsky RL, Venkatakrishnan K, Gaman EA, Houston JB, Tremaine LM. The utility of in vitro cytochrome P450 inhibition data in the prediction of drug-drug interactions. J Pharmacol Exp Ther. 2006;316:336–48. doi: 10.1124/jpet.105.093229. [DOI] [PubMed] [Google Scholar]
- Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Peterkin TCGV, et al. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004;32:1201–8. doi: 10.1124/dmd.104.000794. [DOI] [PubMed] [Google Scholar]
- Walsky RL, Obach RS. Validated assays for human cytochrome p450 activities. Drug Metab Dispos. 2004;32:647–60. doi: 10.1124/dmd.32.6.647. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, et al. The conduct of in vitro and in vivo drug-drug interaction studies: A PhRMA perspective. Drug Metab Dispos. 2003;31:815–32. doi: 10.1124/dmd.31.7.815. [DOI] [PubMed] [Google Scholar]
- Huang SM, Temple R, Throckmorton DC, Lesko LJ. Drug interaction studies: study design, data analysis, and implications for dosing and labeling. Clin Pharmacol Ther. 2007;81:298–304. doi: 10.1038/sj.clpt.6100054. [DOI] [PubMed] [Google Scholar]
- Harris JW, Rahman A, Kim BR, Guengerich FP, Collins JM. Metabolism of taxol by human hepatic microsomes and liver slices: participation of cytochrome P450 3A4 and an unknown P450 enzyme. Cancer Res. 1994;54:4026–35. [PubMed] [Google Scholar]
- Liu HX, He YQ, Hu Y, Liu Y, Zhang JW, Li W, et al. Determination of UDP-glucuronosyltransferase UGT2B7 activity in human liver microsomes by ultra-performance liquid chromatography with MS detection. J Chromatogr B. 2008;870:84–90. doi: 10.1016/j.jchromb.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Liu HX, Liu Y, Zhang JW, Li W, Liu HT, Yang L. UDP-glucuronosyltransferase 1A6 Is the major isozyme responsible for protocatechuic aldehyde glucuronidation in human liver microsomes. Drug Metab Dispos. 2008;36:1562–9. doi: 10.1124/dmd.108.020560. [DOI] [PubMed] [Google Scholar]
- Uchaipichat V, Mackenzie PI, Elliot DJ, Miners JO. Selectivity of substrate (trifluoperazine) and inhibitor (amitriptyline, androsterone, canrenoic acid, hecogenin, phenylbutazone, quinidine, quinine, and sulfinpyrazone) «probes» for human udp-glucuronosyltransferases. Drug Metab Dispos. 2006;34:449–56. doi: 10.1124/dmd.105.007369. [DOI] [PubMed] [Google Scholar]
- Xiong A, Yang L, He Y, Zhang F, Wang J, Han H, et al. Identification of metabolites of adonifoline, a hepatotoxic pyrrolizidine alkaloid, by liquid chromatography/tandem and high-resolution mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:3907–16. doi: 10.1002/rcm.4329. [DOI] [PubMed] [Google Scholar]
- Fu PP, Xia Q, Lin G, Chou MW. Genotoxic pyrrolizidine alkaloids — mechanisms leading to DNA adduct formation and tumorigenicity. Int J Mol Sci. 2002;3:948–64. [Google Scholar]
- King CD, Rios GR, Green MD, Tephly TR. UDP-glucuronosyltransferases. Curr Drug Metab. 2000;1:143–61. doi: 10.2174/1389200003339171. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu W, Innocenti F, Ratain MJ. Searching for tissue-specific expression pattern-linked nucleotides of UGT1A isoforms. PLoS One. 2007;2:e396. doi: 10.1371/journal.pone.0000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler JA, Estabrook R, Murray M, Beaune P. Human cytochromes P450. Mol Aspects Med. 1999;20:1–137. [Google Scholar]
- Chiu SH, Huskey SW. Species differences in N-glucuronidation. Drug Metab Dispos. 1998;26:838–47. [PubMed] [Google Scholar]
- Green MD, Tephly TR. Glucuronidation of amines and hydroxylated xenobiotics and endobiotics catalyzed by expressed human UGT1.4 protein. Drug Metab Dispos. 1996;24:356–63. [PubMed] [Google Scholar]
- Kassahun K, Mattiuz E, Franklin R, Gillespie T. Olanzapine 10-N-glucuronide. A tertiary N-glucuronide unique to humans. Drug Metab Dispos. 1998;26:848–55. [PubMed] [Google Scholar]