Abstract
Aim:
To examine the effects of tanshinone IIA, the main effective component of Salvia miltiorrhiza (known as 'Danshen' in traditional Chinese medicine) on angiotensin II (Ang II)-mediated cardiomyocyte apoptosis.
Methods:
Rat neonatal cardiomyocytes were primarily cultured with Ang II or Ang II plus tanshinone IIA. Myocyte apoptosis was evaluated by caspase-3 activity and DNA strand break level with TdT-mediated dUTP nick-end labeling (TUNEL) staining. Western blot analysis was employed to determine the related protein expression and flow cytometry assay was used to determine the TUNEL positive cells and the intracellular reactive oxygen species (ROS) production. SiRNA targeted to Akt was used.
Results:
Ang II (0.1 μmol/L) remarkably increased caspase-3 activity, TUNEL positive cells, and cleaved caspase-3 and cytochrome c expression, but reduced Bcl-XL expression. These effects were effectively antagonized by pretreatment with tanshione IIA (1−3 μmol/L). Tanshinone IIA had no effect on basal ROS level, while attenuated the ROS production by Ang II. Interestingly, tanshione IIA significantly increased the phosphorylated Akt level, which was countered by the PI3K antagonist wortmannin or LY294002. Knockdown of Akt with Akt siRNA significantly reduced Akt protein levels and tanshinone IIA protective effect.
Conclusion:
Tanshinone IIA prevents Ang II-induced apoptosis, thereby suggesting that tanshinone IIA may be used for the prevention of the cardiac remodeling process.
Keywords: apoptosis, angiotensin II, tanshinone IIA, Akt, neonatal cardiomyocytes, caspase 3, reactive oxygen species
Introduction
Apoptosis or programmed cell death is thought to play a crucial role in a variety of pathological situations1. The importance of apoptosis in heart failure has been recognized for over a decade2. The chronic release of reactive oxygen species (ROS) has been recently linked to the development of left ventricular hypertrophy and progression of heart failure3. Activation of the local and systemic renin-angiotensin system is closely related to increased morbidity in heart failure4. Pharmacological blockade of the renin-angiotensin system is beneficial in patients with heart failure4. Experiments using cultured cardiomyocytes have demonstrated that apoptosis can be stimulated in vitro by angiotensin II (Ang II)5, 6. Ang II induces cardiomyocyte apoptosis, which contributes to heart failure possibly through enhanced ROS production6. Therefore, it is important to develop agents that inhibit cardiomyocyte apoptosis induced by Ang II and, as a result, improve cardiac dysfunction.
Tanshinone IIA extracted from Danshen, a popular medicinal herb used in traditional Chinese medicine, exhibits a variety of cardiovascular activities, including vasorelaxation, and cardioprotective effects7, 8, 9, 10. However, the pretreatment effects and mechanisms of tanshinone IIA on cardioprotection are not well understood. Akt is known to regulate many survival pathways in cardiac cells11 and has been reported to preserve cardiac function and prevent cardiac injury12. Therefore, the present study was set to evaluate the protective effect of tanshinone IIA on Ang II-induced cardiomyocyte apoptosis and to identify whether the underlying mechanisms are associated with the Akt-dependent pathway.
Materials and methods
Chemicals and reagents
Dulbecco's modified Eagle's medium (DMEM), fetal calf serum, and tissue culture reagents were purchased from Invitrogen Corporation (Carlsbad, CA, USA). 5(6)-carboxy-2′ 7′-dichlorofluorescein diacetate (DCFH-DA) was from Molecular Probes Inc (Eugene, OR, USA). All other chemicals of reagent grade were obtained from Sigma-Aldrich Chemical Co (St Louis, MO, USA). Antibodies were purchased from Lab Frontier Co Ltd, Seoul, Korea (anti-GAPDH), Cell Signaling Technology, Inc, Danvers, MA, USA (anti-caspase-3, anti-Ser473 phospho-Akt, anti-Akt), and Santa Cruz Biotechnology, Santa Cruz, CA, USA (anti-cytochrome c, anti-Bcl-xL). Tanshinone IIA (purchased from Santa Cruz Biotechnology) was dissolved in dimethyl sulfoxide (DMSO), and the DMSO content in all groups was 0.1%.
Cell culture
Primary cultures of neonatal rat cardiomyocytes were prepared as previously described13. The study was conducted in accordance with the Declaration of Helsinki and/or with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the United States National Institutes of Health and was approved by the Institutional Animal Care and Use Committee of China Medical University (LAC-94-0069). The purity of the obtained myocyte cultures (>95%) was determined by immunofluorescence microscopy. We counted all nuclei stained by 4′,6-diamidino-2-phenyindole (DAPI) (Sigma-Aldrich) and all cells that stained positive for α-actinin (Sigma-Aldrich). The culture medium was replaced after 24 h with serum-free medium consisting of DMEM (10 μg/mL), insulin (10 μg/mL), and BrdU (0.1 mmol/L) and exposed to the agents as indicated.
Caspase-3 activity assay
For the caspase-3 activity assay, the caspase-3 substrate rhodamine-110 (Z-DEVD-R110) was used as a prefluorescent substrate. The activity of caspase-3 was determined using a commercially available kit (Promega; Madison, WI, USA) according to the manufacturer's instructions. Briefly, after 48-h treatments with Ang II, tanshinone IIA, Ang II+tanshinone IIA, or vehicle, the caspase-3 reagent was added and incubated for 10 h. Levels of release of rhodamine-110 were measured with a luminescence spectrometer LS55 (Perkin-Elmer, Waltham, MA, USA) at an excitation wavelength of 499 nm and an emission wavelength of 521 nm.
TUNEL assay
Ang II-mediated apoptosis in cardiomyocytes was detected with enzymatic labeling of DNA strand breaks, which were identified with terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) stain. TUNEL staining was performed with a Cell Death Detection kit (Roche, Mannheim, Germany) according to the manufacturer's directions. The apoptotic ratio was measured by flow cytometry according to the manufacturer's instructions.
Western blot analysis
Western blot analysis was performed as previously described14. Membranes were blocked in 10 mmol/L Tris (pH 7.5), 100 mmol/L NaCl, and 0.1% Tween 20 containing 5% nonfat dry milk, followed by incubation with the primary antibody. Membranes were washed three times and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (1:5000 dilutions), and enhanced chemiluminescence (Amersham Biosciences Corp, NJ, USA), and bands were quantified with densitometry.
Flow cytometric assay of 2′,7′-dichlorodihydrofluorescein oxidation
The determination of intracellular ROS production was based on the oxidation of 2′,7′-dichlorodihydrofluorescein (DCFH) to fluorescent 2′,7′-dichlorofluorescein (DCF), as described previously15. DCFH was added to the cells at a final concentration of 10 μmol/L and incubated for 30 min at 37 °C. The cells were then washed once with PBS and maintained in 1-mL culture medium. Following drug treatment, the medium was aspirated and the cells were washed twice with PBS, and then dissociated with trypsin. Cellular fluorescence was determined by flow cytometry (FACS-SCAN, Becton-Dickinson, Franklin Lakes, NJ, USA). Cells were excited with an argon laser at 488 nm, and measurements were taken at 510–540 nm.
Short interfering RNA (siRNA) transfection
Akt siRNAs were purchased from Santa Cruz Biotechnology. Akt siRNAs and mock control oligonucleotides were transfected using the Lipofectamine (Invitrogen) reagent according to the manufacturer's instructions. The final concentration of the Akt siRNAs for transfection was 100 nmol/L. Transfected cells were washed with PBS, and then incubated in new culture media for an additional 48 h for Ang II treatment and Western blot assays.
Statistical analysis
Results are expressed as mean±SEM. Statistical analysis was performed using Student's t test or analysis of variance (ANOVA) with the Prism version 3.00 for Windows (GraphPad Software, San Diego, CA, USA). A value of P<0.05 was considered to be statistically significant.
Results
The effect of tanshinone IIA on Ang II-induced cardiomyocyte apoptosis
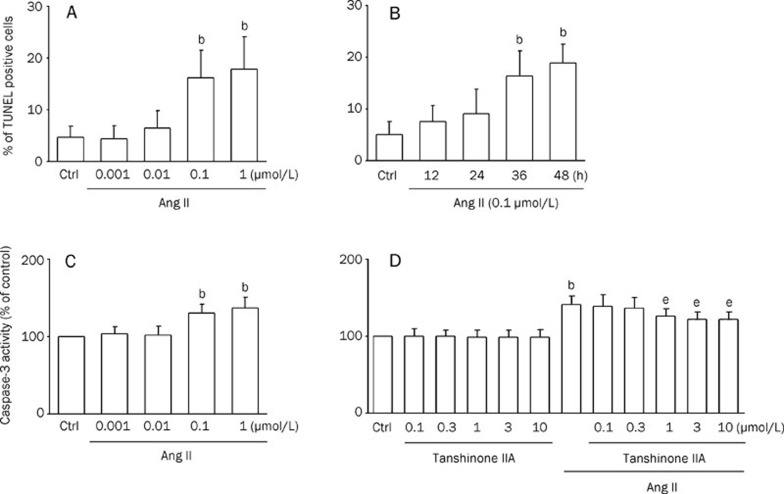
Using TUNEL staining with flow cytometry, we observed the effects of Ang II at a series of concentrations (0.001, 0.01, 0.1, and 1 μmol/L). Ang II at 0.1–1 μmol/L appeared to have the strongest action (Figure 1A). We also observed the effects of Ang II (0.1 μmol/L) at different incubation times (12, 24, 36, and 48 h) and found that Ang II exerted the most significant actions in inducing apoptotic cell death at an incubation time of 48 h (Figure 1B). Therefore, we present the data obtained from 0.1 μmol/L Ang II and 48 h of incubation in this study.
Figure 1.
Caspase-3 activity in cardiomyocytes is inhibited by tanshinone IIA. Results were shown as mean±SEM. bP<0.05 vs control (Ctrl); eP<0.05 vs Ang II. (A) Bar graph showing the percentage of cardiomyocytes undergoing apoptosis in the presence of Ang II (0.001, 0.01, 0.1, and 1 μmol/L) for 48 h. TdT-mediated dUTP nick-end labeling (TUNEL) analysis was performed as described in Materials and methods. (n=5) (B) Bar graph showing the percentage of cardiomyocytes undergoing apoptosis in the presence of Ang II (0.1 μmol/L) at different incubation times (12, 24, 36, and 48 h). (n=4) (C) Effects of angiotensin II (Ang II) (0.001, 0.01, 0.1, and 1 μmol/L) on caspase-3 activity in cardiomyocytes. Caspase-3 activity was measured in lysates prepared from cardiomyocytes. (n=4) (D) Cardiomyocytes pretreated with tanshinone IIA (0.1, 0.3, 1, 3, and 10 μmol/L; for 30 min) in the absence or presence of 0.1 μmol/L Ang II for 48 h. Bars indicate the intensity of R110 from 6 independent experiments, each in triplicate measurements. (n=8)
Recent work has supported a central role for members of the caspase family, especially caspase-3, as effectors of apoptosis16. To examine whether tanshinone IIA attenuates apoptosis induced by Ang II, we measured the caspase-3 activity in cells pretreated with tanshinone IIA. As shown in Figure 1C, the caspase-3 activity in Ang II-treated cells (0.1 and 1 μmol/L; 48 h) was significantly increased compared with vehicle-treated cells. Cardiomyocytes pretreated with tanshinone IIA (1, 3, and 10 μmol/L) for 30 min, followed by 0.1 μmol/L Ang II for 48 h, significantly inhibited the activation of caspase-3 by Ang II (Figure 1D).
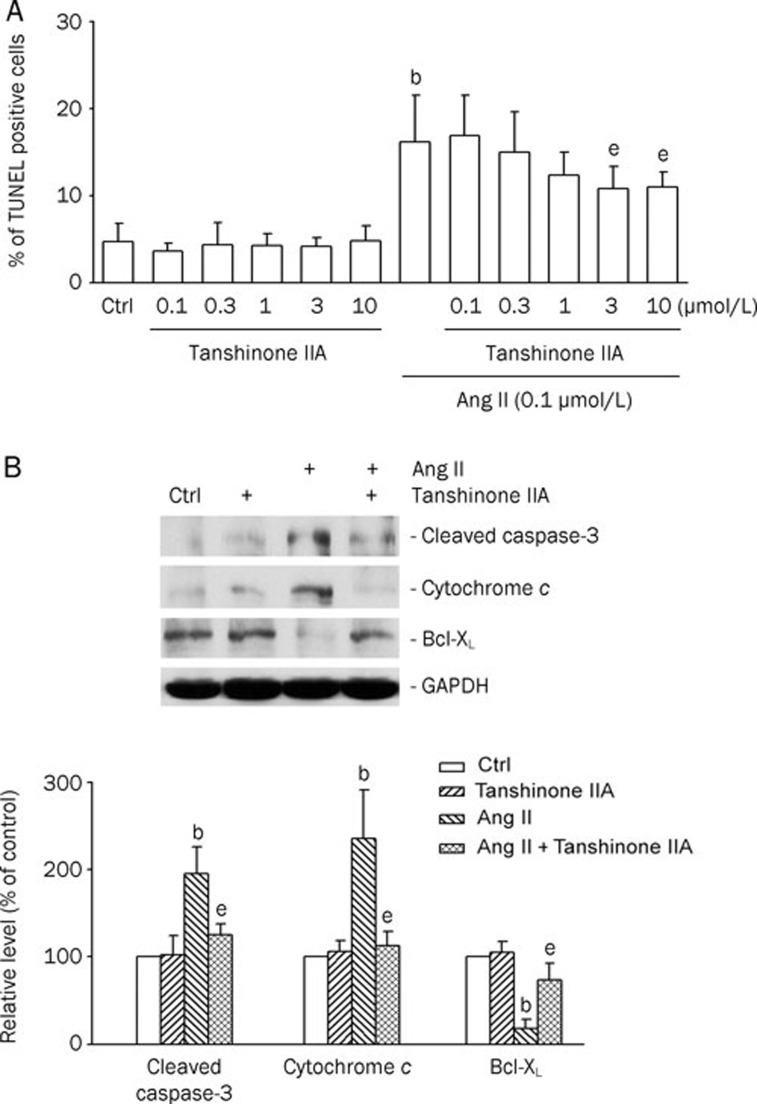
The protective effect of tanshinone IIA against Ang II-induced apoptosis in cardiomyocytes was further examined using TUNEL staining with flow cytometry (Figure 2A). Cardiomyocytes were pretreated with tanshinone IIA for 30 min, followed by 0.1 μmol/L Ang II for 48 h. Treatment with Ang II (0.1 μmol/L) for 48 h increased the percentage of apoptotic cells (Figure 2A). Tanshinone IIA treatment alone did not affect normal cell survival. In contrast, the pretreatment with tanshinone IIA (3, and 10 μmol/L) markedly decreased the number of apoptotic cells induced by Ang II (Figure 2A). The influence of tanshinone IIA on apoptotic markers, such as cleaved caspase, cytochrome c, and Bcl-xL, was further evaluated by Western blot analysis (Figure 2B). As shown in Figure 2B, the levels of cleaved caspase-3 and cytosolic cytochrome c were greatly elevated in the cells treated with 0.1 μmol/L Ang II for 12 h. Pretreatment with tanshinone IIA at 3 μmol/L significantly reduced the amount of cleaved caspase-3 and cytosolic cytochrome c, as compared with that in Ang II-treated alone cells. In contrast, the expression of Bcl-xL was reduced by Ang II treatment, which was also recovered by tanshinone IIA pretreatment. These results indicate that the pretreatment with tanshinone IIA inhibited Ang II-induced cardiomyocyte apoptosis.
Figure 2.
Tanshinone IIA protected cardiomyocytes from angiotensin II (Ang II)-induced apoptosis. (A) Flow cytometric analysis of TdT-mediated dUTP nick-end labeling (TUNEL)-stained cells. Cardiomyocytes pretreated with tanshinone IIA (0.1, 0.3, 1, 3, and 10 μmol/L; for 30 min) in the absence or presence of 0.1 μmol/L Ang II for 48 h. Percentages of apoptotic cardiomyocytes in the different groups. Results were shown as mean±SEM. (n=6). bP<0.05 vs control (Ctrl); eP<0.05 vs Ang II. (B) Effects of tanshinone IIA on apoptotic markers (cleaved caspase-3, released cytochrome c, and BcL-xL) in Ang II-treated cardiomyocytes. The cells were pretreated with tanshinone IIA (3 μmol/L) for 30 min, and then treated with 0.1 μmol/L Ang II for 12 h. Upper panels: Western blotting was carried out with the specific antibody against cleaved caspase-3, cytochrome c, and Bcl-xL. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading control. Lower panels: The quantitative results from the Western blots. Results were shown as mean±SEM. (n=4). bP<0.05 vs control (Ctrl); eP<0.05 vs Ang II.
The influence of tanshinone IIA on Ang II-induced ROS generation in cardiomyocytes
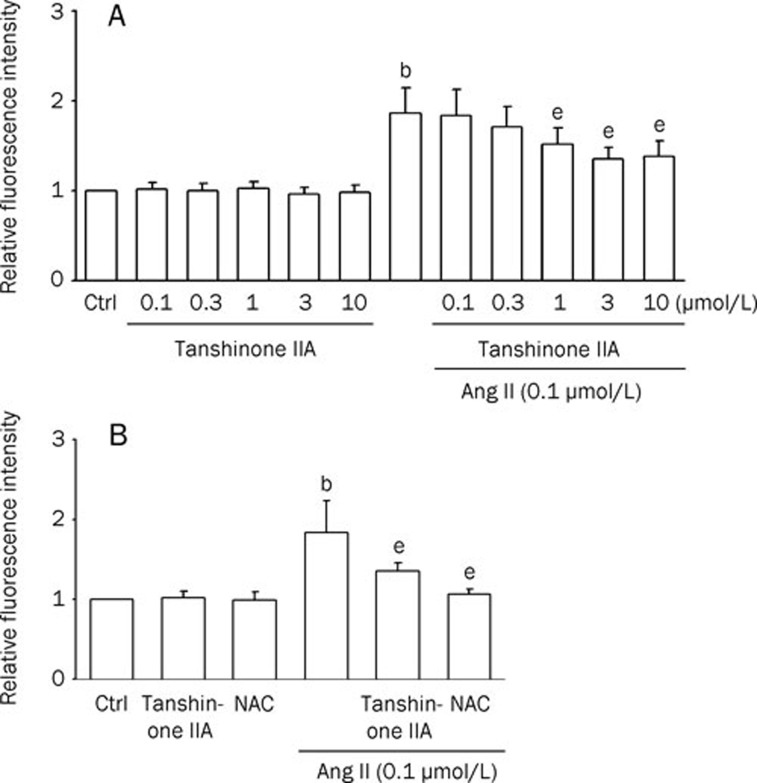
To evaluate the mechanism of the protective effect of tanshinone IIA on Ang II-induced apoptosis, the influence of tanshinone IIA on Ang II-induced ROS generation was monitored. We examined whether tanshinone IIA prevents Ang II-induced ROS formation. Tanshinone IIA-pretreated cells were then treated with 0.1 μmol/L Ang II for 1 h. The Ang II-induced increases in intracellular ROS were revealed by measuring the fluorescent intensities of DCF. As shown in Figure 3A and 3B, pretreatment with tanshinone IIA or the ROS scavenger N-acetylcysteine (NAC; 5 mmol/L) significantly inhibited Ang II-induced ROS production. These results indicate that the pretreatment with tanshinone IIA inhibited Ang II-induced ROS production in cardiomyocytes.
Figure 3.
Effects of tanshinone IIA on angiotensin II (Ang II)-induced ROS generation in cardiomyocytes. Relative fluorescence intensity in rat cardiomyocytes was quantified by flow cytometry using dichlorofluorescin diacetate (DCFH-DA). The fluorescence intensities in untreated control cells are expressed as 100%. Data were presented as relative intensity of the experimental groups compared to untreated control cells. Results were shown as mean±SEM. (n=6). bP<0.05 vs control (Ctrl); eP<0.05 vs Ang II. (A) Column bar graph of mean cell fluorescence for dichlorofluorescein (DCF) evaluated for cardiomyocytes pretreated with tanshinone IIA (0.1, 0.3, 1, 3, and 10 μmol/L; for 30 min) and thereafter in the absence or presence of 0.1 μmol/L Ang II for 1 h. (B) Cells were incubated with tanshinone IIA (3 μmol/L) or N-acetylcysteine (NAC) (5 mmol/L) and thereafter in the absence or presence of 0.1 μmol/L Ang II for 1 h.
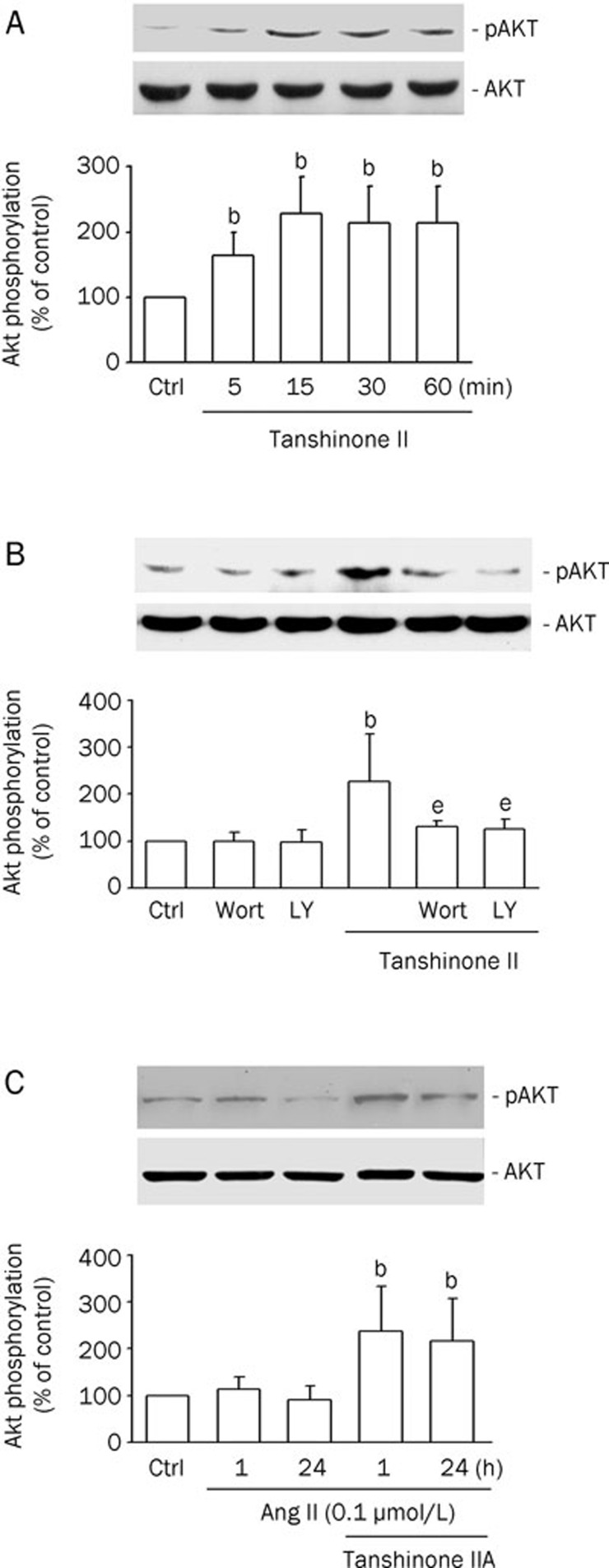
Effects of tanshinone IIA on phospho-Akt in cardiomyocytes
Akt is known to have an inhibitory effect on apoptosis in several cell types including cardiomyocytes12. To determine the effects of tanshinone IIA on Akt phosphorylation in rat cardiomyocytes, the amount of phospho-Akt (for serine 473) was measured. As shown in Figure 4A, tanshinone IIA (3 μmol/L) increased the serine phosphorylation of Akt from 5 to 60 min in cardiomyocytes. Since Akt is one of the downstream effectors of PI3K, we next examined the effects of PI3K inhibitors on Akt phosphorylation. Pretreatment with the PI3K inhibitors wortmannin (Wort; 100 nmol/L) and LY294002 (LY; 10 nmol/L) inhibited the tanshinone IIA-increased Akt phosphorylation (Figure 4B). These findings indicate that tanshinone IIA induces Akt phosphorylation via the PI3K/Akt pathway. To confirm the involvement of Akt signaling pathway in tanshinone IIA survival actions, we next determined whether tanshinone IIA modified their degree of phosphorylation/activation in our experimental conditions. Figure 4C illustrates that incubation with tanshinone IIA enhanced Akt phosphorylation in cultures treated with Ang II. Compared with control cultures, Ang II alone did not modify Akt phosphorylation.
Figure 4.
Tanshinone IIA induces Akt phosphorylation via PI3K. (A) Neonatal cardiomyocytes were incubated with 3 μmol/L tanshinone IIA for the indicated times. (B) Neonatal cardiomyocytes were incubated with wortmannin (Wort; 100 nmol/L) or LY294002 (LY; 10 nmol/L) for 30 min followed by incubation with 3 μmol/L tanshinone IIA for 15 min. (C) Neonatal cardiomyocytes were incubated with tanshinone IIA (3 μmol/L) for 30 min prior to the addition of Ang II (0.1 μmol/L) for 1 or 24 h. Western blot analyses were performed using site- and phospho-specific Akt antibodies against Ser473 (p-Akt, upper blot) or total Akt (lower blot). The results were shown as means±SEM (n=4), expressed as percentage changes in phosphorylation over that in control cells. bP<0.05 vs control (Ctrl). eP<0.05 vs tanshinone IIA.
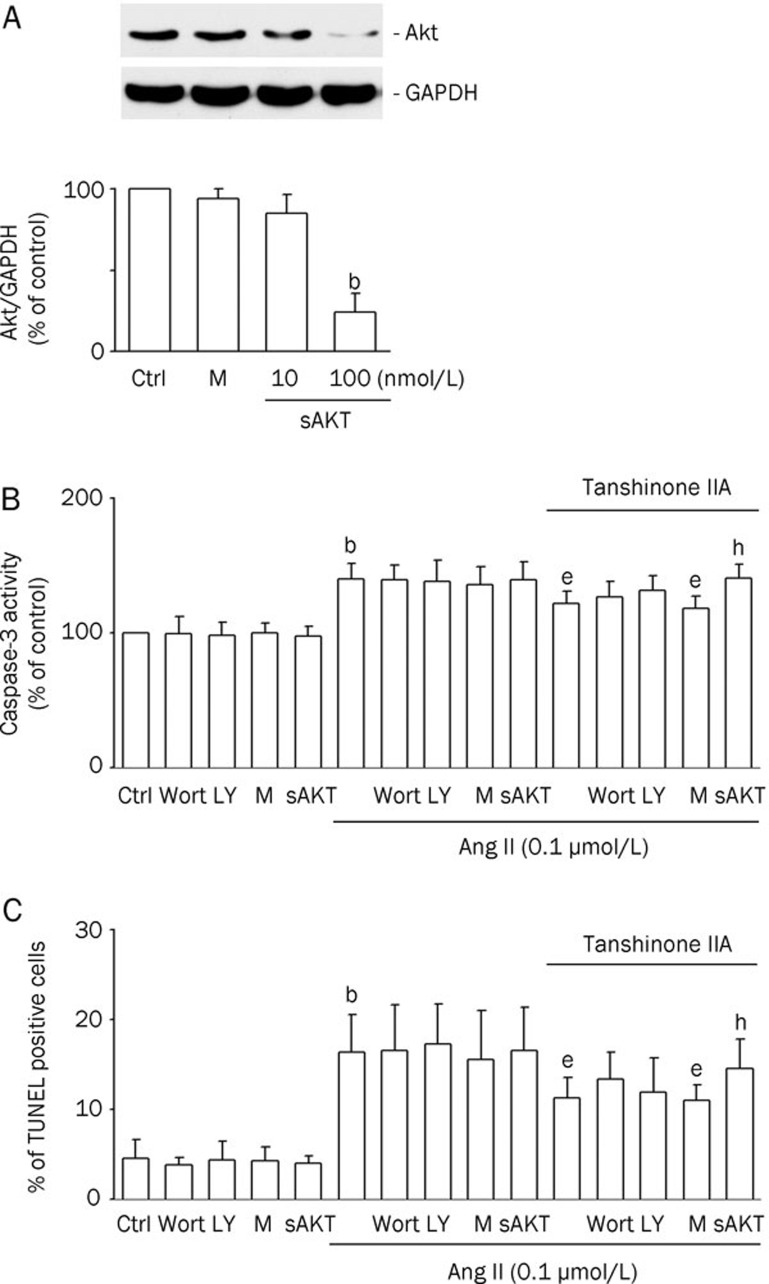
Role of Akt in the protective effect of tanshinone IIA on Ang II-induced cardiomyocyte apoptosis
To identify the signaling pathways involved in the effect of tanshinone IIA, Akt siRNA, which mitigates the kinase activity of Akt, was transfected into cardiomyocytes. The Akt protein levels were noticeably reduced by Akt siRNA transfection (Figure 5A). The inhibitory effect of tanshinone IIA on the Ang II-induced caspase-3 activation was partially reversed by Wort (100 nmol/L), LY (10 nmol/L), and Akt siRNA (Figure 5B). Similarly, the inhibitory effect of tanshinone IIA on Ang II-induced cardiomyocyte apoptosis was reduced by Wort (100 nmol/L), LY (10 nmol/L), and Akt siRNA (Figure 5C). These results revealed the involvement of the Akt signaling pathway in tanshinone IIA's effect on Ang II-induced cardiomyocyte apoptosis.
Figure 5.
Blockage of the Akt pathway attenuated the inhibitory effect of tanshinone IIA on angiotensin II (Ang II)-induced apoptosis. Notes: Ctrl, untransfected control; M, mock control; sAkt, Akt siRNA transfection. bP<0.05 vs the mock control. eP<0.05 vs the Ang II treatment. hP<0.05 vs the tanshinone IIA and Ang II treatment. (A) The effect of Akt siRNA transfection on Akt protein levels in cardiomyocytes. The cells were transfected with Akt siRNA (sAkt; 10, or 100 nmol/L) to get Akt knockdown cells. Control siRNA was also applied as mock controls (M). Western blotting was carried out with the specific antibody against Akt. GAPDH was used as a loading control. Results were shown as mean±SEM (n=3). (B) The effect of wortmannin (Wort), LY294002 (LY), and Akt siRNA on tanshinone IIA-decreased Ang II-induced caspase-3 activity in cardiomyocytes. Transfected cells were pretreated with or without tanshinone IIA (3 μmol/L) for 30 min, and then treated with Ang II (0.1 μmol/L) for 12 h. Results were shown as mean±SEM (n=6). (C) The effect of Wort, LY, and Akt siRNA on tanshinone IIA-decreased Ang II-induced apoptosis in cardiomyocytes. Transfected cells were pretreated with or without tanshinone IIA (3 μmol/L) for 30 min, and then treated with Ang II (0.1 μmol/L) for 48 h. Results were shown as mean±SEM (n=6).
Discussion
The results of this study indicate for the first time that apoptosis in cardiomyocytes induced by Ang II can be considerably reduced (but not totally prevented) by tanshinone IIA. The mechanism involves the inhibition of apoptosis-related increase of ROS, activation of caspase-3, release of cytochrome c, and increased expression of Bcl-xL. We also found that tanshinone IIA upregulated Akt phosphorylation, an interesting self-gain signaling that may possibly enhance the effect of tanshinone IIA. The causal relationship between upregulated Akt phosphorylation and tanshinone IIA action, however, needs further investigations.
The results of our study demonstrated that Ang II caused cardiomyocyte apoptosis; this finding is consistent with that of previous studies and shows that Ang II acts as an efficient inducer of apoptosis in adult and neonatal cardiomyocytes5, 6. A statistically significant reduction of TUNEL-positive cardiomyocytes was observed when tanshinone IIA was added to Ang-II treated cells. Bcl-xL plays important roles in apoptotic cell death, whereas caspase-3 is a key downstream effector of apoptosis. To investigate the underlying mechanism(s) of the antiapoptotic effect of tanshinone IIA, we examined the expression of Bcl-xL and caspase-3. The results showed that tanshinone IIA increased the expression of Bcl-xL. We also found that the caspase-3 activity of myocardial cells was significantly increased when cells were treated with Ang II and that tanshinone IIA greatly reduced this activation. The expression of Bcl-xL and caspase-3 was consistent with the results obtained by TUNEL staining with flow cytometry.
Tanshinone IIA is the main effective component of Salvia miltiorrhiza known as 'Danshen' in traditional Chinese medicine. Clinical evidence has shown that tanshinone IIA increases coronary blood flow and protects the heart against cardiac injury17. On the basis of the cardioprotective action of tanshinone IIA, we investigated the hypothesis that tanshinone IIA may prevent the death of cardiomyocytes. Cardiomyocyte apoptosis is one of the major pathogenic mechanisms underlying myocardial injury. Blocking the apoptotic process could prevent the loss of contractile cells and minimize cardiac injury induced by injury, thereby slowing down or even preventing the occurrence of heart failure18. Therefore, we performed TUNEL staining in order to explore the underlying mechanism responsible for the improvement of the cardiac function induced by tanshinone IIA. The results indicated that tanshinone IIA inhibited cardiomyocyte apoptosis induced by Ang II; this finding was similar to that of previous studies, which reported that tanshinone IIA protected cardiomyocytes against oxidative stress-triggered damage and apoptosis8, 18. The possible mechanisms, which have been proposed for explaining the protective effects of tanshinone IIA, include antioxidant properties involving scavenging of free radicals in cardiomyocytes7. In addition, Akt, a serine/threonine kinase, is a primary mediator of the downstream effects of PI3K, which coordinates a variety of intracellular signals and regulates cell proliferation and survival. Recent studies have also shown that activation of the PI3K/Akt signaling pathway protects the myocardium from myocardial injury and prevents cardiomyocyte apoptosis12. In order to explore whether the protective effects of tanshinone IIA are associated with the Akt pathway, Akt siRNA was employed to compare the effects of co-administration of Akt siRNA and tanshinone IIA with the effects of administration of tanshinone IIA alone. Pretreatment with PI3K inhibitors (Wort and LY) or transfection with Akt siRNA abolished the cardioprotective effects of tanshinone IIA. These results suggest that tanshinone IIA induces cardioprotective effects through the activation of the Akt-pathway. The results of this study suggest that tanshinone IIA may offer a practicable approach to reduce apoptosis of cardiomyocytes and may merit further investigation. The present study strongly demonstrated that tanshinone IIA protects neonatal cardiomyocytes from Ang II-induced apoptosis. Tanshinone IIA might potentially be used to treat heart failure or other apoptosis-related heart diseases if further studies were performed to define and clarify the rationale for its clinical use.
Author contribution
Tzu-hurng CHENG and Paul CHAN designed the study and wrote the paper; Hong-jye HONG and Ju-chi LIU performed the study.
Acknowledgments
This work was supported by a grant to Paul CHAN from the National Science Council, Taiwan, China (NSC 96-2320-B-038-016-MY3) and a grant to Tzu-hurng CHENG from the China Medical University (CMU-98-S-22), Taichung, Taiwan, China.
References
- Masri C, Chandrashekhar Y. Apoptosis: a potentially reversible, meta-stable state of the heart. Heart Fail Rev. 2008;13:175–9. doi: 10.1007/s10741-007-9069-3. [DOI] [PubMed] [Google Scholar]
- Satoh M, Matter CM, Ogita H, Takeshita K, Wang CY, Dorn GW, 2nd, et al. Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation. 2007;115:3197–204. doi: 10.1161/CIRCULATIONAHA.106.657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–40. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Schroder D, Heger J, Piper HM, Euler G. Angiotensin II stimulates apoptosis via TGF-beta1 signaling in ventricular cardiomyocytes of rat. J Mol Med. 2006;84:975–83. doi: 10.1007/s00109-006-0090-0. [DOI] [PubMed] [Google Scholar]
- Qin F, Patel R, Yan C, Liu W. NADPH oxidase is involved in angiotensin II-induced apoptosis in H9C2 cardiac muscle cells: effects of apocynin. Free Radic Biol Med. 2006;40:236–46. doi: 10.1016/j.freeradbiomed.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–59. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- Gao J, Yang G, Pi R, Li R, Wang P, Zhang H, et al. Tanshinone IIA protects neonatal rat cardiomyocytes from adriamycin-induced apoptosis. Transl Res. 2008;151:79–87. doi: 10.1016/j.trsl.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Sun DD, Wang HC, Wang XB, Luo Y, Jin ZX, Li ZC, et al. Tanshinone IIA: a new activator of human cardiac KCNQ1/KCNE1 IKs potassium channels. Eur J Pharmacol. 2008;590:317–21. doi: 10.1016/j.ejphar.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Xu W, Yang J, Wu LM. Cardioprotective effects of tanshinone IIA on myocardial ischemia injury in rats. Pharmazie. 2009;64:332–6. [PubMed] [Google Scholar]
- Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, et al. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res. 2004;94:884–91. doi: 10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]
- Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–5. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- Cheng TH, Shih NL, Chen SY, Wang DL, Chen JJ. Reactive oxygen species modulate endothelin-I-induced c-fos gene expression in cardiomyocytes. Cardiovasc Res. 1999;41:654–62. doi: 10.1016/s0008-6363(98)00275-2. [DOI] [PubMed] [Google Scholar]
- Chao HH, Liu JC, Hong HJ, Lin JW, Chen CH, Cheng TH.L-carnitine reduces doxorubicin-induced apoptosis through a prostacyclin-mediated pathway in neonatal rat cardiomyocytes Int J Cardiol 2009. doi: 10.1016/j.ijcard.2009.06.010 [DOI] [PubMed]
- Chen YL, Liu JC, Loh SH, Chen CH, Hong CY, Chen JJ, et al. Involvement of reactive oxygen species in urotensin II-induced proliferation of cardiac fibroblasts. Eur J Pharmacol. 2008;593:24–9. doi: 10.1016/j.ejphar.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–31. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Jin UH, Suh SJ, Chang HW, Son JK, Lee SH, Son KH, et al. Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J Cell Biochem. 2008;104:15–26. doi: 10.1002/jcb.21599. [DOI] [PubMed] [Google Scholar]
- Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol. 2007;568:213–21. doi: 10.1016/j.ejphar.2007.04.031. [DOI] [PubMed] [Google Scholar]